The Use of 18F-FDG-PET in Systemic Sclerosis with Myocardial Involvement: The Scleroderma Heart Study
Abstract
1. Introduction
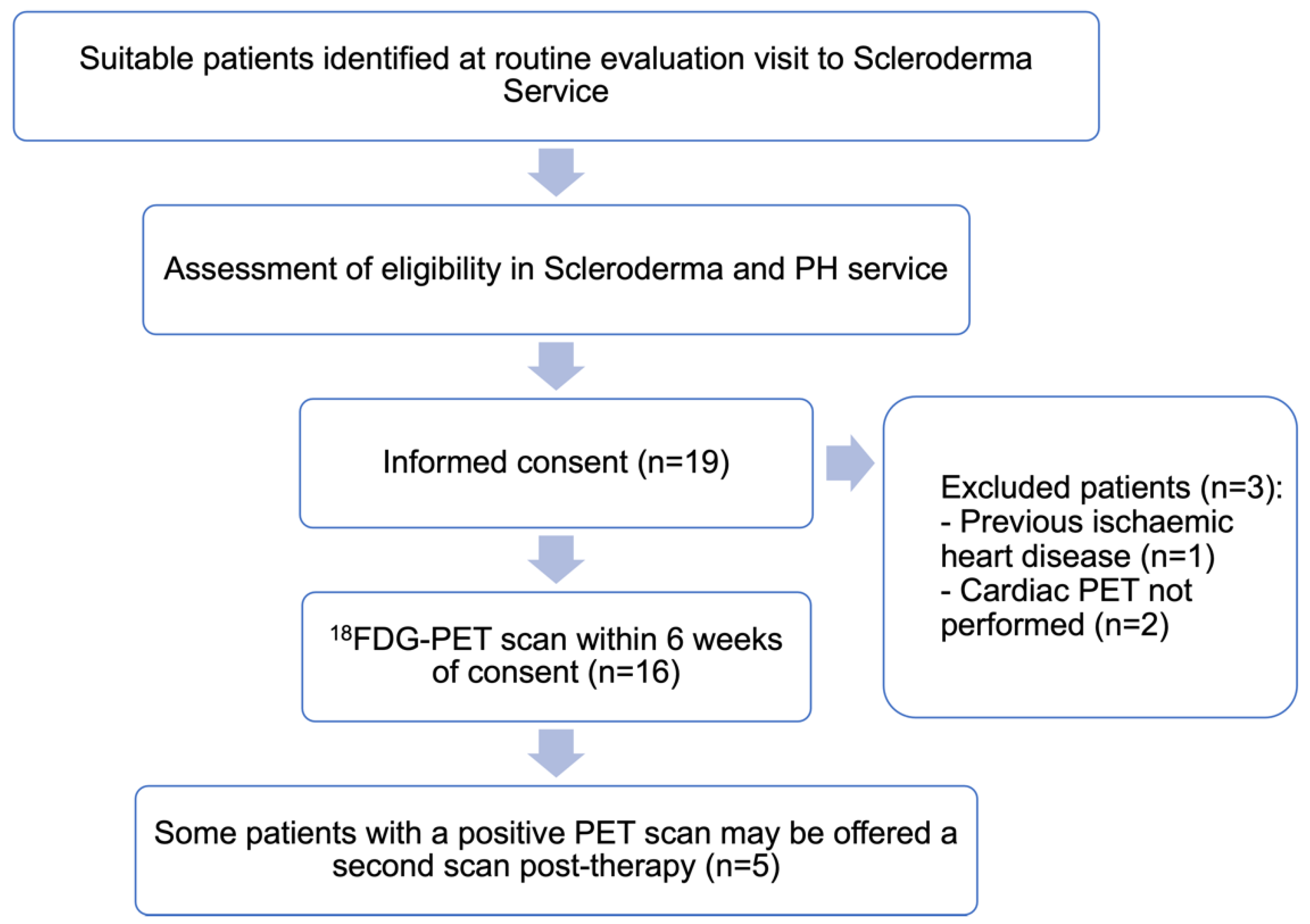
2. Methods
2.1. Study Design
2.2. Patient and Public Involvement
2.3. Patient Eligibility
2.4. Data Collection
2.5. 18F-FDG-PET/CT Protocol
2.6. Study End Points
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics
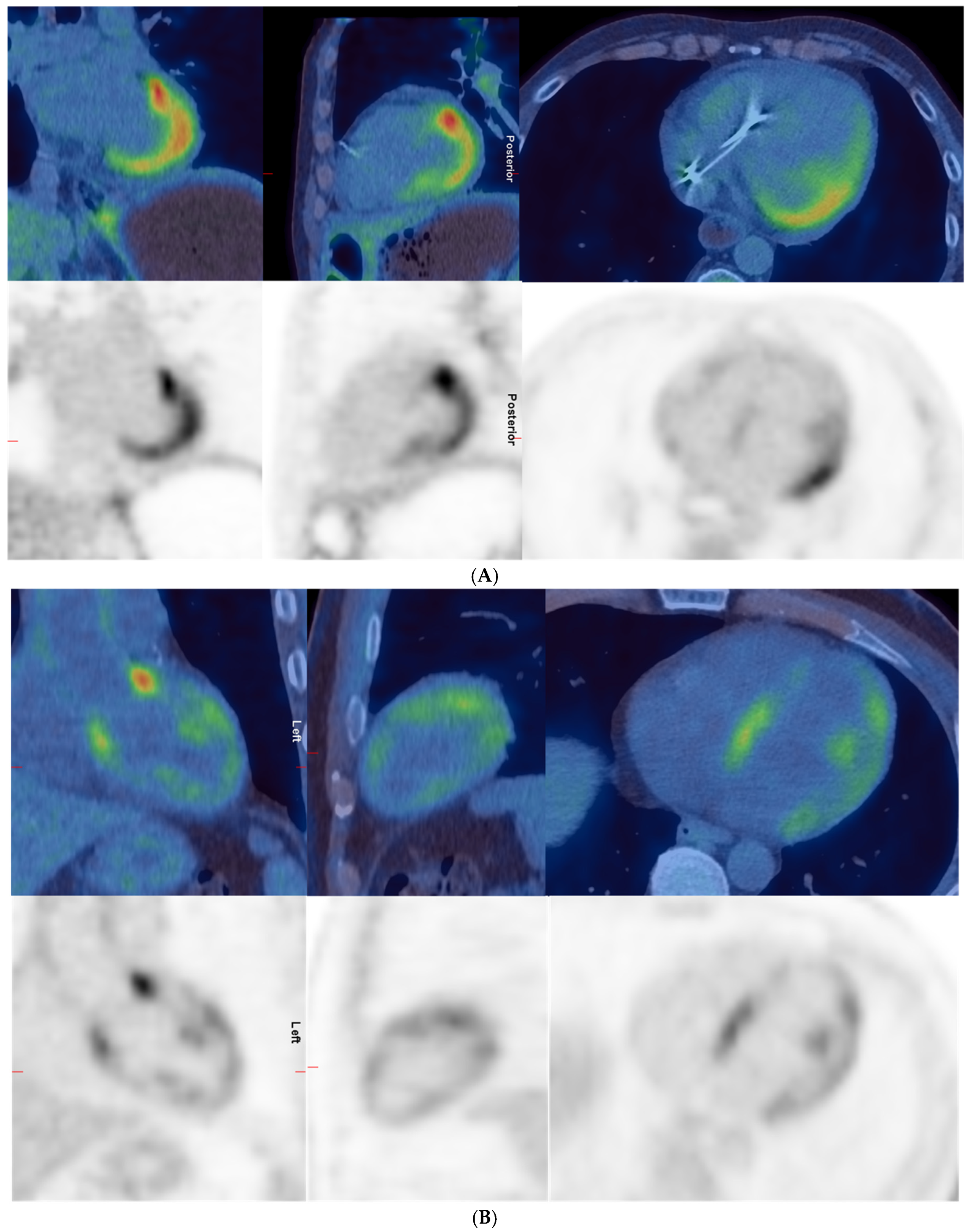
3.2. Positive Versus Negative PETs
3.3. Repeat Cardiac PETs and Immunosuppression During the Interval
4. Discussion
Limitations
5. Conclusions
6. Key Messages
6.1. What Is Already Known on This Topic
- Cardiac involvement in scleroderma due to myocardial inflammation and fibrosis is associated with poor outcomes.
- Existing techniques, such as echocardiogram and cardiac magnetic resonance imaging, only have moderate sensitivity and specificity for cardiac involvement in scleroderma.
- The identification of cardiac scleroderma, prediction of active inflammation, and assessment of response to therapy remains challenging.
6.2. What This Study Adds
- This is the first study to investigate the use of 18F−FDG−PET/CT in scleroderma patients with suspected cardiac involvement.
- Fifty percent of scleroderma patients with cardiac involvement had myocardial uptake on an 18F−FDG−PET/CT, but only 12.5% had patterns definitively consistent with pathological involvement.
- Repeat scanning showed limited utility for treatment monitoring, with stable or increased uptake despite immunosuppression.
6.3. How This Study Might Affect Research, Practice, and Policy
- Cardiac 18F−FDG−PET/CT currently has limited clinical utility in scleroderma due to physiological uptake and a poor correlation with outcomes.
- Future research should focus on alternative tracers, standardised interpretation criteria, and longer-term studies.
- The findings provide important groundwork for future multicentre studies in this understudied area.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahan, A.; Coghlan, G.; McLaughlin, V. Cardiac complications of systemic sclerosis. Rheumatology 2009, 48 (Suppl. S3), iii45–iii48. [Google Scholar] [CrossRef] [PubMed]
- Bissell, L.A.; Md Yusof, M.Y.; Buch, M.H. Primary myocardial disease in scleroderma—A comprehensive review of the literature to inform the UK Systemic Sclerosis Study Group cardiac working group. Rheumatology 2017, 56, 882–895. [Google Scholar]
- BH, B.; RL, R.; WR, S.; Hutchins, G.M. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation 1976, 53, 483–490. [Google Scholar] [CrossRef]
- D’Angelo, W.A.; Fries, J.F.; Masi, A.T.; Shulman, L.E. Pathologic observations in systemic sclerosis (scleroderma): A study of fifty-eight autopsy cases and fifty-eight matched controls. Am. J. Med. 1969, 46, 428–440. [Google Scholar] [CrossRef]
- Bruni, C.; Ross, L. Cardiac involvement in systemic sclerosis: Getting to the heart of the matter. Best. Pract. Res. Clin. Rheumatol. 2021, 35, 101668. [Google Scholar] [CrossRef]
- Ntusi, N.A.B.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Rai, A.B.S.; Matthews, P.M.; Robson, M.D.; Moon, J.; Wordsworth, P.B.; Neubauer, S.; et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—A clinical study using myocardial T1-mapping and extracellular volume quantification. J. Cardiovasc. Magn. Reson. 2014, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.S.; Karia, N.; Cole, A.R.; Maclean, R.H.; Brown, J.T.; Masi, A.; Patel, R.K.; Razvi, Y.; Chacko, L.; Venneri, L.; et al. Distinct cardiovascular phenotypes are associated with prognosis in systemic sclerosis: A cardiovascular magnetic resonance study. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 463–471. [Google Scholar] [CrossRef]
- Webber, M.; Natarajan, A.; Schreiber, B.; Sanjay, P.; Schwaiger, J.; Handler, C.; Coghlan, G. 136 Cardiac Involvement In Systemic Sclerosis: Strengths and Limitations of Cardiac Magnetic Resonance Imaging. Heart 2014, 100 (Suppl. S3), A80. [Google Scholar] [CrossRef]
- Sierra-Sepúlveda, A.; Esquinca-González, A.; Benavides-Suárez, S.A.; Sordo-Lima, D.E.; Caballero-Islas, A.E.; Cabral-Castañeda, A.R.; Rodríguez-Reyna, T.S. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. BioMed Res. Int. 2019, 2019, 4569826. [Google Scholar] [CrossRef]
- Besenyi, Z.; Ágoston, G.; Hemelein, R.; Bakos, A.; Nagy, F.T.; Varga, A.; Kovács, L.; Pávics, L. Detection of myocardial inflammation by 18F-FDG-PET/CT in patients with systemic sclerosis without cardiac symptoms: A pilot study. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S119), 88–96. [Google Scholar] [PubMed]
- Chareonthaitawee, P.; Beanlands, R.S.; Chen, W.; Dorbala, S.; Miller, E.J.; Murthy, V.L.; Birnie, D.H.; Chen, E.S.; Cooper, L.T.; Tung, R.H.; et al. Joint SNMMI–ASNC Expert Consensus Document on the Role of 18F-FDG PET/CT in Cardiac Sarcoid Detection and Therapy Monitoring. J. Nucl. Med. 2017, 58, 1341. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1016–1039. [Google Scholar] [CrossRef]
- De Luca, G.; Campochiaro, C.; De Santis, M.; Sartorelli, S.; Peretto, G.; Sala, S.; Canestrari, G.; De Lorenzis, E.; Basso, C.; Rizzo, S.; et al. Systemic sclerosis myocarditis has unique clinical, histological and prognostic features: A comparative histological analysis. Rheumatology 2020, 59, 2523–2533. [Google Scholar] [CrossRef]
- Hussein, M.R.; Hassan, H.I.; Hofny, E.R.; Elkholy, M.; Fatehy, N.A.; Abd Elmoniem, A.E.; Ezz El-Din, A.M.; Afifi, O.A.; Rashed, H.G. Alterations of mononuclear inflammatory cells, CD4/CD8+ T cells, interleukin 1beta, and tumour necrosis factor alpha in the bronchoalveolar lavage fluid, peripheral blood, and skin of patients with systemic sclerosis. J. Clin. Pathol. 2005, 58, 178–184. [Google Scholar] [CrossRef]
- Love, C.; Tomas, M.B.; Tronco, G.G.; Palestro, C.J. FDG PET of Infection and Inflammation. Radiographics 2005, 25, 1357–1368. [Google Scholar] [CrossRef]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef]
- Peretto, G.; Busnardo, E.; Ferro, P.; Palmisano, A.; Vignale, D.; Esposito, A.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; De Gaspari, M.; et al. Applications of FDG-PET Scan in Arrhythmic Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 1771–1780. [Google Scholar] [CrossRef]
- Agoston, G.; Besenyi, Z.; Hemelein, R.; Palinkas, A.; Kovacs, L.; Pavics, L.; Varga, A. P2424Detection of myocardial involvement in patients with systemic sclerosis by cardiac 18F-FDG PET/CT and speckle tracking echocardiography. Eur. Heart J. 2017, 38 (Suppl. S1), ehx502.P2424. [Google Scholar] [CrossRef]
- Treutlein, C.; Distler, J.H.W.; Tascilar, K.; Fakhouri, S.C.; Györfi, A.H.; Atzinger, A.; Matei, A.-E.; Dees, C.; Büttner-Herold, M.; Kuwert, T.; et al. Assessment of myocardial fibrosis in patients with systemic sclerosis using [(68)Ga]Ga-FAPI-04-PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Meune, C.; Allanore, Y.; Devaux, J.-Y.; Dessault, O.; Duboc, D.; Weber, S.; Kahan, A. High prevalence of right ventricular systolic dysfunction in early systemic sclerosis. J. Rheumatol. 2004, 31, 1941–1945. [Google Scholar]
- Bratis, K.; Lindholm, A.; Hesselstrand, R.; Arheden, H.; Karabela, G.; Stavropoulos, E.; Katsifis, G.; Kolovou, G.; Kitas, G.D.; Sfikakis, P.P.; et al. CMR feature tracking in cardiac asymptomatic systemic sclerosis: Clinical implications. PLoS ONE 2019, 14, e0221021. [Google Scholar] [CrossRef] [PubMed]
- Komócsi, A.; Vorobcsuk, A.; Faludi, R.; Pintér, T.; Lenkey, Z.; Költő, G.; Czirják, L. The impact of cardiopulmonary manifestations on the mortality of SSc: A systematic review and meta-analysis of observational studies. Rheumatology 2012, 51, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Gotschy, A.; Jordan, S.; Stoeck, C.T.; von Deuster, C.; Peer, T.; Gastl, M.; Vishnevskiy, V.; Wissmann, L.; Dobrota, R.; Mihai, C.; et al. Diffuse myocardial fibrosis precedes subclinical functional myocardial impairment and provides prognostic information in systemic sclerosis. Eur. Heart J.—Cardiovasc. Imaging 2022, 24, 373–382. [Google Scholar] [CrossRef]
- Antoniades, L.; Sfikakis, P.P.; Mavrikakis, M. Glucocorticoid effects on myocardial performance in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2001, 19, 431–437. [Google Scholar]
- Pieroni, M.; De Santis, M.; Zizzo, G.; Bosello, S.; Smaldone, C.; Campioni, M.; De Luca, G.; Laria, A.; Meduri, A.; Bellocci, F.; et al. Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: Potential utility of immunosuppressive therapy in cardiac damage progression. Semin. Arthritis Rheum. 2014, 43, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Bissell, L.-A.; Anderson, M.; Burgess, M.; Chakravarty, K.; Coghlan, G.; Dumitru, R.B.; Graham, L.; Ong, V.; Pauling, J.D.; Plein, S.; et al. Consensus best practice pathway of the UK Systemic Sclerosis Study group: Management of cardiac disease in systemic sclerosis. Rheumatology 2017, 56, 912–921. [Google Scholar] [CrossRef]
- Atterton-Evans, V.; Turner, J.; Vivanti, A.; Robertson, T. Variances of dietary preparation for suppression of physiological (18)F-FDG myocardial uptake in the presence of cardiac sarcoidosis: A systematic review. J. Nucl. Cardiol. 2020, 27, 481–489. [Google Scholar] [CrossRef]
- Bravo, P.E.; Bajaj, N.; Padera, R.F.; Morgan, V.; Hainer, J.; Bibbo, C.F.; Harrington, M.; Park, M.A.; Hyun, H.; Robertson, M.; et al. Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: A pilot study. J. Nucl. Cardiol. 2021, 28, 1089–1099. [Google Scholar] [CrossRef]
- Martineau, P.; Pelletier-Galarneau, M.; Juneau, D.; Leung, E.; Nery, P.; DeKemp, R.; Beanlands, R.; Birnie, D. FLT-PET for the assessment of systemic sarcoidosis including cardiac and CNS involvement: A prospective study with comparison to FDG-PET. EJNMMI Res. 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Follansbee, W.P.; Curtiss, E.I.; Medsger, T.A., Jr.; Steen, V.D.; Uretsky, B.F.; Owens, G.R.; Rodnan, G.P. Physiologic abnormalities of cardiac function in progressive systemic sclerosis with diffuse scleroderma. N. Engl. J. Med. 1984, 310, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, C.; Meroni, P.L.; Schreiber, B.E.; Coghlan, J.G.; Denton, C.P.; Ong, V.H. Role of N-terminal pro-brain natriuretic peptide in detecting clinically significant cardiac involvement in systemic sclerosis patients. Clin. Exp. Rheumatol. 2017, 30, S81–S85. [Google Scholar]
- Vacca, A.; Meune, C.; Gordon, J.; Chung, L.; Proudman, S.; Assassi, S.; Allanore, Y. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology 2014, 53, 1172–1177. [Google Scholar] [CrossRef]
- Yiu, K.H.; Schouffoer, A.A.; Marsan, N.A.; Ninaber, M.K.; Stolk, J.; Vlieland, T.V.; Scherptong, R.W.; Delgado, V.; Holman, E.R.; Tse, H.F.; et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: Relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum. 2011, 63, 3969–3978. [Google Scholar] [CrossRef]
- Jung, M.; Bonner, A.; Hudson, M.; Baron, M.; Pope, J.; the Canadian Scleroderma Research Group (CSRG). Myopathy is a poor prognostic feature in systemic sclerosis: Results from the Canadian Scleroderma Research Group (CSRG) cohort. Scand. J. Rheumatol. 2013, 43, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Dobarro, D.; Raphael, C.E.; Baksi, A.J.; Kindler, H.; Mohiaddin, R.; Pennell, D.; Schreiber, B.E.; Prasad, S.K. Prognostic significance of ventricular function and late gadolinium enhancement on CMR in symptomatic patients with scleroderma. J. Cardiovasc. Magn. Reson. 2013, 15, P160. [Google Scholar] [CrossRef]
- Wicks, E.; Menezes, L.; Pantazis, A.; Mohiddin, S.; Porter, J.; Booth, H.; Elliott, P. 135 Novel Hybrid Positron Emission Tomography-Magnetic Resonance (PET-MR) Multi-modality Inflammatory Imaging has Improved Diagnostic Accuracy for Detecting Cardiac Sarcoidosis. Heart 2014, 100, A80. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; et al. The Use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef]
- Skali, H.; Schulman, A.R.; Dorbala, S. 18F-FDG PET/CT for the Assessment of Myocardial Sarcoidosis. Curr. Cardiol. Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, B.A.; Leung, E.; Ohira, H.; Cocker, M.S.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; Beanlands, R.S.; Nery, P.B. The role of F18-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J. Nucl. Cardiol. 2013, 20, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sperry, B.W.; Oldan, J.D.; Hsich, E.M.; Reynolds, J.P.; Tamarappoo, B.K. Infectious myocarditis on FDG-PET imaging mimicking sarcoidosis. J. Nucl. Cardiol. 2015, 22, 840–844. [Google Scholar] [CrossRef]
- Millar, B.C.; Prendergast, B.D.; Alavi, A.; Moore, J.E. 18FDG-positron emission tomography (PET) has a role to play in the diagnosis and therapy of infective endocarditis and cardiac device infection. Int. J. Cardiol. 2013, 167, 1724–1736. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Rudd, J.H. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 443–457. [Google Scholar] [CrossRef] [PubMed]
| No. | Age (Years) | Sex | Ethnicity | Co-Morbidities | Years of Diagnosis | Organ Involvement | Antibodies | Cardiac Signs and Symptoms | Trop T (ng/L) | NT Pro-BNP (ng/L) | ECG | EMB | PET | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | F | Asian | None | 7 | Raynaud’s, telangiectasia, digital ulcers | ANA, U3 RNP | Chest pain, palpitations | 22 | 581 | SR, short PR | Not attempted due to groin haematoma | − | N |
| 2 | 32 | F | White | Small bowel bacterial overgrowth, inflammatory arthritis, migraines, IDA | 6 | NSIP, dysphagia, reflux and constipation, skin, myositis, Raynaud’s digital ulcers | ANA, ACA, Scl-70 | Chest pain, palpitations, presyncope, basal crackles | 41 | 153 | SR, short PR | Not performed as could not position catheter against RV | − | N |
| 4 | 59 | F | White | Narrow complex tachycardias, AF, diverticulosis | 4 | Immotile oesophagus, skin, Raynaud’s, digital ulcers, ILD | ANA, Scl-70 | None | 7 | 269 | SR, LAD, RBBB | N/A | − | N |
| 5 | 35 | F | Black | HTN | 3 | Severe pulmonary hypertension, myositis, NSIP, Raynaud’s, digital ulcers, GI involvement | ANA, Ro, Scl-70 | Chest pain, palpitations, raised JVP, basal crackles, peripheral oedema | 70 | 1196 | SR, RBBB, RVH | N/A | + | Y |
| 6 | 59 | M | White | Bifascicular block, latent TB on isoniazid | 1 | Extensive fibrotic NSIP, vasculitic rash, Raynaud’s | ANA | Basal crackles, peripheral oedema | 7 | 94 | SR, LAD, RBBB | N/A | − | Y |
| 7 | 61 | F | Black | AF, bronchiectasis, OSA, obesity, asthma, hypothyroid, pulmonary HTN | 1 | NSIP, myositis, skin involvement, Raynaud’s, digital ulcers, GI involvement | ANA, ENA, Ro, Scl-70 | None | 146 | 421 | NR | Fibrosis and focal myocarditis | + | N |
| Chest pain, syncope, peripheral oedema | 44 | NR | SR, RBBB | + | ||||||||||
| 8 | 20 | M | Black | SVT | 2 | Microstomia, myositis, myocarditis, Raynaud’s, digital ulcers | ANA, Scl-70, nRNP | Chest pain, palpitations | 692 | 1207 | SR | N/A | + | Y |
| Chest pain, orthopnoea, palpitations, raised JVP | 103 | 889 | SR | + | ||||||||||
| 9 | 40 | F | Black | Discoid lupus | 5 | Myositis, Raynaud’s | ANA, U3 RNP | Palpitations, peripheral oedema | 149 | 211 | SR, LAD | Non-inflamed myocardium with mild interstitial fibrosis | − | N |
| 11 | 34 | F | Black | None | NR | Myositis, mild ILD, digital ulcers | ANA, U3 RNP, Scl-70 | Chest pain, palpitations, raised JVP, peripheral oedema | 19 | 181 | SR | N/A | − | N |
| 12 | 56 | M | White | HTN, hyperlipidaemia, COPD | NR | Heart, digital ulcers | ANA | Orthopnoea, PND, palpitations, raised JVP | 38 | 3707 | Atrial paced | Patchy myocyte hypertrophy and fibrosis | + | Y |
| NR | NR | NR | + | |||||||||||
| 13 | 46 | M | White | Rheumatoid arthritis, SLE overlap, sicca syndrome | 8 | Myocarditis, Raynaud’s, digital ulcers | ANA, PM-Scl, Ro, dsDNA, CCP | None | 838 | 145 | SR | N/A | + | N |
| 14 | 45 | M | Asian | TB lymphadenopathy | 1 | Myositis, sclerodactyly, synovitis, Raynaud’s | ANA, nRNP, Sm, Ro, Ku | None | 190 | 738 | SR | N/A | + | N |
| NR | NR | NR | + | |||||||||||
| 16 | 37 | M | Black | HTN | 4 | Myositis, GORD, Raynaud’s, digital ulcers | U3-RNP, ANA | None | 16 | 106 | SR | N/A | + | N |
| 17 | 57 | F | NR | Familial HTN, hypothyroidism, rectal Ca, migraines, CKD 3, sagittal sinus thrombosis, provoked PE | 20 | Renal crisis, Raynaud’s, digital ulcers, ILD, GI involvement | ANA, RNAP III | Presyncope, peripheral oedema | 19 | 2614 | SR, LAD | No evidence of myocarditis | + | Y |
| NR | NR | NR | + | |||||||||||
| 18 | 66 | F | White | HTN, hyperlipidaemia, asthma | 2 | Myositis, oral neuropathy, Raynaud’s digital ulcers, ILD, GI involvement | ANA | Basal crackles | 292 | 1456 | 1HB, RBBB, LAD | Minor fibrosis, no myocarditis. | − | Y |
| 19 | 70 | F | Asian | HTN, IDA, glaucoma, mixed myelodysplastic/myeloproliferative disorder, CKD | 11 | Pulmonary hypertension, eczema, Raynaud’s, digital ulcers, ILD, reflux, renal crisis | ANA, RNAP III | Basal crackles | 15 | 405 | Sinus arrhythmia | N/A | − | N |
| Variable | Total (n = 16) | PET + ve (n = 8) | PET − ve (n = 8) | MD (95% CI) or OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 46.4 (15.1) | 44.6 (13.7) | 48.3 (17.1) | −3.6 (−20.2, 13.0) | 0.647 |
| Male (%) | 6 (37.5) | 5 (62.5) | 1 (12.5) | 11.7 (0.9, 147.6) | 0.119 |
| Ethnicity | |||||
| White | 6 (40.0) | 2 (28.6) | 4 (50.0) | - | 0.448 |
| Black | 6 (40.0) | 4 (57.1) | 2 (25.0) | - | |
| Asian | 3 (20.0) | 1 (14.3) | 2 (25.0) | - | |
| Inclusion criteria | |||||
| LVEF < 40% on echo | 0 (0) | 0 (0) | 0 (0) | - | - |
| Abnormal heart muscle on cardiac MRI | 7 (50.0) | 3 (42.9) | 4 (57.1) | 0.56 (0.07, 4.67) | 1.00 |
| Diastolic heart failure with no known risk factors | 1 (7.1) | 0 (0) | 1 (14.3) | - | 1.00 |
| Persistently raised troponin | 7 (50.0) | 4 (57.1) | 3 (42.9) | 1.78 (0.21, 14.77) | 1.00 |
| Ventricular arrhythmia | 1 (7.1) | 1 (14.3) | 0 (0) | - | 1.00 |
| Co-morbidities | |||||
| BMI (kg/m2) | 25.1 (5.3) | 26.0 (5.8) | 23.2 (4.5) | 2.8 (−3.1, 8.7) | 0.325 |
| HTN (%) | 7 (43.8) | 4 (50.0) | 3 (37.5) | 1.67 (0.23, 12.22) | 1.00 |
| T2DM (%) | 0 (0) | 0 (0) | 0 (0) | - | − |
| Hyperlipidaemia (%) | 2 (12.5) | 1 (12.5) | 1 (12.5) | 1.00 (0.05, 19.4) | 1.00 |
| AF (%) | 2 (12.5) | 1 (12.5) | 1 (12.5) | 1.00 (0.05, 19.4) | 1.00 |
| Symptoms | |||||
| Chest pain (%) | 5 (31.3) | 2 (25.0) | 3 (37.5) | 0.56 (0.07, 4.76) | 1.00 |
| Orthopnoea (%) | 1 (6.3) | 1 (12.5) | 0 (0) | - | 1.00 |
| PND (%) | 1 (6.3) | 1 (12.5) | 0 (0) | - | 1.00 |
| Palpitations (%) | 7 (43.8) | 3 (37.5) | 4 (50.0) | 0.60 (0.08, 4.40) | 1.00 |
| Presyncope (%) | 2 (12.5) | 1 (12.5) | 1 (12.5) | 1.00 (0.05, 19.36) | 1.00 |
| Syncope (%) | 0 (0) | 0 (0) | 0 (0) | - | - |
| Raised JVP (%) | 3 (18.8) | 1 (12.5) | 2 (25.0) | 2.33 (0.17, 32.58) | 1.00 |
| Basal crackles (%) | 5 (31.3) | 1 (12.5) | 4 (50.0) | 0.14 (0.01−1.76) | 0.282 |
| Peripheral oedema (%) | 5 (31.3) | 2 (25.0) | 3 (37.5) | 0.56 (0.07, 4.76) | 1.00 |
| NYHA Class | 2.75 (0.46) | 2.75 (0.71) | 0 (−0.64, 0.64) | 1.00 | |
| Class I | 1 (6.3) | 0 (0) | 1 (12.5) | - | 1.00 |
| Class II | 2 (12.5) | 2 (25.0) | 0 (0) | - | 0.467 |
| Class III | 13 (81.3) | 6 (75.0) | 7 (87.5) | 0.43 (0.03, 5.99) | - |
| Scleroderma involvement | |||||
| Myositis (%) | 9 (56.3) | 5 (62.5) | 4 (50.0) | 1.67 (0.23, 12.22) | 1.00 |
| Raynaud’s (%) | 14 (87.5) | 7 (87.5) | 7 (87.5) | 1.00 (0.05, 19.4) | 1.00 |
| Telangiectasia (%) | 1 (7.7) | 0 (0) | 1 (16.7) | - | 0.462 |
| Digital ulcers (%) | 13 (81.3) | 7 (87.5) | 6 (75.0) | 2.33 (0.17, 32.58) | 1.00 |
| ILD (%) | 9 (56.3) | 3 (37.5) | 6 (75.0) | 0.20 (0.02, 1.71) | 0.315 |
| GI involvement (%) | 8 (50.0) | 4 (50.0) | 4 (57.1) | 0.75 (0.10−5.77) | 1.00 |
| Duration of scleroderma (years) | 5.36 (5.15) | 5.57 (6.80) | 5.14 (3.34) | 0.43 (−5.81, 6.67) | 0.884 |
| Serology | |||||
| Positive ANA (%) | 16 (100) | 8 (100) | 8 (100) | - | - |
| Positive ENA (%) | 10 (66.7) | 6 (85.7) | 4 (50.0) | 6.00 (0.48, 75.34) | 0.282 |
| Trop T (ng/L) | 160.1 (251.1) | 251.1 (325.4) | 69.0 (101.6) | 182.1 (−93.7, 458.0) | 0.168 |
| NT pro-BNP (ng/L) | 842.8 (1021.6) | 1266.8 (1275.0) | 418.8 (447.3) | 848.0 (−238.4, 1934.4) | 0.111 |
| CRP (mg/L) | 18.6 (21.7) | 21.4 (21.1) | 14.8 (24.0) | 6.5 (−19.8, 32.8) | 0.598 |
| CK (unit/L) | 541.1 (740.3) | 574.3 (578.3) | 508.0 (922.2) | 66.3 (−830,1, 962.7) | 0.875 |
| eGFR (mL/min) | 77.1 (19.1) | 80.9 (19.3) | 73.3 (19.4) | 7.6 (−13.1, 28.4) | 0.444 |
| Fibrosis/myocarditis on biopsy (%) (n = 4) | 3 (75.0) | 1 (50.0) | 2 (100.0) | - | 1.00 |
| Cardiac PET | |||||
| SUV max overall | 6.7 (6.8) | 11.6 (6.7) | 1.8 (0.7) | 9.8 (4.2, 15.4) | 0.004 |
| SUV max septum | 4.9 (4.9) | 8.1 (5.1) | 1.6 (0.6) | 6.5 (2.2, 10.8) | 0.009 |
| TTE LVEF baseline (%) | 59.3 (12.6) | 56.4 (14.4) | 62.1 (10.6) | −5.7 (−19.3, 8.0) | 0.387 |
| TTE LVEF 12 months (%) | 52.3 (15.9) | 55.9 (17.5) | 47.9 (14.4) | 8.0 (−13.8, 29.7) | 0.429 |
| TTE LVEF follow-up (%) | 49.1 (13.1) | 52.0 (12.7) | 45.8 (14.1) | 6.2 (−12.1, 24.5) | 0.463 |
| Decreased LVEF (>5%) in 1 year (%) | 6 (50.0) | 2 (28.6) | 4 (80.0) | 0.10 (0.01, 1.54) | 0.242 |
| Time to 12 months TTE (days) | 334.8 (215.1) | 318.9 (213.4) | 357.0 (240.5) | −38.1 (−331.2, 254.9) | 0.778 |
| Decreased LVEF (>5%) on follow-up (%) | 6 (50.0) | 3 (42.9) | 3 (60.0) | 0.50 (0.05, 5.15) | 1.00 |
| Time to last TTE (days) | 583.1 (482.0) | 466.7 (388.8) | 746.0 (596.0) | −279.3 (−908.8, 350.2) | 0.346 |
| Duration of follow-up (days) | 603.3 (483.3) | 522.4 (285.6) | 684.3 (635.6) | −161.9 (−713.0, 389.2) | 0.526 |
| Mortality (%) | 6 (37.5) | 4 (50) | 2 (25) | 3.00 (0.36, 24.92) | 0.608 |
| No. | No. of PET Scans | Original Report | Re-Report by Blinded Single Nuclear Medicine Physician in This Study | SUVmax Overall | SUVmax Septum |
|---|---|---|---|---|---|
| 5 | 1 | Increased uptake in the heart and intense uptake in the lateral wall, apex, and anterolateral papillary muscle. Cardiac uptake could represent cardiac involvement by scleroderma or physiologic myocardial uptake. | Intense uptake in apex, lateral wall, and right ventricle. Moderate uptake in septum, anterior, and inferior walls. Indeterminate—likely negative. | 11.5 | 5 |
| 7 | 1 | Abnormal uptake in the heart with intense uptake in the base of septum and base of the lateral wall. More moderate uptake in the anterior aspect of the septum and lateral wall. Increased uptake in the LV papillary muscles. Could represent cardiac involvement by scleroderma. | Intense in base of septum and lateral wall, moderate in remainder of lateral and septal walls, moderate in right ventricle, intense anterior to AP trunk; likely physiological uptake. | 10.4 | 10.3 |
| 2 | Diffuse intense heterogeneous uptake in the LV and low-to-moderate uptake in the anterior and lateral walls of the RV. Uptake not significantly changed compared to previous scan. | Diffuse moderate uptake in left and right ventricles, greater than MBP. Likely not suppressed uptake; physiological rather than cardiac inflammation. | 9.6 | 9.6 | |
| 8 | 1 | Diffuse moderate-to-intense uptake in the LV and low-grade uptake in RV; could represent active cardiac myositis. | Moderate diffuse in left ventricle and low-grade in right ventricle; pattern likely physiological. | 7.4 | 5.5 |
| 2 | Intense diffuse homogeneous uptake in the LV and moderate diffuse homogeneous uptake in the RV; unchanged compared to previous scan. | Intense diffuse in left and right myocardium; likely physiological. | 19.5 | 17.7 | |
| 12 | 1 | Increased uptake in the LV, RV, and RA compatible with areas of inflammation due to cardiac involvement by scleroderma. Increased uptake in mediastinal nodes. Abnormal uptake in the larynx. | Moderate in LV lateral wall, base of anterior and inferior walls, low-grade in base of septum and right ventricle. Indeterminate—likely positive. | 7.1 | 2.5 |
| 2 | N/A | Intense diffuse in left myocardium and low-grade in right ventricle; likely physiological. | 11 | 8.2 | |
| 13 | 1 | Patchy myocardial uptake in LV, may represent active myocarditis. Low-grade uptake in hilar lymph nodes is likely inflammatory. | Moderate heterogeneous in left ventricle with intense in base of anterior wall. Indeterminate—likely positive. | 7.4 | 4.2 |
| 14 | 1 | Diffuse uptake in left and right ventricles; could be scleroderma myocarditis or physiological uptake. | Moderate diffuse in left and right ventricles. Indeterminate—likely negative. | 6.2 | 6.2 |
| 2 | More intense uptake in the myocardium, diffuse and a reduction in disease activity elsewhere; likely physiological rather than worsening myocarditis. | Intense diffuse in left ventricle; likely physiological. | 14.6 | 14.6 | |
| 16 | 1 | No active infection or inflammation. | Intense diffuse in left and right ventricles; likely physiological. | 25.6 | 15.2 |
| 17 | 1 | Diffuse intense increased tracer uptake throughout the LV myocardium in keeping with myocarditis. | Intense diffuse homogeneous in left ventricle; indeterminate—likely physiological. | 17.2 | 16 |
| 2 | N/A | Same intense diffuse in LV; indeterminate—likely physiological. | 18 | 13.5 |
| Case no. | Duration Between PETs (Days) | Heart | Extra-Cardiac | SUVmax Overall | SUVmax Septum | Immunosuppression Change |
|---|---|---|---|---|---|---|
| 7 | 211 | Intense in base of septum and of lateral wall, moderate in remainder of lateral and septal walls, moderate in right ventricle, intense anterior to AP trunk. | lungs | 10.4 | 10.3 | Started rituximab, hydroxychloroquine |
| Intense in left myocardium, moderate in right myocardium. | 9.6 | 9.6 | ||||
| 8 | 182 | Moderate diffuse in left ventricle and low-grade in right ventricle; pattern likely physiological. | Low-grade axillary nodes and avid subpleural reticulation LLL | 7.4 | 5.5 | Started steroids, cyclophosphamide, hydroxychloroquine, rituximab |
| Intense diffuse in left and right myocardium; likely physiological. | 19.5 | 17.7 | ||||
| 12 | 284 | Moderate in lateral wall and base of anterior and inferior walls, low-grade in base of septum and right ventricle. | No | 7.1 | 2.5 | No change |
| Intense diffuse in left myocardium and low-grade in right ventricle; likely physiological. | 11 | 8.2 | ||||
| 14 | 258 | Moderate diffuse in left and right ventricles. | Nodes, lungs, spleen | 6.2 | 6.2 | Started MMF, monthly cyclophosphamide, rituximab, increased steroids |
| Intense diffuse in left ventricle; likely physiological. | 14.6 | 14.6 | ||||
| 17 | 212 | Intense diffuse homogeneous in left ventricle. | No | 17.2 | 16 | Started MMF |
| Same intense diffuse in LV; indeterminate— likely physiological. | 18 | 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, J.S.Y.; Wagner, T.; Denton, C.; Coughlan, J.G.; Knight, D.; Kotecha, T.; Schreiber, B. The Use of 18F-FDG-PET in Systemic Sclerosis with Myocardial Involvement: The Scleroderma Heart Study. Sclerosis 2025, 3, 31. https://doi.org/10.3390/sclerosis3030031
Ho JSY, Wagner T, Denton C, Coughlan JG, Knight D, Kotecha T, Schreiber B. The Use of 18F-FDG-PET in Systemic Sclerosis with Myocardial Involvement: The Scleroderma Heart Study. Sclerosis. 2025; 3(3):31. https://doi.org/10.3390/sclerosis3030031
Chicago/Turabian StyleHo, Jamie Sin Ying, Thomas Wagner, Christopher Denton, John Gerry Coughlan, Daniel Knight, Tushar Kotecha, and Benjamin Schreiber. 2025. "The Use of 18F-FDG-PET in Systemic Sclerosis with Myocardial Involvement: The Scleroderma Heart Study" Sclerosis 3, no. 3: 31. https://doi.org/10.3390/sclerosis3030031
APA StyleHo, J. S. Y., Wagner, T., Denton, C., Coughlan, J. G., Knight, D., Kotecha, T., & Schreiber, B. (2025). The Use of 18F-FDG-PET in Systemic Sclerosis with Myocardial Involvement: The Scleroderma Heart Study. Sclerosis, 3(3), 31. https://doi.org/10.3390/sclerosis3030031






