Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability
Abstract
:1. Introduction
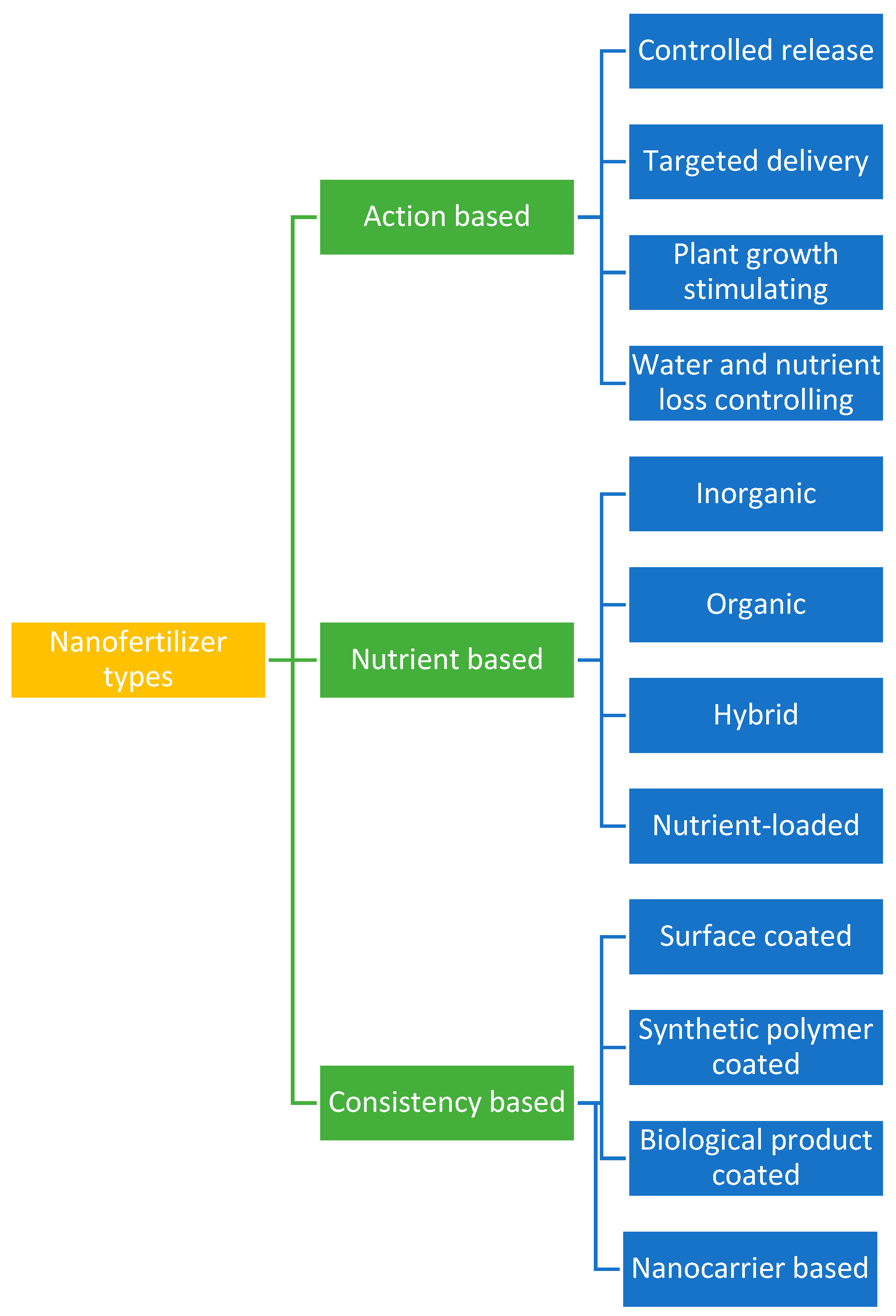
2. Nanofertilizer Types
2.1. Action-Based
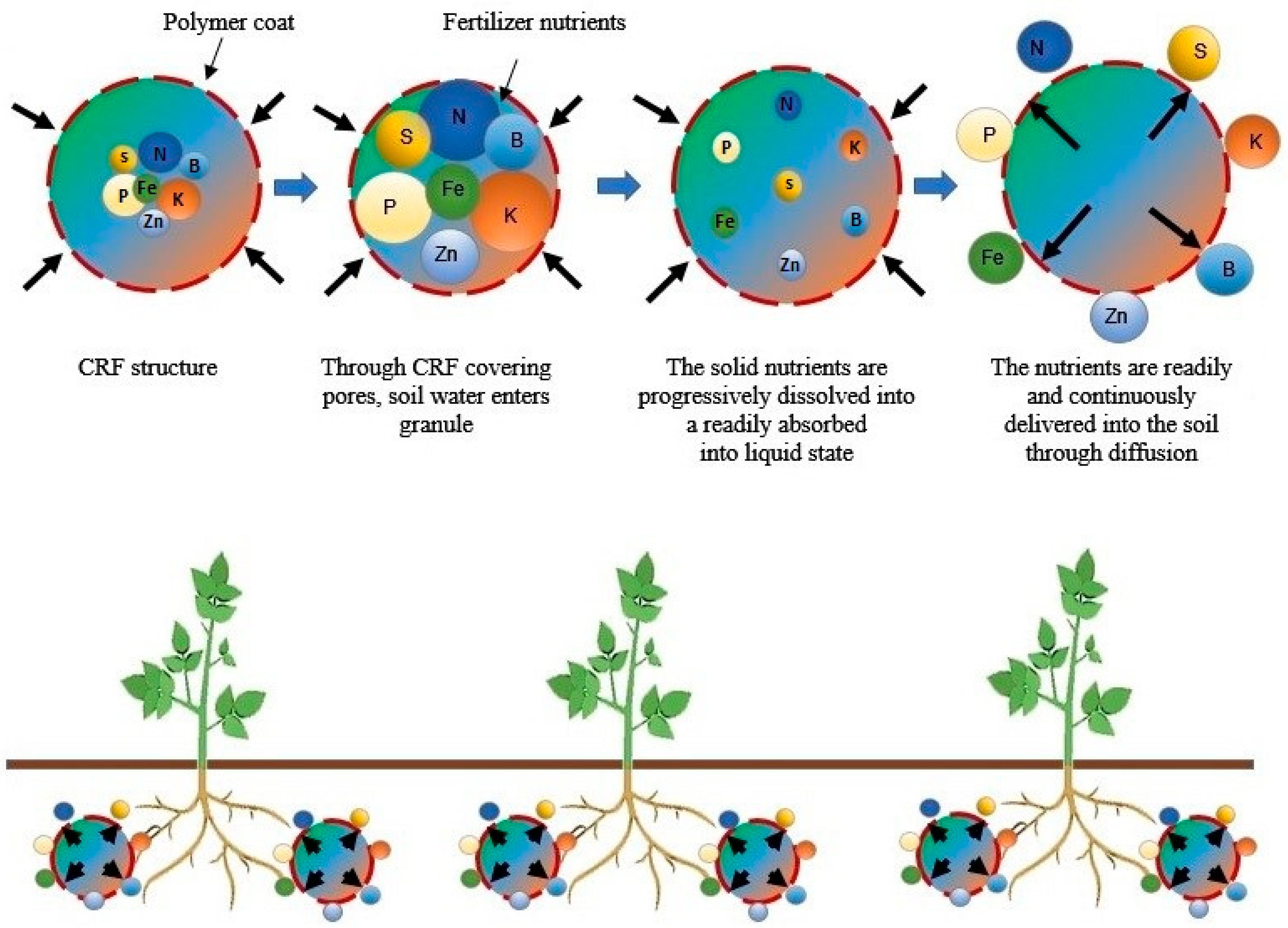
2.1.1. Controlled-Release Nanofertilizers
Carbon-Based
Chitosan-Based
Clay-Based
Layer Double Hydroxides
Nanocapsule-Based
Nanogel-Based
Polyurethane-Based
Starch-Based
Zeolite-Based
2.1.2. Nanofertilizers for Targeted Delivery
Nanoaptamers
Others
2.1.3. Plant Growth-Stimulating Nanofertilizers
2.1.4. Water and Nutrient Loss-Controlling Fertilizers
Nanobeads
Nanoemulsion-Based Fertilizers
2.2. Nutrient Based
2.2.1. Inorganic Nanofertilizers
Macronutrient Nanofertilizers
- (a)
- Nitrogen-based
- (b)
- Phosphorous-based
- (c)
- Potassium-based
- (d)
- Calcium-based
- (e)
- Magnesium-based
- (f)
- Sulfur-based
Micronutrient Nanofertilizers
- (a)
- Boron-based
- (b)
- Copper-based
- (c)
- Iron-based
- (d)
- Nickel-based
- (e)
- Titanium-based
- (f)
- Zinc-based
2.2.2. Organic Nanofertilizers
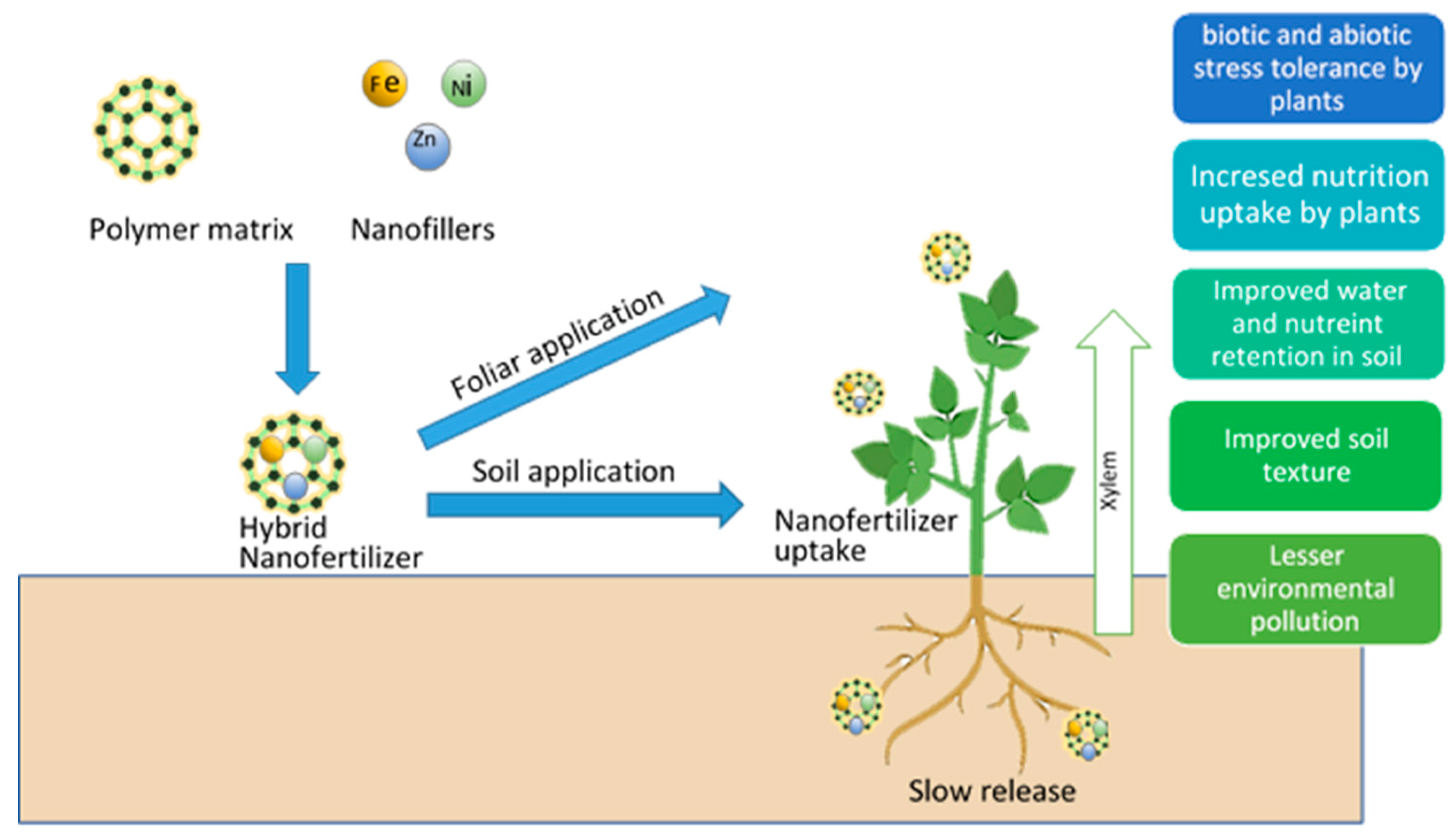
2.2.3. Hybrid Nanofertilizers
2.2.4. Nutrient-Loaded Nanofertilizers
2.3. Consistency-Based Nanofertilizers
2.3.1. Surface-Coated Nanofertilizers
2.3.2. Synthetic Polymer-Coated
2.3.3. Biological Product-Coated
- (a)
- Organic compound-coated
- (b)
- Microbe-coated (Nanobiofertilizers)
2.3.4. Nanocarrier-Based Nanofertilizers
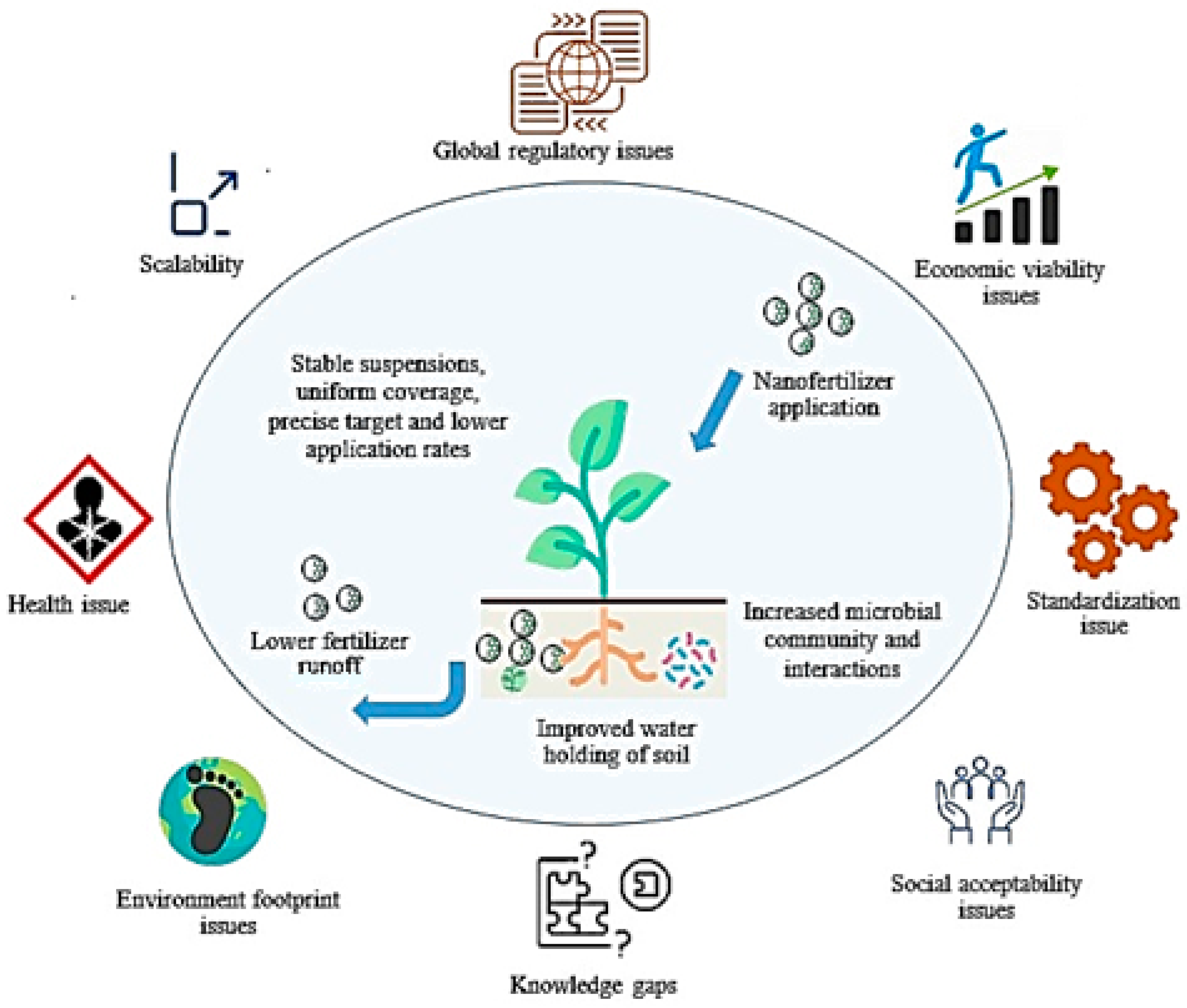
3. Materials and Strategies for the Controlled and Targeted Delivery of NPs
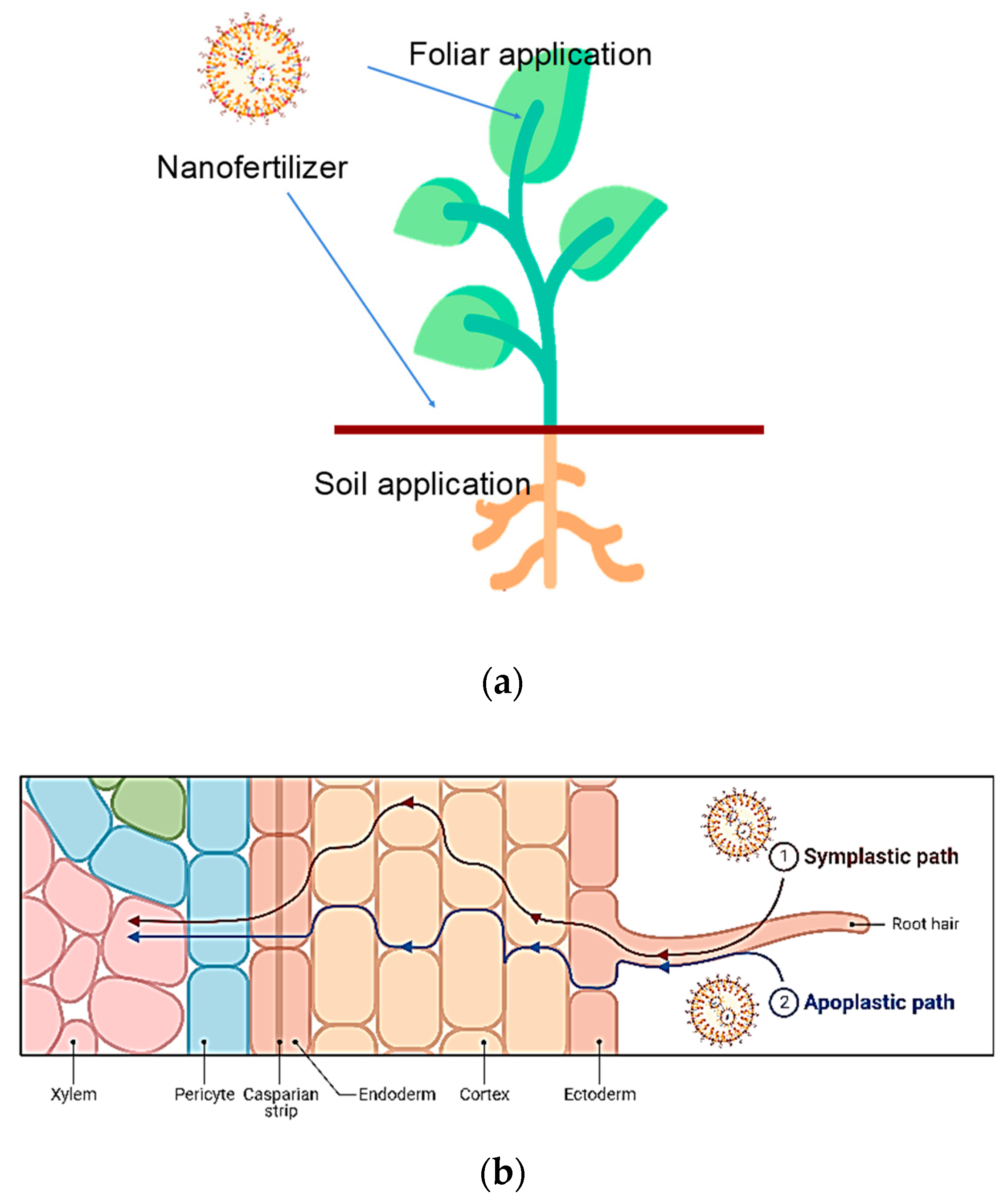
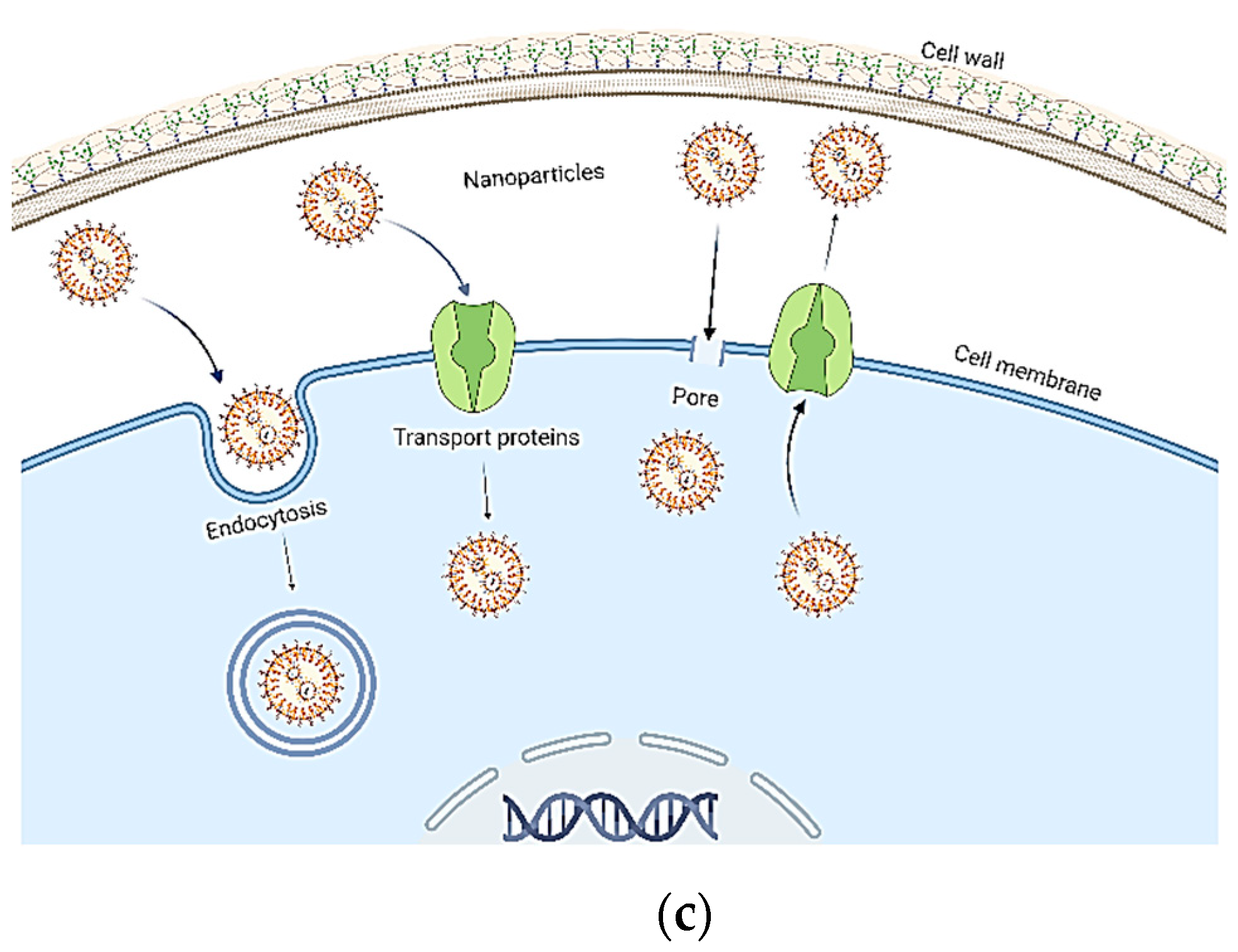
4. Modes of Nanofertilizer Application
4.1. Foliar Spray
4.2. Seed Nanopriming
4.3. Soil Treatment
5. Advantages of Nanofertilizers over Conventional Chemical Fertilizers
5.1. Greater Surface Area
5.2. High Solubility
5.3. Encapsulation of Fertilizers within NPs
5.4. Easy Penetration and Controlled Release of Fertilizers
5.5. High Nutrient Absorption Efficiency
5.6. Effective Duration of Nutrient Release
5.7. Improved Microbial Activity
5.8. Improved Soil Activity
5.9. Improved Soil Water-Holding Capacity
5.10. Ecofriendly Nature
5.11. Low Production Cost
5.12. Fulfills the Goal of Precision Farming
5.13. Improves Plant Stress Tolerance
5.14. Stimulates Plant Growth
6. Limitations and Potential Risks Related to the Application of Nanofertilizers
6.1. Human Health Risks
6.2. Environmental Risks
6.3. Ecological Risks
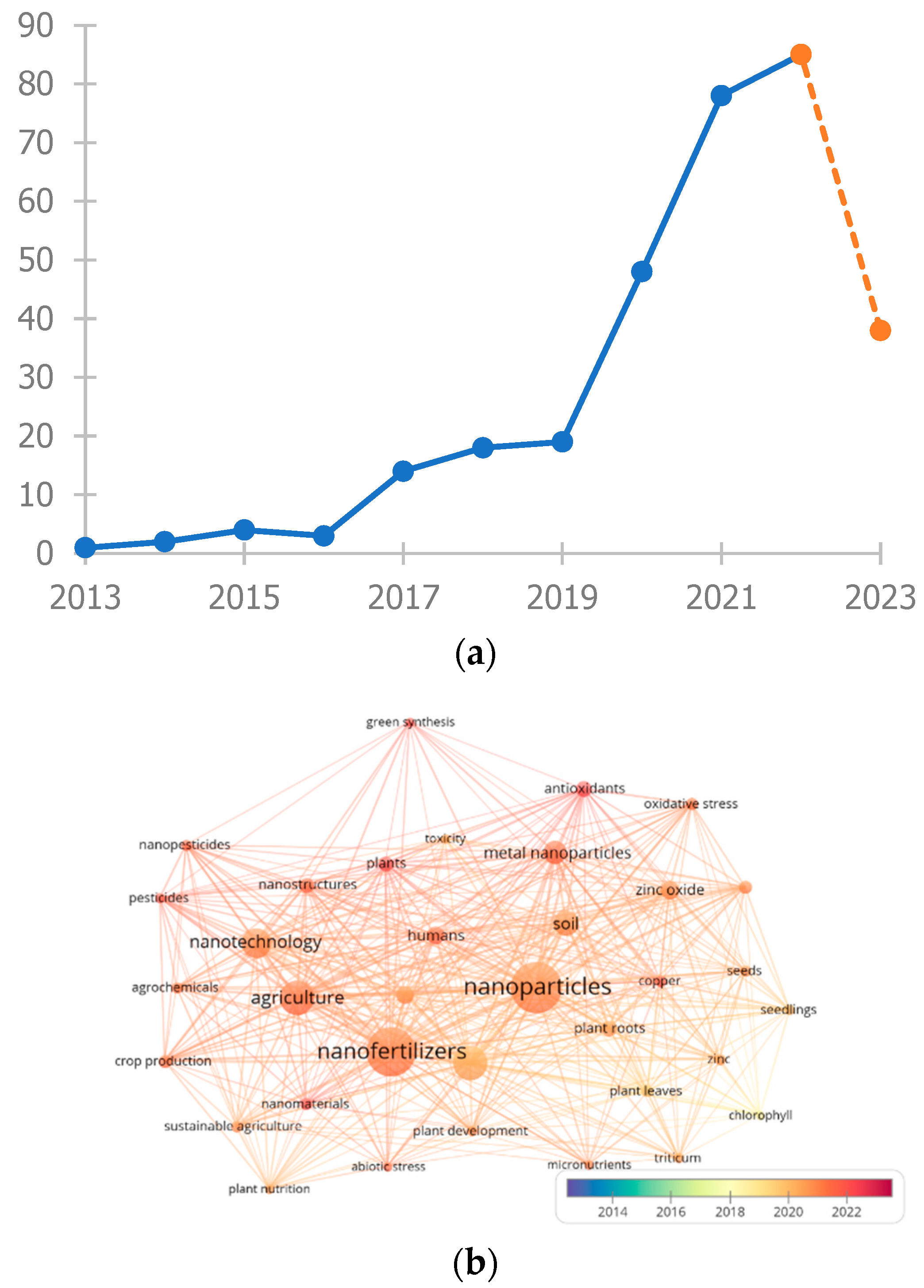
7. Current Status and Future Outlook
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Preetha, P.S.; Balakrishnan, N. A review of nano fertilizers and their use and functions in soil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3117–3133. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Abdalla, Z.F.; El-Sawy, S.; El-Bassiony, A.E.-M.; Jun, H.; Shedeed, S.; Okasha, A.M.; Bayoumi, Y.; El-Ramady, H.; Prokisch, J. Smart Fertilizers vs. Nano-fertilizers: A Pictorial Overview. Environ. Biodivers. Soil Secur. 2022, 6, 191–204. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical applications of carbon nanomaterials: Fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.C.; Oliveira, M. Phosphorous nanofertilizers for precise application in rice cultivation as an adaptation to climate change. Climate 2022, 10, 183. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [Green Version]
- Vaccari, F.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Saxena, M.; Maity, S.; Sarkar, S. Carbon nanoparticles in ‘biochar’boost wheat (Triticum aestivum) plant growth. Rsc. Adv. 2014, 4, 39948–39954. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.; Hussain, I.; Singh, H.; Singh, S. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. 2015, 4, 25–40. [Google Scholar]
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.-O.; Kim, J. Engineering plants with carbon nanotubes: A sustainable agriculture approach. J. Nanobiotechnol. 2022, 20, 1–30. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, H.; Wang, H.; Zhao, J.; Gao, K.; Qiao, J.; Li, J.; Ge, S. The effects of graphene-family nanomaterials on plant growth: A review. Nanomaterials 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; De Moura, M.; Mattoso, L. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degrad. Dev. 2020, 31, 2734–2746. [Google Scholar] [CrossRef]
- Basak, B.; Pal, S.; Datta, S. Use of modified clays for retention and supply of water and nutrients. Curr. Sci. 2012, 102, 1272–1278. [Google Scholar]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous overview on the controlled release fertilizers. Adv. Chem. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Parolo, M.; Fernández, L.; Zajonkovsky, I.; Sánchez, M.; Baschini, M. Antibacterial activity of materials synthesized from clay minerals. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 1, 144–151. [Google Scholar]
- Karpiński, B.; Szkodo, M. Clay minerals–mineralogy and phenomenon of clay swelling in oil & gas industry. Adv. Mater. Sci. 2015, 15, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Rakhimol, K.R.; Thomas, S.; Kalarikkal, N.; Jayachandran, K. Nanotechnology in controlled-release fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 169–181. [Google Scholar] [CrossRef]
- Choy, J.H.; Oh, J.M.; Choi, S.J. Layered Double Hydroxides as Controlled Release Materials. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 545–557. [Google Scholar] [CrossRef]
- Cardoso, L.P.; Celis, R.; Cornejo, J.; Valim, J.B. Layered double hydroxides as supports for the slow release of acid herbicides. J. Agric. Food Chem. 2006, 54, 5968–5975. [Google Scholar] [CrossRef] [PubMed]
- Petosa, A.R.; Rajput, F.; Selvam, O.; Ohl, C.; Tufenkji, N. Assessing the transport potential of polymeric nanocapsules developed for crop protection. Water Res. 2017, 111, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef] [PubMed]
- Krishnani, K.K.; Boddu, V.M.; Chadha, N.K.; Chakraborty, P.; Kumar, J.; Krishna, G.; Pathak, H. Metallic and non-metallic nanoparticles from plant, animal, and fisheries wastes: Potential and valorization for application in agriculture. Environ. Sci. Pollut Res. Int. 2022, 29, 81130–81165. [Google Scholar] [CrossRef] [PubMed]
- Bindra, H.S.; Singh, B. Nanofertilizers and nanopesticides: Future of plant protection. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–84. [Google Scholar] [CrossRef]
- Madzokere, T.C.; Murombo, L.T.; Chiririwa, H. Nano-based slow releasing fertilizers for enhanced agricultural productivity. Mater. Today Proc. 2021, 45, 3709–3715. [Google Scholar] [CrossRef]
- Umarani, R.; Mala, R. Influence of calcium phosphate nano gel fertilizer composite on enzymes, biomolecules and yield of Abelmoschus esculentus. Int. J. Agric. Environ. Biotechnol. 2013, 6, 771. [Google Scholar]
- Thirugnanasambandan, T. Advances of Engineered Nanofertilizers for Modern Agriculture. In Plant-Microbes-Engineered Nano-Particles (PM-ENPs) Nexus in Agro-Ecosystems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 131–152. [Google Scholar] [CrossRef]
- Yuan, S.; Cheng, L.; Tan, Z. Characteristics and preparation of oil-coated fertilizers: A review. J. Control. Release 2022, 345, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xu, J.; Zhao, Q. Biomimetic superhydrophobic biobased polyurethane-coated fertilizer with atmosphere “Outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Y.; Jia, C.; Wang, C.; Li, X.; Zhang, M. Polyurethane from liquefied wheat straw as coating material for controlled release fertilizers. BioResources 2015, 10, 7877–7888. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Anderson, D.P.; Yu, J. Preparation, modification, and application of starch nanocrystals in nanomaterials: A review. J. Nanomater. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ashfaq, M.; Verma, N. Synthesis of novel PVA–starch formulation-supported Cu–Zn nanoparticle carrying carbon nanofibers as a nanofertilizer: Controlled release of micronutrients. J. Mater. Sci. 2018, 53, 7150–7164. [Google Scholar] [CrossRef]
- Jakkula, V.S.; Wani, S. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 1–15. [Google Scholar]
- Sharma, V.; Javed, B.; Byrne, H.; Curtin, J.; Tian, F. Zeolites as carriers of nano-fertilizers: From structures and principles to prospects and challenges. Appl. Nano 2022, 3, 163–186. [Google Scholar] [CrossRef]
- Manikandan, A.; Subramanian, K. Evaluation of zeolite based nitrogen nano-fertilizers on maize growth, yield and quality on inceptisols and alfisols. Int. J. Plant Soil Sci. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Bijali, J.; Acharya, K. Current trends in nano-technological interventions on plant growth and development: A review. IET Nanobiotechnol. 2020, 14, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Gu, J.; Ma, H.; Wu, H. Carbon-Based Nanomaterials for Sustainable Agriculture: Their Application as Light Converters, Nanosensors, and Delivery Tools. Plants 2022, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, K.; Peng, X.; Zhang, L. Chitosan-based drug delivery systems: Current strategic design and potential application in human hard tissue repair. Eur. Polym. J. 2022, 110979. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef]
- Mishra, T.; Mohanty, A.; Tiwari, S. Recent development in clay based functional coating for corrosion protection. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; pp. 93–109. [Google Scholar]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Gupta, A.; Sahu, P.K.; Tiwari, R.K. Nanotechnology in Insect Pest Management. In Molecular Approaches for Sustainable Insect Pest Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 377–394. [Google Scholar]
- Li, J. Protein Nanocapsule Based Protein Carriers for Industrial and Medical Applications; UCLA: Los Angeles, CA, USA, 2015. [Google Scholar]
- Win, Y.Y.; Charoenkanburkang, P.; Limprasutr, V.; Rodsiri, R.; Pan, Y.; Buranasudja, V.; Luckanagul, J.A. In Vivo Biocompatible Self-Assembled Nanogel Based on Hyaluronic Acid for Aqueous Solubility and Stability Enhancement of Asiatic Acid. Polymers 2021, 13, 4071. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [Green Version]
- Aththanayaka, S.; Thiripuranathar, G.; Ekanayake, S. Emerging advances in biomimetic synthesis of nanocomposites and potential applications. Mater. Today Sustain. 2022, 100206. [Google Scholar] [CrossRef]
- Wen, L.-X.; Li, Z.-Z.; Zou, H.-K.; Liu, A.-Q.; Chen, J.-F. Controlled release of avermectin from porous hollow silica nanoparticles. Pest Manag. Sci. 2005, 61, 583–590. [Google Scholar] [CrossRef]
- Mishra, D.; Khare, P. Emerging Nano-agrochemicals for Sustainable Agriculture: Benefits, Challenges and Risk Mitigation. In Sustainable Agriculture Reviews 50: Emerging Contaminants in Agriculture; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 235–257. [Google Scholar]
- Rahman, M.H.; Haque, K.S.; Khan, M.Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Al-Juthery, H.W.; Lahmod, N.R.; Al-Taee, R.A. Intelligent, nano-fertilizers: A new technology for improvement nutrient use efficiency. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Iraq, 21–22 January 2021; p. 735. [Google Scholar]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Rameshaiah, G.; Pallavi, J.; Shabnam, S. Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015, 3, 314–320. [Google Scholar]
- Kaushal, M.; Wani, S.P. Nanosensors: Frontiers in precision agriculture. In Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 279–291. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Naz, M.Y.; Shukrullah, S.; Ghaffar, A. Sensors detecting controlled fertilizer release. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 131–153. [Google Scholar] [CrossRef]
- Raimondi, G.; Maucieri, C.; Toffanin, A.; Renella, G.; Borin, M. Smart fertilizers: What should we mean and where should we go? Ital. J. Agron. 2021, 16. [Google Scholar] [CrossRef]
- Monreal, C.; DeRosa, M.; Mallubhotla, S.; Bindraban, P.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Basu, R.; Das, S.; Nandy, P. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect. J. Nanoparticle Res. 2011, 13, 4519–4528. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 641759, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as nanofertilizers and nanopesticides: An overview. ChemistrySelect 2021, 6, 8645–8663. [Google Scholar] [CrossRef]
- Sivarethinamohan, R.; Sujatha, S. Unlocking the potentials of using nanotechnology to stabilize agriculture and food production. In Proceedings of the AIP Conference Proceedings, Warangal, India, 12–13 February 2021; p. 020022. [Google Scholar]
- Ahmed, S.; Fahmy, A. Environmental Impact for applications of neem cake coated urea and nano iron foliar on rationalization of chemical nitrogen fertilizers and wheat yield. J. Soil Sci. Agric. Eng. 2017, 8, 613–620. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef]
- Oancea, F. Development of Safe Nanoagrochemicals—The Nanoporous Route. Chem. Proc. 2022, 7, 68. [Google Scholar]
- L.E. N-FLEX: New Genetic Solution to Better Nitrogen Efficiency. Available online: https://www.limagrain-europe.com/en/n-flex (accessed on 30 April 2023).
- Nigam, H.; Malik, A.; Singh, V. A novel nanoemulsion-based microalgal growth medium for enhanced biomass production. Biotechnol. Biofuels 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Mala, R.; Selvaraj, R.C.A.; Sundaram, V.B.; Rajan, R.; Gurusamy, U.M. Evaluation of nano structured slow release fertilizer on the soil fertility, yield and nutritional profile of Vigna radiata. Recent Pat. Nanotechnol. 2017, 11, 50–62. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion gel formulation optimization for burn wounds: Analysis of rheological and sensory properties. Processes 2020, 8, 1416. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Gassem, M.A.; Athinarayanan, J.; Periyasamy, V.S.; Prasad, S.; Alshatwi, A.A. Antifungal activity of nanoemulsion from Cleome viscosa essential oil against food-borne pathogenic Candida albicans. Saudi J. Biol. Sci. 2021, 28, 286–293. [Google Scholar] [CrossRef]
- Ravichandran, M.; Samiappan, S.C.; Rangaraj, S.; Murugan, K.; Al-Dhabi, N.A.; Karuppiah, P. Nanoemulsion formulations with plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. In Bio-Based Nanoemulsions for Agri-Food Applications; Abd-Elsalam, K.A., Murugan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–223. [Google Scholar] [CrossRef]
- Chime, S.; Kenechukwu, F.; Attama, A. Nanoemulsions—Advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery; BoD–Books on Demand: Norderstedt, Germany, 2014; Volume 3, pp. 77–126. [Google Scholar]
- Arora, D.; Jaglan, S. Nanocarriers for resveratrol delivery. In Nanoscience in Food and Agriculture 5; Springer: Berlin/Heidelberg, Germany, 2017; pp. 123–138. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, P.; Rastegari, A.; Mottaghitalab, F.; Farokhi, M.; Zarrintaj, P.; Saeb, M.R. Nanoemulsions for intravenous drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 581–601. [Google Scholar] [CrossRef]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-based technologies for delivering natural plant-based antimicrobials in foods. Front. Sustain. Food Syst. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Banerjee, R.; Biswas, S.; McClements, D.J. Application of nanoemulsion-based approaches for improving the quality and safety of muscle foods: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2677–2700. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Kalia, A.; Sharma, S.P. Nanomaterials and vegetable crops: Realizing the concept of sustainable production. In Nanoscience for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2019; pp. 323–353. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Ion, V. Effect of nano-silicon foliar application on safflower growth under organic and inorganic fertilizer regimes. Bot. Lith. 2016, 22, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Mejias, J.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A cutting-edge approach to increase nitrogen use efficiency in grasslands. Front. Environ. Sci. 2021, 9, 52. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Current and future perspectives on the use of nanofertilizers for sustainable agriculture: The case of phosphorus nanofertilizer. 3 Biotech 2021, 11, 357. [Google Scholar] [CrossRef]
- Shilpa, R.S.; Kant, C.; Prashar, N. Role of nanofertilizers in horticulture: A review. Pharma Innov. J. 2022, 11, 831–836. [Google Scholar]
- Sharma, B.; Lakra, U.; Sharma, R.; Sharma, S.R. A comprehensive review on nanopesticides and nanofertilizers—A boon for agriculture. In Nano-Enabled Agrochemicals in Agriculture; Ghorbanpour, M., Ed.; Elsivier: London, UK, 2022; pp. 273–290. [Google Scholar] [CrossRef]
- Rani Sarkar, M.; Rashid, M.H.-O.; Rahman, A.; Kafi, M.A.; Hosen, M.I.; Rahman, M.S.; Khan, M.N. Recent advances in nanomaterials based sustainable agriculture: An overview. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100687. [Google Scholar] [CrossRef]
- Ghumman, A.S.M.; Shamsuddin, R.; Nasef, M.M.; Yahya, W.Z.N.; Abbasi, A.; Almohamadi, H. Sulfur enriched slow-release coated urea produced from inverse vulcanized copolymer. Sci. Total Environ. 2022, 846, 157417. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Thirunavukkarasu, M. Nano-fertilizers and Nutrient Transformations in Soil. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 305–319. [Google Scholar] [CrossRef]
- Dwivedi, B.; Singh, V.; Meena, M.; Dey, A.; Datta, S. Integrated nutrient management for enhancing nitrogen use efficiency. Indian J. Fertil 2016, 12, 62–71. [Google Scholar]
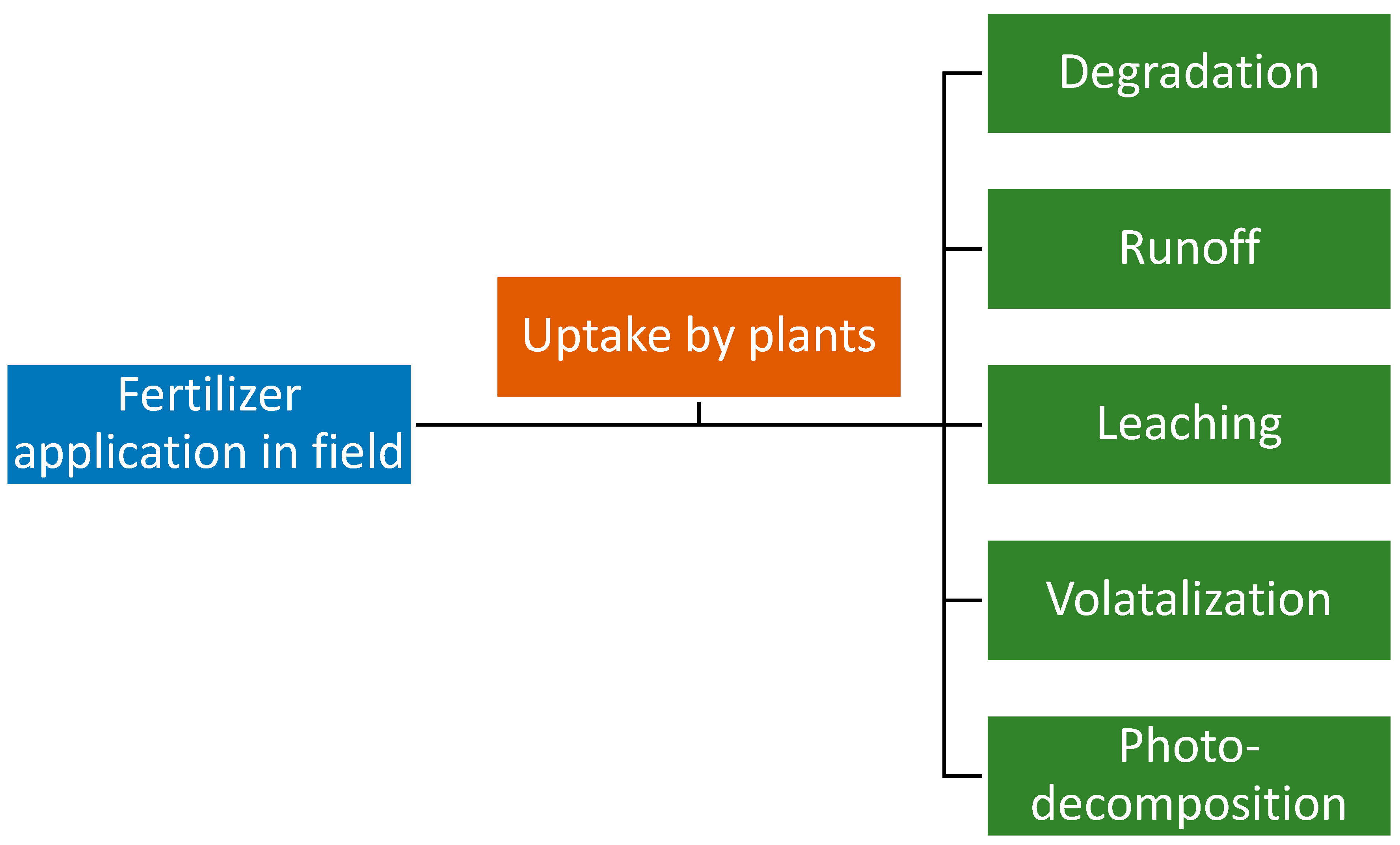
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- Kahrl, F.; Li, Y.; Su, Y.; Tennigkeit, T.; Wilkes, A.; Xu, J. Greenhouse gas emissions from nitrogen fertilizer use in China. Environ. Sci. Policy 2010, 13, 688–694. [Google Scholar] [CrossRef]
- Rahale, S. Nutrient Release Pattern of Nanofertilizer Formulation. Ph.D. Thesis, Tamilnadu Agricultural University, Coimbatore, India, 2011. [Google Scholar]
- Adhikari, T.; Kundu, S.; Meena, V.; Rao, A.S. Utilization of nano rock phosphate by maize (Zea mays L.) crop in a vertisol of Central India. J. Agric. Sci. Technol. A 2014, 4, 384–394. [Google Scholar]
- Tang, S.; Fei, X. Refractory calcium phosphate-derived phosphorus fertilizer based on hydroxyapatite nanoparticles for nutrient delivery. ACS Appl. Nano Mater. 2021, 4, 1364–1376. [Google Scholar] [CrossRef]
- Priyam, A.; Yadav, N.; Reddy, P.M.; Afonso, L.O.B.; Schultz, A.G.; Singh, P.P. Fertilizing benefits of biogenic phosphorous nanonutrients on Solanum lycopersicum in soils with variable pH. Heliyon 2022, 8, e09144. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, T.; Arif, M.S.; Shahzad, S.M.; Riaz, M.; Tufail, M.A.; Mubarik, M.S.; Ahmad, A.; Ali, S.; Albasher, G.; Shakoor, A. Abandoned agriculture soil can be recultivated by promoting biological phosphorus fertility when amended with nano-rock phosphate and suitable bacterial inoculant. Ecotoxicol. Environ. Saf. 2022, 234, 113385. [Google Scholar] [CrossRef]
- Saraiva, R.; Rodrigues, G.; Ferreira, Q.; Oliveira, M.M. The use of nanofertilizers to increase precision in rice production. In 16th Conference on Sustainable Development of Energy, Water and Environment Systems; International Centre for Sustainable Development of Energy, Water and Environment Systems: Dubrovnik, Croatia, 2021. [Google Scholar]
- Sheoran, P.; Goel, S.; Boora, R.; Kumari, S.; Yashveer, S.; Grewal, S. Biogenic synthesis of potassium nanoparticles and their evaluation as a growth promoter in wheat. Plant Gene 2021, 27, 100310. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Jing, P.; Zou, J.; Kong, L.; Hu, S.; Wang, B.; Yang, J.; Xie, G. OsCCD1, a novel small calcium-binding protein with one EF-hand motif, positively regulates osmotic and salt tolerance in rice. Plant Sci. 2016, 247, 104–114. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca(2+) influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Malik, Z.; Afzal, S.; Danish, M.; Abbasi, G.H.; Bukhari, S.A.H.; Khan, M.I.; Dawood, M.; Kamran, M.; Soliman, M.H.; Rizwan, M. Role of nitric oxide and calcium signaling in abiotic stress tolerance in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John and Wiley Sons: Hoboken, NJ, USA, 2020; pp. 563–581. [Google Scholar]
- Ruiqiang, L.; Rattan, L. Nanofertilizers. In Encyclopedia of Soil Science; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 3. [Google Scholar]
- Carmona, F.J.; Dal Sasso, G.; Bertolotti, F.; Ramirez-Rodriguez, G.B.; Delgado-Lopez, J.M.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. The role of nanoparticle structure and morphology in the dissolution kinetics and nutrient release of nitrate-doped calcium phosphate nanofertilizers. Sci. Rep. 2020, 10, 12396. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized calcium phosphates as novel macronutrient nano-fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Perez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-Lopez, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Carmona, F.J.; Dal Sasso, G.; Ramirez-Rodriguez, G.B.; Pii, Y.; Delgado-Lopez, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Huang, Y.; Carvalho, R.; Choudhary, M.; Da Silva, S.; Colee, J.; Huerta, A.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 2021, 55, 13561–13570. [Google Scholar] [CrossRef] [PubMed]
- Delfani, M.; Baradarn Firouzabadi, M.; Farrokhi, N.; Makarian, H. Some Physiological Responses of Black-Eyed Pea to Iron and Magnesium Nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Khordadi Varamin, J.; Fanoodi, F.; Sinaki, J.M.; Rezvan, S.; Damavandi, A. Physiological response of sesame (Sesamum indicum L.) to application of chitosan and magnesium-nano fertilizers under irrigation cut-off in a sustainable agriculture system. Iran. J. Plant Physiol. 2018, 9, 2629–2639. [Google Scholar]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Detrick, J.H. Process for Producing Improved Sulfur-Coated Urea Slow Release Fertilizers. U.S. Patent 5,599,374A, 4 February 1997. [Google Scholar]
- Li, T.; Gao, B.; Tong, Z.; Yang, Y.; Li, Y. Chitosan and graphene oxide nanocomposites as coatings for controlled-release fertilizer. Water Air Soil Pollut. 2019, 230, 1–9. [Google Scholar] [CrossRef]
- Gehlout, S.; Priyam, A.; Afonso, L.; Schultze, G.A.; Singh, P.P. Application of metallic nanoparticles as agri inputs: Modulation in nanoparticle design and application dosage needed. In Nanotechnology in Agriculture and Environmental Science; Deshmukh, S.K., Kochar, M., Kaur, P., Singh, P.P., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2022; pp. 16–54. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, T.; Hebsur, N. Effect of nanofertilizers on growth and yield of selected cereals—A review. Agric. Rev. 2017, 38, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Chhipa, H.; Joshi, P. Nanofertilisers, nanopesticides and nanosensors in agriculture. In Nanoscience in Food and Agriculture 1; Springer: Berlin/Heidelberg, Germany, 2016; pp. 247–282. [Google Scholar] [CrossRef]
- Sharipova, A.; Psakhie, S.; Gotman, I.; Gutmanas, E. Smart nanocomposites based on Fe–Ag and Fe–Cu nanopowders for biodegradable high-strength implants with slow drug release. Phys. Mesomech. 2020, 23, 128–134. [Google Scholar] [CrossRef]
- Liu, A.; Wang, W.; Liu, J.; Fu, R.; Zhang, W.X. Nanoencapsulation of arsenate with nanoscale zero-valent iron (nZVI): A 3D perspective. Sci. Bull. 2018, 63, 1641–1648. [Google Scholar] [CrossRef] [Green Version]
- Pitambara; Archana; Shukla, Y.M. Nanofertilizers: A Recent Approach in Crop Production. In Nanotechnology for Agriculture: Crop Production & Protection; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–58. [Google Scholar] [CrossRef]
- Cui, H.; Yi, Q.; Yang, X.; Wang, X.; Wu, H.; Zhou, J. Effects of hydroxyapatite on leaching of cadmium and phosphorus and their availability under simulated acid rain. J. Environ. Chem. Eng. 2017, 5, 3773–3779. [Google Scholar] [CrossRef]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A micronutrient essential for higher plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A. Plant Nutrients for Citrus Trees; University of Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, EDIS: Gainesville, FL, USA, 2003. [Google Scholar]
- Tsygvintsev, P.N.; Goncharova, L.I.; Manin, K.V.; Rachkova, V.M. Morphophysiological features of wheat (Triticum aestivum L.) seedlings upon exposure to nickel nanoparticles. Sel’skokhozyaistvennaya Biol. 2018, 53, 578–586. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almaary, K.; Ismail, A.; Polizzi, G. Influence of Funneliformis mosseae enhanced with titanium dioxide nanoparticles (TiO2NPs) on Phaseolus vulgaris L. under salinity stress. PLoS ONE 2020, 15, e0235355. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Hortic. Plants 2013, 3, 25–32. [Google Scholar]
- Feizi, H.; Rezvani Moghaddam, P.; Shahtahmassebi, N.; Fotovat, A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace Elem. Res. 2012, 146, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, P.; Grewal, S.; Kumari, S.; Goel, S. Enhancement of growth and yield, leaching reduction in Triticum aestivum using biogenic synthesized zinc oxide nanofertilizer. Biocatal. Agric. Biotechnol. 2021, 32, 101938. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, I.K.; Mishra, R.; Singh, A.; Ramawat, N.; Singh, A. The role of zinc oxide nanoparticles in plants: A critical appraisal. In Nanomaterial Biointeractions at the Cellular, Organismal and System Levels; Springer: Berlin/Heidelberg, Germany, 2021; pp. 249–267. [Google Scholar] [CrossRef]
- Das, A.; Das, B. Nanotechnology a Potential Tool to Mitigate Abiotic Stress in Crop Plants; IntechOpen: London, UK, 2019. [Google Scholar]
- Singh, A.; Rajput, V.D.; Rawat, S.; Sharma, R.; Singh, A.K.; Kumar, P.; Singh, A.K.; Minkina, T.; Singh, R.P.; Singh, S. Geoinformatics and nanotechnological approaches for coping up abiotic and biotic stress in crop plants. Sustain. Agric. Syst. Technol. 2022, 337–359. [Google Scholar] [CrossRef]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Influence of zinc oxide nanoparticles on growth of Sesamum indicum L. in zinc deficient soil. Int. J. Pharm. Pharm. Sci. 2016, 365–371. [Google Scholar]
- Kale, A.P.; Gawade, S.N. Studies on nanoparticle induced nutrient use efficiency of fertilizer and crop productivity. Green Chem. Technol. Lett. 2016, 2, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Tarafdar, J.; Raliya, R.; Mahawar, H.; Rathore, I. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Punitha, V.N.; Vijayakumar, S.; Alsalhi, M.S.; Devanesan, S.; Nilavukkarasi, M.; Vidhya, E.; Kumar, S.P.; Kim, W. Biofabricated ZnO nanoparticles as vital components for agriculture revolutionization—A green approach. Appl. Nanosci. 2023. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Soman, R.; Balachandran, S. Trends and technologies behind controlled-release fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 155–168. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic matter–microorganism–plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Bio/Technol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, L.C.; Choudhary, J.; Kannojiya, S.; Kundu, M.; Kumar, S.; Yadav, A.N. Soil microbiomes for healthy nutrient recycling. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 1–21. [Google Scholar]
- Ekinci, M.; Dursun, A.; Yildirim, E.; Parlakova, F. Effects of nanotechnology liquid fertilizers on the plant growth and yield of cucumber (Cucumis sativus L.). Acta Sci. Pol. Hortorum Cultus 2014, 13, 135–141. [Google Scholar]
- Tarafder, C.; Daizy, M.; Alam, M.M.; Ali, M.R.; Islam, M.J.; Islam, R.; Ahommed, M.S.; Aly Saad Aly, M.; Khan, M.Z.H. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.S.; Manikandan, A.; Thirunavukkarasu, M.; Rahale, C.S. Nano-fertilizers for balanced crop nutrition. In Nanotechnologies in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 69–80. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Sharif, R.; Ali, Q.; Rehman, R.; Khawar, K.M. Biopolymer based nanofertilizers applications in abiotic stress (drought and salinity) control. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–110. [Google Scholar] [CrossRef]
- Kong, X.-P.; Zhang, B.-H.; Wang, J. Multiple roles of mesoporous silica in safe pesticide application by nanotechnology: A review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 81–101. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–116472. [Google Scholar] [CrossRef]
- Badran, A. Management of nano-fertilizers as slow release fertilizers. In Proceedings of the Modern Paradigm of Scientific Knowledge: Relevance and Prospects, Moscow, Russia, 13 April 2016; pp. 52–57. [Google Scholar]
- Singh, P.; Singh, R.; Verma, P.; Bhadouria, R.; Kumar, A.; Kaushik, M. Plant-Microbes-Engineered Nano-Particles (PM-ENPs) Nexus in Agro-Ecosystems: Understanding the Interaction of Plant, Microbes and Engineered Nano-Particles (ENPS); Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Salman, O.A. Polyethylene-coated urea. 1. Improved storage and handling properties. Ind. Eng. Chem. Res. 1989, 28, 630–632. [Google Scholar] [CrossRef]
- Nagar, H.; Badhrachalam, N.; Rao, V.B.; Sridhar, S. A novel microbial fuel cell incorporated with polyvinylchloride/4A zeolite composite membrane for kitchen wastewater reclamation and power generation. Mater. Chem. Phys. 2019, 224, 175–185. [Google Scholar] [CrossRef]
- Iqbal, M.; Umar, S.; Mahmooduzzafar. Nano-fertilization to enhance nutrient use efficiency and productivity of crop plants. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 473–505. [Google Scholar] [CrossRef]
- Wilson, M.A.; Tran, N.H.; Milev, A.S.; Kannangara, G.K.; Volk, H.; Lu, G.M. Nanomaterials in soils. Geoderma 2008, 146, 291–302. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Sobol, M. Oligo-Alginate with Low Molecular Mass Improves Growth and Physiological Activity of Eucomis autumnalis under Salinity Stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A. Nano-fertilizers for sustainable crop production under changing climate: A global perspective. Sustain. Crop Prod. 2019, 8, 1–13. [Google Scholar]
- Kamali, K.; Mirezabadi, M.S.; Zamani, E. Comparison of using some organic and Nano Biofertilizers on morphologic traits and extraction components of Echinacea purpurea as a medicinal plant. Appl. Soil Res. 2023, 10, 1–10. [Google Scholar]
- Gahoi, P.; Omar, R.A.; Verma, N.; Gupta, G.S. Rhizobacteria and Acylated homoserine lactone-based nanobiofertilizer to improve growth and pathogen defense in Cicer arietinum and Triticum aestivum Plants. ACS Agric. Sci. Technol. 2021, 1, 240–252. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, H. Nano-biofertilizers: Harnessing dual benefits of nano-nutrient and bio-fertilizers for enhanced nutrient use efficiency and sustainable productivity. Nanosci. Sustain. Agric. 2019, 51–73. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kumar, R.; Mishra, R.K.; Pandey, A.; Pathak, A.; Zaidi, M.; Srivastava, S.K.; Dikshit, A. Prediction and validation of gold nanoparticles (GNPs) on plant growth promoting rhizobacteria (PGPR): A step toward development of nano-biofertilizers. Nanotechnol. Rev. 2015, 4, 439–448. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, D.; Manyapu, V.; Yadav, M.; Majumder, S. Synergistic impact of iron (III) oxide nano-particles and organic waste on growth and development of Solanum lycopersicum plants: New paradigm in nanobiofertilizer. Plant Arch. 2019, 19, 339–344. [Google Scholar]
- Dutta, S.; Pal, S.; Panwar, P.; Sharma, R.K.; Bhutia, P.L. Biopolymeric Nanocarriers for Nutrient Delivery and Crop Biofortification. ACS Omega 2022, 7, 25909–25920. [Google Scholar] [CrossRef]
- Singh, M.D. Nano-fertilizers is a new way to increase nutrients use efficiency in crop production. Int. J. Agric. Sci. ISSN 2017, 9, 0975–3710. [Google Scholar]
- Zhang, P.; Guo, Z.; Ullah, S.; Melagraki, G.; Afantitis, A.; Lynch, I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Orsiere, T.; De Meo, M.; Thill, A.; Zeyons, O.; Proux, O.; Masion, A.; Chaurand, P.; Spalla, O. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology 2009, 3, 161–171. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, X.; Meng, H.; Xia, T.; Liu, H.; Rolshausen, P.; Roper, C.; McLean, J.E.; Zhang, Y.; Keller, A.A. Cost–benefit analysis of nanofertilizers and nanopesticides emphasizes the need to improve the efficiency of nanoformulations for widescale adoption. Nat. Food 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2018, 66, 6462–6473. [Google Scholar] [CrossRef]
- Azeem, A.; Abbas, N.; Azeem, S.; Iqbal, Z.; Ul-Allah, S. Physiological and Molecular Mechanism of Nanoparticles Induced Tolerance in Plants. In Emerging Contaminants and Plants; Aftab, T., Ed.; Emerging Contaminants and Associated Treatment Technologies; Springer International Publishing: Cham, Switzerland, 2023; pp. 233–248. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef] [PubMed]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Elkafafi, S.; Zedan, A.M.; Dawoud, S.F. Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Prod. 2017, 8, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the soil–plant system: A review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Mishra, M.; Dashora, K.; Srivastava, A.; Fasake, V.D.; Nag, R.H. Prospects, challenges and need for regulation of nanotechnology with special reference to India. Ecotoxicol. Environ. Saf. 2019, 171, 677–682. [Google Scholar] [CrossRef]
- Liscano, J.; Wilson, C.; Norman, R., Jr.; Slaton, N. Zinc Availability to Rice from Seven Granular Fertilizers; Arkansas Agricultural Experiment Station Fayetteville: Fayetteville, CA, USA, 2000; p. 963. [Google Scholar]
- Singh, A.; Singh, N.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Butt, B.Z.; Naseer, I. Nanofertilizers. In Nanoagronomy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 125–152. [Google Scholar] [CrossRef]
- Elsayed, A.A.A.; El-Gohary, A.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, J.; Xiang, Y.; Wang, W.; Dong, Q.; Biswas, P. Nanoparticle synthesis characterization and application to solve some chronic agricultural problems. Appl. Biol. Res. 2012, 14, 138–144. [Google Scholar]
- Sinha, T.; Bhagwatwar, P.; Krishnamoorthy, C.; Chidambaram, R. Polymer based micro-and nanoencapsulation of agrochemicals. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer: Cham, Switzerland, 2019; pp. 5–28. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Arakere, U.C.; Chowdappa, S.; Akbarbasha, R.; Ramachandrappa, N.S. Nanofertilizers and nanopesticides: Recent trends, future prospects in agriculture. In Advances in Nano-Fertilizers and Nano-Pesticides, in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., Lima, R., Eds.; Elsivier Science: Amsterdam, The Netherlands, 2021; pp. 281–330. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Cui, H.X.; Sun, C.J.; Liu, Q.; Jiang, J.; Gu, W. Applications of nanotechnology in agrochemical formulation. In Proceedings of the International Conference on Nanoagri, Sao Pedro, Brazil, 20–25 June 2010; pp. 28–33. [Google Scholar]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostructures 2018, 13, 315–323. [Google Scholar]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency–Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef] [PubMed]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R.A.; Krishnankutty, R.E. Application of encapsulated Bacillus licheniformis supplemented with chitosan nanoparticles and rice starch for the control of Sclerotium rolfsii in Capsicum annuum (L.) seedlings. Curr. Microbiol. 2021, 78, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.U.; Poczai, P. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Sambangi, P.; Srinivas, V.; Gopalakrishnan, S. Crop Microbiome for Sustainable Agriculture in Special Reference to Nanobiology. In Plant Microbiome for Plant Productivity and Sustainable Agriculture; Chhabra, S., Prasad, R., Maddela, N.R., Tuteja, N., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2023; pp. 81–97. [Google Scholar] [CrossRef]
- Yaseen, R.; Ahmed, A.I.S.; Omer, A.M.; Agha, M.K.M.; Emam, T.M. Nano-fertilizers: Bio-fabrication, application and biosafety. Nov. Res. Microbiol. J. 2020, 4, 884–900. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Z.; Hong, A.; Zhang, X.; Kah, M.; Li, L.; Wang, Y. Response of soil enzyme activity and bacterial community to copper hydroxide nanofertilizer and its ionic analogue under single versus repeated applications. Sci. Total Environ. 2021, 796, 148974. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Mineralization of soil carbon, nitrogen, and phosphorus and role of nanofertilizers in soil fertility and plant growth. In Structure and Functions of Pedosphere; Giri, B., Kapoor, R., Wu, Q., Varma, A., Eds.; Springer: Singapore, 2022; pp. 393–409. [Google Scholar] [CrossRef]
- Das, P.; Davis, K.; Penton, C.R.; Westerhoff, P.; Bi, Y. Impacts of graphitic nanofertilizers on nitrogen cycling in a sandy, agricultural soil. J. Nanoparticle Res. 2022, 24, 1–21. [Google Scholar] [CrossRef]
- Pide, J.L.V.; Organo, N.D.; Cruz, A.F.; Fernando, L.M.; Villegas, L.C.; Delfin, E.F.; Calubaquib, M.A.M.; Madayag, R.E.; Paterno, E.S. Effects of nanofertilizer and nano-plant hormone on soil chemical properties and microbial community in two different soil types. Pedosphere 2022. [Google Scholar] [CrossRef]
- Mastronardi, E.; Tsae, P.; Zhang, X.; Monreal, C.; DeRosa, M.C. Strategic role of nanotechnology in fertilizers: Potential and limitations. In Nanotechnologies in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 25–67. [Google Scholar] [CrossRef]
- León-Silva, S.; Arrieta-Cortes, R.; Fernández-Luqueño, F.; López-Valdez, F. Design and Production of Nanofertilizers. In Agricultural Nanobiotechnology; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 17–31. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Joshi, A.; Tian, D.D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.R. Recent Trends in Nano-Fertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Shoults-Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.V.; Unrine, J.M. Role of particle size and soil type in toxicity of silver nanoparticles to earthworms. Soil Sci. Soc. Am. J. 2011, 75, 365–377. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bradu, P.; Biswas, A.; Nair, C.; Sreevalsakumar, S.; Patil, M.; Kannampuzha, S.; Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; et al. Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ. Sci. Pollut. Res. Int. 2022, 1–32. [Google Scholar] [CrossRef]
- Toksha, B.; Sonawale, V.A.M.; Vanarase, A.; Bornare, D.; Tonde, S.; Hazra, C.; Kundu, D.; Satdive, A.; Tayde, S.; Chatterjee, A. Nanofertilizers: A review on synthesis and impact of their use on crop yield and environment. Environ. Technol. Innov. 2021, 24, 101986. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munne-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Saul, A. From the green revolution to the green chemistry revolution: In pursuit of a paradigm shift in agricultural sustainability. In Go Green for Environmental Sustainability; CRC Press: Boca Raton, FL, USA, 2021; pp. 47–66. [Google Scholar] [CrossRef]
- Lombi, E.; Donner, E.; Dusinska, M.; Wickson, F. A One Health approach to managing the applications and implications of nanotechnologies in agriculture. Nat. Nanotechnol. 2019, 14, 523–531. [Google Scholar] [CrossRef] [PubMed]
| Nanofertilizer Type | Advantages | Ref. | Disadvantages | Ref. |
|---|---|---|---|---|
| Carbon-based | promote plant growth, increase water and nutrient retention, help during drought | [46] | time consuming synthesis methods | [47] |
| Chitosan-based | biodegradable, adjustable in size, easy to modify, protect biomolecules from environmental factors | [48,49] | hydrophilicity, weak mechanical properties, low gas permeability, low encapsulation efficiency | [50] |
| Clay-based | large surface area, nanolayer reactivity, regulate the release of anions | [51] | can inhibit leaf growth and transpiration | [52] |
| Nanocapsule-based | controlled nutrient release, efficient nutrient delivery, reduced risk of leaching | [53,54] | require complex synthesis processes, subject to material limitations | |
| Nanogel-based | highly soluble, biodegradable, non-toxic, improves water retention | [55] | limitations regarding the optimization of biodistribution, degradation mechanism, and component toxicity | [56] |
| Polyurethane-based | controlled nutrient release, improved water-holding capacity, reduced soil erosion | [57] | weak chemical and thermal stability, rapid elimination, lower polymer life span due to the formation of acid monomers in polymer matrix | [58] |
| Starch-based | renewable energy source, effective nutrient delivery, minimal chemical waste | [59] | expensive and time-consuming, unstable nature | [60] |
| Zeolite-based | improved nutrient delivery, tailored nutrient provision, reduced fertilization cost | [45,61] | require specific formulations and synthesis processes for optimal results, not useful in the management of anionic nutrients and need to be complemented with biopolymers and biopolymer complexes | [62] |
| Nanofertilizer Type | Advantage | Reference | Disadvantages | References |
|---|---|---|---|---|
| Controlled-release nanofertilizers (CRNFs) | Gradual release of nutrients, reducing nutrient leaching and losses to the environment | [7] | More complex manufacturing processes, potentially increase production costs | [8] |
| Improved nutrient use efficiency, resulting in higher crop yields | [31] | Limited availability and high cost may hinder widespread adoption | [157] | |
| Nanofertilizers for targeted delivery | Precise delivery of nutrients to specific plant tissues, enhancing nutrient uptake | [69] | Potential risks to non-target organisms due to high specificity | [69] |
| Reduced application rates, minimized environmental impact and conserving resources | [180] | Further research is needed to fully understand the long-term effects on soil health and ecosystems | [181] | |
| Plant growth-stimulating nanofertilizers (PGSNFs) | Enhanced plant growth, leading to increased crop yields | [182] | Possible unintended effects on plant physiology and gene expression | [183] |
| Reduced dependency on chemical fertilizers, lower environmental pollution | [173] | Long-term impacts on plant health and soil ecosystems not fully understood | [8] | |
| Water and nutrient loss-controlling fertilizers | Improved water use efficiency, reducing irrigation requirements | [69,184] | Limited research on the long-term impacts of WNLCFs on soil health | [157] |
| Prevention of nutrient leaching, minimized environmental pollution | [8,31,150,157,185] | Potential for increased production costs due to more complex formulations | [186] |
| Properties | Nano Fertilizers | Conventional Fertilizers |
|---|---|---|
| Nutrient uptake efficiency | Increases fertilizer utilization efficiency and the ratio of plant nutrient uptake while saving fertilizers. | Less effective since its bulk composites are poorly absorbed by plants. |
| Control release modes | Encapsulation, in conjunction with a covering of polymer resin, waxes, and sulfur, permits precise control over the release of nutrients. | Excessive release results in toxicity and undermines ecological balance. |
| Solubility and dispersion of nutrients | Increases the solubility and dispersion of insoluble mineral components in soil, making them more bioavailable to plants. | Less available to plants due to lower solubility and larger particle size |
| Effective duration of release | Improves and prolongs the plant’s nutrient acquisition rate | During delivery, nutrients required by plants are lost as insoluble salts. |
| Low rate of fertilizer needed | Reduces nutrient losses resulting from leaching, runoff, and drift. | High fertilizer levels are lost due to leaching, runoff, and drift. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals 2023, 2, 296-336. https://doi.org/10.3390/agrochemicals2020019
Yadav A, Yadav K, Abd-Elsalam KA. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals. 2023; 2(2):296-336. https://doi.org/10.3390/agrochemicals2020019
Chicago/Turabian StyleYadav, Anurag, Kusum Yadav, and Kamel A. Abd-Elsalam. 2023. "Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability" Agrochemicals 2, no. 2: 296-336. https://doi.org/10.3390/agrochemicals2020019
APA StyleYadav, A., Yadav, K., & Abd-Elsalam, K. A. (2023). Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals, 2(2), 296-336. https://doi.org/10.3390/agrochemicals2020019







