Investigating Path Integration Cues in Sand Scorpion Homing Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Collection Details, Storage, and Maintenance
2.2. Pilot Studies
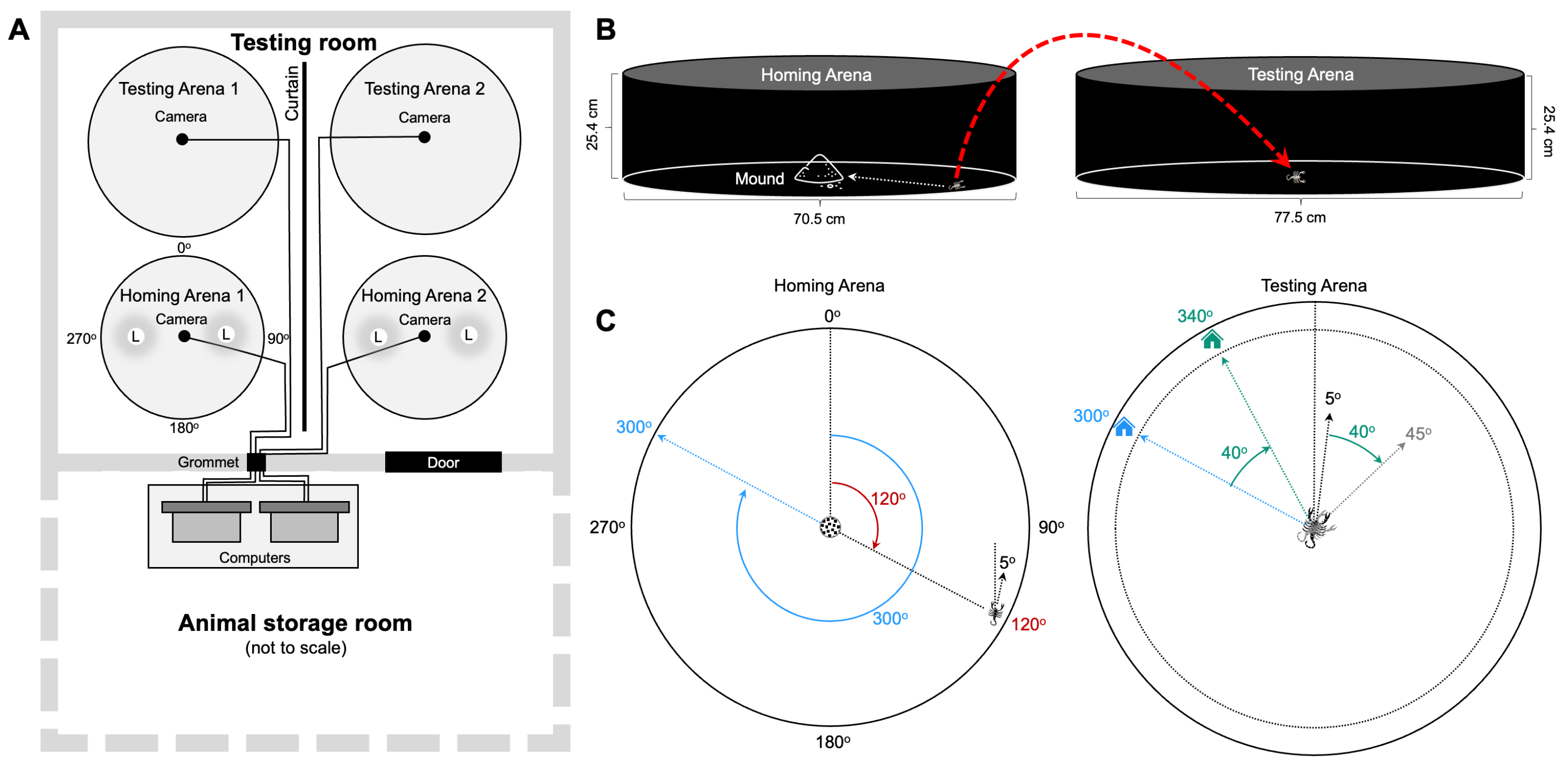
2.3. Experimental Room Setup
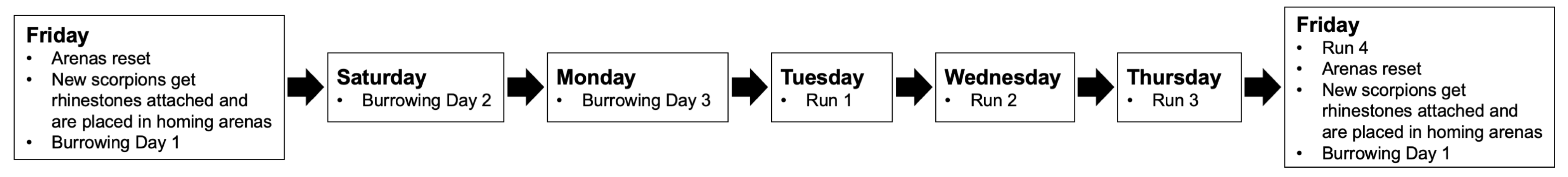
2.4. Procedure
2.5. Video Recording, Data Processing, and Calculations
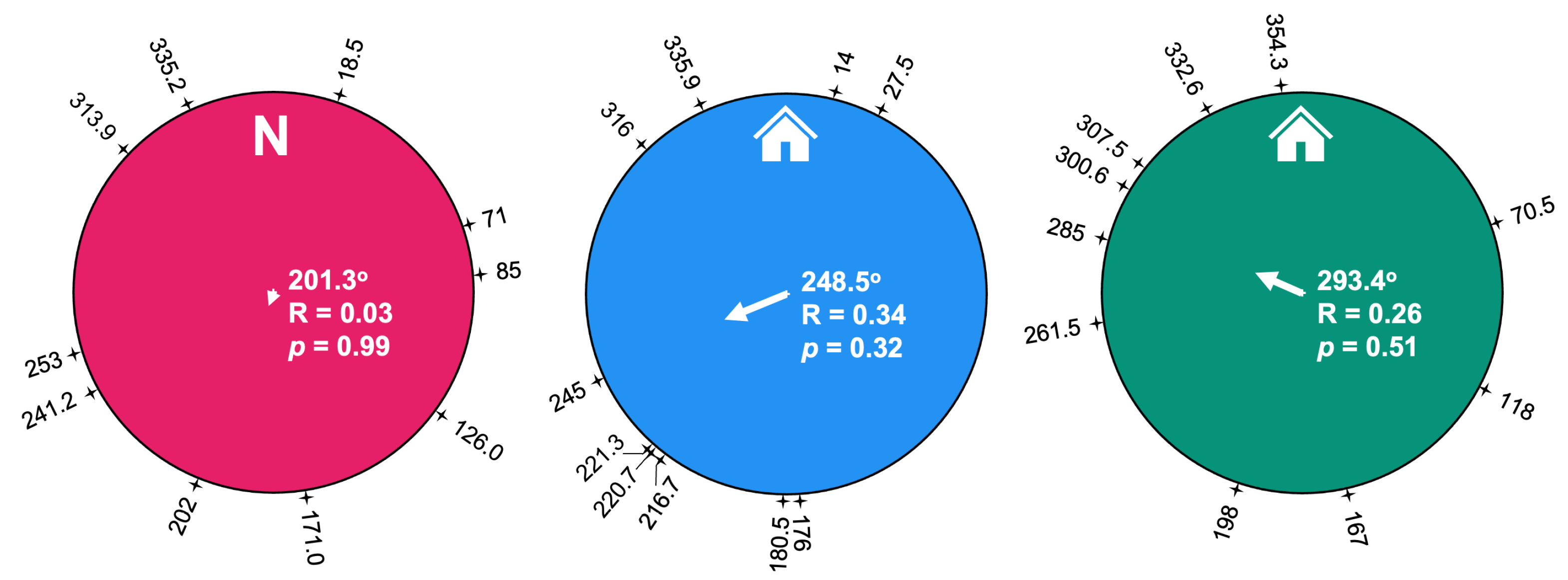
3. Results
3.1. Summary of Runs and Trials
3.2. Path Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polis, G.A. Ecology. In The Biology of Scorpions; Stanford University Press: Redwood City, CA, USA, 1990; pp. 247–293. [Google Scholar]
- Polis, G.A.; McReynolds, C.N.; Ford, R.G. Home range geometry of the desert scorpion Paruroctonus mesaensis. Oecologia 1985, 67, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Heinze, S.; Narendra, A.; Cheung, A. Principles of insect path integration. Curr. Biol. 2018, 28, R1043–R1058. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, D.D.; Muñoz, M.G.; Hoefnagels, M.H. Evidence of learning walks related to scorpion home burrow navigation. J. Exp. Biol. 2022, 225, jeb243947. [Google Scholar] [CrossRef] [PubMed]
- Prévost, E.D.; Stemme, T. Non-visual homing and the current status of navigation in scorpions. Anim. Cogn. 2020, 23, 1215–1234. [Google Scholar] [CrossRef]
- Wehner, R. Arthropods. In Animal Homing; Papi, F., Ed.; Chapman & Hall: London, UK, 1992; pp. 45–144. [Google Scholar]
- Gaffin, D.D.; Curry, C.M. Arachnid navigation—A review of classic and emerging models. J. Arachnol. 2020, 48, 1–25. [Google Scholar] [CrossRef]
- Müller, M.; Wehner, R. Path integration in desert ants, Cataglyphis fortis. Proc. Natl. Acad. Sci. USA 1988, 85, 5287–5290. [Google Scholar] [CrossRef]
- Kirchner, W.H.; Braun, U. Dancing honey bees Indicate the location of food sources using path integration rather than cognitive maps. Anim. Behav. 1994, 48, 1437–1441. [Google Scholar] [CrossRef]
- Seyfarth, E.A.; Barth, F.G. Compound slit sense organs on the spider leg: Mechanoreceptors involved in kinesthetic orientation. J. Comp. Physiol. 1972, 78, 176–191. [Google Scholar] [CrossRef]
- Ortega-Escobar, J. Evidence that the wolf-spider Lycosa tarentula (Araneae, Lycosidae) Needs Vis. Input Path Integr. J. Arachnol. 2002, 30, 481–486. [Google Scholar] [CrossRef]
- Ortega-Escobar, J. Role of the anterior lateral eyes of the wolf spider Lycosa tarentula (Araneae, Lycosidae) Path Integr. J. Arachnol. 2006, 34, 51–61. [Google Scholar] [CrossRef]
- Hebets, E.A.; Aceves-Aparicio, A.; Aguilar-Argüello, S.; Bingman, V.P.; Escalante, I.; Gering, E.J.; Nelsen, D.R.; Rivera, J.; Sánchez-Ruiz, J.Á.; Segura-Hernández, L.; et al. Multimodal sensory reliance in the nocturnal homing of the amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi)? Behav. Process. 2014, 108, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wehner, R.; Srinivasan, M.V. Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J. Comp. Physiol. 1981, 142, 315–338. [Google Scholar] [CrossRef]
- Ortega-Escobar, J.; Hebets, E.A.; Bingman, V.P.; Wiegmann, D.D.; Gaffin, D.D. Comparative biology of spatial navigation in three arachnid orders (Amblypygi, Araneae, and Scorpiones). J. Comp. Physiol. A 2023. [Google Scholar] [CrossRef] [PubMed]
- Drozd, D.; Wolf, H.; Stemme, T. Mechanosensory pathways of scorpion pecten hair sensillae—Adjustment of body height and pecten position. J. Comp. Neurol. 2022, 530, 2918–2937. [Google Scholar] [CrossRef]
- Foelix, R.F.; Müller-Vorholt, G. The fine structure of scorpion sensory organs. II. Pecten sensilla. Bull. Br. Arachnol. Soc. 1983, 6, 68–74. [Google Scholar]
- Gaffin, D.D.; Brownell, P.H. Response properties of chemosensory peg sensilla on the pectines of scorpions. J. Comp. Physiol. A 1997, 181, 291–300. [Google Scholar] [CrossRef]
- Knowlton, E.D.; Gaffin, D.D. Functionally redundant peg sensilla on the scorpion pecten. J. Comp. Physiol. A 2011, 197, 895. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Brayfield, B.P. Exploring the chemo-textural familiarity hypothesis for scorpion navigation. J. Arachnol. 2017, 45, 265–270. [Google Scholar] [CrossRef]
- Musaelian, A.; Gaffin, D.D. High-throughput simulations indicate feasibility of navigation by familiarity with a local sensor such as scorpion pectines. bioRxiv 2020, 2020.06.17.156612. [Google Scholar] [CrossRef]
- Vinnedge, J.E.; Gaffin, D.D. Determination of in-lab site fidelity and movement patterns of Paruroctonus utahensis (Scorpiones: Vaejovidae). J. Arachnol. 2015, 43, 54–58. [Google Scholar] [CrossRef]
- Ortega-Escobar, J.; Muñoz-Cuevas, A. Anterior median eyes of Lycosa tarentula (Araneae, Lycosidae) detect polarized light: Behavioral experiments and electroretinographic analysis. J. Arachnol. 1999, 27, 663–671. [Google Scholar]
- Barth, F.G.; Seyfarth, E.A. Cupiennius salei Keys. (Araneae) in the highlands of central Guatemala. J. Arachnol. 1979, 7, 255–263. [Google Scholar]
- Bartels, M. Sinnesphysiologische und psychologische Untersuchungen an der Trichterspinne Agelena labyrinthica(Cl.). Z. Vgl. Physiol. 1929, 10, 527–593. [Google Scholar] [CrossRef]
- Görner, P. Über die Koppelung der optischen und kinästhetischen Orientierung bei den Trichterspinnen Agelena labyrinthica (Clerck) Und Agelena gracilens C. L. Koch. Z. Vgl. Physiol. 1966, 53, 253–276. [Google Scholar] [CrossRef]
- Görner, P. Homing behavior of funnel web spiders (Agelenidae) by means of web-related cues. Naturwissenschaften 1988, 75, 209–211. [Google Scholar] [CrossRef]
- Moller, P. Die systematischen Abweichungen bei der optischen Richtungsorientierung der Trichterspinne Agelena labyrinthica. Z. Vgl. Physiol. 1970, 66, 78–106. [Google Scholar] [CrossRef]
- Reyes-Alcubilla, C.; Ruiz, M.A.; Ortega-Escobar, J. Homing in the wolf spider Lycosa tarantula (Araneae, Lycosidae): Role Act. Locomot. Vis. Landmarks. Naturwissenschaften 2009, 96, 485–494. [Google Scholar] [CrossRef]
- Duelli, P.; Wehner, R. The spectral sensitivity of polarized light orientation in Cataglyphis bicolor (Formicidae, Hymenoptera). J. Comp. Physiol. A 1973, 86, 37–53. [Google Scholar] [CrossRef]
- Horváth, G.; Varjú, D. (Eds.) Polarization sensitivity in spiders and scorpions. In Polarized Light in Animal Vision: Polarization Patterns in Nature; Springer: Berlin/Heidelberg, Germany, 2004; pp. 243–246. [Google Scholar] [CrossRef]
- Freas, C.A.; Spetch, M.L. Role of the pheromone for navigation in the group foraging ant, Veromessor pergandei. J. Comp. Physiol. A 2021, 207, 353–367. [Google Scholar] [CrossRef]
- Brownell, P. Sensory ecology and orientational behaviors. In Scorpion Biology and Research; Oxford University Press: Oxford, UK, 2001; pp. 159–183. [Google Scholar]
- Locket, A. Eyes and vision. In Scorpion Biology and Research; Brownell, P.H., Polis, G.A., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 79–106. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchant, A.B.; Gaffin, D.D. Investigating Path Integration Cues in Sand Scorpion Homing Behavior. Arthropoda 2023, 1, 49-59. https://doi.org/10.3390/arthropoda1020008
Merchant AB, Gaffin DD. Investigating Path Integration Cues in Sand Scorpion Homing Behavior. Arthropoda. 2023; 1(2):49-59. https://doi.org/10.3390/arthropoda1020008
Chicago/Turabian StyleMerchant, Alexis B., and Douglas D. Gaffin. 2023. "Investigating Path Integration Cues in Sand Scorpion Homing Behavior" Arthropoda 1, no. 2: 49-59. https://doi.org/10.3390/arthropoda1020008
APA StyleMerchant, A. B., & Gaffin, D. D. (2023). Investigating Path Integration Cues in Sand Scorpion Homing Behavior. Arthropoda, 1(2), 49-59. https://doi.org/10.3390/arthropoda1020008





