Antithrombotic and Anti-Inflammatory Health Promoting Pharmacological Properties of Chalcones and Their Derivatives Against Atherosclerosis and CVD
Abstract
1. Introduction
2. Chalcones, Origin, Sources, Structure, and General Uses/Applications
2.1. Origin of Chalcones
2.2. Structural Features
2.3. Occurrence of Chalcones in Natural Sources
2.4. Initial Uses/Applications of Chalcones in Agriculture and Plant Protection
2.4.1. Chalcones as Herbicides and Plant Growth Regulators
2.4.2. Chalcones as Fungicides
2.4.3. Chalcones as Insecticides
3. Recent Advances on the Observed Health Promoting Properties of Chalcones
3.1. Antioxidant Properties of Chalcones
3.1.1. Butein
3.1.2. Panduratin A
3.1.3. 4’-OH-Flurbiprofen-Chalcone
3.2. Health Promoting Properties of Chalcones Against Atherosclerosis and CVDs
3.2.1. Anti-Atherosclerotic and Cardioprotective Effects of Chalcones
| Structure | Name | Biological Activity |
|---|---|---|
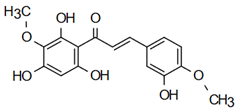 | 3,2’,4’,6’-tetrahydroxy-4,3’-dimethoxy chalcone | Anti-cardiovascular diseases Sivasankaran et al. [58] |
 | 2-hydroxychalcone | Anti-cardiovascular diseases Lecour, K. T. Lamont [59] |
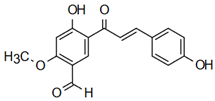 | Neobavachalcone | Anti-cardiovascular diseases Annapurna et al. [60] |
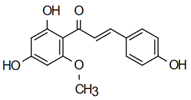 | 2’,4’-dihydroxy-6’-methoxychalcone | Anti-cardiovascular diseases Jian et al. [57] |
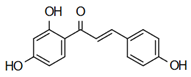 | Isoliquiritigenin | Anti-cardiovascular diseases Zhou et al. [61] |
 | Curcumin | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
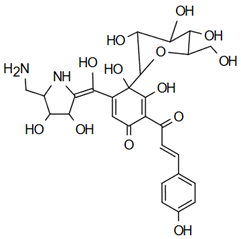 | Tinctormine | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
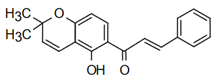 | Lonchocarpin | Anti-cardiovascular diseases Mahapatra, K. Bharti [49] |
 | Derricin | Anti-cardiovascular diseases Mahapatra και S. K. Bharti [49] |
3.2.2. Health Benefits of Chalcones Against Heart Failure
3.3. Chalcones in Clinical Trials Against CVDs and Other Inflammation-Related Disorders
4. Conclusions—Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tukur, A.R.; Habila, J.; Ayo, R.; Lyun, O. Synthesis Reactions and Pharmacological Applications of Chalcones and Their Derivatives—A Mini Review. J. Chem. Rev. 2022, 4, 100–119. [Google Scholar] [CrossRef]
- Pereira, R.; Silva, A.M.S.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Bis-chalcones: A review of synthetic methodologies and anti-inflammatory effects. Eur. J. Med. Chem. 2023, 252, 115280. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Marzocchi, A.; Keivani, N.; Piccolo, V.; Summa, V.; Tenore, G.C. Natural Chalcones for the Management of Obesity Disease. Int. J. Mol. Sci. 2023, 24, 15929. [Google Scholar] [CrossRef]
- Liu, C.-S.; Chang, C.-C.; Du, Y.-C.; Chang, F.-R.; Wu, Y.-C.; Chang, W.-C.; Hsieh, T.-J. 2-Hydroxy-4ʼ-Methoxychalcone Inhibits Proliferation and Inflammation of Human Aortic Smooth Muscle Cells by Increasing the Expression of Peroxisome Proliferator–Activated Receptor Gamma. J. Cardiovasc. Pharmacol. 2012, 59, 339–351. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Mah, S.H. Chalcones in Diets. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 273–324. ISBN 978-981-15-4147-6. [Google Scholar]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Tekale, S.; Mashele, S.; Pooe, O.; Thore, S.; Kendrekar, P.; Pawar, R. Biological Role of Chalcones in Medicinal Chemistry. In Vector-Borne Diseases—Recent Developments in Epidemiology and Control; Claborn, D., Bhattacharya, S., Roy, S., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-021-5. [Google Scholar]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Biological activities and novel applications of chalcones. Planta Daninha 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Hijova, E. Bioavailability of chalcones. Bratisl. Lek. Listy 2006, 107, 80–84. [Google Scholar]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, Identification Techniques, Attributes, and Application in Agriculture. Molecules 2024, 29, 2247. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Fang, X.; Yang, B.; Cheng, Z.; Zhang, P.; Yang, M. Synthesis and antimicrobial activity of novel chalcone derivatives. Res. Chem. Intermed. 2014, 40, 1715–1725. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, S.J.; Zeng, L. Biological effects and mechanisms of dietary chalcones: Latest research progress, future research strategies, and challenges. Food Funct. 2024, 15, 10582–10599. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Molinillo, J.M.G.; Torres, A.; Varela, R.M.; Castellano, D. Bioactive flavonoids from Helianthus annuus cultivars. Phytochemistry 1997, 45, 683–687. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Ngadjui, B.T.; Dongo, E.; Ngameni, B.; Nindi, M.N.; Bezabih, M. Chalcones and other constituents of Dorstenia prorepens and Dorstenia zenkeri. Phytochemistry 2002, 59, 877–883. [Google Scholar] [CrossRef]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a Chalcone Constituent of Angelica keiskei, Induces Apoptosis in Neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal Chalcones and New Caffeic Acid Esters from Zuccagnia punctata Acting against Soybean Infecting Fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Balasooriya, B.A.I.S.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Malhotra, B.; Elder, M.; French, C.J.; Towers, G.H.N. Comparative studies of inhibitory activities of chalcones on tomato ringspot virus (ToRSV). Can. J. Plant Pathol. 1997, 19, 133–137. [Google Scholar] [CrossRef]
- Nunes, A.S.; Campos, V.P.; Mascarello, A.; Stumpf, T.R.; Chiaradia-Delatorre, L.D.; Machado, A.R.T.; Santos Júnior, H.M.; Yunes, R.A.; Nunes, R.J.; Oliveira, D.F. Activity of chalcones derived from 2,4,5-trimethoxybenzaldehyde against Meloidogyne exigua and in silico interaction of one chalcone with a putative caffeic acid 3-O-methyltransferase from Meloidogyne incognita. Exp. Parasitol. 2013, 135, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J.; Blaney, W.M.; Delle Monache, F.; Marini Bettolo, G.B. Insect antifeedant activity associated with compounds isolated from species of Lonchocarpus and Tephrosia. J. Chem. Ecol. 1990, 16, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activity of Flavokawains and Related trans-Chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Sotelo, T.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012, 35, 1500–1517. [Google Scholar] [CrossRef]
- Smailagić, D.; Banjac, N.; Ninković, S.; Savić, J.; Ćosić, T.; Pěnčík, A.; Ćalić, D.; Bogdanović, M.; Trajković, M.; Stanišić, M. New Insights Into the Activity of Apple Dihydrochalcone Phloretin: Disturbance of Auxin Homeostasis as Physiological Basis of Phloretin Phytotoxic Action. Front. Plant Sci. 2022, 13, 875528. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114. [Google Scholar] [CrossRef]
- Badaracco, P.; Sortino, M.; Pioli, R.N. Estudio de compuestos vegetales con potencial acción antifúngica sobre patógenos de plantas cultivadas. Chil. J. Agric. Anim. Sci. 2020, 36, 244–252. [Google Scholar] [CrossRef]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Ż.; Oszust, K.; Frąc, M. Phytochemicals of Apple Pomace as Prospect Bio-Fungicide Agents against Mycotoxigenic Fungal Species—In Vitro Experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Feng, S.; Gong, C.; Zhou, Y.; Xing, L.; He, B.; Wu, Y.; Xue, W. Design, synthesis and biological activity of chalcone derivatives containing pyridazine. Arab. J. Chem. 2023, 16, 104852. [Google Scholar] [CrossRef]
- Gafner, S.; Wolfender, J.-L.; Mavi, S.; Hostettmann, K. Antifungal and Antibacterial Chalcones from Myrica serrata. Planta Med. 1996, 62, 67–69. [Google Scholar] [CrossRef]
- Stompor, M.; Dancewicz, K.; Gabryś, B.; Anioł, M. Insect Antifeedant Potential of Xanthohumol, Isoxanthohumol, and Their Derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Shakil, N.A.; Saxena, D.B. Isolation and Structure of Cordifolin, A Novel Insecticidal Oxygenated Chalcone, from the Stem of Tinospora cordifolia Miers. Nat. Prod. Commun. 2006, 1, 1934578X0600100707. [Google Scholar] [CrossRef]
- Ruiz Hidalgo, J.; Santillán, M.; Parellada, E.A.; Khyaliya, P.; Neske, A.; Ameta, K.L. Synthetic bis- and mono-chalcones with insecticide effects on Spodoptera frugiperda (Lepidoptera: Noctuidae). Int. J. Pest Manag. 2020, 66, 116–121. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Shard, A.; Tewary, D.K.; Nadda, G.; Sinha, A.K. Chalcones as promising pesticidal agents against diamondback moth (Plutella xylostella): Microwave-assisted synthesis and structure–activity relationship. Med. Chem. Res. 2012, 21, 922–931. [Google Scholar] [CrossRef]
- Dresel, M.; Dunkel, A.; Hofmann, T. Sensomics Analysis of Key Bitter Compounds in the Hard Resin of Hops (Humulus lupulus L.) and Their Contribution to the Bitter Profile of Pilsner-Type Beer. J. Agric. Food Chem. 2015, 63, 3402–3418. [Google Scholar] [CrossRef]
- Vanhoecke, B.; Derycke, L.; Van Marck, V.; Depypere, H.; De Keukeleire, D.; Bracke, M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int. J. Cancer 2005, 117, 889–895. [Google Scholar] [CrossRef]
- Nawrot, J.; Dams, I.; Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Villa, S.M.; Heckman, J.; Bandyopadhyay, D. Medicinally Privileged Natural Chalcones: Abundance, Mechanisms of Action, and Clinical Trials. Int. J. Mol. Sci. 2024, 25, 9623. [Google Scholar] [CrossRef]
- Annie-Mathew, A.S.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D.V.L. The pivotal role of Nrf2 activators in adipocyte biology. Pharmacol. Res. 2021, 173, 105853. [Google Scholar] [CrossRef]
- Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory Effects of Butein on Adipogenesis through Upregulation of the Nrf2/HO-1 Pathway in 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2017, 22, 306–311. [Google Scholar] [CrossRef]
- Pi, J.; Leung, L.; Xue, P.; Wang, W.; Hou, Y.; Liu, D.; Yehuda-Shnaidman, E.; Lee, C.; Lau, J.; Kurtz, T.W.; et al. Deficiency in the Nuclear Factor E2-related Factor-2 Transcription Factor Results in Impaired Adipogenesis and Protects against Diet-induced Obesity. J. Biol. Chem. 2010, 285, 9292–9300. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, Q.; Yan, J.; Du, Z.; Huang, L.; Li, X. A novel series of tacrine–selegiline hybrids with cholinesterase and monoamine oxidase inhibition activities for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 62, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Klein, W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 668–680. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent Development of Multifunctional Agents as Potential Drug Candidates for the Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2014, 22, 373–404. [Google Scholar] [CrossRef]
- Chen, L.W.; Tsai, M.; Chern, C.; Tsao, T.; Lin, F.; Chen, S.; Tsui, P.; Liu, Y.; Lu, H.; Wu, W.; et al. A chalcone derivative, 1m-6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation-induced endothelial dysfunction. Br. J. Pharmacol. 2020, 177, 5375–5392. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Yu, B.; Wang, Y.; Ren, H.; Zhang, B.; Wang, Y.; Zheng, Q. Cardioprotection against Ischemia/Reperfusion by Licochalcone B in Isolated Rat Hearts. Oxidative Med. Cell. Longev. 2014, 2014, 134862. [Google Scholar] [CrossRef]
- Kiatsoonthon, K.; Phimthong, N.; Potikanond, S.; Wikan, N.; Nimlamool, W. Panduratin A Inhibits TNF Alpha-Stimulated Endothelial Cell Activation Through Suppressing the NF-κB Pathway. Biomolecules 2024, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- WHO Cardiovascular Data. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 31 July 2025).
- Böhm, H.; Boeing, H.; Hempel, J.; Raab, B.; Kroke, A. Falvonole, Flavone und Anthocyane als natürliche Antioxidantien der Nahrung und ihre mögliche Rolle bei der Prävention chronischer Erkrankungen. Z. Ernährungswiss 1998, 37, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Jolobe, O.M.P. Cardioprotective therapeutics—Drugs used in hypertension, hyperlipidaemia, thromboembolization, arrhythmias, postmenopausal state and as anti-oxidants. Postgrad. Med. J. 1994, 70, 767–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jian, J.; Qing, F.; Zhang, S.; Huang, J.; Huang, R. The Effect of 17-methoxyl-7-hydroxy-benzene-furanchalcone Isolated from Millettia pulchra on Myocardial Ischemia In Vitro and In Vivo. Planta Med. 2012, 78, 1324–1331. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential therapeutic agents for cardiovascular disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, R.; Zuo, W.; Ji, Z.; Fan, Z.; Chen, X.; Huang, R.; Li, X.; Ma, G. Association between dietary intake of anthocyanidins and heart failure among American adults: NHANES (2007–2010 and 2017–2018). Front. Nutr. 2023, 10, 1107637. [Google Scholar] [CrossRef]
- Annapurna, A.; Mudagal, M.P.; Ansari, A.; Rao, A.S. Cardioprotective activity of chalcones in ischemia/reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2012, 17, 110–114. [Google Scholar]
- Stein, J.M. The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells. Biochem. Pharmacol. 1975, 24, 1659–1662. [Google Scholar] [CrossRef]
- Ye, F.; He, J.; Wu, X.; Xie, J.; Chen, H.; Tang, X.; Lai, Z.; Huang, R.; Huang, J. The regulatory mechanisms of Yulangsan MHBFC reversing cardiac remodeling in rats based on eNOS-NO signaling pathway. Biomed. Pharmacother. 2019, 117, 109141. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Pella, D.; Fatima, G.; Elkilany, G.; Moshiri, M.; Hristova, K.; Jakabcin, P.; Vaňova, N. High Exogenous Antioxidant, Restorative Treatment (Heart) for Prevention of the Six Stages of Heart Failure: The Heart Diet. Antioxidants 2022, 11, 1464. [Google Scholar] [CrossRef]
- Jung, F.; Staltner, R.; Tahir, A.; Baumann, A.; Burger, K.; Halilbasic, E.; Hellerbrand, C.; Bergheim, I. Oral intake of xanthohumol attenuates lipoteichoic acid-induced inflammatory response in human PBMCs. Eur. J. Nutr. 2022, 61, 4155–4166. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Kim, C.; Hwang, J. Oral intake of Boesenbergia pandurata extract improves skin hydration, gloss, and wrinkling: A randomized, double-blind, and placebo-controlled study. J. Cosmet. Dermatol. 2017, 16, 512–519. [Google Scholar] [CrossRef]
- Benchabane, S.; Belguendouz, H.; Behairi, N.; Arroul-Lammali, A.; Boudjelida, A.; Youinou, P.; Touil-boukoffa, C. Cardamonin inhibits pro-inflammatory cytokine production and suppresses NO pathway in PBMCs from patients with primary Sjögren’s syndrome. Immunopharmacol. Immunotoxicol. 2018, 40, 126–133. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Piao, C.; Zhang, Z.; Kong, C.; Yin, L.; Liu, X. Flavokawain A is a natural inhibitor of PRMT5 in bladder cancer. J. Exp. Clin. Cancer Res. 2022, 41, 293. [Google Scholar] [CrossRef]
- Phillpotts, R.J.; Higgins, P.G.; Willman, J.S.; Tyrrell, D.A.J.; Lenox-Smith, I. Evaluation of the antirhinovirus chalcone Ro 09-0415 given orally to volunteers. J. Antimicrob. Chemother 1984, 14, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, M.; Worthmann, A.-C.; Holtzmann, U.; Buck, B.; Jung, K.A.; Schoelermann, A.M.; Rippke, F.; Stäb, F.; Wenck, H.; Neufang, G.; et al. Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. Acad. Dermatol. Venereol. 2016, 30, 9–17. [Google Scholar] [CrossRef]
- Buckett, L.; Sus, N.; Spindler, V.; Rychlik, M.; Schoergenhofer, C.; Frank, J. The Pharmacokinetics of Individual Conjugated Xanthohumol Metabolites Show Efficient Glucuronidation and Higher Bioavailability of Micellar than Native Xanthohumol in a Randomized, Double-Blind, Crossover Trial in Healthy Humans. Mol. Nutr. Food Res. 2023, 67, 2200684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gu, S.; Wang, X.; Cai, Y.; Zhou, Z. The Safety, Tolerability, and Pharmacokinetics of Active Ingredients From Hydroxysafflor Yellow A in Healthy Chinese Volunteers. Clin. Pharm. Drug Dev. 2025, 14, 79–86. [Google Scholar] [CrossRef]
- Chularojanamontri, L.; Tuchinda, P.; Kulthanan, K.; Varothai, S.; Winayanuwattikun, W. A double-blinded, randomized, vehicle-controlled study to access skin tolerability and efficacy of an anti-inflammatory moisturizer in treatment of acne with 0.1% adapalene gel. J. Dermatol. Treat. 2016, 27, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S.; et al. The Oral Bioavailability of 8-Prenylnaringenin from Hops (Humulus lupulus L.) in Healthy Women and Men is Significantly Higher than that of its Positional Isomer 6-Prenylnaringenin in a Randomized Crossover Trial. Mol. Nutr. Food Res. 2018, 62, 1700838. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef] [PubMed]


| Structure | Name | Biological Activity |
|---|---|---|
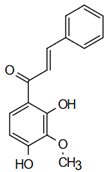 | 2’,4’-dihydroxy-3’-methoxychalcone | Herbicidal Action Svetaz et al. [21] |
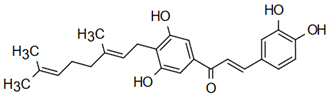 | 2’,3,4,4’-tetrahydroxy-3’-geranylchalcone | Herbicidal Action Jayasinghe et al. [22] |
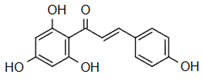 | 2’,3’,4’,4-tetrahydroxychalcone | Antimicrobial Action Onyilagha [23] |
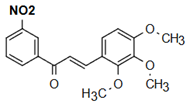 | (2E)-1-(4’-nitrophenyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one | Fungicidal Action Nunes et al. [24] |
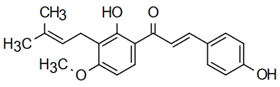 | (E)-1-[2-hydroxy-4-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-phenylprop-2-en-1-one | Insect Repellent Action Simmonds et al. [25] |
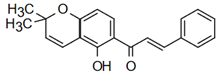 | (E)-1-(5-hydroxy-2,2-dimethylchromen-6-yl)-3-phenylprop-2-en-1-one | Insect Repellent Action Simmonds et al. [25] |
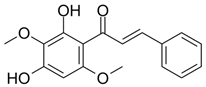 | 2’,4-Dihydroxy-3’,4’-dimethoxychalcon | Phytotoxic Action Chotsaeng et al. [26] |
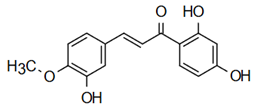 | 2’,4’,4-Trihydroxy-3’-methoxyxchalcone | Phytotoxic Action Chotsaeng et al. [26] |
| Structure | Name | Biological Activity |
|---|---|---|
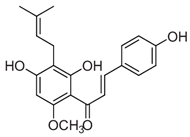 | Xanthohumol, 2’,4,4’-Trihydroxy-6’-methoxy-3’-(3-methylbut-2-en-1-yl)chalcone | Insecticidal Action Díaz-Tielas et al. [12] |
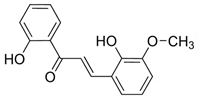 | 2’,4’-dihydroxy-3’-methoxychalcone | Fungicidal Action Molecules et al. [14] |
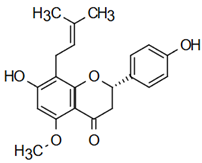 | Isoxanthoumol 7-hydroxy-2-(4-hydroxyphenyl)-5-methoxy-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | Díaz-Tielas et al. [12] |
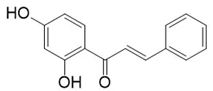 | 2’,4’-dihydroxychalcone | Fungicidal Action Molecules et al. [12] |
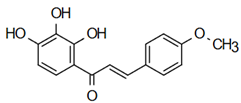 | Cordifolin, 1-(2’,3’,4’-trihydroxyphenyl)-3-(4”- methoxyphenyl)-propen-1-one] | Insecticidal Action Díaz-Tielas et al. [12] |
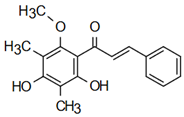 | 2’,4’-dihydroxy-3’,5’-dimethyl-6’-methoxychalcone | Fungicidal Action Oleszek et al. [31] |
| Structure | Name | Biological Activity |
|---|---|---|
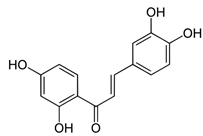 | 3,4,2’,4’-tetrahydroxychalcone, Butaine | Antioxidant Action, Annie-Mathew et al. [43] |
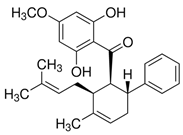 | Panduratin A | Antioxidant Action Maisto [4] |
 | 4-(Diethylamino)chalcone | Antioxidant Action, N. Guzior et al. [50] |
 | 4-(Dimethylamino)chalcone | Antioxidant Action, N. Guzior et al. [50] |
| Name of Chalcone | Aim of the Study | Type of Organism Used/Number and Type of Volunteers | Study Design | Main Findings | Reference/Year of Study |
|---|---|---|---|---|---|
| Xanthohumol |
|
| Quantity (of Chalcone)
|
| [64] (July 2020) |
| Panduratin A |
|
|
|
| [65] (April 2017) |
| Cardamonin |
|
|
|
| [66] (January 2018) |
| Flavokawain A |
|
|
|
| [67] (October 2022) |
| 4’ ethoxy-2’-hydroxy-4, 6’ dimethoxy- chalcone (Ro 09-0410) |
|
|
|
| [68] (1984) |
| licochalcone A in combination with 4-t-butylcyclohexanol |
|
|
|
| [69] (February 2016) |
| Xanthohumol |
|
|
|
| [70] (September 2018) |
| Hydroxysafflor Yellow A |
|
|
|
| [71] (January 2025) |
| Licochalcone A, l-carnitine and 1,2-decanediol |
|
|
|
| [72] (September 2015) |
| 6-prenylnaringenin (6-PN) 8-prenylnaringenin (8-PN) |
|
|
|
| [73] (March 2018) |
| Xanthohumol |
|
|
|
| (November 2017) [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsoti, V.; Ofrydopoulou, A.; Tsoupras, A. Antithrombotic and Anti-Inflammatory Health Promoting Pharmacological Properties of Chalcones and Their Derivatives Against Atherosclerosis and CVD. Sclerosis 2025, 3, 33. https://doi.org/10.3390/sclerosis3040033
Katsoti V, Ofrydopoulou A, Tsoupras A. Antithrombotic and Anti-Inflammatory Health Promoting Pharmacological Properties of Chalcones and Their Derivatives Against Atherosclerosis and CVD. Sclerosis. 2025; 3(4):33. https://doi.org/10.3390/sclerosis3040033
Chicago/Turabian StyleKatsoti, Valeria, Anna Ofrydopoulou, and Alexandros Tsoupras. 2025. "Antithrombotic and Anti-Inflammatory Health Promoting Pharmacological Properties of Chalcones and Their Derivatives Against Atherosclerosis and CVD" Sclerosis 3, no. 4: 33. https://doi.org/10.3390/sclerosis3040033
APA StyleKatsoti, V., Ofrydopoulou, A., & Tsoupras, A. (2025). Antithrombotic and Anti-Inflammatory Health Promoting Pharmacological Properties of Chalcones and Their Derivatives Against Atherosclerosis and CVD. Sclerosis, 3(4), 33. https://doi.org/10.3390/sclerosis3040033







