Investigating Black Soldier Fly Larval (Hermetia illucens) Frass Applications as a Partial Peat Replacement and Liquid Fertilizer in Brassicaceae Crop Production
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study 1 Frass Amended Soilless Substrate
3.1.1. Plant Growth Characteristics and Other Parameters
3.1.2. Macronutrient Concentrations
3.1.3. Micronutrient Concentrations
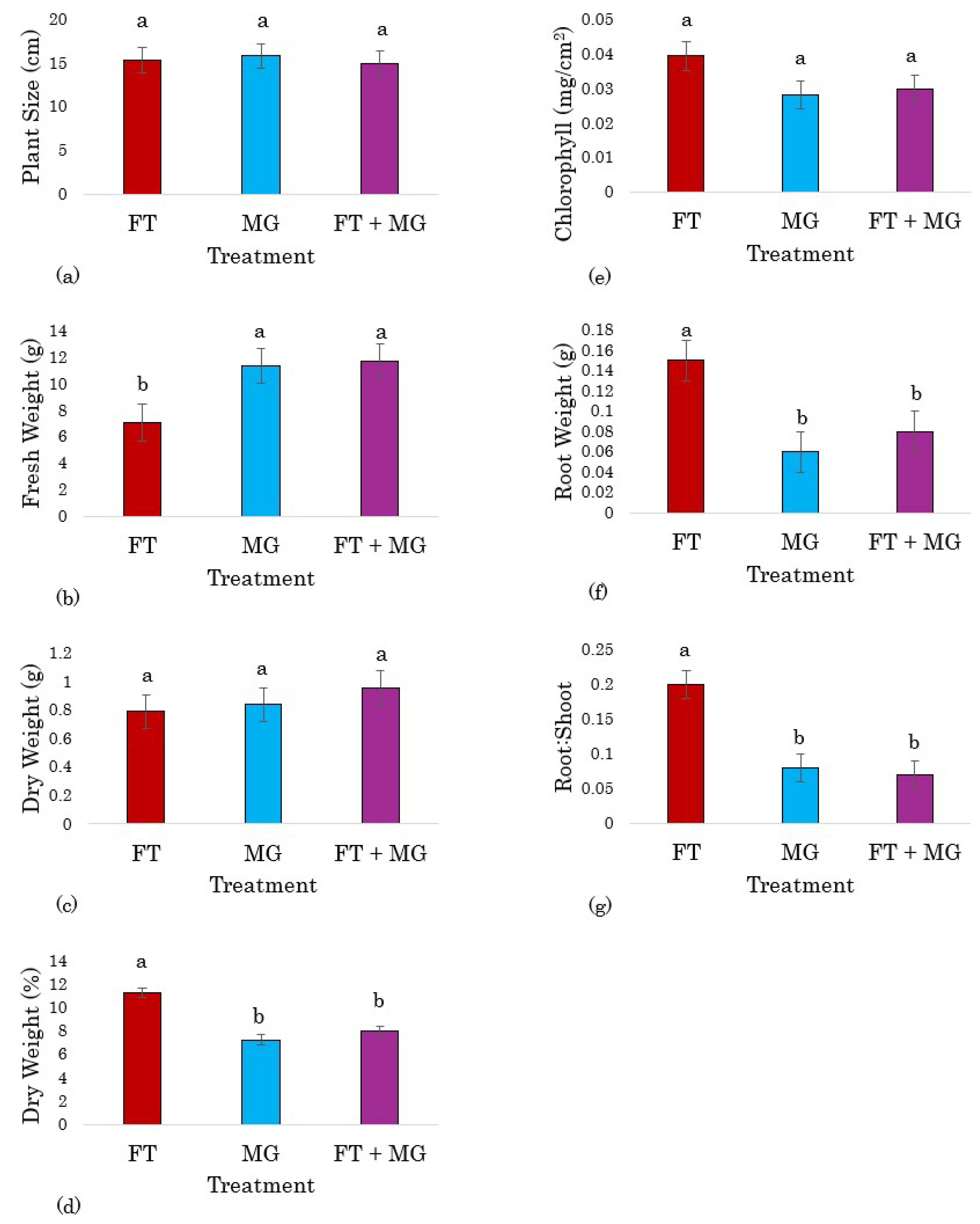
3.2. Study 2 Frass Tea Fertilizer Applications
4. Discussion
4.1. Study 1 Frass Amended Potting Media
4.2. Study 2 Frass Tea Liquid Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez, M. The sustainability of industrial insect mass rearing for food and feed production: Zero waste goals through by-product utilization. Curr. Opin. Insect Sci. 2021, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Uchanski, M. Insect left-over substrate as plant fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Life-history traits of the black soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), reared on three manure types. Animals 2019, 9, 281. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Schmitt, E. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Chavez, M.Y.; Uchanski, M.; Tomberlin, J.K. Impacts of black soldier fly, Hermetia illucens, larval frass on lettuce and arugula production. Front. Sustain. Food Syst. 2024, 8, 1399932. [Google Scholar] [CrossRef]
- Chavez, M.Y.; Uchanski, M.; Tomberlin, J.K. Impacts of black soldier fly, (Diptera: Stratiomyidae) larval frass on tomato production. J. Econ. Entomol. 2023, 116, 1490–1495. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Klammsteiner, T.; Turan, V.; Oberegger, S.; Insam, H.; Juárez, M.F.D. Black soldier Fly (Hermetia illucens) Frass as plant fertilizer. In Proceedings of the 7th International Conference on Sustainable Solid Waste Management, Heraklion, Crete Island, Greece, 26–29 June 2019. [Google Scholar]
- Kagata, H.; Ohgushi, T. Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Popul. Ecol. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 1, 4659. [Google Scholar] [CrossRef]
- Fernández-Romero, M.L.; Clark, J.M.; Collins, C.D.; Parras-Alcántara, L.; Lozano-García, B. Evaluation of optical techniques for characterising soil organic matter quality in agricultural soils. Soil Tillage Res. 2016, 155, 450–460. [Google Scholar] [CrossRef]
- Esteves, C.; Fareleira, P.; Castelo-Branco, M.A.; Lopes, I.G.; Mota, M.; Murta, D.; Menino, R. Black soldier fly larvae frass increases the soil’s residual nutrient content and enzymatic activity—A lettuce production trial. J. Insects Food Feed 2022, 8, 1431–1440. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, Q.; Li, X.; Zhang, S. Compost tea-mediated induction of resistance in biocontrol of strawberry Verticillium wilt. J. Plant Dis. Prot. 2020, 127, 257–268. [Google Scholar] [CrossRef]
- Mmbone, S.; Wanjala, F.F.; Gohole, L. Exploring Desert Locust (S. gregaria) Frass as an Organic Fertilizer for the Growth of Kales (Brassica oleracea L.) under Open Field Conditions. J. Crops Livest. Pest. Manag. 2023, 1, 72–78. [Google Scholar]
- Abiya, A.A.; Kupesa, D.M.; Beesigamukama, D.; Kassie, M.; Mureithi, D.; Thairu, D.; Wesonga, J.; Tanga, C.M.; Niassy, S. Agronomic performance of kale (Brassica oleracea) and swiss chard (Beta vulgaris) grown on soil amended with black soldier fly frass fertilizer under wonder multistorey gardening system. Agronomy 2022, 2, 2211. [Google Scholar] [CrossRef]
- Romano, N.; Datta, S.N.; Sinha, A.K.; Pande, G.S. Partially replacing synthetic fertilizer with black soldier fly (Hermetia illucens) larvae frass enhances kale (Brassica oleracea var. sabellica) production. Technol. Hortic. 2023, 3, 8. [Google Scholar] [CrossRef]
- Chia, S.Y.; van Loon, J.J.; Dicke, M. Effects of frass from larvae of black soldier fly (Hermetia illucens) and yellow mealworm (Tenebrio molitor) on growth and insect resistance in field mustard (Brassica rapa): Differences between insect species and frass treatments. Entomologia Experimen. App. 2024, 172, 394–408. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Rusman, Q.; Mertens, D.; Weldegergis, B.T.; Peller, J.; Polder, G.; Dicke, M. Insect exuviae as soil amendment affect flower reflectance and increase flower production and plant volatile emission. Plant Cell Environ. 2023, 46, 931–945. [Google Scholar] [CrossRef]
- Brousseau, V.D.; Leroux, D.; Martel, S.; Tikasz, P.; Debbagh, M.; Giguère, T.; Tazi, I.; MacPherson, S.; Lefsrud, M.G. Animal-Waste Based Organic Liquid Fertilizer as a Replacement for Synthetic Nitrogen in Basil Production: A Case Study. In Proceedings of the CSBE/SCGAB AGM and Technical Conference 2022, Charlottetown, PE, Canada, 24–27 July 2022; Canadian Soc. Bio. CSBE22-175. pp. 1–11. [Google Scholar]
- Camberato, D.M.; Lopez, R.G.; Mickelbart, M.V. pH and electrical conductivity measurements in soilless substrates. Purdue Univ. Ext. Serv. Bul. 2009, HO-237-W, 1–6. [Google Scholar]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar] [CrossRef]
- Chavez, M.Y.; Uchanski, M.; Tomberlin, J.K. Impacts of black soldier fly, Hermetia illucens, larval frass on the emergence and seedling vigor of three vegetable crop species. J. Insects Food Feed. 2024, 1, 819–832. [Google Scholar] [CrossRef]
- Wendling, M.; Büchi, L.; Amossé, C.; Sinaj, S.; Walter, A.; Charles, R. Influence of root and leaf traits on the uptake of nutrients in cover crops. Plant Soil. 2016, 409, 419–434. [Google Scholar] [CrossRef]
- Mendoza-Tafolla, R.O.; Juarez-Lopez, P.; Ontiveros-Capurata, R.E.; Sandoval-Villa, M.; Iran, A.T.; Alejo-Santiago, G. Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and at LEAF readings. Not. Bot. Horti Agrobot. 2019, 47, 751–756. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Quinche, M.; Reid, B.; Hotte, N.; Fortin, A.; Normandin, É.; Renaud, G.D.L.R.; Zadeh, A.R.; Deschamps, M.H.; Vandenberg, G. Opportunities and challenges in upcycling agri-food byproducts to generate insect manure(frass): A literature review. Waste Manag. 2024, 176, 169–191. [Google Scholar] [CrossRef]
- McFarland, C.; Huggins, D.R. Acidification in the inland Pacific Northwest. Crops Soils 2015, 48, 4–12. [Google Scholar] [CrossRef]
- Fielding, D.J.; Trainor, E.; Zhang, M. Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadavers. Biol. Fertil. Soils 2013, 49, 537–544. [Google Scholar] [CrossRef]
- Rummel, P.S.; Beule, L.; Hemkemeyer, M.; Schwalb, S.A.; Wichern, F. Black soldier fly diet impacts soil greenhouse gas emissions from frass applied as fertilizer. Front. Sustain. Food Syst. 2021, 5, 709993. [Google Scholar] [CrossRef]
- Ghimire, R.; Machado, S. Soil acidity limits wheat yield in dryland regions of the Inland Pacific Northwest. Crops Soils 2017, 50, 14–16. [Google Scholar] [CrossRef]
- Davis, A.G.; Huggins, D.R.; Reganold, J.P. Linking soil health and ecological resilience to achieve agricultural sustainability. Front. Ecol. Environ. 2023, 21, 131–139. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Reid, B.; Hotte, N.; Paris, N.; Quinche, M.; Lachance, C.; Fortin, A.; Normandin, É.; Laderriere, V.; Vandenberg, G. Growth trials on vegetables, herbs, and flowers using mealworm frass, chicken manure, and municipal compost. ACS Agric. Sci. Technol. 2023, 3, 249–259. [Google Scholar] [CrossRef]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; van Huis, A.; Yu, Z.; Fasulo, S.; et al. Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L.(Diptera: Strati-omyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.L.; Davis, J.G.; Waskom, R.M.; Self, J.R.; Christensen, D.K. Phosphorus Fertilizers for Organic Farming Systems. CSU Extension 2007, Fact Sheet 0.569. Available online: https://extension.colostate.edu/docs/pubs/crops/00569.pdf (accessed on 5 February 2025).
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 19, 30–50. [Google Scholar] [CrossRef]
- Luu, T.T.; Le, T.L.; Nguyen, V.T.; Nguyen, T.D.; Green, I.D. Effect of three types of growing media and vermicompost tea on the growth and individual weight of Chinese Kale (Brassica oleracea var. alboglabra Bailey). Tra Vinh Univ. J. Sci. 2023, 13, 52–59. [Google Scholar] [CrossRef]
- Smart, S.M.; Glanville, H.C.; Blanes, M.D.C.; Mercado, L.M.; Emmett, B.A.; Jones, D.L.; Cosby, B.J.; Marrs, R.H.; Butler, A.; Marshall, M.R.; et al. Leaf dry matter content is better at predicting above-ground net primary production than specific leaf area. Funct. Ecol. 2017, 31, 1336–1344. [Google Scholar] [CrossRef]
- Romano, N.; Powell, A.; Islam, S.; Fischer, H.; Renukdas, N.; Sinha, A.K.; Francis, S. Supplementing aquaponics with black soldier fly (Hermetia illucens) larvae frass tea: Effects on the production and composition of sweetpotato slips and sweet banana peppers. Aquaculture 2022, 555, 738160. [Google Scholar] [CrossRef]
- Shaban, H.; Fazeli-Nasab, B.; Alahyari, H.; Alizadeh, G.; Shahpesandi, S. An Overview of the Benefits of Compost tea on Plant and Soil Structure. Adv. Biores. 2015, 6, 154–158. [Google Scholar]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Tan, J.K.; Lee, J.T.; Chiam, Z.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T. Applications of food waste-derived black soldier fly larval frass as incorporated compost, side-dress fertilizer and frass-tea drench for soilless cultivation of leafy vegetables in biochar-based growing media. Waste Manag. 2021, 130, 155–166. [Google Scholar] [CrossRef]
- Romano, N.; Webster, C.; Datta, S.N.; Pande, G.S.J.; Fischer, H.; Sinha, A.K.; Huskey, G.; Rawles, S.D.; Francis, S. Black soldier Fly (Hermetia illucens) Frass on sweet-potato (Ipomea batatas) slip production with aquaponics. Horticulturae 2023, 9, 1088. [Google Scholar] [CrossRef]

| Treatment | BSFL Frass | Peat |
|---|---|---|
| CP 100% | 0% (0 mL) | 100% (500 mL) |
| BSFL 10% | 10% (50 mL) | 90% (450 mL) |
| BSFL 20% | 20% (100 mL) | 80% (400 mL) |
| BSFL 30% | 30% (150 mL) | 70% (350 mL) |
| Parameter | Dry Basis | Fertilizer |
|---|---|---|
| Dry Matter-Total Solids (%) | 46.26 | - |
| Moisture (%) | 53.74 | - |
| Soluble Salts (mmhos/cm) | 4.00 | - |
| pH | 8.80 | - |
| Organic Nitrogen (%) | 20.20 | - |
| Ammonium (%) | 0.05 | - |
| Nitrate (%) | 0.00 | - |
| Total Nitrogen (%) | 20.26 | 24 |
| Phosphorus (%P2O5) | 1.30 | 8 |
| Potassium (%K2O) | 1.70 | 16 |
| Sulfur (%) | 3.15 | - |
| Calcium (%) | 4.23 | - |
| Magnesium (%) | 2.68 | - |
| Sodium (%) | 0.08 | - |
| Zinc (ppm) | 0.001 | 0.12% |
| Iron (ppm) | 0.65 | 0.3% |
| Manganese (ppm) | 0.02 | 0.1% |
| Boron (%) | - | 0.02 |
| Copper (%) | - | 0.14 |
| Treatment | Frass Tea (mL) | Inorganic Fertilizer (mL) |
|---|---|---|
| Frass tea (FT) | 200 | 0 |
| Miracle Gro (MG) | 0 | 200 |
| FT + MG | 100 | 100 |
| Treatment | N (%) | P (%) | K (%) | S (%) | Ca (%) | Mg (%) |
|---|---|---|---|---|---|---|
| Kale | ||||||
| CP 100% | 5.29 ± 0.08 | 0.63 ± 0.15 | 3.17 ± 0.28 a | 0.91 ± 0.06 | 1.53 ± 0.10 | 1.08 ± 0.05 |
| BSFL 10% | 5.7 ± 0.08 | 0.52 ± 0.15 | 2.42 ± 0.28 ab | 0.97 ± 0.06 | 1.08 ± 0.10 | 0.48 ± 0.05 |
| BSFL 20% | 6.23 ± 0.08 | 0.47 ± 0.15 | 0.73 ± 0.28 b | 1.05 ± 0.06 | 1.15 ± 0.10 | 0.45 ± 0.05 |
| Mustard | ||||||
| CP 100% | 5.2 ± 0.04 | 0.44 ± 0.04 y | 2.65 ± 0.09 y | 1.05 ± 0.05 | 1.11 ± 0.09 x | 0.37 ± 0.06 |
| BSFL 10% | 5.61 ± 0.04 | 0.50 ± 0.04 x | 5.29 ± 0.09 x | 0.99 ± 0.05 | 0.76 ± 0.09 y | 0.36 ± 0.06 |
| Treatment | Na (%) | B (ppm) | Cu (ppm) | Fe (ppm) | Mn (ppm) | Zn (ppm) | Mo (ppm) |
|---|---|---|---|---|---|---|---|
| Kale | |||||||
| CP 100% | 0.36 ± 0.07 b | 111.50 ± 40.94 | 7.55 ± 0.29 | 139.65 ± 16.86 a | 65.17 ± 4.37 | 118.19 ± 6.83 a | 0.01 ± 0.003 |
| BSFL 10% | 1.17 ± 0.07 a | 58.52 ± 40.94 | 8.48 ± 0.29 | 74.45 ± 16.86 b | 73.15 ± 4.37 | 92.99 ± 6.83 ab | 0.002 ± 0.003 |
| BSFL 20% | 1.37 ± 0.07 a | 98.24 ± 40.94 | 8.06 ± 0.29 | 82.17 ± 16.86 ab | 66.27 ± 4.37 | 90.06 ± 6.83 b | 0.006 ± 0.003 |
| Mustard | |||||||
| CP 100% | 0.38 ± 0.05 y | 134.30 ± 50.09 | 15.60 ± 2.64 | 304.10 ± 80.78 | 50.52 ± 5.08 | 147.75 ± 6.15 | 0.028 ± 0.01 |
| BSFL 10% | 1.19 ± 0.05 x | 65.18 ± 50.09 | 12.94 ± 2.64 | 124.36 ± 80.78 | 52.76 ± 5.08 | 158.88 ± 6.15 | 0.008 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez, M.Y.; Villa Ignacio, A.; Craver, J.K.; Bousselot, J. Investigating Black Soldier Fly Larval (Hermetia illucens) Frass Applications as a Partial Peat Replacement and Liquid Fertilizer in Brassicaceae Crop Production. Agrochemicals 2025, 4, 8. https://doi.org/10.3390/agrochemicals4020008
Chavez MY, Villa Ignacio A, Craver JK, Bousselot J. Investigating Black Soldier Fly Larval (Hermetia illucens) Frass Applications as a Partial Peat Replacement and Liquid Fertilizer in Brassicaceae Crop Production. Agrochemicals. 2025; 4(2):8. https://doi.org/10.3390/agrochemicals4020008
Chicago/Turabian StyleChavez, Maria Y., Armando Villa Ignacio, Joshua K. Craver, and Jennifer Bousselot. 2025. "Investigating Black Soldier Fly Larval (Hermetia illucens) Frass Applications as a Partial Peat Replacement and Liquid Fertilizer in Brassicaceae Crop Production" Agrochemicals 4, no. 2: 8. https://doi.org/10.3390/agrochemicals4020008
APA StyleChavez, M. Y., Villa Ignacio, A., Craver, J. K., & Bousselot, J. (2025). Investigating Black Soldier Fly Larval (Hermetia illucens) Frass Applications as a Partial Peat Replacement and Liquid Fertilizer in Brassicaceae Crop Production. Agrochemicals, 4(2), 8. https://doi.org/10.3390/agrochemicals4020008







