Isolated Myelopathy in Occult Breast Carcinoma with Negative Paraneoplastic Antibodies: A Case Report of a Rare Condition
Abstract
1. Introduction
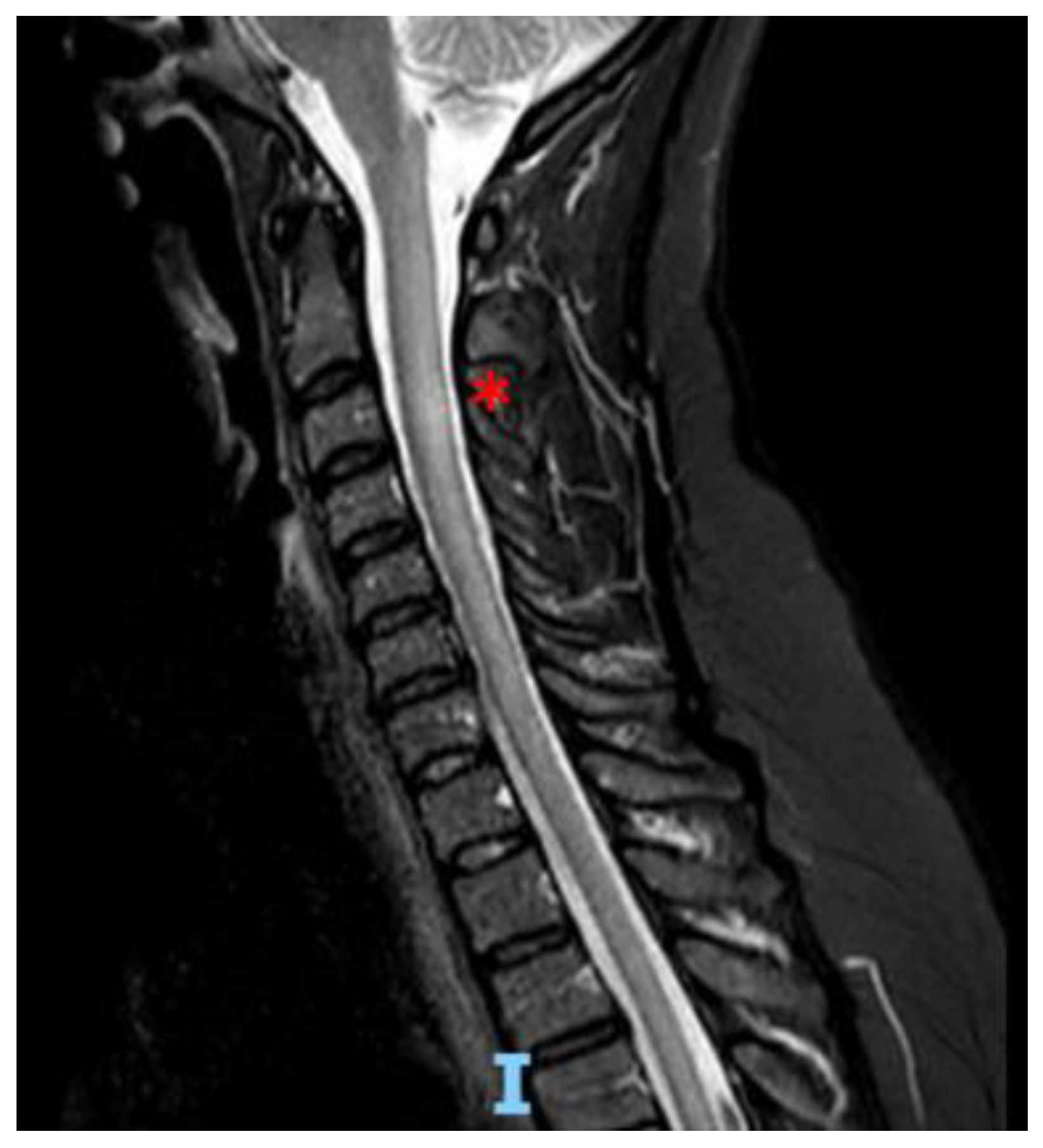
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Vecchio, D.; Virgilio, E.; Naldi, P.; Comi, C.; Cantello, R. MOG-antibody demyelinating diseases: A case of post-partum severe rhombencephalitis and transverse myelitis. Mult. Scler. Relat. Disord. 2018, 21, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; Vecchio, D.; Vercellino, M.; Naldi, P.; Tesser, F.; Cantello, R.; Cavalla, P.; Comi, C. Paraneoplastic neuromyelitis optica spectrum disorders: A case series. Neurol. Sci. 2021, 42, 2519–2522. [Google Scholar] [CrossRef] [PubMed]
- Cacciaguerra, L.; Sechi, E.; Rocca, M.A.; Filippi, M.; Pittock, S.J.; Flanagan, E.P. Neuroimaging features in inflammatory myelopathies: A review. Front. Neurol. 2022, 13, 993645. [Google Scholar] [CrossRef] [PubMed]
- Brinar, V.V.; Habek, M.; Brinar, M.; Malojcić, B.; Boban, M. The differential diagnosis of acute transverse myelitis. Clin. Neurol. Neurosurg. 2006, 108, 278–283. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Pittock, S.J.; Lennon, V.A. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology 2011, 76, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; McKeon, A.; Lennon, V.A.; Kearns, J.; Weinshenker, B.G.; Krecke, K.N.; Matiello, M.; Keegan, B.M.; Mokri, B.; Aksamit, A.J.; et al. Paraneoplastic isolated myelopathy: Clinical course and neuroimaging clues. Neurology 2011, 76, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Keegan, B.M. Paraneoplastic myelopathy. Neurol. Clin. 2013, 31, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol. Neuroimmunol. Neuroinflamm 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Keegan, B.M.; Pittock, S.J.; Lennon, V.A. Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann. Neurol. 2008, 63, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Dalmau, J. Paraneoplastic neurological syndromes. Curr. Opin. Neurol. 2012, 25, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Tirthani, E.; Said, M.S.; Smith, R.G.; Jadhav, N.; Shanina, E. Paraneoplastic Encephalomyelitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Flanagan, E.P. Autoimmune myelopathies. Handb. Clin. Neurol. 2016, 133, 327–351. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Delattre, J.Y.; Antoine, J.C.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.E. Treatment of paraneoplastic neurologic disorders. Curr. Treat. Options Neurol. 2010, 12, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Mahendraker, N.; Ramphul, K. Paraneoplastic Syndromes. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciolla, L.; Galli, G.; Vecchio, D.; Padelli, S.; Comi, C.; Cantello, R.; Virgilio, E. Isolated Myelopathy in Occult Breast Carcinoma with Negative Paraneoplastic Antibodies: A Case Report of a Rare Condition. Sclerosis 2023, 1, 60-66. https://doi.org/10.3390/sclerosis1010007
Paciolla L, Galli G, Vecchio D, Padelli S, Comi C, Cantello R, Virgilio E. Isolated Myelopathy in Occult Breast Carcinoma with Negative Paraneoplastic Antibodies: A Case Report of a Rare Condition. Sclerosis. 2023; 1(1):60-66. https://doi.org/10.3390/sclerosis1010007
Chicago/Turabian StylePaciolla, Loredana, Giulia Galli, Domizia Vecchio, Samuel Padelli, Cristoforo Comi, Roberto Cantello, and Eleonora Virgilio. 2023. "Isolated Myelopathy in Occult Breast Carcinoma with Negative Paraneoplastic Antibodies: A Case Report of a Rare Condition" Sclerosis 1, no. 1: 60-66. https://doi.org/10.3390/sclerosis1010007
APA StylePaciolla, L., Galli, G., Vecchio, D., Padelli, S., Comi, C., Cantello, R., & Virgilio, E. (2023). Isolated Myelopathy in Occult Breast Carcinoma with Negative Paraneoplastic Antibodies: A Case Report of a Rare Condition. Sclerosis, 1(1), 60-66. https://doi.org/10.3390/sclerosis1010007






