Lifestyle, Cognition, and Disability Outcomes in Multiple Sclerosis: A Comprehensive Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Procedures and Assessment
2.4. Clinical Classification Criteria
2.5. Statistical Analysis
3. Results
3.1. Sample Characterization
3.2. Clinical, Cognitive, and Disability Changes
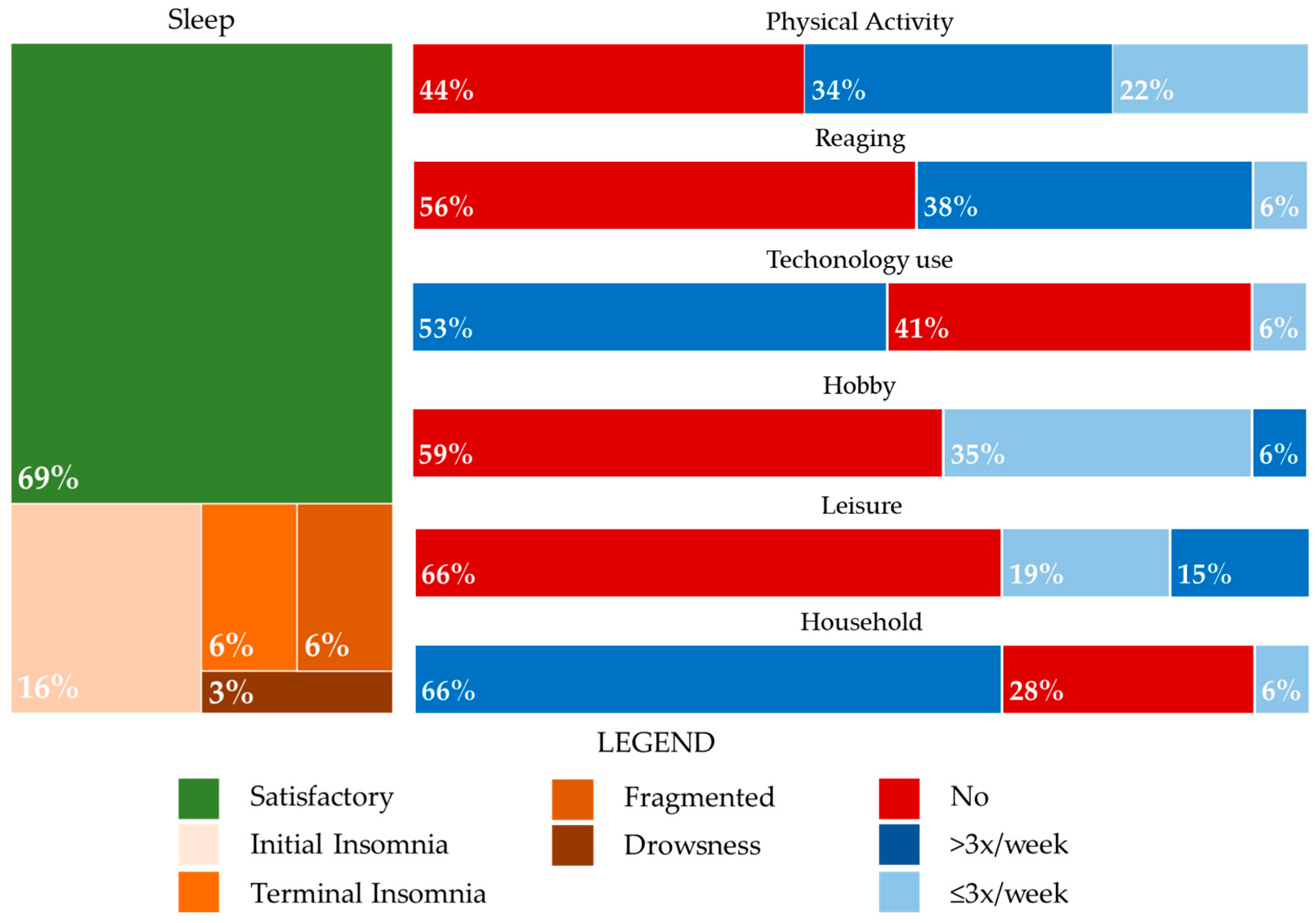
3.3. Lifestyle and Associations with Clinical, Cognitive, and Functional Disabilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of Multiple Sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, D.; Bamer, A.M.; Kim, J.; Chung, H.; Salem, R. People with Multiple Sclerosis Report Significantly Worse Symptoms and Health Related Quality of Life than the US General Population as Measured by PROMIS and NeuroQoL Outcome Measures. Disabil. Health J. 2018, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lobentanz, I.S.; Asenbaum, S.; Vass, K.; Sauter, C.; Klosch, G.; Kollegger, H.; Kristoferitsch, W.; Zeitlhofer, J. Factors Influencing Quality of Life in Multiple Sclerosis Patients: Disability, Depressive Mood, Fatigue and Sleep Quality. Acta Neurol. Scand. 2004, 110, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Morales-Gonzáles, J.M.; Benito-León, J.; Rivera-Navarro, J.; Mitchell, A.J.; GEDMA Study Group. A Systematic Approach to Analyse Health-Related Quality of Life in Multiple Sclerosis: The GEDMA Study. Mult. Scler. J. 2004, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ruet, A.; Deloire, M.; Hamel, D.; Ouallet, J.-C.; Petry, K.; Brochet, B. Cognitive Impairment, Health-Related Quality of Life and Vocational Status at Early Stages of Multiple Sclerosis: A 7-Year Longitudinal Study. J. Neurol. 2013, 260, 776–784. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, N.G. Impact of Walking Impairment in Multiple Sclerosis: Perspectives of Patients and Care Partners. Patient Patient-Centered Outcomes Res. 2011, 4, 189–201. [Google Scholar] [CrossRef]
- Motl, R.W.; Snook, E.M.; McAuley, E.; Scott, J.A.; Hinkle, M.L. Demographic Correlates of Physical Activity in Individuals with Multiple Sclerosis. Disabil. Rehabil. 2007, 29, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain Health: Time Matters in Multiple Sclerosis; Oxford PharmaGenesis Ltd.: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Janssens, A.C.J.W.; Van Doorn, P.A.; De Boer, J.B.; Van Der Meché, F.G.A.; Passchier, J.; Hintzen, R.Q. Impact of Recently Diagnosed Multiple Sclerosis on Quality of Life, Anxiety, Depression and Distress of Patients and Partners: Quality of Life and Emotional Well-Being in MS. Acta Neurol. Scand. 2003, 108, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Riise, T.; Bjørnevik, K.; Bhan, A.; Farbu, E.; Grytten, N.; Hogenesch, I.; Midgard, R.; Smith Simonsen, C.; Telstad, W.; et al. Preclinical Disease Activity in Multiple Sclerosis: A Prospective Study of Cognitive Performance Prior to First Symptom. Ann. Neurol. 2016, 80, 616–624. [Google Scholar] [CrossRef]
- DiGiuseppe, G.; Blair, M.; Morrow, S.A. Short Report: Prevalence of Cognitive Impairment in Newly Diagnosed Relapsing-Remitting Multiple Sclerosis. Int. J. MS Care 2018, 20, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Rashid, W.; Cercignani, M.; Langdon, D. Cognitive Impairment among Patients with Multiple Sclerosis: Associations with Employment and Quality of Life. Postgrad. Med. J. 2017, 93, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Oset, M.; Stasiolek, M.; Matysiak, M. Cognitive Dysfunction in the Early Stages of Multiple Sclerosis—How Much and How Important? Curr. Neurol. Neurosci. Rep. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Fenu, G.; Fronza, M.; Lorefice, L.; Arru, M.; Coghe, G.; Frau, J.; Marrosu, M.G.; Cocco, E. Performance in Daily Activities, Cognitive Impairment and Perception in Multiple Sclerosis Patients and Their Caregivers. BMC Neurol. 2018, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Zipoli, V.; Portaccio, E. Multiple Sclerosis-Related Cognitive Changes: A Review of Cross-Sectional and Longitudinal Studies. J. Neurol. Sci. 2006, 245, 41–46. [Google Scholar] [CrossRef]
- Goretti, B.; Portaccio, E.; Zipoli, V.; Hakiki, B.; Siracusa, G.; Sorbi, S.; Amato, M.P. Coping Strategies, Psychological Variables and Their Relationship with Quality of Life in Multiple Sclerosis. Neurol. Sci. 2009, 30, 15–20. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B.M.; DeLuca, J. Exercise Training and Cognitive Rehabilitation: A Symbiotic Approach for Rehabilitating Walking and Cognitive Functions in Multiple Sclerosis? Neurorehabil. Neural Repair 2016, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Goverover, Y.; Chiaravalloti, N.; DeLuca, J. Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) and Performance of Everyday Life Tasks: Actual Reality. Mult. Scler. J. 2016, 22, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Guclu-Gunduz, A.; Eldemir, K.; Apaydin, Y.; Yazici, G.; Irkec, C. Combined Exercise Training Improves Cognitive Functions in Multiple Sclerosis Patients with Cognitive Impairment: A Single-Blinded Randomized Controlled Trial. Mult. Scler. Relat. Disord. 2020, 45, 102419. [Google Scholar] [CrossRef]
- Motl, R.W.; Pilutti, L.A. The Benefits of Exercise Training in Multiple Sclerosis. Nat. Rev. Neurol. 2012, 8, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Klaren, R.E.; Pilutti, L.A.; Dlugonski, D.; Benedict, R.H.B.; Motl, R.W. Randomized Controlled Trial of Physical Activity, Cognition, and Walking in Multiple Sclerosis. J. Neurol. 2014, 261, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Balto, J.M.; Klaren, R.E.; Sommer, S.K.; DeLuca, J.; Motl, R.W. Systematically Developed Pilot Randomized Controlled Trial of Exercise and Cognition in Persons with Multiple Sclerosis. Neurocase 2016, 22, 443–450. [Google Scholar] [CrossRef]
- Briken, S.; Gold, S.; Patra, S.; Vettorazzi, E.; Harbs, D.; Tallner, A.; Ketels, G.; Schulz, K.; Heesen, C. Effects of Exercise on Fitness and Cognition in Progressive MS: A Randomized, Controlled Pilot Trial. Mult. Scler. J. 2014, 20, 382–390. [Google Scholar] [CrossRef]
- Feys, P.; Moumdjian, L.; Van Halewyck, F.; Wens, I.; Eijnde, B.O.; Van Wijmeersch, B.; Popescu, V.; Van Asch, P. Effects of an Individual 12-Week Community-Located “Start-to-Run” Program on Physical Capacity, Walking, Fatigue, Cognitive Function, Brain Volumes, and Structures in Persons with Multiple Sclerosis. Mult. Scler. J. 2019, 25, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Campanholo, K.; Pitombeira, M.; Rimkus, C.; Mendes, M.; Apóstolos-Pereira, S.; Busatto Filho, G.; Callegaro, D.; Buchpiguel, C.; Duran, F.; De Paula Faria, D. Myelin Imaging Measures as Predictors of Cognitive Impairment in MS Patients: A Hybrid PET-MRI Study. Mult. Scler. Relat. Disord. 2022, 57, 103331. [Google Scholar] [CrossRef] [PubMed]
- Pitombeira, M.S.; Koole, M.; Campanholo, K.R.; Souza, A.M.; Duran, F.L.S.; Solla, D.J.F.; Mendes, M.F.; Pereira, S.L.A.; Rimkus, C.M.; Busatto, G.F.; et al. Innate Immune Cells and Myelin Profile in Multiple Sclerosis: A Multi-Tracer PET/MR Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4551–4566. [Google Scholar] [CrossRef]
- Horton, M.; Rudick, R.A.; Hara-Cleaver, C.; Marrie, R.A. Validation of a Self-Report Comorbidity Questionnaire for Multiple Sclerosis. Neuroepidemiology 2010, 35, 83–90. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Rodrigues, J.d.L.; Ferreira, F.d.O.; Haase, V.G. Perfil do Desempenho Motor e Cognitivo na Idade Adulta e Velhice. Gerais Rev. Interinstitucional Psicol. 2008, 1, 20–33. Available online: https://pepsic.bvsalud.org/pdf/gerais/v1n1/v1n1a04.pdf (accessed on 6 November 2024).
- Damasceno, A.; Amaral, J.M.S.D.S.; Barreira, A.A.; Becker, J.; Callegaro, D.; Campanholo, K.R.; Damasceno, L.A.; Diniz, D.S.; Fragoso, Y.D.; Franco, P.S.; et al. Normative Values of the Brief Repeatable Battery of Neuropsychological Tests in a Brazilian Population Sample: Discrete and Regression–Based Norms. Arq. Neuropsiquiatr. 2018, 76, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.; Pereira, L.D. TRADUÇÃO, VALIDAÇÃO CULTURAL E SEMÂNTICA DO COGNITIVE RESERVE INDEX QUESTIONNAIRE (criq) PARA O PORTUGUÊS BRASILEIRO. Estud. Interdiscip. Sobre O Envelhec. 2022, 27. [Google Scholar] [CrossRef]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How Patients with Multiple Sclerosis Acquire Disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Tomic, D.; Bright, J.R.; Havrdová, E. “No Evident Disease Activity”: The Use of Combined Assessments in the Management of Patients with Multiple Sclerosis. Mult. Scler. J. 2017, 23, 1179–1187. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Rimkus, C.D.M.; Avolio, I.M.B.; Miotto, E.C.; Pereira, S.A.; Mendes, M.F.; Callegaro, D.; Leite, C.D.C. The Protective Effects of High-Education Levels on Cognition in Different Stages of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kania, K.; Pawlak, M.A.; Forycka, M.; Wiłkość-Dębczyńska, M.; Michalak, S.; Łukaszewska, A.; Wyciszkiewicz, A.; Wypych, A.; Serafin, Z.; Marcinkowska, J.; et al. Predicting Clinical Progression and Cognitive Decline in Patients with Relapsing-Remitting Multiple Sclerosis: A 6-Year Follow-up Study. Neurol. Neurochir. Pol. 2024, 58, 176–184. [Google Scholar] [CrossRef]
- Boa, I.N.F.; Rimkus, C.D.M.; Campanholo, K.R.; Pereira, S.L.A.; Junqueira, T.D.F.; Machado, M.D.A.R.; Callegaro, D.; Otaduy, M.C.G.; Leite, C.D.C.; Miotto, E.C. Longitudinal Analysis of Verbal Episodic Memory in Patients with Relapsing-Remitting Multiple Sclerosis. Arq. Neuropsiquiatr. 2018, 76, 302–309. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in Multiple Sclerosis: State of the Field and Priorities for the Future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Van Horssen, J.; Mahad, D. Progressive Multiple Sclerosis: Pathology and Pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Harding, K.E.; Clarkson, H.; Pickersgill, T.P.; Wardle, M.; Robertson, N.P. Demographic and Clinical Factors Associated with Changes in Employment in Multiple Sclerosis. Mult. Scler. J. 2013, 19, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Fantoni-Quinton, S.; Kwiatkowski, A.; Vermersch, P.; Roux, B.; Hautecoeur, P.; Leroyer, A. Impact of Multiple Sclerosis on Employment and Use of Job-Retention Strategies: The Situation in France in 2015. J. Rehabil. Med. 2016, 48, 535–540. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Chan, F.; Edward Sharp, S.; Dutta, A.; Hartman, E.; Bezyak, J. Employment as a Health Promotion Intervention for Persons with Multiple Sclerosis. Work 2015, 52, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Bøe Lunde, H.M.; Telstad, W.; Grytten, N.; Kyte, L.; Aarseth, J.; Myhr, K.-M.; Bø, L. Employment among Patients with Multiple Sclerosis-A Population Study. PLoS ONE 2014, 9, e103317. [Google Scholar] [CrossRef]
- Raggi, A.; Covelli, V.; Schiavolin, S.; Scaratti, C.; Leonardi, M.; Willems, M. Work-Related Problems in Multiple Sclerosis: A Literature Review on Its Associates and Determinants. Disabil. Rehabil. 2016, 38, 936–944. [Google Scholar] [CrossRef]
- Salter, A.; Thomas, N.; Tyry, T.; Cutter, G.; Marrie, R.A. Employment and Absenteeism in Working-Age Persons with Multiple Sclerosis. J. Med. Econ. 2017, 20, 493–502. [Google Scholar] [CrossRef]
- Da Silva, N.L.; Takemoto, M.L.S.; Damasceno, A.; Fragoso, Y.D.; Finkelsztejn, A.; Becker, J.; Gonçalves, M.V.M.; Tilbery, C.; De Oliveira, E.M.L.; Callegaro, D.; et al. Cost Analysis of Multiple Sclerosis in Brazil: A Cross-Sectional Multicenter Study. BMC Health Serv. Res. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Neate, S.; Nag, N.; Bevens, W.; Jelinek, G.; Simpson-Yap, S.; Davenport, R.A.; Fidao, A.; Reece, J. Baseline Engagement with Healthy Lifestyles and Their Associations with Health Outcomes in People with Multiple Sclerosis Enrolled in an Online Multimodal Lifestyle Course. Eur. J. Neurol. 2024, 31, e16429. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Skjerbæk, A.G.; Nørgaard, M.; Boesen, F.; Hvid, L.G.; Dalgas, U. Associations between Fatigue Impact and Lifestyle Factors in People with Multiple Sclerosis – The Danish MS Hospitals Rehabilitation Study. Mult. Scler. Relat. Disord. 2021, 50, 102799. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Prestipino, E.; Bellinvia, A.; Niccolai, C.; Razzolini, L.; Pastò, L.; Fratangelo, R.; Tudisco, L.; Fonderico, M.; Mattiolo, P.L.; et al. Cognitive Impairment in Multiple Sclerosis: An Exploratory Analysis of Environmental and Lifestyle Risk Factors. PLoS ONE 2019, 14, e0222929. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, K.L.J.; Jeng, B.; Sasaki, J.E.; Motl, R.W. Physical Activity and Sedentary Behavior Timing in Fatigued and Nonfatigued Adults With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2022, 103, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pandigama, D.H.; Wrigglesworth, J.; Owen, A.; Woods, R.L.; Chong, T.T.-J.; Orchard, S.G.; Shah, R.C.; Sheets, K.M.; McNeil, J.J.; et al. Lifestyle Enrichment in Later Life and Its Association With Dementia Risk. JAMA Netw. Open 2023, 6, e2323690. [Google Scholar] [CrossRef]

| Baseline | Follow-Up (7 Years) | p | ||||
|---|---|---|---|---|---|---|
| Age (years–median–IQR) | 38 | (35, 48) | 44 | (41, 54) | <0.001 † | |
| Years of education (median–IQR) | 11 | (11, 15) | 12 | (11, 15) | 0.720 † | |
| Sex | Female—n (%) | 22 (69%) | 16 RRMS + 6 PMS | 13 RRMS + 9 PMS | ||
| Race or ethnicity | White—n (%) | 22 (69%) | 13 RRMS + 9 PMS | - | ||
| Non-white—n (%) | 10 (31%) | 6 RRMS + 4 PMS | - | |||
| Occupation | Unskilled worker—n (%) | 5 (16%) | 1 (3%) | 0.072 †† | ||
| Skilled worker—n (%) | 2 (6%) | 3 (9%) | ||||
| Merchant/religious, musician—n (%) | 1 (3%) | 5 (16%) | ||||
| Micro-businessman, self-employed—n (%) | 4 (13%) | 4 (13%) | ||||
| Businessman—n (%) | 1 (3%) | 0 | ||||
| Housewife—n (%) | 2 (6%) | 0 | ||||
| Government beneficiary—n (%) | 8 (25%) | 14 (44%) | ||||
| Unemployed—n (%) | 9 (28%) | 5 (16%) | ||||
| Clinical | Phenotype—n (%) | 19 (59%) RRMS 13 (41%) PMS | 16 (50%) RRMS 16 (50%) PMS | 0.451 †† | ||
| Comorbidities number (median–IQR) | 1 | (1, 2) | 1 | (1, 2) | 0.565 † | |
| Current use of high-efficacy DMT—n (%) | 10 | (31%) | 15 | (47%) | 0.227 | |
| Disease duration (years–median–IQR) | 10 | (7, 15) | 17 | (13, 21) | <0.001 † | |
| Diagnose duration (years–median–IQR) | 4 | (2, 7) | 10 | (8, 13) | <0.001 † | |
| Relapses (median–IQR) | 4 | (2, 5) | 5 | (2, 6) | 0.001 † | |
| EDSS (median–IQR) | 3.5 | (2.5, 6.5) | 4.8 | (2.5, 6.5) | 0.021 ††† | |
| 9HPT (median–IQR) | 27.0 | (21.8, 34.2) | 24.2 | (20.8, 42.5) | <0.001 ††† | |
| 25FWT (median–IQR) | 7.2 | (5.3, 12.1) | 6.4 | (4.1, 180.0) | <0.001 ††† | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanholo, K.R.; Faria, G.A.S.; Pitombeira, M.S.; Apóstolos-Pereira, S.L.; Callegaro, D.; Buchpiguel, C.A.; de Paula Faria, D. Lifestyle, Cognition, and Disability Outcomes in Multiple Sclerosis: A Comprehensive Cohort Study. Sclerosis 2024, 2, 394-404. https://doi.org/10.3390/sclerosis2040026
Campanholo KR, Faria GAS, Pitombeira MS, Apóstolos-Pereira SL, Callegaro D, Buchpiguel CA, de Paula Faria D. Lifestyle, Cognition, and Disability Outcomes in Multiple Sclerosis: A Comprehensive Cohort Study. Sclerosis. 2024; 2(4):394-404. https://doi.org/10.3390/sclerosis2040026
Chicago/Turabian StyleCampanholo, Kenia R., Graziella A. S. Faria, Milena S. Pitombeira, Samira L. Apóstolos-Pereira, Dagoberto Callegaro, Carlos Alberto Buchpiguel, and Daniele de Paula Faria. 2024. "Lifestyle, Cognition, and Disability Outcomes in Multiple Sclerosis: A Comprehensive Cohort Study" Sclerosis 2, no. 4: 394-404. https://doi.org/10.3390/sclerosis2040026
APA StyleCampanholo, K. R., Faria, G. A. S., Pitombeira, M. S., Apóstolos-Pereira, S. L., Callegaro, D., Buchpiguel, C. A., & de Paula Faria, D. (2024). Lifestyle, Cognition, and Disability Outcomes in Multiple Sclerosis: A Comprehensive Cohort Study. Sclerosis, 2(4), 394-404. https://doi.org/10.3390/sclerosis2040026






