Abstract
The quantification of endocannabinoids in biological fluids is becoming increasingly popular as an indicator of psychological and physiological function. Numerous methods to quantify the endocannabinoid ligands have been published so far, yet their concentrations and responses often exhibit significant variability across studies. Endocannabinoids regulate and interact with a wide range of biomolecules, causing their concentrations to vary between cohorts of individuals, and sensitivities to them depend on pre-experimental behaviours and activities. Moreover, matrix effects produced by the complex nature of biofluids necessitate rigorous sample preparation techniques, all of which introduce opportunities for both inter- and intra-assay variability. This review aims to address the causes of variability prior to mass spectrometric analysis, including biofluid choice, human variability, sample collection and extraction methods. If these factors are fully considered and standardised methods are introduced, endocannabinoid concentrations may become more reliable, allowing their utility as clinical markers to progress.
1. Introduction
The endocannabinoid system is a lipid signalling system that has key roles in regulating various physiological and psychological processes, including pain, stress, appetite, metabolism, immunity, as well as reproductive function [1]. The two major endocannabinoid ligands include the arachidonic acid-derived N-arachidonoyl-ethanolamine (AEA) [2] and 2-arachidonoylglycerol (2-AG) [3] (Figure 1), which are synthesised and degraded via distinct biochemical pathways [4]. The enzyme primarily responsible for AEA synthesis is NAPE-specific phospholipase D [5], whereas it is degraded via fatty acid amide hydrolase (FAAH) [6]. In contrast, 2-AG is synthesised through diacylglycerol lipases and primarily degraded via the enzyme monoacylglycerol lipase [7]. AEA and 2-AG have different affinities for the receptors of the endocannabinoid system, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), and operate in tonic and phasic manners depending on their localisation [8]. Several other ligands are often studied in conjunction with the AEA and 2-AG, which include several N-acylethanolamines (NAEs), such as Oleoylethanolamide (OEA), Palmitoylethanolamide (PEA) and N-docosahexaenoylethanolamine (DHEA) [9]. Although these ligands do not bind to the classical cannabinoid receptors, they often interact with the endocannabinoid system and regulate similar processes. Collectively, the status of these endocannabinoids acts as a tone that becomes disrupted in pathological conditions; thus, they can potentially be used as biomarkers of certain diseases [10,11]. As human tissue samples are not readily available, exploratory and preclinical studies have mainly relied on analysing the endocannabinoid ligands and other NAEs in various biofluids.
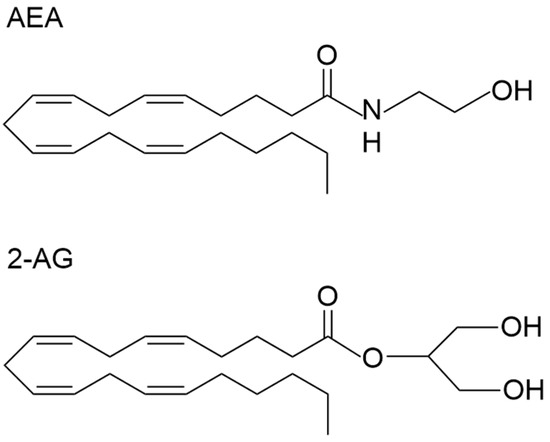
Figure 1.
The chemical structure of N-arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG).
Endocannabinoids and other NAEs in biological fluids can be quantified using multiple methods, including enzyme-linked immunosorbent assays, radioimmunoassay and gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry. Immunoassay kits are commercially available; however, these kits lack sensitivity and have high levels of cross-reactivity, especially for 2-AG, which has a similar structure to other monoacylglycerols. Instead, the most common method to date is mass spectrometry, which offers high levels of selectivity and sensitivity and enables the simultaneous quantification of multiple analytes [12]. So far, endocannabinoids and various different NAEs have been quantified in various human biofluids, including plasma [13], serum [14], cerebrospinal fluid (CSF) [15,16], saliva [17,18,19], hair [20,21] and urine [22]. The concentrations in these fluids range from a low picomolar to nanomolar range; however, the studies that have quantified their basal levels or responses so far have not found consistent results. These discrepancies may stem from variations in experimental setups and biofluid processing methods before mass spectrometric analysis.
Endocannabinoid regulation is complex and often involves their interaction with numerous other biomolecules, including sex hormones [23,24], cytokines [25], stress hormones [26,27] and neuropeptides [28]. These interactions contribute to variability in endocannabinoid concentrations across cohorts of individuals at baseline and during various physiological responses. Obtaining biofluid samples in an uncontrolled manner or not considering these differences during analysis can introduce noise and obscure meaningful signals. Furthermore, due to their rapid uptake into cells, AEA and 2-AG concentrations in biofluids are extremely low, whereas the concentrations of other constituents, like lipids, proteins and DNA, are high [29]. These factors can create interfering signals during ionisation during mass spectrometry; thus, robust lipid extraction techniques are needed to purify samples before detection. Common forms of sample preparation include protein precipitation, solid-phase extraction (SPE) and liquid–liquid extraction (LLE), yet there is a current lack of standardised procedures between laboratories [30]. Endocannabinoids and their congeners are unstable molecules, especially 2-AG, which undergoes spontaneous isomerisation at the physiological pH; thus, variations in sample processing can drastically alter their final concentrations and further exacerbate discrepancies in reported concentrations [31,32]. To quantify endocannabinoid concentrations to the accuracy needed for biomarker use, strategies to minimise variation during sample collection, preparation and analysis need to be introduced [33].
This review will update previous literature that examines pre-analytical considerations of endocannabinoid quantification in human matrices [33,34]. This review will add additional emphasis on considerations that need to be addressed to reduce variability before sample preparation, during the experimental design and sample collection (Figure 2), as well as during sample processing (Figure 3) and chromatography (Figure 4). By addressing individual variability and refining analytical methodologies, this review aims to advance the use of endocannabinoids as biomarkers for physiological and pathological processes.
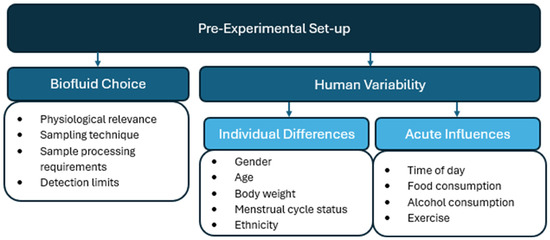
Figure 2.
Pre-experimental considerations for endocannabinoid quantification in human biomatrices.
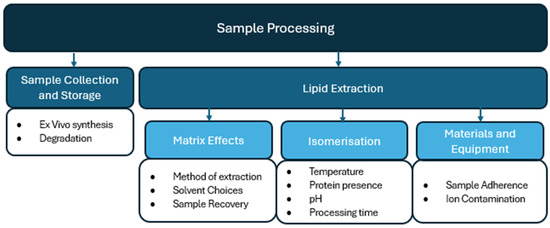
Figure 3.
Sample processing considerations for endocannabinoid quantification in human biomatrices.
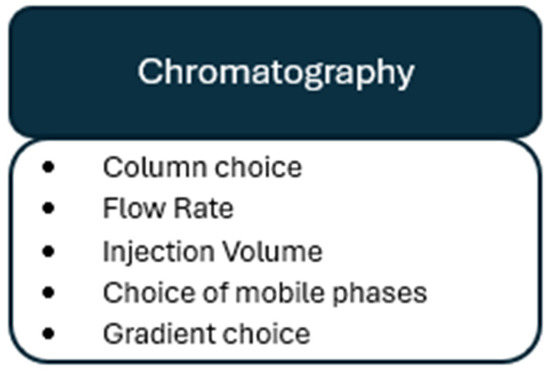
Figure 4.
Chromatographical considerations for endocannabinoid quantification in human biomatrices.
2. Endocannabinoid Structure
The structure and chemical properties of endocannabinoids need to be considered to ensure the best sample processing methods for mass spectrometric detection are applied (Figure 1). AEA is considered both a fatty acid amide and an eicosanoid. It consists of an ethanolamine head group connected via an amide bond to a long lipophilic hydrocarbon tail. The amide bond is polar, allowing AEA to interact in aqueous environments; however, the hydrocarbon tail dominates the molecule’s overall chemical properties. AEA has a molecular mass of 347.53 g/mol and the molecular formula C22H37NO2 [35]. The compound 2-AG is also considered an eicosanoid and contains a glycerol backbone as well as the same long lipophilic hydrocarbon tail as AEA. The compound 2-AG contains an ester bond formed between the hydroxyl group of glycerol and the carboxylic acid group of arachidonic acid, which is chemically unstable and prone to hydrolysis. While 2-AG has some polar characteristics due to containing several hydroxyl groups, it is an overall highly lipophilic molecule. The compound 2-AG has a molecular mass of 378.3 g/mol and has the chemical formula C23H38O4 [36]. OEA, PEA and DHEA all form a linkage of ethanolamine to oleic acid, palmitic acid and docosahexaenoic acid, respectively. Like the endocannabinoid ligands, OEA, PEA and DHEA are highly lipophilic and have similar chemical properties to AEA and 2-AG; therefore, they are commonly stored and extracted using the same techniques [34].
Due to these lipophilic characteristics of each molecule, endocannabinoid ligands and other NAEs are soluble in organic compounds, including methanol, acetonitrile and isopropanol. Processing samples in these polar organic solvents reduces degradation via non-enzymatic hydrolysis [37], which readily occurs under basic and aqueous conditions [38]. Maintaining a slightly acidic pH by using additives such as 0.1% formic acid, triethylamine or trifluoroacetic acid (TFA) to organic solvents can increase stability and improve recovery further [39], whereas processing samples in highly basic conditions should be avoided as it causes rapid degradation due to saponification reactions. The lipophilic nature of endocannabinoids also leads to their association with various proteins, which requires separation during sample preparation for efficient analysis. The presence of long fatty acid chains provides low volatility, while the presence of double bonds makes these molecules prone to oxidation. Derivatisation of these molecules is therefore necessary to increase volatility and thermal stability, which is essential for their analysis via gas chromatography methods. Their unsaturated bonds make AEA and 2-AG particularly susceptible to degradation during storage or freeze–thaw conditions, emphasising the need for careful sample handling.
3. Pre-Experimental Considerations
3.1. Biofluid Choice
The choice of biofluid is an important consideration during endocannabinoid analysis. AEA and 2-AG synthesis occurs in many diverse tissue types, and it remains unclear how they transfer into and between different biofluids [40]. While cerebrospinal fluid is thought to reflect the brain activity of endocannabinoids, this biofluid is invasive to collect, has to be collected by a specialist and allows for only a limited sample volume [41]. Consequently, many studies have opted to use blood samples without fully understanding whether concentrations in this biofluid reflect endocannabinoid production from nervous tissue, peripheral tissue or production by blood cells. Studies that have compared different biofluids within the same population have reported a lack of linear relationships between each one, including Meier et al. [42], who found no significant correlations between serum and CSF concentrations of AEA. Similarly, Ney et al. [43] reported no correlations between plasma and salivary concentrations of AEA and 2-AG in response to stress, and Valdivieso Barba et al. [44] found no correlations between hair and plasma or hair and urine concentrations. These findings suggest that the physiological functions represented by endocannabinoid responses may differ across biofluids; thus, the matrix chosen for a study should be carefully considered depending on the aims of the experiment.
Additionally, the collection of biofluids themselves can have implications for endocannabinoid concentrations. The collection of serum or plasma requires venipuncture during blood draw, which can trigger a stress response, or activate nociceptors, both of which incite endocannabinoid responses and can skew baseline concentrations [8,45]. In contrast, less invasive methods, such as saliva and hair sampling, may provide a more reliable baseline measurement during studies, while also allowing a larger number of samples to be taken from a single participant. Matrices like CSF, blood or saliva can represent current endocannabinoid tone, whereas the collection of hair can potentially reflect a longer-term endocannabinoid functioning, which may be more useful for detecting chronic changes [44]. However, it should also be noted that there is a lack of standardised procedures for the collection of endocannabinoids in biofluids like hair and saliva so far. Factors such as salivary flow rate [46] or a change in the length or section of hair sampled [47] can alter concentrations and introduce variability between studies. Collectively, the sampling of biofluids should be standardised and methods to minimise the induction of endocannabinoid responses during sampling should be considered.
The choice of biofluid can also influence the quantification of endocannabinoids and other NAEs due to differences in sample processing requirements and detection limits. Serum is produced from whole blood samples by activating the coagulation cascade, which is an enzymatic process that takes around 20 min at room temperature. Significant enhancement of AEA occurs during this time, as blood cells carry on synthesising endocannabinoids ex vivo [48]. Obtaining plasma, on the other hand, does not require this coagulation step, meaning cells can be immediately removed from samples, and endocannabinoid concentrations more accurately reflect physiological levels. However, it also means that plasma contains lower overall concentrations, which may be harder to detect [49,50]. The range of physiological concentration of endocannabinoids and their congeners naturally varies between biofluids. Biofluids like CSF and saliva have concentrations in the lower range [17,41], which results in a smaller magnitude of change in their responses or between clinical groups. Alternatively, serum and plasma yield higher concentrations of endocannabinoids, thus having a higher signal-to-noise ratio, improving the statistical power and robustness of results. Overall, the choice of biofluid can have certain implications for endocannabinoid analysis and should be carefully thought out before obtaining samples.
3.2. Human Variability
Individual Differences. Variability of endocannabinoid concentrations has been observed between cohorts of individuals. Higher baseline levels of AEA and 2-AG have been found in males in serum [30,51] and saliva [52]; thus, separating results based on gender reduces variability and allows clearer trends to be discovered. It is not only beneficial to account for gender differences during data interpretation but also when considering sample size. Meier et al. [42] examined endocannabinoid levels in the CSF of 74 Multiple-Sclerosis (MS) and 80 non-neuroinflammatory control participants and did not initially find any changes between these two clinical groups. Once male and female results were separated, increases in 2-AG in male MS patients compared to controls were found. This result was not detected in previous studies with smaller sample sizes [16,53], demonstrating that larger sample sizes should be considered, especially when moderating factors, such as participant gender, are included.
Other individual differences influencing endocannabinoid concentrations include age and body weight. Amir Hamzah et al. [51] discovered that basal AEA and 2-AG concentrations were significantly higher in older age groups, and Engeli et al. [54] found that peripheral endocannabinoids were significantly increased in women with obesity. In addition to observable differences in baseline tonic endocannabinoid levels, phasic endocannabinoid responses can also vary due to individual differences. Dlugos et al. [55] discovered differences in AEA responses to stress depending on the sex and menstrual cycle status of the participant. They additionally found ethnic differences, with participants of Asian and African American descent exhibiting distinct endocannabinoid stress response patterns compared to those of Caucasian descent. Carefully accounting for these differences in experimental design, data analysis or matched control groups can reveal significant results that would otherwise be masked by noise.
Acute Influences. External influences, such as the time of day and a subject’s behaviour before sample collection, can alter endocannabinoid levels. Both AEA and 2-AG concentrations in serum show daily oscillations governed by the circadian rhythm [56,57]. AEA shows a biphasic 24 h rhythm, peaking in the late afternoon, whereas 2-AG shows a monophasic rhythm, peaking in the early to mid-afternoon. Samples collected at different time points in the day can differ significantly; thus, maintaining a consistent sampling time frame is necessary to reduce variability and enhance data reliability within a study.
The endocannabinoid system regulates hunger, satiety and reward mechanisms associated with eating [58], and, accordingly, endocannabinoid levels are influenced by food intake. Concentrations of 2-AG, in particular, increase after consuming high-fat or palatable meals [59,60]. Although by different mechanisms, endocannabinoid levels are also mobilised after alcohol consumption [61] and exercise [62]. These factors show that implementing detailed screening protocols or establishing restrictions prior to sample collection can minimise the variability introduced by subjects’ pre-experimental behaviours or activities.
4. Sample Processing Considerations
4.1. Sample Collection and Storage
Sample handling procedures vary depending on the matrix of interest. Since cells can synthesise and release endocannabinoids ex vivo [13,63], biofluids that contain cells should be placed on ice immediately after collection and centrifuged as quickly as possible. This is particularly critical after blood draws due to the high abundance of leukocytes and platelets in this matrix, which can contribute to ex vivo endocannabinoid production [64]. Significant increases in AEA have been found in blood samples after one hour, even when stored at low temperatures and treated with an anticoagulant [65]. Simultaneous to synthesis, active degradation of endocannabinoids may also occur due to the presence of the enzymes MAGL and FAAH within cellular membranes [66]; thus, the addition of enzyme inhibitors can also slow down these ex vivo concentration changes. It should be noted that biofluids, such as CSF [67], saliva [68] and urine [69], can contain low numbers of cells; thus, the same treatment of these biofluids may also be beneficial. Although endocannabinoid levels are a lot more stable after the removal of cells, AEA and 2-AG can still degrade in cell-free biofluids due to their poor stability and short half-life [32]. Processing samples at low temperatures, reducing processing times and freezing samples soon after collection can reduce degradation in this manner. Once frozen at −80 °C, AEA and 2-AG levels have been found to remain stable for at least three months [70] Freeze–thawing also accelerates endocannabinoid degradation, and 2-AG has been found to be particularly sensitive to repeated freeze–thaw cycles [60,65]. Aliquoting samples according to the volume desired for analysis can, therefore, preserve the original physiological concentration. Collectively, combining careful sampling handling techniques and treating all samples in the same manner can prevent ex vivo changes in endocannabinoid concentrations and limit inter-assay variability.
4.2. Lipid Extraction
Matrix Effects. Certain constituents in biofluids can affect the ion sources used in mass spectrometry, causing ion suppression or enhancement [71]. These matrix effects lead to the inaccurate reporting of endocannabinoid concentrations; thus, robust lipid extraction and purification of matrix samples are necessary. However, these steps are also associated with sample loss, which should also be considered when comparing extraction methods. Prior to further clean-up methods, performing protein precipitation can lead to increased recovery of samples due to the removal of excess proteins. This method involves the addition of organic solvents, such as acetonitrile [72] or methanol [73], into the sample and removing proteins by centrifugation. While some studies have opted to use protein precipitation alone, excluding further extraction steps can lead to high levels of matrix interference and potential blockage and contamination of LC or MS equipment.
The Folch [74], Bligh and Dyer [75], methods are the most well-known form of LLE. These methods involve using different ratios of chloroform, methanol and water to create organic and aqueous phases, and endocannabinoids are then extracted from the corresponding organic layer due to their solubility and non-polar nature [76]. However, these methods involve toxic chemicals and can co-extract phospholipids, which cause significant matrix effects [77]; therefore, the use of alternative solvents, including ethyl acetate [78], ethyl acetate/hexane [79], or toluene [32], is recommended for endocannabinoid extraction specifically. Another popular method of lipid extraction is SPE, which involves purifying samples by retaining and eluting lipids from columns using specific solvents. Reverse-, mixed- and normal-phase columns have all been employed for endocannabinoid extraction [72,80,81]; however, the use of C8 or C18 reverse-phase columns can improve yields due to these column’s compatibility with their hydrophobic nature [82]. LLE tends to be the most favourable method for endocannabinoid extraction, potentially due to being faster and cheaper than SPE, which requires the purchase of SPE cartridges. Nonetheless, SPE is more efficient at removing interferences, thus reducing the matrix effect and potentially preserving the equipment in downstream processes. SPE methods can also be automated and scaled up [83], which enables high-throughput processing of many samples at once. Each of these methods can also lead to loss of analyte while performing lipid extraction; therefore, each should be tested using isotope-labelled standards for their effect on analyte recovery.
Solvent evaporation is almost always required while performing lipid extraction methods; however, this process can lead to accelerated isomerisation, degradation and oxidation of AEA and 2-AG [84]. To reduce any loss during this step, samples should be evaporated at low temperatures under a nitrogen stream [85], and the addition of antioxidants should be tested to increase the stability of AEA and 2-AG during heating [86]. Ensuring samples are swiftly reconstituted will also minimise loss during evaporation and prepare samples for mass spectrometry analysis. Due to their solubility in methanol and acetonitrile, endocannabinoids are commonly eluted in methanol/water and acetonitrile/water mixtures, which complements the corresponding chromatographic separation [87]. Collectively, there are a range of sample purification and extraction techniques, which results in a chance of introducing variability between samples at each stage. Different procedures should be tested and optimised for those that produce good yields and low levels of variability between samples.
Isomerisation. A common challenge when preparing 2-AG for analysis is the ex vivo isomerisation of 2-AG to 1-AG, which occurs spontaneously under normal sample processing conditions. Factors such as temperature, protein presence, pH, centrifugal force, and solvent choices affect the rate of isomerisation [65,84,88], leading to varying ratios of 2-AG to 1-AG between samples. Techniques such as continuously keeping samples at low temperatures, reducing processing times and using non-protic solvents like toluene or tert-butyl methyl ether, can minimise its rate [32,88]. A common approach post-quantification is to combine 2-AG and 1-AG concentrations [54]; however, as 2-AG represents the active form of the molecule, this practice may obscure important physiological information [89]. Even the addition of isotopically-labelled standards cannot fully address this issue, as commercially available 2-AG is not isotopically pure and can also undergo isomerisation during sample processing. Keeping sample processing protocols consistent and standardised can ensure all samples isomerise in a similar manner to limit variability.
AEA can isomerise to virodhamine (O-AEA) under a highly acidic pH and vice versa at a highly basic pH [90]. This form of isomerisation is not usually an issue during sample processing, as the amide bond in AEA is more stable than the ester bond in 2-AG and does not occur spontaneously. AEA and O-AEA should nevertheless be separated during quantification due to differences in the physiological functions of these isomers. Importantly, as the O-AEA and 1-AG isomers have identical molecular weights to AEA and 2-AG, the separation of these molecules cannot be achieved through mass spectrometry alone and must be performed at the chromatography stage [78].
Materials and Equipment. Due to their highly hydrophobic nature, AEA and 2-AG readily adhere to plastic and glass equipment used during the above sample processing steps [91]. The use of treated laboratory materials, minimising contact with plastic or glass and limiting the number of transfers between containers can help reduce analyte loss and improve recovery [92]. Additionally, spiking samples with isotopically-labelled internal standards early into sample preparation can also account for any analyte loss due to attachment to laboratory materials, as well as accounting for any degradation over time [93]. The commercially available standards include deuterated versions of AEA and 2-AG (e.g., d8-AEA, d4-AEA, d8-2AG, d5-2AG), which are chemically identical to their native versions but have slightly higher masses [39]. These properties allow the standards to be co-extracted and ionised with the native analytes during sample processing and ensure that they can be separated and distinguished during analysis. Sodium ions (Na+) can also be introduced into samples from contaminated solvents or glass and plasticware. Na+ readily forms adducts with AEA and 2-AG, which reduces ionisation and shifts in mass/charge values, further diminishing the already-low signal of endocannabinoids [94] and complicating chromatographic interpretation. Na+ contamination can be reduced by using clean, sodium-free laboratory materials, preparing fresh solvents and ensuring the use of mass-spectrometry grade solvents. De-salting samples via SPE can reduce naturally occurring sodium from within biofluids, and acidifying or adding ammonium acetate to the mobile phases can reduce the formation of the sodium adducts [95]. Consideration of the solvents and equipment used during sample processing is vital to retain physiological endocannabinoid levels and reduce variability.
5. Chromatography
After their extraction from biofluids, both LC and GC can separate endocannabinoids and other N-acylethanolamines for detection via mass spectrometry. Gas chromatography requires longer retention times and an additional derivatisation step during sample preparation [60]; as a result, LC methods, including high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC) [96,97], are more commonly used. For LC methods, reverse-phase C18 columns are almost exclusively used as the stationary phase, given the nonpolar and lipophilic characteristics of endocannabinoids. However, other column and chromatography parameters are less consistent between studies, especially between different biomatrices. The efficient separation of the low concentrations of endocannabinoids and similar molecules has been achieved with varying column particle sizes, including 1.7 µm [98] and as large as 4 µm [14]. Separation of endocannabinoid and multiple additional analogues has been achieved in blood samples using 50 mm in length [99], however, other methods adopted 100 mm or 150 mm columns for this purpose [65,100]. These columns efficiently separate similar lipids to a high resolution when paired with commonly used mobile phases like methanol or acetonitrile and water [87]. Other mobile phases have been reported to work well with C18 columns, including isopropanol or different ratios of these solvents [100,101]. Various modifiers such as formic acid, acetic acid, ammonium acetate and ammonium formate [41,102] have been included within mobile phases at various concentrations and can be added to improve ionisation and increase signal strength in samples with low endocannabinoid concentrations [102,103]. Sample injection volumes can vary between 2 and 400 µL [20,104] depending on the accompanying sample preparation procedures, and flow rates also vary from as little as 0.2 mL/min [105] to 0.8 mL/min [106]. Both AEA and 2-AG elute at higher percentages of organic solvent. Therefore, a gradient with a gradual increase in the organic solvent over a period of 10 min allows for a good resolution; however, to efficiently separate the endocannabinoid isomers, holding the gradient at 75–79% organic phase for 4.5 min may be required [32]. The lack of a common chromatography method both between and within different biofluids for the quantification of endocannabinoids and other NAEs suggests that different methodologies should be tested for robust separation of analytes depending on the biofluid and analytes of interest. Nevertheless, the employment of a robust chromatography method is necessary for effective downstream detection via mass spectrometry and is needed to accurately quantify endocannabinoid concentrations.
6. Conclusions
Endocannabinoid and NEA detection in different biofluids is a potentially valuable tool for the diagnosis, monitoring or prevention of disease. Due to the advancement of highly sensitive analytical techniques, accurate quantification in biofluids is possible; however, high levels of variability are apparent between studies. If endocannabinoid analysis is to progress to clinical studies, experimental and pre-analytical challenges that contribute to this variability must be addressed.
As the mechanism by which endocannabinoids end up in biofluids is not fully understood, it can be difficult to select the right biofluid for analysis. When selecting a biofluid, it is important to weigh up a number of factors, such as the ease of collection, biomolecule abundance and the biological relevance of each ligand in that matrix. Before sample collection, it is also important to consider differences between subjects, as cohorts of subjects that differ in their age, ethnicity and body weight can create noise between baseline measurements and responses. These factors should be accounted for during sampling and analysis, otherwise, potentially significant results can be lost, leading to different results being reported between studies. Moreover, AEA and 2-AG become mobilised depending on the time of day, upon food or alcohol consumption, upon exercising or during sampling procedures. Sampling times and screening conditions should be tightly controlled, as these confounding variables can inflate or deflate endocannabinoid levels so that any main effects are lost. Collectively, understanding the biology of endocannabinoids can reduce variation within a study and lead to more significant results being discovered and reported, reducing inter-assay variability.
Variability can also be introduced after sample collection and during the processing of samples. Robust purification techniques are needed to ensure other lipids and constituents that interfere with ionisation in mass spectrometry are not co-isolated with endocannabinoids. This includes using methods like protein precipitation, liquid–liquid extraction or SPE. However, employing these methods in different ways can lead to the loss, degradation or isomerisation of these samples. Cells should be removed immediately after collection and samples should be processed in a timely manner at low temperatures to avoid variability introduced by ex vivo synthesis or degradation. The use of toluene appears to be the most ideal extraction solvent for endocannabinoids specifically, as it yields a high recovery, low levels of matrix effects and reduced 2-AG to 1-AG isomerisation compared to other solvents [32]. However, although more expensive, reverse-phase SPE methods should also be strongly considered as they can further reduce matrix effects and have the potential to be high throughput. Whatever the method chosen for endocannabinoid extraction, the use of mass-spectrometry grade solvents and clean laboratory equipment will minimise contamination which can interfere with the following analysis. Finally, a reliable and robust chromatography method must also be employed before mass spectrometry, so that analytes can be adequately separated. Reverse-phase columns perform this separation well when paired with an appropriate mobile phase and an optimised gradient. These factors must be carefully adjusted based on the available equipment, to ensure isomers that cannot be isolated during mass spectrometry are separated. The addition of commercially available stable isotope standards early into the sample processing procedure can account for minor variances in methods, however, samples should still be processed in a similar manner.
If the above factors are carefully considered, then endocannabinoid and NEA detection via proceeding mass spectrometric analysis should yield low levels of variability, high levels of inter-assay reliability, and accurately reflect physiological concentrations. As endocannabinoid quantification for biomarker use advances, standard pre-analytical procedures need to be validated and employed to gain inter-assay reproducibility and increase consistent results between laboratories.
Author Contributions
Writing—original draft preparation, J.H.; writing—review and editing, L.N.; supervision, L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AEA | N-arachidonoyl-ethanolamine |
| 2-AG | 2-arachidonoylglycerol |
| FAAH | Fatty acid amide hydrolase |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| GC | Gas chromatography |
| LC | Liquid chromatography |
| CSF | Cerebrospinal fluid |
| SPE | Solid-phase extraction |
| LLE | Liquid–liquid extraction |
| HPLC | High-performance liquid chromatography |
| UHPCL | Ultra-high-performance liquid chromatography |
References
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Simard, M.; Archambault, A.-S.; Lavoie, J.-P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef]
- PSchmid, C.; Reddy, P.V.; Natarajan, V.; Schmid, H.H. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J. Biol. Chem. 1983, 258, 9302–9306. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Lichtman, A.H. Fatty acid amide hydrolase: An emerging therapeutic target in the endocannabinoid system. Curr. Opin. Chem. Biol. 2003, 7, 469–475. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Jurado-Barba, R.; Rubio, G.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry 2020, 11, 315. [Google Scholar] [CrossRef]
- Skaper, S.D.; Di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Shevchenko, A. Quantification of Endogenous Endocannabinoids by LC-MS/MS. In Lipidomics; Wood, P., Ed.; Springer New York: New York, NY, USA, 2017; pp. 99–107. [Google Scholar]
- Röhrig, W.; Achenbach, S.; Deutsch, B.; Pischetsrieder, M. Quantification of 24 circulating endocannabinoids, endocannabinoid-related compounds, and their phospholipid precursors in human plasma by UHPLC-MS/MS. J. Lipid Res. 2019, 60, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Couttas, T.A.; Boost, C.; Pahlisch, F.; Sykorova, E.B.; Leweke, J.E.; Koethe, D.; Endepols, H.; Rohleder, C.; Leweke, F.M. Simultaneous Assessment of Serum Levels and Pharmacologic Effects of Cannabinoids on Endocannabinoids and N-Acylethanolamines by Liquid Chromatography-Tandem Mass Spectrometry. Cannabis Cannabinoid Res. 2023, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Cebo, M.; Mielnik, J.; Richter, H.; Schüle, R.; Sievers-Engler, A.; Młynarz, P.; Lämmerhofer, M. UHPLC-ESI-MS/MS assay for quantification of endocannabinoids in cerebrospinal fluid using surrogate calibrant and surrogate matrix approaches. J. Pharm. Biomed. Anal. 2023, 222, 115090. [Google Scholar] [CrossRef]
- Di Filippo, M.; Pini, L.A.; Pelliccioli, G.P.; Calabresi, P.; Sarchielli, P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1224–1229. [Google Scholar] [CrossRef]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Simultaneous quantification of endocannabinoids, oleoylethanolamide and steroid hormones in human plasma and saliva. J. Chromatogr. B 2020, 1152, 122252. [Google Scholar] [CrossRef]
- Matias, I.; Gatta-Cherifi, B.; Tabarin, A.; Clark, S.; Leste-Lasserre, T.; Marsicano, G.; Piazza, P.V.; Cota, D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS ONE 2012, 7, e42399. [Google Scholar] [CrossRef]
- Jarvis, M.; Hamzah, K.A.; Nichols, D.; Ney, L.J. Hair and Saliva Endocannabinoid and Steroid Hormone Analysis by Liquid Chromatography Paired with Tandem Mass Spectrometry. Methods Mol. Biol. 2025, 2868, 135–147. [Google Scholar]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Chloroform-based liquid-liquid extraction and LC-MS/MS quantification of endocannabinoids, cortisol and progesterone in human hair. J. Pharm. Biomed. Anal. 2021, 201, 114103. [Google Scholar] [CrossRef]
- Gao, W.; Walther, A.; Wekenborg, M.; Penz, M.; Kirschbaum, C. Determination of endocannabinoids and N-acylethanolamines in human hair with LC-MS/MS and their relation to symptoms of depression, burnout, and anxiety. Talanta 2020, 217, 121006. [Google Scholar] [CrossRef]
- Vago, R.; Ravelli, A.; Bettiga, A.; Casati, S.; Lavorgna, G.; Benigni, F.; Salonia, A.; Montorsi, F.; Orioli, M.; Ciuffreda, P.; et al. Urine Endocannabinoids as Novel Non-Invasive Biomarkers for Bladder Cancer at Early Stage. Cancers 2020, 12, 870. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and gonadal hormones: Bidirectional interactions in physiology and behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018, 94, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Barrie, N.; Manolios, N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur. J. Rheumatol. 2017, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2016, 7, 1–15. [Google Scholar] [PubMed]
- Balsevich, G.; Petrie, G.N.; Hill, M.N. Endocannabinoids: Effectors of glucocorticoid signaling. Front. Neuroendocrinol. 2017, 47, 86–108. [Google Scholar] [CrossRef]
- Vähätalo, L.H.; Ruohonen, S.T.; Mäkelä, S.; Ailanen, L.; Penttinen, A.-M.; Stormi, T.; Kauko, T.; Piscitelli, F.; Silvestri, C.; Savontaus, E.; et al. Role of the endocannabinoid system in obesity induced by neuropeptide Y overexpression in noradrenergic neurons. Nutr. Diabetes 2015, 5, e151. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Di Lallo, V.D.; Belluomo, I.; De Iasio, R.; Baccini, M.; Casadio, E.; Gasparini, D.I.; Colavita, M.; Gambineri, A.; Grossi, G.; et al. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. J. Lipid Res. 2012, 53, 481–493. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Johnson, C.T. Measuring the Content of Endocannabinoid-Like Compounds in Biological Fluids: A Critical Overview of Sample Preparation Methodologies. Methods Mol. Biol. 2023, 2576, 21–40. [Google Scholar]
- Zoerner, A.A.; Batkai, S.; Suchy, M.T.; Gutzki, F.M.; Engeli, S.; Jordan, J.; Tsikas, D. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J. Chromatogr. B 2012, 883–884, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Gutzki, F.-M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta 2011, 1811, 706–723. [Google Scholar] [CrossRef] [PubMed]
- Kratz, D.; Thomas, D.; Gurke, R. Endocannabinoids as potential biomarkers: It’s all about pre-analytics. J. Mass Spectrom. Adv. Clin. Lab 2021, 22, 56–63. [Google Scholar] [CrossRef]
- Di Scala, C.; Fantini, J.; Yahi, N.; Barrantes, F.J.; Chahinian, H. Anandamide Revisited: How Cholesterol and Ceramides Control Receptor-Dependent and Receptor-Independent Signal Transmission Pathways of a Lipid Neurotransmitter. Biomolecules 2018, 8, 31. [Google Scholar] [CrossRef]
- Ottria, R.; Casati, S.; Rota, P.; Ciuffreda, P. 2-Arachidonoylglycerol Synthesis: Facile and Handy Enzymatic Method That Allows to Avoid Isomerization. Molecules 2022, 27, 5190. [Google Scholar] [CrossRef] [PubMed]
- Buczynski, M.W.; Parsons, L.H. Quantification of brain endocannabinoid levels: Methods, interpretations and pitfalls. Br. J. Pharmacol. 2010, 160, 423–442. [Google Scholar] [CrossRef]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavón, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef]
- Keereetaweep, J.; Chapman, K.D. Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification. Neural Plast. 2016, 2016, 2426398. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef]
- He, B.; Di, X.; Guled, F.; Harder, A.V.; Maagdenberg, A.M.v.D.; Terwindt, G.M.; Krekels, E.H.; Kohler, I.; Harms, A.; Ramautar, R.; et al. Quantification of endocannabinoids in human cerebrospinal fluid using a novel micro-flow liquid chromatography-mass spectrometry method. Anal. Chim. Acta 2022, 1210, 339888. [Google Scholar] [CrossRef]
- Meier, P.; Glasmacher, S.; Salmen, A.; Chan, A.; Gertsch, J. Comparative targeted lipidomics between serum and cerebrospinal fluid of multiple sclerosis patients shows sex and age-specific differences of endocannabinoids and glucocorticoids. Acta Neuropathol. Commun. 2024, 12, 160. [Google Scholar] [CrossRef]
- Ney, L.; Stone, C.; Nichols, D.; Felmingham, K.; Bruno, R.; Matthews, A. Endocannabinoid reactivity to acute stress: Investigation of the relationship between salivary and plasma levels. Biol. Psychol. 2021, 159, 108022. [Google Scholar] [CrossRef] [PubMed]
- Barba, S.V.; Kirschbaum, C.; Gao, W. Endocannabinoid and perceived stress: Association analysis of endocannabinoid levels in hair versus levels in plasma and urine. Biol. Psychol. 2023, 178, 108541. [Google Scholar]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 15781. [Google Scholar] [CrossRef]
- LeBeau, M.A.; Montgomery, M.A.; Brewer, J.D. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci. Int. 2011, 210, 110–116. [Google Scholar] [CrossRef]
- Kratz, D.; Sens, A.; Schäfer, S.M.G.; Hahnefeld, L.; Geisslinger, G.; Thomas, D.; Gurke, R. Pre-analytical challenges for the quantification of endocannabinoids in human serum. J. Chromatogr. B 2022, 1190, 123102. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; Konje, J.C. Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2089–2097. [Google Scholar] [CrossRef]
- Hillard, C.J.; Weinlander, K.M.; Stuhr, K.L. Contributions of endocannabinoid signaling to psychiatric disorders in humans: Genetic and biochemical evidence. Neuroscience 2012, 204, 207–229. [Google Scholar] [CrossRef]
- Hamzah, K.A.; Toms, L.M.; Kucharski, N.; Orr, J.; Turner, N.P.; Hobson, P.; Nichols, D.S.; Ney, L.J. Sex-dimorphism in human serum endocannabinoid and n-acyl ethanolamine concentrations across the lifespan. Sci. Rep. 2023, 13, 23059. [Google Scholar] [CrossRef]
- Ney, L.J.; Cooper, J.; Lam, G.N.; Moffitt, K.; Nichols, D.S.; Mayo, L.M.; Lipp, O.V. Hair endocannabinoids predict physiological fear conditioning and salivary endocannabinoids predict subjective stress reactivity in humans. Psychoneuroendocrinology 2023, 154, 106296. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; De Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 2007, 130, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef]
- Dlugos, A.; Childs, E.; Stuhr, K.L.; Hillard, C.J.; de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2012, 37, 2416–2427. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; de Wit, H.; Hillard, C.J.; Van Cauter, E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J. Clin. Endocrinol. Metab. 2015, 100, 220–226. [Google Scholar] [CrossRef]
- Hanlon, E.C. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 2020, 111, 104471. [Google Scholar] [CrossRef]
- Vasquez, N.A.; Nielsen, D.E. The Endocannabinoid System and Eating Behaviours: A Review of the Current State of the Evidence. Curr. Nutr. Rep. 2022, 11, 665–674. [Google Scholar] [CrossRef]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; Di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012, 97, E917–E924. [Google Scholar] [CrossRef] [PubMed]
- Lanz, C.; Mattsson, J.; Stickel, F.; Dufour, J.F.; Brenneisen, R. Determination of the Endocannabinoids Anandamide and 2-Arachidonoyl Glycerol with Gas Chromatography-Mass Spectrometry: Analytical and Preanalytical Challenges and Pitfalls. Med. Cannabis Cannabinoids 2018, 1, 9–18. [Google Scholar] [CrossRef]
- Serrano, A.; Natividad, L.A. Alcohol-Endocannabinoid Interactions: Implications for Addiction-Related Behavioral Processes. Alcohol Res. 2022, 42, 9. [Google Scholar] [CrossRef]
- Gupta, S.; Bharatha, A.; Cohall, D.; Rahman, S.; Haque, M.; Majumder, M.A.A. Aerobic Exercise and Endocannabinoids: A Narrative Review of Stress Regulation and Brain Reward Systems. Cureus 2024, 16, e55468. [Google Scholar] [CrossRef]
- Jian, W.; Edom, R.; Weng, N.; Zannikos, P.; Zhang, Z.; Wang, H. Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibition in human plasma. J. Chromatogr. B 2010, 878, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007, 152, 671–675. [Google Scholar] [CrossRef]
- Gurke, R.; Thomas, D.; Schreiber, Y.; Schäfer, S.M.G.; Fleck, S.C.; Geisslinger, G.; Ferreirós, N. Determination of endocannabinoids and endocannabinoid-like substances in human K3EDTA plasma—LC-MS/MS method validation and pre-analytical characteristics. Talanta 2019, 204, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gkini, E.; Anagnostopoulos, D.; Mavri-Vavayianni, M.; Siafaka-Kapadai, A. Metabolism of 2-acylglycerol in rabbit and human platelets. Involvement of monoacylglycerol lipase and fatty acid amide hydrolase. Platelets 2009, 20, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.C.; Antone, J.; Alsop, E.; Jayakumar, R.; Parikh, K.; Chiot, A.; Sanchez-Molina, P.; Ajami, B.; Arnold, S.E.; Jensen, K.; et al. Cryopreservation of cerebrospinal fluid cells preserves the transcriptional landscape for single-cell analysis. J. Neuroinflamm. 2024, 21, 71. [Google Scholar] [CrossRef]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Ström, J. Cellular elements in the urine in health and in acute infectious diseases, especially with respect to the presence of haematuria. A study with application of millipore procedure and Papanicolaou staining. Scand. J. Infect. Dis. 1975, 7, 97–102. [Google Scholar] [CrossRef]
- Schreiber, D.; Harlfinger, S.; Nolden, B.M.; Gerth, C.W.; Jaehde, U.; Schömig, E.; Klosterkötter, J.; Giuffrida, A.; Astarita, G.; Piomelli, D.; et al. Determination of anandamide and other fatty acyl ethanolamides in human serum by electrospray tandem mass spectrometry. Anal. Biochem. 2007, 361, 162–168. [Google Scholar] [CrossRef]
- Dubbelman, A.C.; van Wieringen, B.; Arias, L.R.; van Vliet, M.; Vermeulen, R.; Harms, A.C.; Hankemeier, T. Strategies for Using Postcolumn Infusion of Standards to Correct for Matrix Effect in LC-MS-Based Quantitative Metabolomics. J. Am. Soc. Mass Spectrom. 2024, 35, 3286–3295. [Google Scholar] [CrossRef]
- Balvers, M.G.; Verhoeckx, K.C.; Witkamp, R.F. Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2009, 877, 1583–1590. [Google Scholar] [CrossRef]
- Huang, S.M.; Strangman, N.M.; Walker, J.M. Liquid chromatographic-mass spectrometric measurement of the endogenous cannabinoid 2-arachidonylglycerol in the spinal cord and peripheral nervous system. Zhongguo Yao Li Xue Bao 1999, 20, 1098–1102. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Tagliamonte, S.; Gill, C.I.R.; Pourshahidi, L.K.; Slevin, M.M.; Price, R.K.; Ferracane, R.; Lawther, R.; O’Connor, G.; Vitaglione, P. Endocannabinoids, endocannabinoid-like molecules and their precursors in human small intestinal lumen and plasma: Does diet affect them? Eur. J. Nutr. 2021, 60, 2203–2215. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Jemal, M. Phospholipids in liquid chromatography/mass spectrometry bioanalysis: Comparison of three tandem mass spectrometric techniques for monitoring plasma phospholipids, the effect of mobile phase composition on phospholipids elution and the association of phospholipids with matrix effects. Rapid Commun. Mass Spectrom. 2009, 23, 2125–2138. [Google Scholar]
- Bobrich, M.; Schwarz, R.; Ramer, R.; Borchert, P.; Hinz, B. A simple LC-MS/MS method for the simultaneous quantification of endocannabinoids in biological samples. J. Chromatogr. B 2020, 1161, 122371. [Google Scholar] [CrossRef]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An Analysis of Endocannabinoid Concentrations and Mood Following Singing and Exercise in Healthy Volunteers. Front. Behav. Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef]
- Schmid, P.C.; Schwartz, K.D.; Smith, C.N.; Krebsbach, R.J.; Berdyshev, E.V.; Schmid, H.H. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem. Phys. Lipids 2000, 104, 185–191. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Lam, P.M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. A solid-phase method for the extraction and measurement of anandamide from multiple human biomatrices. Anal. Biochem. 2009, 384, 106–113. [Google Scholar] [CrossRef]
- Hardison, S.; Weintraub, S.T.; Giuffrida, A. Quantification of endocannabinoids in rat biological samples by GC/MS: Technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006, 81, 106–112. [Google Scholar] [CrossRef]
- Sergi, M.; Battista, N.; Montesano, C.; Curini, R.; Maccarrone, M.; Compagnone, D. Determination of the two major endocannabinoids in human plasma by μ-SPE followed by HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 785–793. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Ghebreselasie, K.; Marnett, L.J. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem. Phys. Lipids 2002, 119, 69–82. [Google Scholar] [CrossRef]
- Zoerner, A.A.; Gutzki, F.M.; Suchy, M.T.; Beckmann, B.; Engeli, S.; Jordan, J.; Tsikas, D. Targeted stable-isotope dilution GC-MS/MS analysis of the endocannabinoid anandamide and other fatty acid ethanol amides in human plasma. J. Chromatogr. B 2009, 877, 2909–2923. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Hasselstrøm, J.B. The effect of antioxidants on the long-term stability of THC and related cannabinoids in sampled whole blood. Drug Test Anal. 2018, 10, 301–309. [Google Scholar] [CrossRef]
- Hamzah, K.A.; Turner, N.; Nichols, D.; Ney, L.J. Advances in targeted liquid chromatography-tandem mass spectrometry methods for endocannabinoid and N-acylethanolamine quantification in biological matrices: A systematic review. Mass Spectrom. Rev. 2024, 43, 350–372. [Google Scholar] [CrossRef]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; de la Torre, R. Analysis of ECs and related compounds in plasma: Artifactual isomerization and ex vivo enzymatic generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef]
- Dócs, K.; Mészár, Z.; Gonda, S.; Kiss-Szikszai, A.; Holló, K.; Antal, M.; Hegyi, Z. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front. Cell. Neurosci. 2017, 11, 39. [Google Scholar] [CrossRef]
- Markey, S.P.; Dudding, T.; Wang, T.C. Base- and acid-catalyzed interconversions of O-acyl- and N-acyl-ethanolamines: A cautionary note for lipid analyses. J. Lipid Res. 2000, 41, 657–662. [Google Scholar] [CrossRef]
- Fowler, C.J.; Tiger, G.; Ligresti, A.; López-Rodríguez, M.L.; Di Marzo, V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis--a difficult issue to handle. Eur. J. Pharmacol. 2004, 492, 1–11. [Google Scholar] [CrossRef]
- Djilali, E.; Pappalardo, L.; Posadino, A.M.; Giordo, R.; Pintus, G. Effects of the Storage Conditions on the Stability of Natural and Synthetic Cannabis in Biological Matrices for Forensic Toxicology Analysis: An Update from the Literature. Metabolites 2022, 12, 801. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Campo, P.; Suidan, M.T.; Chai, Y.; Davis, J. A liquid chromatography-electrospray ionization-tandem mass spectrometry study of ethanolamines in high salinity industrial wastewaters. Talanta 2010, 80, 1110–1115. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; El-Talatini, M.; Finney, M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal. Biochem. 2008, 380, 195–201. [Google Scholar] [CrossRef]
- Marchioni, C.; de Souza, I.D.; Grecco, C.F.; Crippa, J.A.; Tumas, V.; Queiroz, M.E.C. A column switching ultrahigh-performance liquid chromatography-tandem mass spectrometry method to determine anandamide and 2-arachidonoylglycerol in plasma samples. Anal. Bioanal. Chem. 2017, 409, 3587–3596. [Google Scholar] [CrossRef]
- Mwanza, C.; Chen, Z.; Zhang, Q.; Chen, S.; Wang, W.; Deng, H. Simultaneous HPLC-APCI-MS/MS quantification of endogenous cannabinoids and glucocorticoids in hair. J. Chromatogr. B 2016, 1028, 1–10. [Google Scholar] [CrossRef]
- Dong, X.; Li, L.; Ye, Y.; Zhang, D.; Zheng, L.; Jiang, Y.; Shen, M. Surrogate analyte-based quantification of main endocannabinoids in whole blood using liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4439. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Di, X.; Kohler, I.; Osuna-Prieto, F.J.; Acosta, F.M.; Vilchez-Vargas, R.; Link, A.; Plaza-Díaz, J.; van der Stelt, M.; et al. Plasma Levels of Endocannabinoids and Their Analogues Are Related to Specific Fecal Bacterial Genera in Young Adults: Role in Gut Barrier Integrity. Nutrients 2022, 14, 2143. [Google Scholar] [CrossRef]
- Pedersen, T.L.; Gray, I.J.; Newman, J.W. Plasma and serum oxylipin, endocannabinoid, bile acid, steroid, fatty acid and nonsteroidal anti-inflammatory drug quantification in a 96-well plate format. Anal. Chim. Acta 2021, 1143, 189–200. [Google Scholar] [CrossRef]
- De Luca, L.; Ferracane, R.; Ramírez, N.C.; Vitaglione, P. N-Acylphosphatidylethanolamines and N-acylethanolamines increase in saliva upon food mastication: The influence of the individual nutritional status and fat type in food. Food Funct. 2020, 11, 3382–3392. [Google Scholar] [CrossRef]
- Junior, V.R.A.; Goméz-Ríos, G.A.; Tascon, M.; Queiroz, M.E.C.; Pawliszyn, J. Analysis of endocannabinoids in plasma samples by biocompatible solid-phase microextraction devices coupled to mass spectrometry. Anal. Chim.Acta 2019, 1091, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Inamassu, C.H.; Raspini, E.S.L.; Marchioni, C. Recent advances in the chromatographic analysis of endocannabinoids and phytocannabinoids in biological samples. J. Chromatogr. A 2024, 1732, 465225. [Google Scholar] [CrossRef]
- Sempio, C.; Klawitter, J.; Jackson, M.; Freni, F.; Shillingburg, R.; Hutchison, K.; Bidwell, L.C.; Christians, U.; Klawitter, J. Analysis of 14 endocannabinoids and endocannabinoid congeners in human plasma using column switching high-performance atmospheric pressure chemical ionization liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Kantae, V.; Ogino, S.; Noga, M.; Harms, A.C.; van Dongen, R.M.; Onderwater, G.L.; van den Maagdenberg, A.M.; Terwindt, G.M.; van der Stelt, M.; Ferrari, M.D.; et al. Quantitative profiling of endocannabinoids and related N-acylethanolamines in human CSF using nano LC-MS/MS. J. Lipid Res. 2017, 58, 615–624. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; de Castro, M.D.L.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC-MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).