Abstract
Chalcones, a class of flavonoid compounds, are recognized for their unique biological properties, and especially for their antithrombotic, anti-inflammatory, and antioxidant health-promoting properties against inflammation-related disorders. Chalcones are phytochemicals naturally found in plants, fruits, and vegetables, such as tomatoes, apples, and licorice. Their characteristic chemical structure, which includes two aromatic rings and an α,β-unsaturated carbonyl group, makes them particularly versatile for pharmaceutical use. At the same time, chalcones exhibit strong antioxidant activity by neutralizing free radicals and enhancing endogenous antioxidant defense systems, such as glutathione. Structural modifications have improved their biological activity, leading to important applications in the treatment of atherosclerosis and cardiovascular diseases, cancer, neurodegenerative diseases, and inflammatory disorders. In addition, they have been successfully used in agriculture as natural pesticides and in the food industry as antioxidant additives. This review demonstrates the interdisciplinary importance of chalcones, highlighting the need for further research into their molecular mechanisms of action. A deeper understanding of their properties may open new avenues for the development of innovative drugs and environmentally friendly applications. In this way, chalcones can be a decisive factor in improving human health and environmental sustainability.
Keywords:
chalcones; flavonoids; natural products; uses; applications; antioxidants; cardiovascular action 1. Introduction
Chalcones, as a subclass of flavonoids, have attracted considerable scientific interest due to their diverse pharmacological activities, including antioxidant, anti-inflammatory, and antifibrotic effects. These properties are not only relevant to atherosclerosis and cardiovascular diseases but also highly pertinent to sclerosis-related disorders. In multiple sclerosis (MS), chalcones have been reported to reduce neuroinflammation, modulate oxidative stress, and exert neuroprotective actions. Similarly, in systemic sclerosis, chalcones may downregulate fibrotic mediators, inhibit fibroblast proliferation, and improve endothelial function, thereby addressing the key pathological features of the disease [1].
The history of chalcones begins with the first synthetic attempts that took place in the 19th century. Stanisław Kostanecki and Josef Tambor, who are known as the pioneers in the synthesis of synthetic chalcones, introduced their chemical name and developed production methods that remain useful even today [1]. Chalcones naturally occur both in their free (aglycone) form and as glycosides. They have been detected in a wide range of plants, fruits, and vegetables, including apples, tomatoes, citrus species, and licorice. Their broad presence in nature has encouraged extensive research, since these compounds play a significant role in the human diet and in various traditional medicinal systems [2].
The antithrombotic and anti-inflammatory actions of chalcones are two of the most interesting aspects of their pharmacological significance. The antithrombotic effects of these compounds are attributed to their capacity to inhibit platelet aggregation and enhance blood circulation, while their anti-inflammatory actions are attributed to the inhibition of inflammatory pathways, such as the inhibition of cyclooxygenase (COX) and lipoxygenase (LOX). Furthermore, chalcones have demonstrated efficacy in modulating reactive oxygen species (ROS) and alleviating oxidative stress, traits that are closely linked to the prevention and management of various chronic diseases [3]. The chemical structure of chalcones is key to their biological properties. They are derivatives of 1,3-diphenyl-2-propen-1-ones, which include two aromatic rings connected via an α,β-unsaturated carbonyl group. This structure makes chalcones extremely versatile, allowing them to undergo various chemical modifications to enhance their pharmacological properties. In particular, the presence of phenolic groups in their rings enhances their action as antioxidants, while variations in substituents can increase biological activity [4].
Chalcones have been found to modulate various molecular mechanisms, notably by preventing the activation of nuclear factor kappa-B (NF-κB) and reducing the production of pro-inflammatory cytokines like IL-6 and TNF-α. These mechanisms are critical for the management of inflammatory diseases, including autoimmune disorders and rheumatoid arthritis. At the same time, the ability of chalcones to interfere with the signaling of the MAPK and PI3K/Akt pathways reinforces their importance in research into treatments for cancer and chronic inflammation. In conclusion, the analysis provided in this paper highlights the interdisciplinary nature of chalcones and their variety of applications [5]. Copper ions appear to serve a key role in controlling inflammatory processes and thrombotic processes, making them candidates for use in pharmaceutical products. In particular, studies focusing on structural modifications of chalcones show that these changes can dramatically improve their biological performance, while providing new opportunities for the development of environmentally friendly agrochemicals and therapies. Extensive research on chalcones has resulted in the identification of numerous synthetic and natural derivatives with specialized properties. These derivatives have been exploited in various fields, such as agriculture (as pesticides and plant growth regulators), pharmaceuticals (for the development of new drugs), and the food industry (as natural additives). At the same time, chalcones have proven useful in addressing diseases, including cancer, cardiovascular disease, and neurodegenerative diseases, by targeting numerous molecular mechanisms. In addition, the use of chalcones as natural antioxidants has been extensively studied. Their capacity to scavenge free radicals and strengthen endogenous antioxidant mechanisms, including glutathione, renders them important for the prevention of degenerative disorders. Furthermore, their ability to reduce oxidative stress in the brain suggests potential therapeutic applications for diseases such as Alzheimer’s and Parkinson’s.
This review aims to deepen our understanding of the antithrombotic and anti-inflammatory properties of chalcones by examining their chemical, biological, and pharmacological aspects. Furthermore, it seeks to highlight the importance of structural modifications of chalcones in enhancing their therapeutic properties. By systematically reviewing the latest research, this paper seeks to offer a thorough overview of the role of chalcones in contemporary pharmacology and their potential for clinical application.
2. Chalcones, Origin, Sources, Structure, and General Uses/Applications
2.1. Origin of Chalcones
The name chalcone is derived from the Greek word chalcos, meaning “copper,” in reference to the yellow–orange coloration characteristic of many chalcone compounds. This etymology reflects their appearance rather than any chemical connection with the metal copper [1]. The first studies on chalcones focused on their synthetic preparation. Stanisław Kostanecki and Josef Tambor, pioneers in flavonoid chemistry, successfully synthesized chalcones in the late 19th century and introduced the term chalcone to describe this class of open-chain flavonoids. Their work established the basic methods of chalcone synthesis, many of which remain in use today [2]. By contrast, naturally occurring chalcones were not isolated until 1910, when plant-derived chalcones were first identified. This marked the beginning of systematic research into chalcones as secondary metabolites with biological roles in plants and, later, as pharmacologically active natural products in humans [6].
As early as the 19th century, several researchers worked on synthesizing chalcones, with Kostanecki and Tambor acknowledged as the pioneers who successfully prepared synthetic chalcones by treating o-acetoxychalcone dibromides with alcoholic alkalis [6]. In fact, Kostanecki and Tambor coined the term chalcone [1], and the word was first introduced in 1899 based on the discovery of mono-oxychalcone [7]. Other widely known chemical names for chalcone include benzyl acetophenone or benzylideneacetophenone [8], phenylstyryl ketone, β-phenyl-acrylophenone, α-phenyl-β-benzoyl-ethylene, etc., and form the central core of biologically active heterocyclic compounds [9]. It was not until 1910 that naturally occurring chalcones were first isolated [8].
2.2. Structural Features
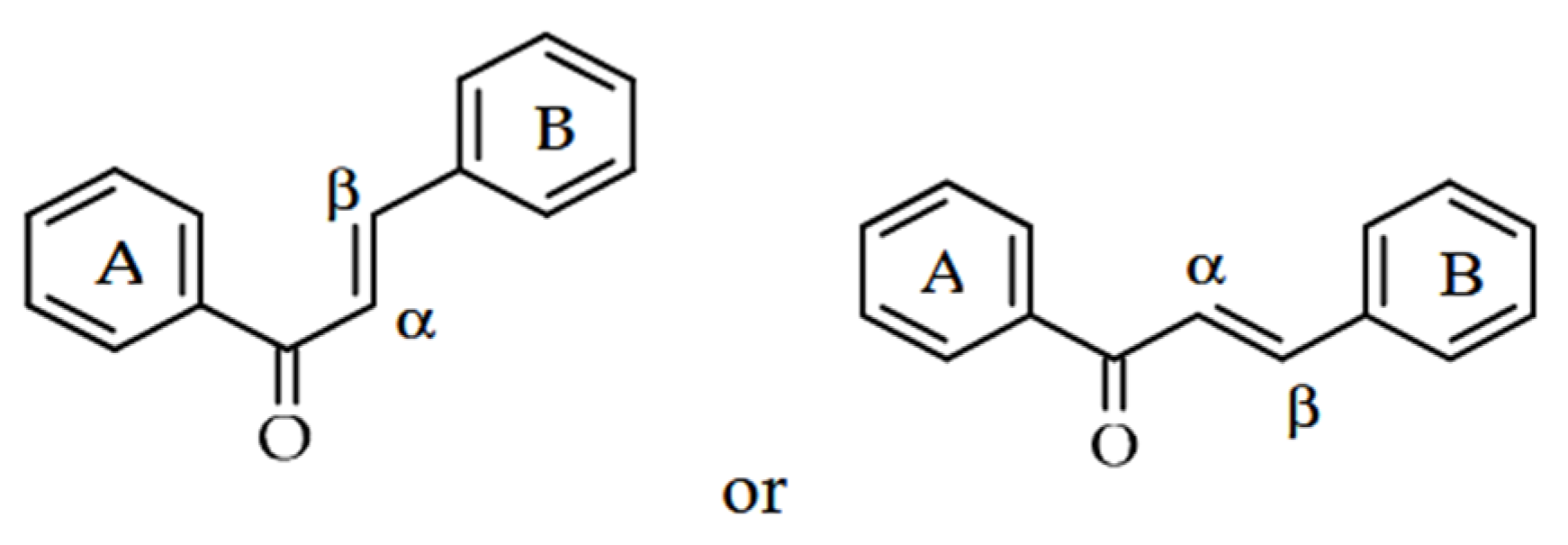
Chalcones are classified within the flavonoid group of phenolic compounds [6]. As open-chain flavonoids, they are derivatives of 1,3-diphenyl-2-propen-1-ones and consist of two aromatic rings connected by an α,β-unsaturated carbonyl system (Figure 1). Due to the double bond in this system, chalcones can exist in two stereoisomeric forms: the trans (E)-isomer, which is the most stable and commonly found in nature, and the less stable cis (Z)-isomer. Both configurations share the same core structural features but differ in the relative orientation of the substituents around the C=C bond [1]. They are based on two aryl units bridged by an α,β-unsaturated carbonyl group [10] and are not only important precursors for synthetic manipulations, but also constitute an important component of natural products [11]. The chalcones in their aromatic rings have a delocalized p-electron order [10]. Specifically, their biological activities are believed to be attributed to the presence of this double (π) bond, in conjunction with the carbonyl property, as the removal of the carbonyl makes them inactive substances [11].
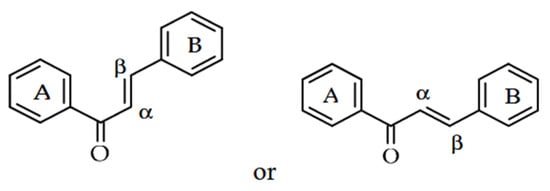
Figure 1.
General structures of chalcone alloys [6].
Due to the inclusion of a ketoethylenic CO-CH=CH- structure and the reactive α,β-unsaturated carbonyl group, copper derivatives are valued for their broad range of properties [10]. The coloration of these compounds is attributed to the chromophore (-CO-CH=CH-), whose effect depends on the presence of additional auxochromes [1]. These are polyphenolic compounds that change color and are usually yellow or orange pigments in plants due to the conjugated bonds in their structure [1,7]. Owing to the presence of two aromatic rings linked by a three-carbon α,β-unsaturated carbonyl system, these compounds can exist as either trans (E) or cis (Z) isomers [1]. Chalcones typically exist in either cis or trans configurations because of the double bond in their structure, and they generally adopt a nearly planar shape [7].
2.3. Occurrence of Chalcones in Natural Sources
In nature, chalcones are most commonly present as chalcone aglycones and chalcone glycosides, although they may also undergo modifications such as hydroxylation, condensation, or methylation [12]. Importantly, chalcones are involved in plant defense mechanisms, helping to combat reactive oxygen species and thereby ensuring plant survival while preventing molecular damage and injury caused by microorganisms, insects, and animals [8].
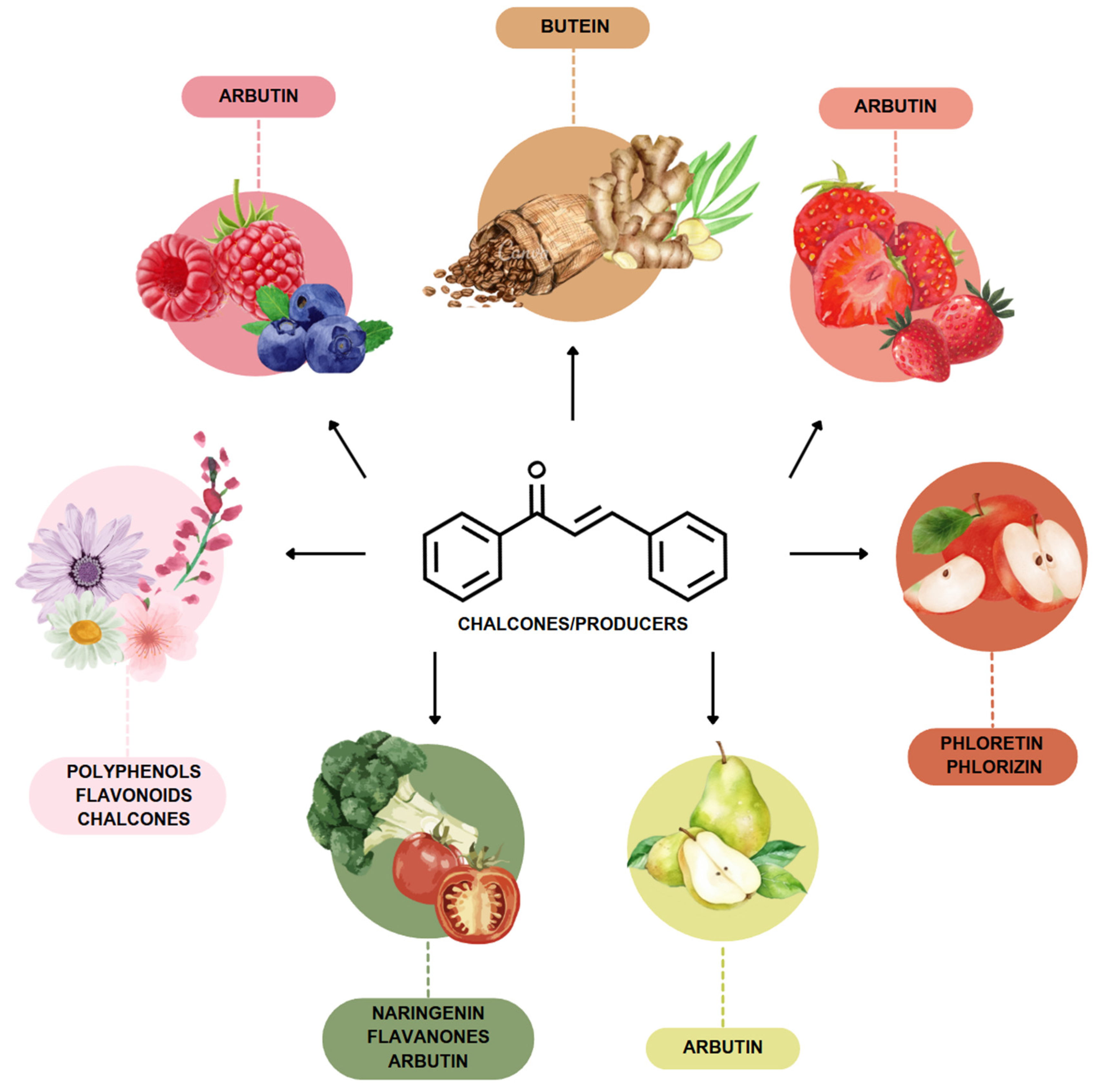
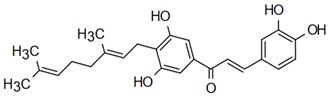
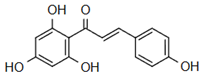
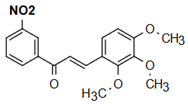
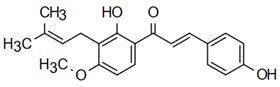
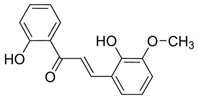
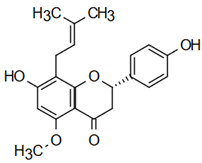
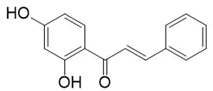
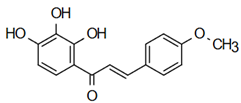
The most prevalent chalcones in food include glucoside, phlorizin (floretin 2’-O-glucose), naringenin (a flavonoid), and arbutin (Figure 2). Flavone and phlorizin are typically associated with apples, while naringenin is characteristic of tomatoes. Arbutin is notably present in pears, and is also found in strawberries, bearberry, wheat and wheat products, as well as in trace amounts in tea, coffee, red wine, and broccoli [13]. Prominent examples of chalcones also include butein. These compounds are widely distributed in strawberries, various berries, certain wheat products, tomatoes, pears, apples, citrus fruits, and hops (Humulus lupulus) [14].
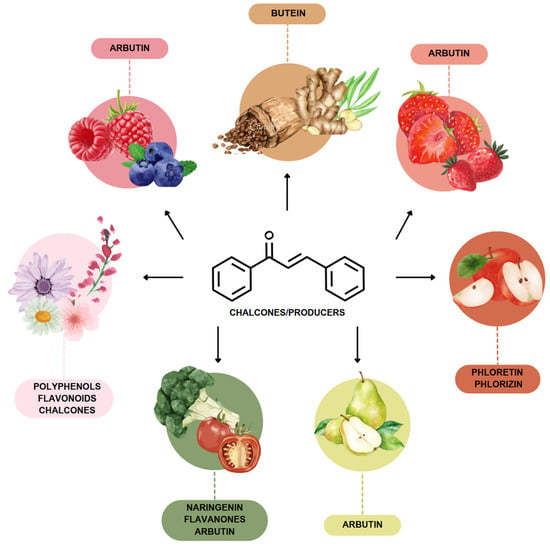
Figure 2.
Chalcones in natural products.
More specifically, they are found in a variety of fruits and vegetables, such as apples (Malus domestica) and other citrus fruits, tomatoes (Solanum lycopersicum), various plants and spices, such as licorice (Glycyrrhiza inflata), many of which have been used in traditional herbal medicine for millennia [1,15]. Flavanones, dihydroxycinnamates, and chalcones, along with their glycosides, are the main categories of polyphenolic compounds found in tomatoes [7]. Licorice is also a type of legume, rich in bioactive chalcones and belonging to the Leguminosae family. This plant is widely used in the manufacture of pharmaceutical products, such as iron pills and cough suppressants, to mask the bitter taste of other treatments [7]. In fact, such derivatives have even been found in soy-based foods [16]. Various members of the Bidens and Coreopsis genera of the Asteraceae family are also noted for their high chalcone content [17]. Their occurrence is particularly significant in the Heliantheae tribe, and several chalcones have been isolated from Helianthus annuus [18].
Chalcones are widely distributed in various parts of plants, including roots, rhizomes, leaves, and seeds [1]. They are abundant in nature, from ferns to higher plants, and many of them have polyhydroxylic acrylic rings [1]. They have also been found in the aerial roots of Ficus microcarpa, the stems and leaves of the genus Morus, the leaves or fruits of the genus Artocarpus, and members of the genus Dorstenia, all belonging to the Moraceae family [19]. In the Fabaceae family, chalcones are found in Desmodium renifolium, the roots of the Sophora genus, the Glycyrrhiza genus, and the Dalbergia genus [12]. Finally, chalcones are also found in plant species of other families, sometimes in very high concentrations, such as in the yellow juice of Angelica keiskei [20] or in members of the genus Scutellaria [12].
These edible plants are an integral part of the human diet, and the various naturally active compounds they contain, especially polyphenols, polysaccharides, and amino acids, have always been a notable topic of research among nutritionists. As precursors of polyphenolic compounds in edible plants, chalcones are not only widespread but also have diverse biological activities due to their unique structure [17].
2.4. Initial Uses/Applications of Chalcones in Agriculture and Plant Protection
With the European Union’s “From Farm to Fork” strategy aiming to reduce the use of synthetic pesticides by 2030, bioinsecticides are becoming increasingly popular not only for their different modes of action but also for their increased environmental sustainability and their applications in “copper farming” [14]. Due to their numerous biological activities, the future practical application of chalcogenides as environmentally friendly plant growth regulators and defensive agents in agriculture and plant production is noteworthy and worth discussing. In terms of pest defense and weed control, the most interesting biological properties of chalcones are their bactericidal, antifungal, anthelmintic, insecticidal, antiviral, and phytotoxic properties [12]. Modifying chalcones by adding specific functional groups can enhance their desirable properties, while at the same time, due to their unique structure, chalcones also serve as intermediates in the synthesis of therapeutically valuable compounds [14].
The chemical structures of some chalcones with potential use in agriculture and plant protection are presented in Table 1.

Table 1.
Chemical structures and biological activities of some chalcones important for agriculture and plant protection.
However, because plants produce chalcones in limited amounts, extracting them from natural sources remains difficult, and their relatively short half-life further complicates their study and application as pesticides. These challenges have driven extensive research into the synthesis and development of chalcones. There is now an urgent need for comprehensive studies to clarify their mechanisms of action, assess their effectiveness under real agricultural conditions, and evaluate their environmental and human safety [18]. Below are categories of environmentally friendly products based on chalcones.
2.4.1. Chalcones as Herbicides and Plant Growth Regulators
Exploring the chemistry of molecules naturally selected to function in ecological defense provides a promising starting point for discovering new pest control agents. Chalcones are gaining recognition in agriculture for their phytotoxic properties, which support the development of novel herbicides. Studies indicate that numerous chalcones exhibit strong herbicidal effects while showing low toxicity toward crops. As noted earlier, their activity depends on factors such as the substituents on the A and B rings of their structure (Figure 1), the concentrations applied, and the targeted plant species and organs. Derivatives with functional groups like phenoxyacetic acid, 4-(N,N-dimethylamino)phenyl, and N-methylpyrrole have shown particularly significant inhibitory effects [14].
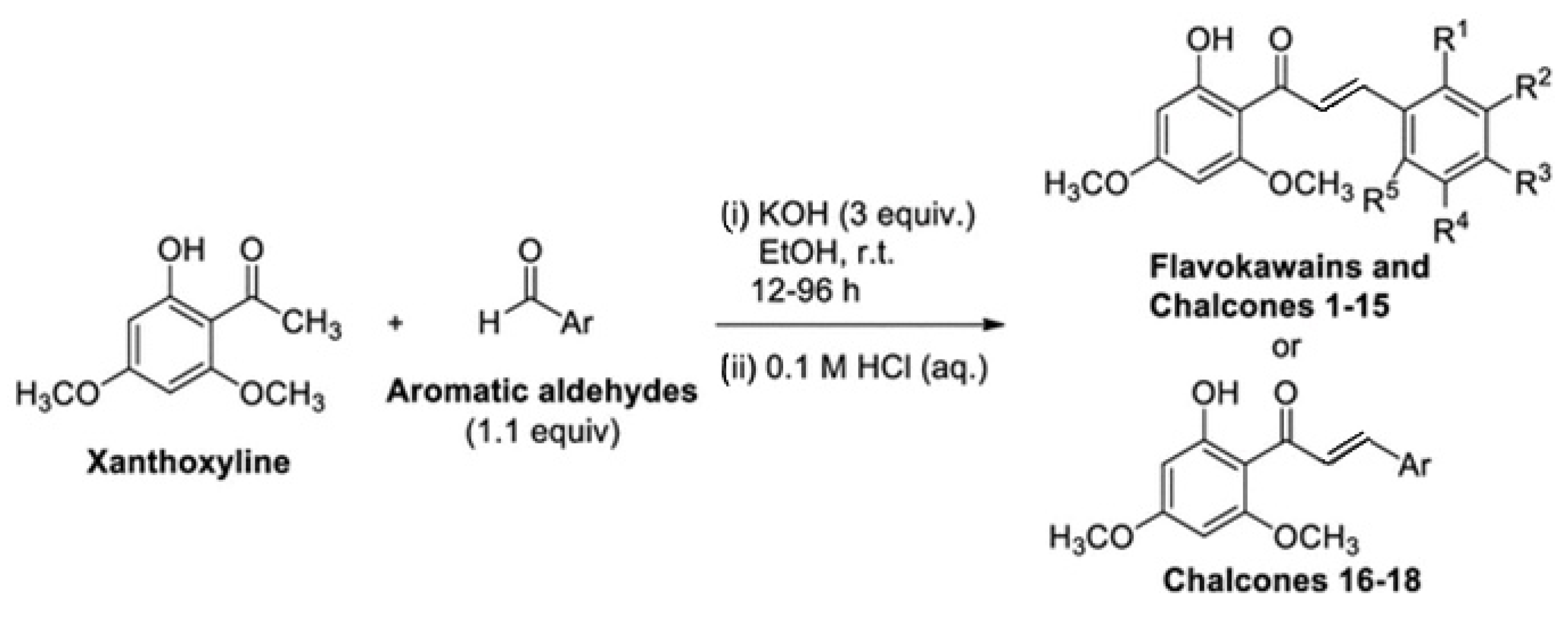
A study by Chotsaeng [26] found that flabocavaines, xanthoxylin derivatives related to chalcones through the Claisen–Schmidt reaction as shown in Figure 3, significantly inhibited the growth of Chinese amaranth and weed [14].
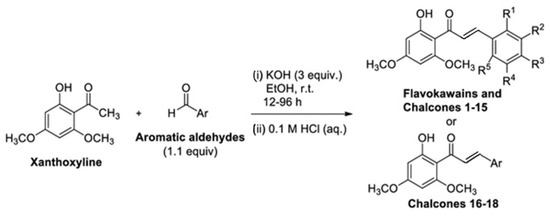
Figure 3.
Claisen–Schmidt condensation of xanthoxyline with aromatic aldehydes to synthesize flavokawains and chalcones, reproduced from [26].
In addition, Diaz-Tielaz in another study [27] examined the selective phytotoxic effects of chalcones on crops and weeds. Their study evaluated spray and irrigation applications on mature Arabidopsis and found that trans-chalcone adversely affected germination and early root development in certain weeds and crops, while exhibiting beneficial effects in others [14]. In additional research [28], phloretin, a well-known dihydrochalcone, demonstrated marked dose-dependent growth inhibition, pronounced morphological abnormalities, and altered behavior in Arabidopsis plants. These results further emphasize the potential of chalcones as effective and selective agents for controlling plant growth and weeds [14]. Indeed, chalcones exhibit bioactivity against nearly all eukaryotic organisms and some prokaryotic species, with numerous molecular targets. Over recent decades, intensive agriculture has relied heavily on synthetic pesticides for crop protection, often in an uncontrolled manner. This excessive use of synthetic herbicides has resulted in the emergence of agrochemical-resistant weeds, leading to significant economic losses, potential health hazards, and environmental contamination. These studies aim to identify compounds with novel mechanisms of action distinct from those of conventional synthetic agrochemicals, in order to develop effective agents with reduced environmental impact [12].
2.4.2. Chalcones as Fungicides
Numerous studies have demonstrated the efficacy of natural chalcones against a wide variety of plant pathogens responsible for serious agronomic and economic losses worldwide [12,14]. Their natural occurrence inspired the development of synthetic chalcones with enhanced antifungal properties [14], which have since become widely recognized for their activity against both plant and human fungal pathogens. Mechanistically, chalcones inhibit β(1,3)-glucan and chitin synthase, enzymes essential for the synthesis of fungal cell wall polymers, thereby compromising fungal integrity and growth [29].
Several experimental studies highlight the breadth of these effects. For example, Svetaz [21] reported that Phomopsis longicolla was highly sensitive to chalcones derived from Zuccagnia punctata. The chloroform fraction of an ethanolic extract, containing compounds such as 2’,4’-dihydroxy-3’-methoxychalcone and 2’,4’-dihydroxychalcone, exhibited strong antifungal activity against P. longicolla and Colletotrichum truncatum, two pathogens that severely affect soybean quality and yield. In a related study, Badaracco [30] demonstrated that 1,3-diphenyl-2-propen-1-one inhibited several agriculturally important fungi, including Alternaria sp., P. longicolla, Fusarium proliferatum, and Fusarium subglutinans. Likewise, Oleszek [31] showed that methanolic extracts of apple cores, particularly those enriched in phlorizin (a chalcone), were strongly active against Botrytis sp., Fusarium oxysporum, Petriella setifera, and Neosartorya fischeri [14].
Synthetic derivatives have also proven effective. Chen [32] found that pyridazine-containing chalcones displayed stronger antifungal activity than azoxystrobin, disrupting fungal cell membranes and inhibiting growth. The mechanism was associated with abnormal mycelial growth, surface rupture, and structural damage, underscoring the structural versatility of chalcones in antifungal design. Furthermore, chalcones isolated from the leaves of Myrica serrata—notably 2’,4’-dihydroxy-3’,5’-dimethyl-6’-methoxychalcone and stekurenesin—showed potent inhibition against Cladosporium cucumerinum, a common cucumber pathogen [33]. Additional chalcones from Z. punctata were also active against P. longicolla and Alternaria alternata [12]. Similarly, five chalcones isolated from Artocarpus nobilis (Moraceae) inhibited Cladosporium cladosporioides, which affects wheat, and Aspergillus niger, the causative agent of black mold in fruits and vegetables [22].
Together, these findings highlight both the natural and synthetic potential of chalcones as antifungal agents, with activities spanning multiple pathogens of agricultural importance. A summary of chalcones with reported fungicidal properties is provided in Table 2.

Table 2.
Chalcones mentioned above used as insecticides are summarized in the table.
2.4.3. Chalcones as Insecticides
Significant research has been devoted to the synthesis and development of new chalcones and their derivatives with insecticidal properties, emphasizing structural modifications and the choice of appropriate substituents [14]. Many studies have confirmed the effectiveness of both natural and synthetic chalcones as insecticidal agents [12].
Among naturally occurring chalcones, xanthohumol and isoxanthohumol, isolated from hops (Humulus lupulus L.), serve as notable examples. These compounds have shown considerable insecticidal activity against the peach–potato aphid (Myzus persicae) [34].
The research of Shakil and Saxena [35] reported the isolation of a novel chalcone, cordifoline, from the woody stem of Tinospora cordifolia and assessed its effects on Spodoptera litura larvae. Their results indicated that cordifoline delayed larval development, extended the larval period, and reduced larval weight [14]. Meanwhile, Hidalgo [36] investigated both bis- and mono-chalcones against Spodoptera frugiperda, finding that two mono-chalcones containing brominated and hydroxyl groups on the A ring and an N,N-dimethyl group on the B ring caused larval mortality rates of 40% and 60%, respectively [14].
The research conducted by Kumar et al. [37] is especially notable as it represents the first documented case of chalcones synthesized via microwave irradiation exhibiting pesticidal activity against Plutella xylostella [14]. Their study revealed that an electron-withdrawing group on the A ring is essential for pesticidal effectiveness, while the B ring can accommodate either electron-withdrawing or electron-donating substituents. Chlorine (-Cl) substituents and their positions within both rings were found to be particularly important. Among the compounds tested, 1,3-bis(4-chlorophenyl)prop-2-en-1-one exhibited the highest pesticidal activity, laying the groundwork for further structural optimization and the development of chalcone-based pesticides targeting P. xylostella and related insect pests [14].
Moreover, the common hop, Humulus lupulus L. (Cannabaceae), contains more than 1000 distinct chemical compounds [38]. The flavonoids present in hops (H. lupulus L.) are the focus of extensive research due to their beneficial health effects [34]. Xanthohumol, the principal chalcone in hop cones at an approximate concentration of 1%, possesses numerous valuable biological activities [39].
Growing concerns about the health risks associated with the extensive use of pesticides and synthetic fertilizers have driven increased interest in safer and more eco-friendly plant protection solutions. As a result, agricultural practices increasingly favor the use of naturally occurring pesticidal compounds from plants as alternatives to harmful chemical pesticides [40]. These compounds not only eliminate harmful microflora and pests but are also safe for both humans and the environment, which is a primary concern. Being natural substances, they are completely biodegradable. Furthermore, they exhibit activity against specific insect groups. This makes them environmentally friendly agents that can influence insect taste receptors, deterring feeding on plants and ultimately causing starvation and death [34].
3. Recent Advances on the Observed Health Promoting Properties of Chalcones
Over the last ten years, there has been a significant increase in interest in copper nitrates due to their interesting biological activities. As mentioned above, chalcones have a fantastic compound that allows them to produce a wide variety of new heterocyclic compounds with interesting pharmacological effects. In fact, they have shown promising therapeutic efficacy in treating a variety of disorders due to a wide range of structural modifications. This is because, in fact, scientific studies have confirmed that chalcone derivatives, based on their structure, have a special connection with such a wide range of pharmacological actions [14]. Structural modifications of copper rings have led to a high degree of diversity that has proven useful for the development of these new pharmaceutical agents, making them a subject of ongoing interest in both academia and industry [6]. Over recent decades, extensive research has been carried out on the pharmacological properties of both natural and synthetic chalcones, including their anti-inflammatory, antioxidant, anti-infective (such as anti-leukemic, anti-malarial, and anti-tuberculosis), antiviral, and particularly anticancer activities. Indeed, several chalcones have been successfully developed into commercial drugs for treating certain digestive system disorders, while others are currently undergoing clinical trials for applications in cancer therapy, cardiovascular disease management, and the treatment of viral infections [12,41]. Some of the main useful properties of chalcones are listed below.
3.1. Antioxidant Properties of Chalcones
As noted, the high presence of chalcones in foods such as fruits, vegetables, and medicinal plants underscores their potential role as natural antioxidants. Antioxidants are compounds that slow down or prevent oxidation, a process that generates free radicals. These free radicals can trigger chain reactions that damage cells, contributing to oxidative stress. Such stress is linked to the development of chronic conditions, including heart disease, strokes, cancer, arthritis, respiratory disorders, Parkinson’s disease, and various inflammatory ailments [9]. Chalcones demonstrate notable antioxidant potential owing to their chemical composition and effectiveness, with their electron-rich phenolic structure making them particularly well-suited as antioxidant agents. Among their various properties, antioxidant activity is arguably the most straightforward feature of chalcones [42].
Chalcones have been shown to influence the activity of antioxidant enzymes and regulate gene expression, thereby strengthening their capacity to protect against oxidative damage. Their ability to interact with multiple pathways linked to oxidative stress positions them as promising candidates for therapeutic approaches in health maintenance and disease prevention. Oxidative stress inflicts significant damage on nucleic acids, lipids, proteins, and vital enzymes, contributing to the development of conditions such as cancer, arthritis, cardiovascular disorders, and neurodegenerative diseases [42].
Antioxidant chalcones act through multiple cellular targets and mechanisms. They boost enzymatic antioxidant defenses, including superoxide dismutase, catalase, and glutathione peroxidase, and activate the Nrf2-ARE pathway to enhance the expression of antioxidant and detoxification genes. Additionally, chalcones can suppress the generation of reactive oxygen species (ROS) by inhibiting enzymes such as NADPH oxidases and xanthine oxidases, and they possess metal-chelating abilities that help prevent ROS formation catalyzed by metals [3,4].
There are many chalcones with different antioxidant properties that even follow different metabolic pathways [43].
3.1.1. Butein
3,4,2’,4’-tetrahydroxycoumarin, also known as butein, is a natural coumarin isolated mainly from the tree Toxicodendron vernicifluum, which is traditional and widely known in China [43]. The ability of butein to positively modulate the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), a critical transcription factor involved in the regulation of many cellular pathways [44]. Primarily, Nrf2 directly influences key regulators such as C/EBP-α and PPARγ, which, as mentioned earlier, contribute to preserving the differentiated state of preadipocytes [45]. Conversely, Nrf2 serves as a vital transcription factor essential for sustaining the redox balance in tissues [44].
Specifically, Nrf2 controls the expression of numerous enzymes linked to oxidative stress, including antioxidant enzymes such as thioredoxin, glutathione, heme oxygenase-1 (HO-1), and quinone oxidoreductase (NQO1), as well as detoxifying enzymes like glutathione S-transferases (GSTs) [46]. Overall, HO-1 is recognized as a crucial enzyme in antioxidant processes. It catalyzes the rate-limiting step in the breakdown of free heme into iron, carbon monoxide, and biliverdin, all of which are metabolites possessing significant antioxidant properties [47]. Recently, several studies have highlighted the critical role of HO-1 in the management of metabolic homeostasis, given its high level of expression in the white adipose tissue of genetically obese mice induced by a high-fat diet (HFD) [48]. Consequently, Nrf2 and its downstream enzyme HO-1 could serve as potential therapeutic targets for obesity-related disorders. In this context, Yang et al. demonstrated that treating 3T3-L1 cells with butein results in elevated Nrf2 expression, which subsequently modulates HO-1 mRNA expression levels [44]. A similar experimental design was followed by Wang et al., who also confirmed the ability of butein to induce increased expression of HO-1 mRNA and related protein expression in the 3T3-L1 adipocyte cell line [49]. Furthermore, Song et al. carried out a comparative investigation into the anti-obesity potential of several natural compounds derived from Rhus verniciflua Stokes, a lacquer tree long valued in traditional medicine.
3.1.2. Panduratin A
Panduratin A (PAN A) is a flavonoid featuring a chalcone backbone with three oxygenated groups located solely on the B ring and a geranyl substituent at the C2–C3 position formed via the Diels–Alder reaction. This natural chalcone is primarily obtained from Boesenbergia pandurata, a traditional medicinal plant renowned for its antioxidant and anti-inflammatory effects. In this regard, a study explored the potential of PAN A as a novel AMPK activator [4]. Modulating AMPK represents a promising strategy for addressing obesity, given its critical role as an energy sensor in mammalian cells. In their study, the authors observed that treating various cell models (including 3T3-L1 adipocytes, HepG2 liver carcinoma cells, and L6 skeletal muscle cells) with PAN A led to AMPK-dependent inhibition of ACC, thereby reducing endogenous lipid synthesis. Specifically, AMPK activation can be triggered by several regulators such as LKB1, CaMKKβ, sirtuin (SIRT1), and NAD(P)H. To elucidate the molecular mechanism behind PAN A’s activation of AMPK, the researchers examined its effects on the expression of these activators. They discovered that PAN A treatment enhanced the translocation of LKB1 from the nucleus to the cytoplasm and increased its binding affinity to AMPK, resulting in rapid enzyme activation [4,45].
3.1.3. 4’-OH-Flurbiprofen-Chalcone
Alzheimer’s disease (AD) is the most common type of dementia, which is a fatal, chronic, neurodegenerative disease of the brain [48]. However, because Alzheimer’s disease (AD) involves complex pathological mechanisms, current therapeutic approaches—such as cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists—primarily address symptoms and are unable to effectively halt or reverse the progression of neurodegeneration [46].
Although the exact pathogenesis of Alzheimer’s disease (AD) remains unclear, several factors—including β-amyloid (Aβ) accumulation, oxidative stress, and reduced acetylcholine levels—have been identified as key contributors to its development. A sustained imbalance between the production and clearance of Aβ1-42 results in the buildup of Aβ1-42 monomers, oligomers, and eventually large insoluble amyloid fibrils [47]. Amyloid deposits can therefore lead to cerebrovascular damage, neuroinflammation, abnormal calcium homeostasis, and neurodegeneration. Therefore, reducing the accumulation of Ab1-42 is a potential therapeutic strategy for treating AD [50].
A recent study demonstrated that chalcones possess properties suitable for Aβ imaging tracers, exhibiting high brain uptake and strong affinity for Aβ aggregates. Moreover, multifunctional agents containing phenolic hydroxyl groups showed enhanced antioxidant activity [48]. Consequently, 4’-OH-flurbiprofen was chosen for combination with chalcones to create a series of 4’-OH-flurbiprofen–chalcone hybrids, designed to function as multifunctional agents with both inhibitory effects on Aβ aggregation and antioxidant properties [50].
The in vitro antioxidant activities of the 4’-OH-flurbiprofen–chalcone hybrids were evaluated using the ORAC-FL method (oxygen radical absorbance capacity with fluorescein). Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a water-soluble analogue of vitamin E, served as the standard, and antioxidant activity was expressed in Trolox equivalents. Among the tested compounds, 4’-hydroxy-flurbiprofen and 4-(diethylamino)chalcone exhibited the strongest antioxidant activities. Notably, 4-(diethylamino)chalcone also demonstrated significant radical-scavenging potential. All hybrid compounds showed higher ORAC-FL values compared to flurbiprofen alone, indicating that the introduction of the chalcone unit and the free 4’-OH group into flurbiprofen was crucial for enhanced antioxidant activity. Hybrids containing two phenolic hydroxyl groups displayed greater antioxidant capacity than those with only one. Furthermore, hybrids with dimethylamino or diethylamino groups at position 4’ of the chalcone moiety exhibited remarkable antioxidant properties. Thus, both the phenolic hydroxyl groups and the amino substituents at position 4’ were essential for improving antioxidant activity [50].
Chalcones with antioxidant properties are summarized in Table 3 below.

Table 3.
Chalcones with antioxidant properties.
3.2. Health Promoting Properties of Chalcones Against Atherosclerosis and CVDs
Accumulating preclinical data indicate that natural and synthetic chalcones exert cardioprotective effects across complementary mechanisms relevant to atherothrombosis. In vascular and macrophage models, representative chalcones (e.g., xanthohumol, licochalcone A, cardamonin, panduratin A) reduce NF-κB-driven inflammation, attenuate ROS generation via Nrf2/HO-1 activation, and modulate lipid handling (↓oxLDL uptake; ↑cholesterol efflux) [51]. In animal models of atherosclerosis and cardiometabolic risk, chalcones have been associated with improvements in endothelial function (↑eNOS/NO bioavailability), reductions in vascular oxidative stress and cytokines (TNF-α, IL-6), and decreases in lesion burden or lipid indices, alongside antiplatelet/antithrombotic actions (inhibition of platelet activation/aggregation and thrombus formation) [5]. Additional benefits have been reported in ischemia–reperfusion and cardiac remodeling settings, where chalcones can limit infarct size, fibrosis, and hypertrophy via modulation of PI3K/Akt, MAPK, and apoptotic signaling [52]. Early human data remain limited; small exploratory studies and nutraceutical formulations suggest acceptable short-term tolerability and signal-level effects on inflammatory/oxidative markers, but robust randomized trials evaluating hard cardiovascular outcomes are still lacking [53]. Overall, the weight of evidence supports chalcones as promising multi-target leads for anti-inflammatory, antioxidant, endothelial-protective, and antithrombotic strategies in atherosclerosis and CVD, while underscoring the need for standardized pharmacokinetic optimization, dose-ranging, and controlled clinical evaluation [52].
3.2.1. Anti-Atherosclerotic and Cardioprotective Effects of Chalcones
In recent years, coronary heart disease has accounted for around 7.3 million deaths, while high blood pressure and stroke have caused approximately 9.4 million and 6.2 million deaths, respectively. Obesity and atherosclerosis continue to be the primary underlying pathological factors contributing to these conditions [49]. Projections indicate that by 2030, cardiovascular-related deaths could rise to approximately 23.3 million. The majority of these diseases are attributed to modifiable risk factors, including smoking, poor dietary habits, lack of physical activity, hypertension, obesity, diabetes, and elevated lipid levels [54].
The growing prevalence of fast food consumption, unhealthy dietary habits, and sedentary lifestyles has made cardiac disorders—especially atherosclerosis and associated progressive diseases—a major concern within the medical community [49]. Lower-income, middle-income countries are disproportionately affected by cardiovascular disease (~75%), and the number of deaths is almost equal in men and women. The reason for the decline in cardiovascular disease in developed countries may be improved healthcare facilities and better access to newer drugs [54]. The global demand for novel and more effective cardiovascular agents remains a top priority in managing heart disease, as cardioprotection has been a central focus of research over the past 10–15 years. However, an ideal cardio-protective compound or drug has yet to be clearly identified [49].
Several natural and semi-synthetic chalcones have emerged as promising candidates for the inhibition of various cardiovascular disorders. Chalcone-based compounds that exhibit strong biological activity and possess well-defined mechanisms of action (MOAs) and structure–activity relationships (SARs) can serve as valuable prototypes for developing antihypertensive, anti-angiogenic, antiarrhythmic, and cardioprotective drugs. By leveraging insights into these molecular targets, structural characteristics, and SARs, researchers can focus on designing chalcone derivatives that are more potent, selective, safe, and cost-effective for cardiovascular therapy [49].
Over 4000 polyphenolic compounds have existed in the plant kingdom for more than a billion years. These compounds are widely present in fruits, vegetables, tea, and wine, and are generally classified into nine subgroups, including flavonols, flavones, flavanones, flavanols, isoflavones, anthocyanidins, and proanthocyanidins, many of which exhibit promising cardioprotective properties [55]. Several chalcones, such as tinctorimine, lonchocarpin, xanthohumol, xanthohumol B, desmethylxanthohumol, xanthoangelol, xanthoangelol E, isobachalcone, derricin, hydroxysaflor yellow A, 4-hydroxyderricin, hydroxylated chalcones, substituted chalcone imides, sulfonamide-substituted chalcones, and lupulone-based chalcones, have been identified as bioactive agents targeting cardiovascular systems [49].
A list of chalcones, their cardiovascular targets, and their physicochemical properties is presented in Table 4. The cardioprotective effects of chalcones are closely linked to their structural features. The α,β-unsaturated carbonyl group is essential for bioactivity, as it enables Michael addition reactions with nucleophilic sites on target proteins involved in oxidative and inflammatory pathways. Substitutions on the aromatic rings strongly influence potency: hydroxyl groups, particularly in ortho and para positions, enhance antioxidant and radical-scavenging properties, while methoxy groups can increase lipophilicity and membrane permeability. Prenylated chalcones, such as xanthohumol and its derivatives, show improved bioavailability and stronger inhibition of platelet aggregation and vascular inflammation. Halogenated chalcones have demonstrated superior activity in ischemia–reperfusion models, likely due to increased stability and interactions with cardiomyocyte signaling proteins. In contrast, glycosylation often reduces activity, reflecting diminished cell permeability. Collectively, these SAR insights highlight how relatively small modifications of the chalcone scaffold can markedly alter their antioxidant, anti-inflammatory, and antithrombotic activities, offering guidance for the rational design of novel chalcone-based cardiovascular agents.
It has recently been found that a few natural compounds (such as curcumin (bis-chalcone), quercetin (flavonoid), provide adequate protection against myocardial infarction (MI) and other cardiovascular diseases such as hypertension, hyperlipidemia, thromboembolism, and arrhythmia through their anti-dyslipidemic and antioxidant action, the attenuation/inhibition of lipid peroxidation, increased expression of cardioprotective proteins, and through various other pathways [56]. Among these compounds, a few chalcones and their hybrids are reported to exert a protective effect against myocardial infarction caused by ischemia/reperfusion. These chalcones significantly reduce the size of the infarction, moderate lipid peroxidation, and reduce/inhibit protein expression [49].
A study evaluated the cardioprotective potential of halogenated chalcones (Cl- and F-substituted chalcones) against myocardial infarction caused by ischemia/reperfusion (I/R) in rats using 2,3,5-triphenyl tetrazolium chloride as a staining agent. The study revealed that chalcones significantly reduced infarct size and lipid peroxidation, supporting their cardioprotective activity against MI [56]. Similar cardioprotective effects were observed with YLSC. The authors reported that YLSC reduced infarct size, serum LDH, and AST. It also reduced the apoptosis rate and protein expression of GRP78 and caspase 12 in the myocardium [57].
In addition to existing and established chalcones, a few 1,3-diphenyl-2-E-propen-1-one derivatives have been found to exhibit remarkable activity in inhibiting various cardiovascular targets. Chalcones such as 3,2’,4’,6’-tetrahydroxy-4,3’-dimethoxychalcone, 2-hydroxychalcone, and neovavachalcone have shown usefulness in reperfusion of ischemic injuries, response to vascular damage, regression of vascular damage, and prevention of coronary atherosclerosis. 2’,4’-dihydroxy-6’-methoxychalcone, lycopene B, and isoliquiritigenin are promising candidates for the management of hypertension and the prevention of MI and stroke through their β-adrenergic receptor blocking action. Similarly, mixed receptor blocking action (alpha- and beta-adrenergic receptors) has been demonstrated by glypalichalcone and licochalcone A. Interestingly, antithrombotic properties have been reported for licochalcone G, which effectively inhibits coagulation factor Xa. Licochalcone A and G, glypalichalcone, neobavachalkone, 3,2’,4’,6’-tetrahydroxy-4,3’-dimethoxychalcone, and 2’,4’-dihydroxy-6’-methoxychalcone are molecules with impressive anti-obesity activity. Table 4 summarizes some chalcones with anti-atherosclerotic activity [57].

Table 4.
List of various copper compounds with cardiovascular action.
Table 4.
List of various copper compounds with cardiovascular action.
| Structure | Name | Biological Activity |
|---|---|---|
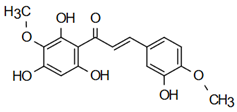 | 3,2’,4’,6’-tetrahydroxy-4,3’-dimethoxy chalcone | Anti-cardiovascular diseases Sivasankaran et al. [58] |
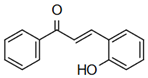 | 2-hydroxychalcone | Anti-cardiovascular diseases Lecour, K. T. Lamont [59] |
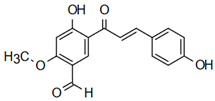 | Neobavachalcone | Anti-cardiovascular diseases Annapurna et al. [60] |
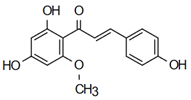 | 2’,4’-dihydroxy-6’-methoxychalcone | Anti-cardiovascular diseases Jian et al. [57] |
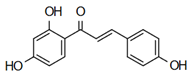 | Isoliquiritigenin | Anti-cardiovascular diseases Zhou et al. [61] |
 | Curcumin | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
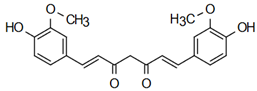
 | Tinctormine | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
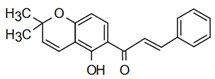 | Lonchocarpin | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
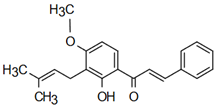 | Derricin | Anti-cardiovascular diseases Mahapatra και S. K. Bharti [49] |
3.2.2. Health Benefits of Chalcones Against Heart Failure
Almost all cardiovascular diseases lead to changes in the structure and function of the heart [58]. The systolic and diastolic functions of the myocardium are essential for maintaining the heart’s pumping ability, with calcium serving as a critical ion in initiating electromechanical coupling in the heart [59]. In end-stage heart failure, environmental disturbances disrupt the stability of intracellular ion channels, leading to calcium accumulation that can trigger malignant arrhythmias. Mitochondria, as the main source of cellular energy, are particularly affected by calcium overload, which induces oxidative stress and impairs mitochondrial functions, thereby compromising cardiomyocyte performance [61]. Flavonoids help regulate the expression of Cav1.2 and NCX1 plasma membrane transporters, maintaining calcium homeostasis in cardiomyocytes and protecting against mitochondrial apoptosis. Through reducing oxidative dysfunction and preventing mitochondrial permeability transition, flavonoids can counteract the progression of heart failure [61]. Myocardial fibrosis is a hallmark of advanced heart failure, characterized by collagen fiber deposition and interstitial cell proliferation that disrupts the heart’s structure and accelerates pump failure. Transforming growth factor beta (TGF-β) plays a significant role in fibrosis pathogenesis, with TGF-β1 closely associated with collagen production in the heart. Flavonoids exhibit therapeutic effects akin to antioxidants, offering anti-fibrotic benefits by regulating key factors such as Nrf2 and NF-ĸB. For instance, quercetin modulates the expression of the antioxidant enzyme Prx-3 by regulating Nrf2 transcription factors, thereby reducing oxidative damage to mitochondria [55,56].
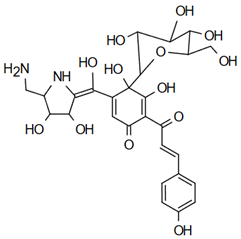
In addition, the monomer 17-methoxy-7-hydroxy-benzoin-furachalcone plays an important role in alleviating heart failure by targeting key mechanisms involved in cardiac remodeling and dysfunction. This chalcone promotes the expression of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) by activating the PI3K/Akt pathway, which is vital for maintaining vascular health and reducing oxidative stress. By enhancing NO production, chalcone helps improve endothelial function, reduce inflammation, and prevent structural remodeling of the heart [62]. Cardiac remodeling, a hallmark of advanced heart failure, often involves fibrosis, oxidative stress, and reduced myocardial function [63]. 17-Methoxyl-7-hydroxy-benzene-furanchalcone counteracts these effects by maintaining mitochondrial function, inhibiting oxidative damage, and maintaining calcium homeostasis within cardiomyocytes. These actions collectively mitigate mitochondrial dysfunction, calcium overload, and inflammation, which contribute significantly to the progression of heart failure [62].
There is sufficient evidence for the positive effects of flavonoids on cardiac function in patients with heart failure, as well as their ability to reverse drug-related heart damage, supporting the therapeutic potential of flavonoids for clinical heart failure.
3.3. Chalcones in Clinical Trials Against CVDs and Other Inflammation-Related Disorders
Over recent decades, chalcones have attracted significant attention as bioactive compounds due to their diverse pharmacological properties, which include anti-inflammatory, anticancer, antimicrobial, antiviral, and dermatological effects. Structurally characterized by an α,β-unsaturated carbonyl system, chalcones act as key precursors in the biosynthesis of flavonoids and are naturally present in a wide range of edible plants, herbs, and traditional medicinal sources. Given their structural versatility and biological relevance, increasing efforts have been made to investigate chalcones not only in vitro and in vivo but also in human clinical settings.
The following Table 5 presents a detailed summary of clinical and ex vivo studies conducted on different chalcone derivatives. It highlights the purpose of each study, the type and number of participants or test organisms, the experimental design, and the key outcomes observed. Compounds such as xanthohumol, cardamonin, panduratin A, flavokawain A, and others have been evaluated for their effects on inflammation, cancer progression, viral infections, skin health, metabolic disorders, and more. These findings contribute significantly to understanding the translational potential of chalcones from bench to bedside. The diversity of methods—ranging from double-blind placebo-controlled trials to targeted molecular pathway analysis—illustrates the growing scientific interest in integrating chalcone-based compounds into therapeutic and nutraceutical applications.

Table 5.
Clinical Trials with Chalcones against CVDs and other inflammation-related disorders.
4. Conclusions—Future Perspectives
Chalcones constitute a structurally diverse group of flavonoids with well-documented antithrombotic, anti-inflammatory, and antioxidant properties that contribute to cardiovascular protection. By modulating key molecular pathways such as NF-κB, Nrf2/HO-1, and PI3K/Akt, chalcones can suppress vascular inflammation, inhibit platelet aggregation, and enhance endothelial function—mechanisms that are central to the prevention and management of atherosclerosis and cardiovascular diseases.
Structure–activity relationship (SAR) studies have shown that subtle chemical modifications, including hydroxylation, methoxylation, and prenylation, significantly influence biological activity and pharmacokinetic behavior. These findings underscore the potential of chalcones as multi-target molecular scaffolds for the rational design of new cardioprotective and anti-inflammatory therapeutics.
Despite encouraging preclinical results, clinical evidence remains limited. Future research should therefore prioritize: (i) well-designed clinical trials to validate efficacy and safety in humans; (ii) improvement of bioavailability and stability through nanocarrier and formulation technologies; (iii) structure-guided optimization to enhance potency, selectivity, and pharmacokinetic profiles; and (iv) expanded investigation of chalcones in sclerosis-related and other inflammation-driven disorders, where their antifibrotic and antioxidant actions may offer additional benefits.
In summary, chalcones represent a promising class of bioactive compounds with significant potential in the development of next-generation therapeutic and nutraceutical agents targeting atherosclerosis, cardiovascular disease, and other chronic inflammatory conditions. Advancing this field will require integrated interdisciplinary efforts that bridge synthetic chemistry, molecular pharmacology, and clinical research to fully translate their pharmacological promise into clinical reality.
Author Contributions
Conceptualization, A.T.; methodology, V.K. and A.T.; software, all authors; validation, A.T.; investigation, V.K. and A.T.; writing—original draft preparation, V.K.; writing—review and editing, A.O. and A.T.; visualization, A.T.; supervision, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the School of Chemistry, Faculty of Sciences of the Democritus University of Thrace.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tukur, A.R.; Habila, J.; Ayo, R.; Lyun, O. Synthesis Reactions and Pharmacological Applications of Chalcones and Their Derivatives—A Mini Review. J. Chem. Rev. 2022, 4, 100–119. [Google Scholar] [CrossRef]
- Pereira, R.; Silva, A.M.S.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Bis-chalcones: A review of synthetic methodologies and anti-inflammatory effects. Eur. J. Med. Chem. 2023, 252, 115280. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Marzocchi, A.; Keivani, N.; Piccolo, V.; Summa, V.; Tenore, G.C. Natural Chalcones for the Management of Obesity Disease. Int. J. Mol. Sci. 2023, 24, 15929. [Google Scholar] [CrossRef]
- Liu, C.-S.; Chang, C.-C.; Du, Y.-C.; Chang, F.-R.; Wu, Y.-C.; Chang, W.-C.; Hsieh, T.-J. 2-Hydroxy-4ʼ-Methoxychalcone Inhibits Proliferation and Inflammation of Human Aortic Smooth Muscle Cells by Increasing the Expression of Peroxisome Proliferator–Activated Receptor Gamma. J. Cardiovasc. Pharmacol. 2012, 59, 339–351. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Mah, S.H. Chalcones in Diets. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 273–324. ISBN 978-981-15-4147-6. [Google Scholar]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Tekale, S.; Mashele, S.; Pooe, O.; Thore, S.; Kendrekar, P.; Pawar, R. Biological Role of Chalcones in Medicinal Chemistry. In Vector-Borne Diseases—Recent Developments in Epidemiology and Control; Claborn, D., Bhattacharya, S., Roy, S., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-021-5. [Google Scholar]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Biological activities and novel applications of chalcones. Planta Daninha 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Hijova, E. Bioavailability of chalcones. Bratisl. Lek. Listy 2006, 107, 80–84. [Google Scholar]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, Identification Techniques, Attributes, and Application in Agriculture. Molecules 2024, 29, 2247. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Fang, X.; Yang, B.; Cheng, Z.; Zhang, P.; Yang, M. Synthesis and antimicrobial activity of novel chalcone derivatives. Res. Chem. Intermed. 2014, 40, 1715–1725. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, S.J.; Zeng, L. Biological effects and mechanisms of dietary chalcones: Latest research progress, future research strategies, and challenges. Food Funct. 2024, 15, 10582–10599. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Molinillo, J.M.G.; Torres, A.; Varela, R.M.; Castellano, D. Bioactive flavonoids from Helianthus annuus cultivars. Phytochemistry 1997, 45, 683–687. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Ngadjui, B.T.; Dongo, E.; Ngameni, B.; Nindi, M.N.; Bezabih, M. Chalcones and other constituents of Dorstenia prorepens and Dorstenia zenkeri. Phytochemistry 2002, 59, 877–883. [Google Scholar] [CrossRef]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a Chalcone Constituent of Angelica keiskei, Induces Apoptosis in Neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal Chalcones and New Caffeic Acid Esters from Zuccagnia punctata Acting against Soybean Infecting Fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Balasooriya, B.A.I.S.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Malhotra, B.; Elder, M.; French, C.J.; Towers, G.H.N. Comparative studies of inhibitory activities of chalcones on tomato ringspot virus (ToRSV). Can. J. Plant Pathol. 1997, 19, 133–137. [Google Scholar] [CrossRef]
- Nunes, A.S.; Campos, V.P.; Mascarello, A.; Stumpf, T.R.; Chiaradia-Delatorre, L.D.; Machado, A.R.T.; Santos Júnior, H.M.; Yunes, R.A.; Nunes, R.J.; Oliveira, D.F. Activity of chalcones derived from 2,4,5-trimethoxybenzaldehyde against Meloidogyne exigua and in silico interaction of one chalcone with a putative caffeic acid 3-O-methyltransferase from Meloidogyne incognita. Exp. Parasitol. 2013, 135, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J.; Blaney, W.M.; Delle Monache, F.; Marini Bettolo, G.B. Insect antifeedant activity associated with compounds isolated from species of Lonchocarpus and Tephrosia. J. Chem. Ecol. 1990, 16, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activity of Flavokawains and Related trans-Chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Sotelo, T.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012, 35, 1500–1517. [Google Scholar] [CrossRef]
- Smailagić, D.; Banjac, N.; Ninković, S.; Savić, J.; Ćosić, T.; Pěnčík, A.; Ćalić, D.; Bogdanović, M.; Trajković, M.; Stanišić, M. New Insights Into the Activity of Apple Dihydrochalcone Phloretin: Disturbance of Auxin Homeostasis as Physiological Basis of Phloretin Phytotoxic Action. Front. Plant Sci. 2022, 13, 875528. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114. [Google Scholar] [CrossRef]
- Badaracco, P.; Sortino, M.; Pioli, R.N. Estudio de compuestos vegetales con potencial acción antifúngica sobre patógenos de plantas cultivadas. Chil. J. Agric. Anim. Sci. 2020, 36, 244–252. [Google Scholar] [CrossRef]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Ż.; Oszust, K.; Frąc, M. Phytochemicals of Apple Pomace as Prospect Bio-Fungicide Agents against Mycotoxigenic Fungal Species—In Vitro Experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Feng, S.; Gong, C.; Zhou, Y.; Xing, L.; He, B.; Wu, Y.; Xue, W. Design, synthesis and biological activity of chalcone derivatives containing pyridazine. Arab. J. Chem. 2023, 16, 104852. [Google Scholar] [CrossRef]
- Gafner, S.; Wolfender, J.-L.; Mavi, S.; Hostettmann, K. Antifungal and Antibacterial Chalcones from Myrica serrata. Planta Med. 1996, 62, 67–69. [Google Scholar] [CrossRef]
- Stompor, M.; Dancewicz, K.; Gabryś, B.; Anioł, M. Insect Antifeedant Potential of Xanthohumol, Isoxanthohumol, and Their Derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Shakil, N.A.; Saxena, D.B. Isolation and Structure of Cordifolin, A Novel Insecticidal Oxygenated Chalcone, from the Stem of Tinospora cordifolia Miers. Nat. Prod. Commun. 2006, 1, 1934578X0600100707. [Google Scholar] [CrossRef]
- Ruiz Hidalgo, J.; Santillán, M.; Parellada, E.A.; Khyaliya, P.; Neske, A.; Ameta, K.L. Synthetic bis- and mono-chalcones with insecticide effects on Spodoptera frugiperda (Lepidoptera: Noctuidae). Int. J. Pest Manag. 2020, 66, 116–121. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Shard, A.; Tewary, D.K.; Nadda, G.; Sinha, A.K. Chalcones as promising pesticidal agents against diamondback moth (Plutella xylostella): Microwave-assisted synthesis and structure–activity relationship. Med. Chem. Res. 2012, 21, 922–931. [Google Scholar] [CrossRef]
- Dresel, M.; Dunkel, A.; Hofmann, T. Sensomics Analysis of Key Bitter Compounds in the Hard Resin of Hops (Humulus lupulus L.) and Their Contribution to the Bitter Profile of Pilsner-Type Beer. J. Agric. Food Chem. 2015, 63, 3402–3418. [Google Scholar] [CrossRef]
- Vanhoecke, B.; Derycke, L.; Van Marck, V.; Depypere, H.; De Keukeleire, D.; Bracke, M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int. J. Cancer 2005, 117, 889–895. [Google Scholar] [CrossRef]
- Nawrot, J.; Dams, I.; Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Villa, S.M.; Heckman, J.; Bandyopadhyay, D. Medicinally Privileged Natural Chalcones: Abundance, Mechanisms of Action, and Clinical Trials. Int. J. Mol. Sci. 2024, 25, 9623. [Google Scholar] [CrossRef]
- Annie-Mathew, A.S.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D.V.L. The pivotal role of Nrf2 activators in adipocyte biology. Pharmacol. Res. 2021, 173, 105853. [Google Scholar] [CrossRef]
- Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory Effects of Butein on Adipogenesis through Upregulation of the Nrf2/HO-1 Pathway in 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2017, 22, 306–311. [Google Scholar] [CrossRef]
- Pi, J.; Leung, L.; Xue, P.; Wang, W.; Hou, Y.; Liu, D.; Yehuda-Shnaidman, E.; Lee, C.; Lau, J.; Kurtz, T.W.; et al. Deficiency in the Nuclear Factor E2-related Factor-2 Transcription Factor Results in Impaired Adipogenesis and Protects against Diet-induced Obesity. J. Biol. Chem. 2010, 285, 9292–9300. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, Q.; Yan, J.; Du, Z.; Huang, L.; Li, X. A novel series of tacrine–selegiline hybrids with cholinesterase and monoamine oxidase inhibition activities for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 62, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Klein, W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 668–680. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent Development of Multifunctional Agents as Potential Drug Candidates for the Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2014, 22, 373–404. [Google Scholar] [CrossRef]
- Chen, L.W.; Tsai, M.; Chern, C.; Tsao, T.; Lin, F.; Chen, S.; Tsui, P.; Liu, Y.; Lu, H.; Wu, W.; et al. A chalcone derivative, 1m-6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation-induced endothelial dysfunction. Br. J. Pharmacol. 2020, 177, 5375–5392. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Yu, B.; Wang, Y.; Ren, H.; Zhang, B.; Wang, Y.; Zheng, Q. Cardioprotection against Ischemia/Reperfusion by Licochalcone B in Isolated Rat Hearts. Oxidative Med. Cell. Longev. 2014, 2014, 134862. [Google Scholar] [CrossRef]
- Kiatsoonthon, K.; Phimthong, N.; Potikanond, S.; Wikan, N.; Nimlamool, W. Panduratin A Inhibits TNF Alpha-Stimulated Endothelial Cell Activation Through Suppressing the NF-κB Pathway. Biomolecules 2024, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- WHO Cardiovascular Data. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 31 July 2025).
- Böhm, H.; Boeing, H.; Hempel, J.; Raab, B.; Kroke, A. Falvonole, Flavone und Anthocyane als natürliche Antioxidantien der Nahrung und ihre mögliche Rolle bei der Prävention chronischer Erkrankungen. Z. Ernährungswiss 1998, 37, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Jolobe, O.M.P. Cardioprotective therapeutics—Drugs used in hypertension, hyperlipidaemia, thromboembolization, arrhythmias, postmenopausal state and as anti-oxidants. Postgrad. Med. J. 1994, 70, 767–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jian, J.; Qing, F.; Zhang, S.; Huang, J.; Huang, R. The Effect of 17-methoxyl-7-hydroxy-benzene-furanchalcone Isolated from Millettia pulchra on Myocardial Ischemia In Vitro and In Vivo. Planta Med. 2012, 78, 1324–1331. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential therapeutic agents for cardiovascular disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, R.; Zuo, W.; Ji, Z.; Fan, Z.; Chen, X.; Huang, R.; Li, X.; Ma, G. Association between dietary intake of anthocyanidins and heart failure among American adults: NHANES (2007–2010 and 2017–2018). Front. Nutr. 2023, 10, 1107637. [Google Scholar] [CrossRef]
- Annapurna, A.; Mudagal, M.P.; Ansari, A.; Rao, A.S. Cardioprotective activity of chalcones in ischemia/reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2012, 17, 110–114. [Google Scholar]
- Stein, J.M. The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells. Biochem. Pharmacol. 1975, 24, 1659–1662. [Google Scholar] [CrossRef]
- Ye, F.; He, J.; Wu, X.; Xie, J.; Chen, H.; Tang, X.; Lai, Z.; Huang, R.; Huang, J. The regulatory mechanisms of Yulangsan MHBFC reversing cardiac remodeling in rats based on eNOS-NO signaling pathway. Biomed. Pharmacother. 2019, 117, 109141. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Pella, D.; Fatima, G.; Elkilany, G.; Moshiri, M.; Hristova, K.; Jakabcin, P.; Vaňova, N. High Exogenous Antioxidant, Restorative Treatment (Heart) for Prevention of the Six Stages of Heart Failure: The Heart Diet. Antioxidants 2022, 11, 1464. [Google Scholar] [CrossRef]
- Jung, F.; Staltner, R.; Tahir, A.; Baumann, A.; Burger, K.; Halilbasic, E.; Hellerbrand, C.; Bergheim, I. Oral intake of xanthohumol attenuates lipoteichoic acid-induced inflammatory response in human PBMCs. Eur. J. Nutr. 2022, 61, 4155–4166. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Kim, C.; Hwang, J. Oral intake of Boesenbergia pandurata extract improves skin hydration, gloss, and wrinkling: A randomized, double-blind, and placebo-controlled study. J. Cosmet. Dermatol. 2017, 16, 512–519. [Google Scholar] [CrossRef]
- Benchabane, S.; Belguendouz, H.; Behairi, N.; Arroul-Lammali, A.; Boudjelida, A.; Youinou, P.; Touil-boukoffa, C. Cardamonin inhibits pro-inflammatory cytokine production and suppresses NO pathway in PBMCs from patients with primary Sjögren’s syndrome. Immunopharmacol. Immunotoxicol. 2018, 40, 126–133. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Piao, C.; Zhang, Z.; Kong, C.; Yin, L.; Liu, X. Flavokawain A is a natural inhibitor of PRMT5 in bladder cancer. J. Exp. Clin. Cancer Res. 2022, 41, 293. [Google Scholar] [CrossRef]
- Phillpotts, R.J.; Higgins, P.G.; Willman, J.S.; Tyrrell, D.A.J.; Lenox-Smith, I. Evaluation of the antirhinovirus chalcone Ro 09-0415 given orally to volunteers. J. Antimicrob. Chemother 1984, 14, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, M.; Worthmann, A.-C.; Holtzmann, U.; Buck, B.; Jung, K.A.; Schoelermann, A.M.; Rippke, F.; Stäb, F.; Wenck, H.; Neufang, G.; et al. Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. Acad. Dermatol. Venereol. 2016, 30, 9–17. [Google Scholar] [CrossRef]
- Buckett, L.; Sus, N.; Spindler, V.; Rychlik, M.; Schoergenhofer, C.; Frank, J. The Pharmacokinetics of Individual Conjugated Xanthohumol Metabolites Show Efficient Glucuronidation and Higher Bioavailability of Micellar than Native Xanthohumol in a Randomized, Double-Blind, Crossover Trial in Healthy Humans. Mol. Nutr. Food Res. 2023, 67, 2200684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gu, S.; Wang, X.; Cai, Y.; Zhou, Z. The Safety, Tolerability, and Pharmacokinetics of Active Ingredients From Hydroxysafflor Yellow A in Healthy Chinese Volunteers. Clin. Pharm. Drug Dev. 2025, 14, 79–86. [Google Scholar] [CrossRef]
- Chularojanamontri, L.; Tuchinda, P.; Kulthanan, K.; Varothai, S.; Winayanuwattikun, W. A double-blinded, randomized, vehicle-controlled study to access skin tolerability and efficacy of an anti-inflammatory moisturizer in treatment of acne with 0.1% adapalene gel. J. Dermatol. Treat. 2016, 27, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S.; et al. The Oral Bioavailability of 8-Prenylnaringenin from Hops (Humulus lupulus L.) in Healthy Women and Men is Significantly Higher than that of its Positional Isomer 6-Prenylnaringenin in a Randomized Crossover Trial. Mol. Nutr. Food Res. 2018, 62, 1700838. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).