Experimental and Pre-Analytical Considerations of Endocannabinoid Quantification in Human Biofluids Prior to Mass Spectrometric Analysis
Abstract
1. Introduction
2. Endocannabinoid Structure
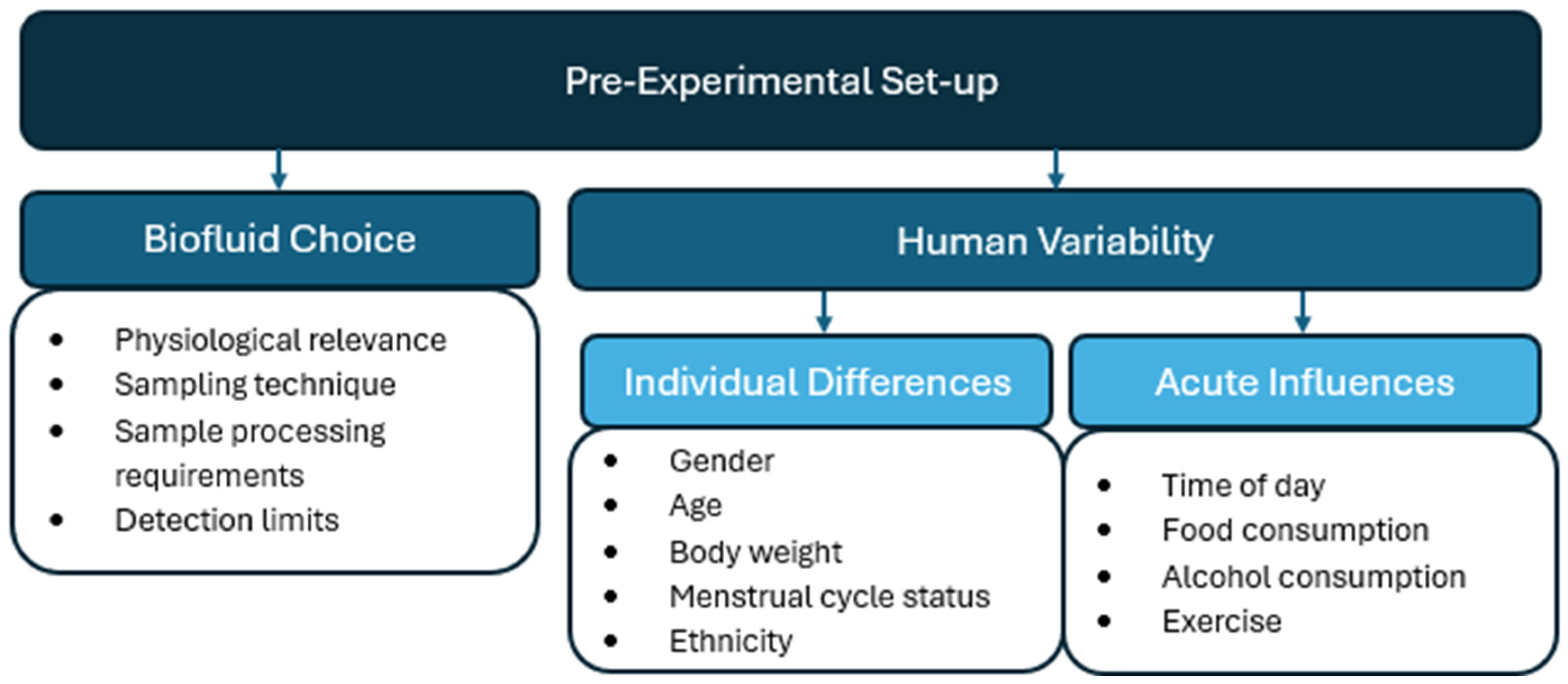
3. Pre-Experimental Considerations
3.1. Biofluid Choice
3.2. Human Variability
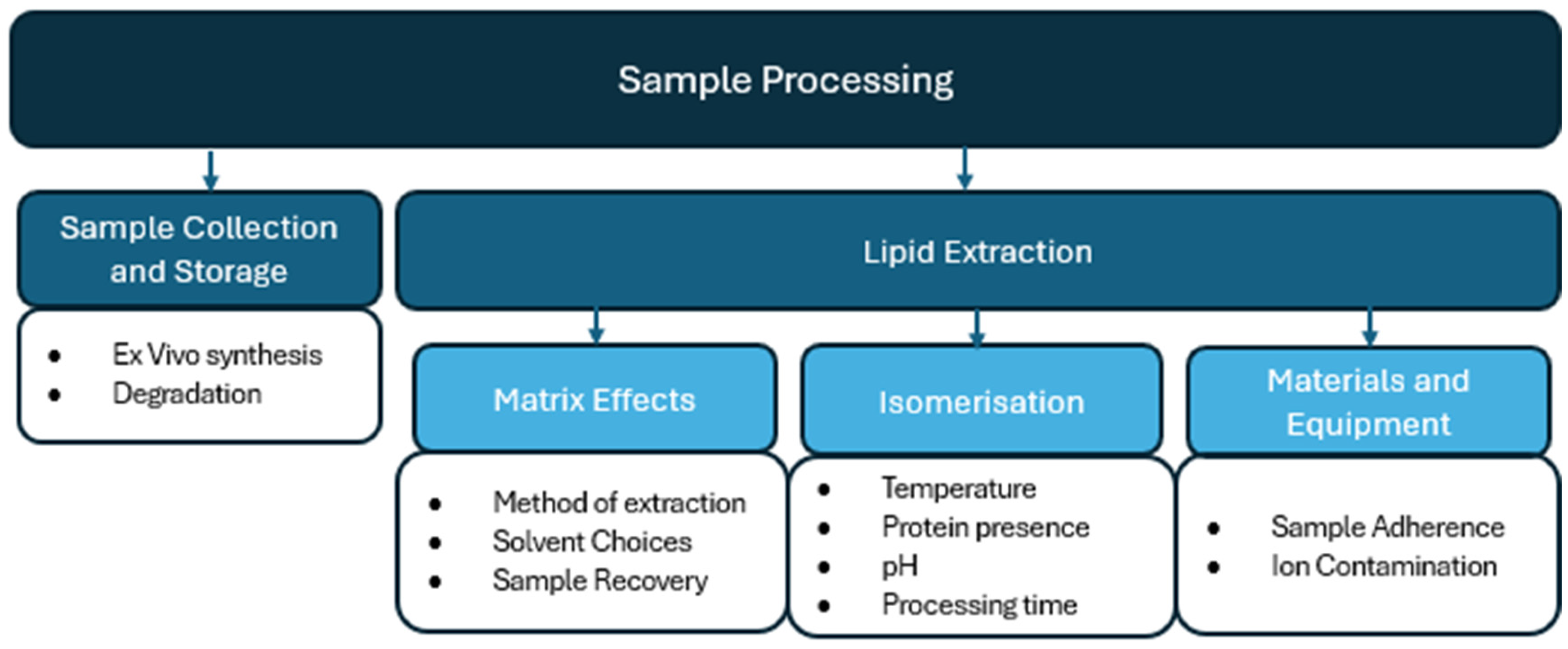
4. Sample Processing Considerations
4.1. Sample Collection and Storage
4.2. Lipid Extraction
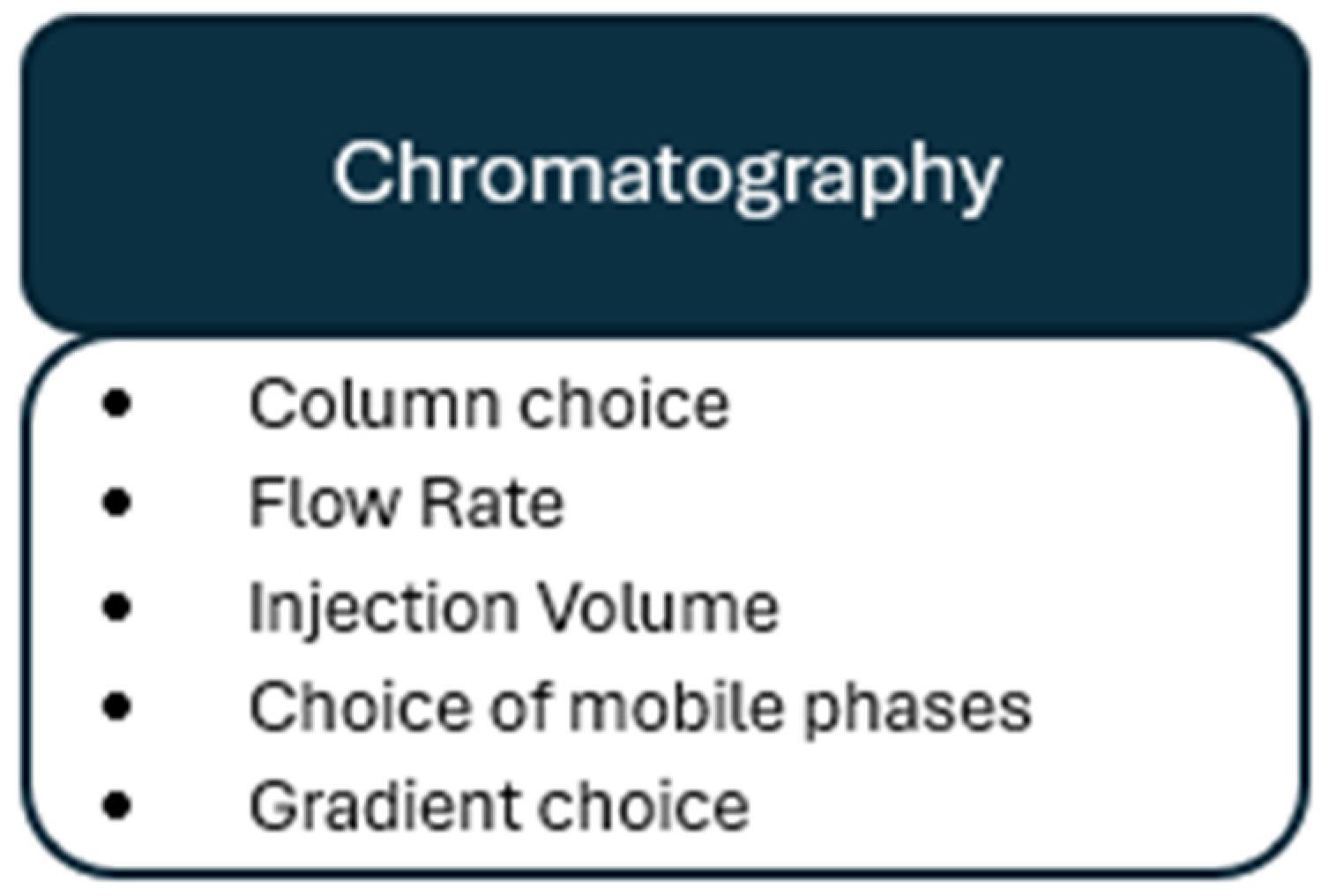
5. Chromatography
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEA | N-arachidonoyl-ethanolamine |
| 2-AG | 2-arachidonoylglycerol |
| FAAH | Fatty acid amide hydrolase |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| GC | Gas chromatography |
| LC | Liquid chromatography |
| CSF | Cerebrospinal fluid |
| SPE | Solid-phase extraction |
| LLE | Liquid–liquid extraction |
| HPLC | High-performance liquid chromatography |
| UHPCL | Ultra-high-performance liquid chromatography |
References
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Simard, M.; Archambault, A.-S.; Lavoie, J.-P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef]
- PSchmid, C.; Reddy, P.V.; Natarajan, V.; Schmid, H.H. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J. Biol. Chem. 1983, 258, 9302–9306. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Lichtman, A.H. Fatty acid amide hydrolase: An emerging therapeutic target in the endocannabinoid system. Curr. Opin. Chem. Biol. 2003, 7, 469–475. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Jurado-Barba, R.; Rubio, G.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry 2020, 11, 315. [Google Scholar] [CrossRef]
- Skaper, S.D.; Di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Shevchenko, A. Quantification of Endogenous Endocannabinoids by LC-MS/MS. In Lipidomics; Wood, P., Ed.; Springer New York: New York, NY, USA, 2017; pp. 99–107. [Google Scholar]
- Röhrig, W.; Achenbach, S.; Deutsch, B.; Pischetsrieder, M. Quantification of 24 circulating endocannabinoids, endocannabinoid-related compounds, and their phospholipid precursors in human plasma by UHPLC-MS/MS. J. Lipid Res. 2019, 60, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Couttas, T.A.; Boost, C.; Pahlisch, F.; Sykorova, E.B.; Leweke, J.E.; Koethe, D.; Endepols, H.; Rohleder, C.; Leweke, F.M. Simultaneous Assessment of Serum Levels and Pharmacologic Effects of Cannabinoids on Endocannabinoids and N-Acylethanolamines by Liquid Chromatography-Tandem Mass Spectrometry. Cannabis Cannabinoid Res. 2023, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Cebo, M.; Mielnik, J.; Richter, H.; Schüle, R.; Sievers-Engler, A.; Młynarz, P.; Lämmerhofer, M. UHPLC-ESI-MS/MS assay for quantification of endocannabinoids in cerebrospinal fluid using surrogate calibrant and surrogate matrix approaches. J. Pharm. Biomed. Anal. 2023, 222, 115090. [Google Scholar] [CrossRef]
- Di Filippo, M.; Pini, L.A.; Pelliccioli, G.P.; Calabresi, P.; Sarchielli, P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1224–1229. [Google Scholar] [CrossRef]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Simultaneous quantification of endocannabinoids, oleoylethanolamide and steroid hormones in human plasma and saliva. J. Chromatogr. B 2020, 1152, 122252. [Google Scholar] [CrossRef]
- Matias, I.; Gatta-Cherifi, B.; Tabarin, A.; Clark, S.; Leste-Lasserre, T.; Marsicano, G.; Piazza, P.V.; Cota, D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS ONE 2012, 7, e42399. [Google Scholar] [CrossRef]
- Jarvis, M.; Hamzah, K.A.; Nichols, D.; Ney, L.J. Hair and Saliva Endocannabinoid and Steroid Hormone Analysis by Liquid Chromatography Paired with Tandem Mass Spectrometry. Methods Mol. Biol. 2025, 2868, 135–147. [Google Scholar]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Chloroform-based liquid-liquid extraction and LC-MS/MS quantification of endocannabinoids, cortisol and progesterone in human hair. J. Pharm. Biomed. Anal. 2021, 201, 114103. [Google Scholar] [CrossRef]
- Gao, W.; Walther, A.; Wekenborg, M.; Penz, M.; Kirschbaum, C. Determination of endocannabinoids and N-acylethanolamines in human hair with LC-MS/MS and their relation to symptoms of depression, burnout, and anxiety. Talanta 2020, 217, 121006. [Google Scholar] [CrossRef]
- Vago, R.; Ravelli, A.; Bettiga, A.; Casati, S.; Lavorgna, G.; Benigni, F.; Salonia, A.; Montorsi, F.; Orioli, M.; Ciuffreda, P.; et al. Urine Endocannabinoids as Novel Non-Invasive Biomarkers for Bladder Cancer at Early Stage. Cancers 2020, 12, 870. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and gonadal hormones: Bidirectional interactions in physiology and behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018, 94, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Barrie, N.; Manolios, N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur. J. Rheumatol. 2017, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2016, 7, 1–15. [Google Scholar] [PubMed]
- Balsevich, G.; Petrie, G.N.; Hill, M.N. Endocannabinoids: Effectors of glucocorticoid signaling. Front. Neuroendocrinol. 2017, 47, 86–108. [Google Scholar] [CrossRef]
- Vähätalo, L.H.; Ruohonen, S.T.; Mäkelä, S.; Ailanen, L.; Penttinen, A.-M.; Stormi, T.; Kauko, T.; Piscitelli, F.; Silvestri, C.; Savontaus, E.; et al. Role of the endocannabinoid system in obesity induced by neuropeptide Y overexpression in noradrenergic neurons. Nutr. Diabetes 2015, 5, e151. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Di Lallo, V.D.; Belluomo, I.; De Iasio, R.; Baccini, M.; Casadio, E.; Gasparini, D.I.; Colavita, M.; Gambineri, A.; Grossi, G.; et al. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. J. Lipid Res. 2012, 53, 481–493. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Johnson, C.T. Measuring the Content of Endocannabinoid-Like Compounds in Biological Fluids: A Critical Overview of Sample Preparation Methodologies. Methods Mol. Biol. 2023, 2576, 21–40. [Google Scholar]
- Zoerner, A.A.; Batkai, S.; Suchy, M.T.; Gutzki, F.M.; Engeli, S.; Jordan, J.; Tsikas, D. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J. Chromatogr. B 2012, 883–884, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Gutzki, F.-M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta 2011, 1811, 706–723. [Google Scholar] [CrossRef] [PubMed]
- Kratz, D.; Thomas, D.; Gurke, R. Endocannabinoids as potential biomarkers: It’s all about pre-analytics. J. Mass Spectrom. Adv. Clin. Lab 2021, 22, 56–63. [Google Scholar] [CrossRef]
- Di Scala, C.; Fantini, J.; Yahi, N.; Barrantes, F.J.; Chahinian, H. Anandamide Revisited: How Cholesterol and Ceramides Control Receptor-Dependent and Receptor-Independent Signal Transmission Pathways of a Lipid Neurotransmitter. Biomolecules 2018, 8, 31. [Google Scholar] [CrossRef]
- Ottria, R.; Casati, S.; Rota, P.; Ciuffreda, P. 2-Arachidonoylglycerol Synthesis: Facile and Handy Enzymatic Method That Allows to Avoid Isomerization. Molecules 2022, 27, 5190. [Google Scholar] [CrossRef] [PubMed]
- Buczynski, M.W.; Parsons, L.H. Quantification of brain endocannabinoid levels: Methods, interpretations and pitfalls. Br. J. Pharmacol. 2010, 160, 423–442. [Google Scholar] [CrossRef]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavón, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef]
- Keereetaweep, J.; Chapman, K.D. Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification. Neural Plast. 2016, 2016, 2426398. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef]
- He, B.; Di, X.; Guled, F.; Harder, A.V.; Maagdenberg, A.M.v.D.; Terwindt, G.M.; Krekels, E.H.; Kohler, I.; Harms, A.; Ramautar, R.; et al. Quantification of endocannabinoids in human cerebrospinal fluid using a novel micro-flow liquid chromatography-mass spectrometry method. Anal. Chim. Acta 2022, 1210, 339888. [Google Scholar] [CrossRef]
- Meier, P.; Glasmacher, S.; Salmen, A.; Chan, A.; Gertsch, J. Comparative targeted lipidomics between serum and cerebrospinal fluid of multiple sclerosis patients shows sex and age-specific differences of endocannabinoids and glucocorticoids. Acta Neuropathol. Commun. 2024, 12, 160. [Google Scholar] [CrossRef]
- Ney, L.; Stone, C.; Nichols, D.; Felmingham, K.; Bruno, R.; Matthews, A. Endocannabinoid reactivity to acute stress: Investigation of the relationship between salivary and plasma levels. Biol. Psychol. 2021, 159, 108022. [Google Scholar] [CrossRef] [PubMed]
- Barba, S.V.; Kirschbaum, C.; Gao, W. Endocannabinoid and perceived stress: Association analysis of endocannabinoid levels in hair versus levels in plasma and urine. Biol. Psychol. 2023, 178, 108541. [Google Scholar]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 15781. [Google Scholar] [CrossRef]
- LeBeau, M.A.; Montgomery, M.A.; Brewer, J.D. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci. Int. 2011, 210, 110–116. [Google Scholar] [CrossRef]
- Kratz, D.; Sens, A.; Schäfer, S.M.G.; Hahnefeld, L.; Geisslinger, G.; Thomas, D.; Gurke, R. Pre-analytical challenges for the quantification of endocannabinoids in human serum. J. Chromatogr. B 2022, 1190, 123102. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; Konje, J.C. Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2089–2097. [Google Scholar] [CrossRef]
- Hillard, C.J.; Weinlander, K.M.; Stuhr, K.L. Contributions of endocannabinoid signaling to psychiatric disorders in humans: Genetic and biochemical evidence. Neuroscience 2012, 204, 207–229. [Google Scholar] [CrossRef]
- Hamzah, K.A.; Toms, L.M.; Kucharski, N.; Orr, J.; Turner, N.P.; Hobson, P.; Nichols, D.S.; Ney, L.J. Sex-dimorphism in human serum endocannabinoid and n-acyl ethanolamine concentrations across the lifespan. Sci. Rep. 2023, 13, 23059. [Google Scholar] [CrossRef]
- Ney, L.J.; Cooper, J.; Lam, G.N.; Moffitt, K.; Nichols, D.S.; Mayo, L.M.; Lipp, O.V. Hair endocannabinoids predict physiological fear conditioning and salivary endocannabinoids predict subjective stress reactivity in humans. Psychoneuroendocrinology 2023, 154, 106296. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; De Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 2007, 130, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef]
- Dlugos, A.; Childs, E.; Stuhr, K.L.; Hillard, C.J.; de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2012, 37, 2416–2427. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; de Wit, H.; Hillard, C.J.; Van Cauter, E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J. Clin. Endocrinol. Metab. 2015, 100, 220–226. [Google Scholar] [CrossRef]
- Hanlon, E.C. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 2020, 111, 104471. [Google Scholar] [CrossRef]
- Vasquez, N.A.; Nielsen, D.E. The Endocannabinoid System and Eating Behaviours: A Review of the Current State of the Evidence. Curr. Nutr. Rep. 2022, 11, 665–674. [Google Scholar] [CrossRef]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; Di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012, 97, E917–E924. [Google Scholar] [CrossRef] [PubMed]
- Lanz, C.; Mattsson, J.; Stickel, F.; Dufour, J.F.; Brenneisen, R. Determination of the Endocannabinoids Anandamide and 2-Arachidonoyl Glycerol with Gas Chromatography-Mass Spectrometry: Analytical and Preanalytical Challenges and Pitfalls. Med. Cannabis Cannabinoids 2018, 1, 9–18. [Google Scholar] [CrossRef]
- Serrano, A.; Natividad, L.A. Alcohol-Endocannabinoid Interactions: Implications for Addiction-Related Behavioral Processes. Alcohol Res. 2022, 42, 9. [Google Scholar] [CrossRef]
- Gupta, S.; Bharatha, A.; Cohall, D.; Rahman, S.; Haque, M.; Majumder, M.A.A. Aerobic Exercise and Endocannabinoids: A Narrative Review of Stress Regulation and Brain Reward Systems. Cureus 2024, 16, e55468. [Google Scholar] [CrossRef]
- Jian, W.; Edom, R.; Weng, N.; Zannikos, P.; Zhang, Z.; Wang, H. Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibition in human plasma. J. Chromatogr. B 2010, 878, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007, 152, 671–675. [Google Scholar] [CrossRef]
- Gurke, R.; Thomas, D.; Schreiber, Y.; Schäfer, S.M.G.; Fleck, S.C.; Geisslinger, G.; Ferreirós, N. Determination of endocannabinoids and endocannabinoid-like substances in human K3EDTA plasma—LC-MS/MS method validation and pre-analytical characteristics. Talanta 2019, 204, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gkini, E.; Anagnostopoulos, D.; Mavri-Vavayianni, M.; Siafaka-Kapadai, A. Metabolism of 2-acylglycerol in rabbit and human platelets. Involvement of monoacylglycerol lipase and fatty acid amide hydrolase. Platelets 2009, 20, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.C.; Antone, J.; Alsop, E.; Jayakumar, R.; Parikh, K.; Chiot, A.; Sanchez-Molina, P.; Ajami, B.; Arnold, S.E.; Jensen, K.; et al. Cryopreservation of cerebrospinal fluid cells preserves the transcriptional landscape for single-cell analysis. J. Neuroinflamm. 2024, 21, 71. [Google Scholar] [CrossRef]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Ström, J. Cellular elements in the urine in health and in acute infectious diseases, especially with respect to the presence of haematuria. A study with application of millipore procedure and Papanicolaou staining. Scand. J. Infect. Dis. 1975, 7, 97–102. [Google Scholar] [CrossRef]
- Schreiber, D.; Harlfinger, S.; Nolden, B.M.; Gerth, C.W.; Jaehde, U.; Schömig, E.; Klosterkötter, J.; Giuffrida, A.; Astarita, G.; Piomelli, D.; et al. Determination of anandamide and other fatty acyl ethanolamides in human serum by electrospray tandem mass spectrometry. Anal. Biochem. 2007, 361, 162–168. [Google Scholar] [CrossRef]
- Dubbelman, A.C.; van Wieringen, B.; Arias, L.R.; van Vliet, M.; Vermeulen, R.; Harms, A.C.; Hankemeier, T. Strategies for Using Postcolumn Infusion of Standards to Correct for Matrix Effect in LC-MS-Based Quantitative Metabolomics. J. Am. Soc. Mass Spectrom. 2024, 35, 3286–3295. [Google Scholar] [CrossRef]
- Balvers, M.G.; Verhoeckx, K.C.; Witkamp, R.F. Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2009, 877, 1583–1590. [Google Scholar] [CrossRef]
- Huang, S.M.; Strangman, N.M.; Walker, J.M. Liquid chromatographic-mass spectrometric measurement of the endogenous cannabinoid 2-arachidonylglycerol in the spinal cord and peripheral nervous system. Zhongguo Yao Li Xue Bao 1999, 20, 1098–1102. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Tagliamonte, S.; Gill, C.I.R.; Pourshahidi, L.K.; Slevin, M.M.; Price, R.K.; Ferracane, R.; Lawther, R.; O’Connor, G.; Vitaglione, P. Endocannabinoids, endocannabinoid-like molecules and their precursors in human small intestinal lumen and plasma: Does diet affect them? Eur. J. Nutr. 2021, 60, 2203–2215. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Jemal, M. Phospholipids in liquid chromatography/mass spectrometry bioanalysis: Comparison of three tandem mass spectrometric techniques for monitoring plasma phospholipids, the effect of mobile phase composition on phospholipids elution and the association of phospholipids with matrix effects. Rapid Commun. Mass Spectrom. 2009, 23, 2125–2138. [Google Scholar]
- Bobrich, M.; Schwarz, R.; Ramer, R.; Borchert, P.; Hinz, B. A simple LC-MS/MS method for the simultaneous quantification of endocannabinoids in biological samples. J. Chromatogr. B 2020, 1161, 122371. [Google Scholar] [CrossRef]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An Analysis of Endocannabinoid Concentrations and Mood Following Singing and Exercise in Healthy Volunteers. Front. Behav. Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef]
- Schmid, P.C.; Schwartz, K.D.; Smith, C.N.; Krebsbach, R.J.; Berdyshev, E.V.; Schmid, H.H. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem. Phys. Lipids 2000, 104, 185–191. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Lam, P.M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. A solid-phase method for the extraction and measurement of anandamide from multiple human biomatrices. Anal. Biochem. 2009, 384, 106–113. [Google Scholar] [CrossRef]
- Hardison, S.; Weintraub, S.T.; Giuffrida, A. Quantification of endocannabinoids in rat biological samples by GC/MS: Technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006, 81, 106–112. [Google Scholar] [CrossRef]
- Sergi, M.; Battista, N.; Montesano, C.; Curini, R.; Maccarrone, M.; Compagnone, D. Determination of the two major endocannabinoids in human plasma by μ-SPE followed by HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 785–793. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Ghebreselasie, K.; Marnett, L.J. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem. Phys. Lipids 2002, 119, 69–82. [Google Scholar] [CrossRef]
- Zoerner, A.A.; Gutzki, F.M.; Suchy, M.T.; Beckmann, B.; Engeli, S.; Jordan, J.; Tsikas, D. Targeted stable-isotope dilution GC-MS/MS analysis of the endocannabinoid anandamide and other fatty acid ethanol amides in human plasma. J. Chromatogr. B 2009, 877, 2909–2923. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Hasselstrøm, J.B. The effect of antioxidants on the long-term stability of THC and related cannabinoids in sampled whole blood. Drug Test Anal. 2018, 10, 301–309. [Google Scholar] [CrossRef]
- Hamzah, K.A.; Turner, N.; Nichols, D.; Ney, L.J. Advances in targeted liquid chromatography-tandem mass spectrometry methods for endocannabinoid and N-acylethanolamine quantification in biological matrices: A systematic review. Mass Spectrom. Rev. 2024, 43, 350–372. [Google Scholar] [CrossRef]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; de la Torre, R. Analysis of ECs and related compounds in plasma: Artifactual isomerization and ex vivo enzymatic generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef]
- Dócs, K.; Mészár, Z.; Gonda, S.; Kiss-Szikszai, A.; Holló, K.; Antal, M.; Hegyi, Z. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front. Cell. Neurosci. 2017, 11, 39. [Google Scholar] [CrossRef]
- Markey, S.P.; Dudding, T.; Wang, T.C. Base- and acid-catalyzed interconversions of O-acyl- and N-acyl-ethanolamines: A cautionary note for lipid analyses. J. Lipid Res. 2000, 41, 657–662. [Google Scholar] [CrossRef]
- Fowler, C.J.; Tiger, G.; Ligresti, A.; López-Rodríguez, M.L.; Di Marzo, V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis--a difficult issue to handle. Eur. J. Pharmacol. 2004, 492, 1–11. [Google Scholar] [CrossRef]
- Djilali, E.; Pappalardo, L.; Posadino, A.M.; Giordo, R.; Pintus, G. Effects of the Storage Conditions on the Stability of Natural and Synthetic Cannabis in Biological Matrices for Forensic Toxicology Analysis: An Update from the Literature. Metabolites 2022, 12, 801. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Campo, P.; Suidan, M.T.; Chai, Y.; Davis, J. A liquid chromatography-electrospray ionization-tandem mass spectrometry study of ethanolamines in high salinity industrial wastewaters. Talanta 2010, 80, 1110–1115. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; El-Talatini, M.; Finney, M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal. Biochem. 2008, 380, 195–201. [Google Scholar] [CrossRef]
- Marchioni, C.; de Souza, I.D.; Grecco, C.F.; Crippa, J.A.; Tumas, V.; Queiroz, M.E.C. A column switching ultrahigh-performance liquid chromatography-tandem mass spectrometry method to determine anandamide and 2-arachidonoylglycerol in plasma samples. Anal. Bioanal. Chem. 2017, 409, 3587–3596. [Google Scholar] [CrossRef]
- Mwanza, C.; Chen, Z.; Zhang, Q.; Chen, S.; Wang, W.; Deng, H. Simultaneous HPLC-APCI-MS/MS quantification of endogenous cannabinoids and glucocorticoids in hair. J. Chromatogr. B 2016, 1028, 1–10. [Google Scholar] [CrossRef]
- Dong, X.; Li, L.; Ye, Y.; Zhang, D.; Zheng, L.; Jiang, Y.; Shen, M. Surrogate analyte-based quantification of main endocannabinoids in whole blood using liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4439. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Di, X.; Kohler, I.; Osuna-Prieto, F.J.; Acosta, F.M.; Vilchez-Vargas, R.; Link, A.; Plaza-Díaz, J.; van der Stelt, M.; et al. Plasma Levels of Endocannabinoids and Their Analogues Are Related to Specific Fecal Bacterial Genera in Young Adults: Role in Gut Barrier Integrity. Nutrients 2022, 14, 2143. [Google Scholar] [CrossRef]
- Pedersen, T.L.; Gray, I.J.; Newman, J.W. Plasma and serum oxylipin, endocannabinoid, bile acid, steroid, fatty acid and nonsteroidal anti-inflammatory drug quantification in a 96-well plate format. Anal. Chim. Acta 2021, 1143, 189–200. [Google Scholar] [CrossRef]
- De Luca, L.; Ferracane, R.; Ramírez, N.C.; Vitaglione, P. N-Acylphosphatidylethanolamines and N-acylethanolamines increase in saliva upon food mastication: The influence of the individual nutritional status and fat type in food. Food Funct. 2020, 11, 3382–3392. [Google Scholar] [CrossRef]
- Junior, V.R.A.; Goméz-Ríos, G.A.; Tascon, M.; Queiroz, M.E.C.; Pawliszyn, J. Analysis of endocannabinoids in plasma samples by biocompatible solid-phase microextraction devices coupled to mass spectrometry. Anal. Chim.Acta 2019, 1091, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Inamassu, C.H.; Raspini, E.S.L.; Marchioni, C. Recent advances in the chromatographic analysis of endocannabinoids and phytocannabinoids in biological samples. J. Chromatogr. A 2024, 1732, 465225. [Google Scholar] [CrossRef]
- Sempio, C.; Klawitter, J.; Jackson, M.; Freni, F.; Shillingburg, R.; Hutchison, K.; Bidwell, L.C.; Christians, U.; Klawitter, J. Analysis of 14 endocannabinoids and endocannabinoid congeners in human plasma using column switching high-performance atmospheric pressure chemical ionization liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Kantae, V.; Ogino, S.; Noga, M.; Harms, A.C.; van Dongen, R.M.; Onderwater, G.L.; van den Maagdenberg, A.M.; Terwindt, G.M.; van der Stelt, M.; Ferrari, M.D.; et al. Quantitative profiling of endocannabinoids and related N-acylethanolamines in human CSF using nano LC-MS/MS. J. Lipid Res. 2017, 58, 615–624. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; de Castro, M.D.L.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC-MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargreaves, J.; Ney, L. Experimental and Pre-Analytical Considerations of Endocannabinoid Quantification in Human Biofluids Prior to Mass Spectrometric Analysis. Targets 2025, 3, 11. https://doi.org/10.3390/targets3010011
Hargreaves J, Ney L. Experimental and Pre-Analytical Considerations of Endocannabinoid Quantification in Human Biofluids Prior to Mass Spectrometric Analysis. Targets. 2025; 3(1):11. https://doi.org/10.3390/targets3010011
Chicago/Turabian StyleHargreaves, Jessica, and Luke Ney. 2025. "Experimental and Pre-Analytical Considerations of Endocannabinoid Quantification in Human Biofluids Prior to Mass Spectrometric Analysis" Targets 3, no. 1: 11. https://doi.org/10.3390/targets3010011
APA StyleHargreaves, J., & Ney, L. (2025). Experimental and Pre-Analytical Considerations of Endocannabinoid Quantification in Human Biofluids Prior to Mass Spectrometric Analysis. Targets, 3(1), 11. https://doi.org/10.3390/targets3010011





