Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms
Abstract
1. Introduction
2. Pharmacological Applications of Glycyrrhizin
2.1. Effect on Different Inflammatory Conditions
2.1.1. Effect on HMGB1 Secretion and Its Cytokine Activities
2.1.2. Effect on Prostaglandin E2 Production
2.1.3. Effect on Neutrophil Functions and ROS Generation
2.1.4. Effect on Neutrophil-Induced Generation of Alternatively Activated Macrophages
2.1.5. Effect on Inflammatory Responses Mediated by Toll-like Receptors
2.1.6. Effect on Salmonella enterica Serovar Typhimurium-Induced Injury
2.1.7. Effect on Traumatic Spinal Cord Injury
2.1.8. Effect on Ischemia/Reperfusion Injury
2.1.9. Effect on Subarachnoid Hemorrhage and Inflammatory Brain Injury
2.1.10. Effect on Lipopolysaccharide-Induced Inflammation
2.1.11. Effect on Thermal and Burn Injury
2.1.12. Effect on Inflammation and Cytokine Expression
2.1.13. Effect on Inflammatory Pain
2.1.14. Effect on Radiation-Induced Tissue Damage
2.1.15. Effects Against Reproductive and Autoimmune Inflammatory Conditions
2.1.16. Effects Against Colitis and Mucosal Inflammation
2.1.17. Effect Against Granulomatous Inflammation
2.2. Effect of Glycyrrhizin on Central Nervous System
2.2.1. Effect Against Intracerebral Hemorrhage
2.2.2. Effects Against Neuronal Cell Damage
2.2.3. Effects Against Ischemia–Reperfusion-Induced Brain Injury
2.2.4. Effects Against Traumatic Brain Injury
2.2.5. Effects Against Post-Traumatic Stress Disorder
2.2.6. Effects Against Sciatic Nerve Injury
2.2.7. Effects Against Neuro-Toxicity
2.2.8. Effects Against Status Epilepticus and Seizures
2.2.9. Effects Against Neuroinflammation and Cognitive Deficits
2.2.10. Effects Against Subarachnoid Hemorrhage
2.2.11. Effects Against Anxiety and Stress
2.2.12. Effects on Intracellular Calcium Mobilization and Neuromuscular Transmission
2.2.13. Effects Against Virus-Born Neurological Disorders
2.2.14. Effect Against Neonatal Hypoxic–Ischemic Brain Damage
2.3. Effect of Glycyrrhizin on Respiratory System
2.3.1. Effect Against Severe Acute Respiratory Syndrome
2.3.2. Effect Against Upper Respiratory Tract Infections
2.3.3. Effect Against Acute Lung Injury
2.3.4. Effect Against Asthma and Chronic Obstructive Pulmonary Disease
2.3.5. Effects Against Nitrogen Species-Mediated Lung Cell Damage
2.3.6. Effects Against Allergic Rhinitis
2.4. Effect of Glycyrrhizin on Cardiovascular System
2.4.1. Anti-Hypertensive Effect
2.4.2. Anti-Thrombotic Effect
2.4.3. Effect Against Myocardial Ischemia–Reperfusion Induced Injury
2.4.4. Effect Against 3-Nitropropionic Acid-Induced Neurotoxicity
2.4.5. Effect Against Coronary Microembolization-Induced Myocardial Dysfunction
2.4.6. Effect Against Viral Myocarditis
2.4.7. Effect Against Doxorubicin-Induced Cardiotoxicity
2.5. Effect of Glycyrrhizin on Various Liver Injuries and Conditions
2.5.1. Effect on Hepatectomy and Liver Regeneration
2.5.2. Effect on Liver Fibrosis and Hepatic Stellate Cell Activation
2.5.3. Effect on Hepatotoxins-Induced Liver Injury
2.5.4. Effect on Acetaminophen (APAP)-Induced Liver Injury
2.5.5. Effect on LPS/D-Galactosamine and HMGB1-Mediated Liver Injury
2.5.6. Effect on Ischemia–Reperfusion Injury
2.5.7. Effect on Nonalcoholic Fatty Liver Disease and NASH
2.5.8. Effect on Ferroptosis
2.5.9. Effect on Bile Acid Regulation and PXR Activation
2.5.10. Effect on Viral Hepatitis and Related Conditions
2.5.11. Effect on Chronic Hepatitis B
2.5.12. Effect on Chronic Hepatitis C
2.5.13. Effect on Autoimmune, Drug-Induced, and Acute Hepatitis
2.5.14. Effect Against Hepatocellular Damage in Metabolic Syndrome
2.6. Effect of Glycyrrhizin on Urinary System
2.6.1. Effect on Acute Kidney Injury and Nephrotoxicity
2.6.2. Effect on Kidney Inflammation
2.6.3. Effect on Adriamycin-Induced Nephropathy and Glomerulosclerosis
2.6.4. Effect on Hemodialysis-Associated Oxidative Stress
2.6.5. Effect on Renal Disorders
2.7. Effect of Glycyrrhizin on Endocrine System
2.7.1. Effect on Pancreas and Its Function
2.7.2. Mineralocorticoid Activity
2.7.3. Anti-Androgen Activity
2.8. Effect of Glycyrrhizin on Skin and Skin-Related Disorders
2.8.1. Effect Against Psoriasis
2.8.2. Effect Against Alopecia Areata
2.8.3. Effect Against Eczema and Keloids
2.8.4. Effect Against Hyperpigmentation
2.8.5. Effect Against Vitiligo
2.8.6. Effect Against Facial Rosacea and Acne Vulgaris
2.9. Effect of Glycyrrhizin on Ocular Diseases
2.10. Effect of Glycyrrhizin on Metabolic Disorders
2.10.1. Effect on Obesity and Associated Complications
2.10.2. Effect on Diabetes and Associated Complications
2.11. Effect of Glycyrrhizin on Gastrointestinal System
2.12. Effect on Different Cancers and Tumors
2.13. Effect of Glycyrrhizin on Immune System
2.13.1. Effect on T-Cell Regulation, Apoptosis, and IL-2 Signaling
2.13.2. Effect on Macrophage Activation and Innate Immune Modulation
2.13.3. Effect on Immunocompromised Models
2.13.4. Effect on Dendritic Cell Maturation and Adaptive Immune Biasing
2.13.5. Effect on Antigen-Induced Histamine Release
2.13.6. Effect on Unique Regulatory T Cell Subtypes
2.13.7. Effect on Humoral Immune Response
2.14. Effect of Glycyrrhizin on Arthritis
2.14.1. Effect on Osteoarthritis
2.14.2. Effect on Rheumatoid Arthritis
2.14.3. Effect on Collagen-Induced Arthritis
2.14.4. Effect on Osteoclastogenesis and Osteoporosis
2.15. Effect of Glycyrrhizin on Various Microbial Pathogens
2.15.1. Effect on Bacterial Pathogens
2.15.2. Effect on Fungal Pathogens
2.15.3. Effect on Different Viruses
2.16. Other Pharmacological Activities of Glycyrrhizin
2.16.1. Effects on Reproductive Health
2.16.2. Effects in Aplastic Anemia
2.16.3. Effect Against Chemotherapy-Induced Toxicity
2.16.4. Effects in Precocious Puberty
2.16.5. Effect on Oral and Periodontal Health
2.16.6. Antivenom Activity
3. Toxicity Studies
4. Pharmacokinetic Studies
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HMGB1 | high-mobility group box 1 |
| Bcl2 | B-cell lymphoma 2 |
| Bax | Bcl-2-associated X protein |
| GSH | glutathione |
| SOD | superoxide dismutase |
| CAT | catalase |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| TNF-α | tumor necrosis factor-alpha |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| MAPK | mitogen-activated protein kinase |
| GL | glycyrrhizin |
| ROS | reactive oxygen species |
| LPS | lipopolysaccharide |
| PGE2 | prostaglandin E2 |
| STAT3 | signal transducer and activator of transcription 3 |
| I/R | ischemia–reperfusion |
References
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Nascimento, M.H.M.; de Araújo, D.R. Exploring the pharmacological potential of glycyrrhizic acid: From therapeutic applications to trends in nanomedicine. Future Pharmacol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, T.; Zang, C.; Xu, Z.; Sun, W.; Luo, H.; Yang, M.; Song, J.; Chen, S.; Yao, H. Glycyrrhiza, a commonly used medicinal herb: Review of species classification, pharmacology, active ingredient biosynthesis, and synthetic biology. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef]
- Ding, Y.; Brand, E.; Wang, W.; Zhao, Z. Licorice: Resources, applications in ancient and modern times. J. Ethnopharmacol. 2022, 298, 115594. [Google Scholar] [CrossRef]
- Taarji, N.; Bouhoute, M.; Fainassi, F.; Hafidi, A.; Kobayashi, I.; Neves, M.A.; Tominaga, K.; Isoda, H.; Nakajima, M. Interfacial and emulsifying properties of purified glycyrrhizin and non-purified glycyrrhizin-rich extracts from liquorice root (Glycyrrhiza glabra). Food Chem. 2021, 337, 127949. [Google Scholar] [CrossRef]
- Hennell, J.R.; Lee, S.; Khoo, C.S.; Gray, M.J.; Bensoussan, A. The determination of glycyrrhizic acid in Glycyrrhiza uralensis Fisch. ex DC. (Zhi Gan Cao) root and the dried aqueous extract by LC-DAD. J. Pharm. Biomed. Anal. 2008, 47, 494–500. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Analysis of Glycyrrhizin Binding to Protein HMGB1. Med. Drug Discov. 2020, 7, 100058. [Google Scholar] [CrossRef]
- Stecanella, L.A.; Bitencourt, A.P.R.; Vaz, G.R.; Quarta, E.; Silva Júnior, J.O.C.; Rossi, A. Glycyrrhizic acid and its hydrolyzed metabolite 18β-glycyrrhetinic acid as specific ligands for targeting nanosystems in the treatment of liver cancer. Pharmaceutics 2021, 13, 1792. [Google Scholar] [CrossRef]
- Schmid, C.; Brockhoff, A.; Shoshan-Galeczki, Y.B.; Kranz, M.; Stark, T.D.; Erkaya, R.; Meyerhof, W.; Niv, M.Y.; Dawid, C.; Hofmann, T. Comprehensive structure–activity–relationship studies of sensory active compounds in licorice (Glycyrrhiza glabra). Food Chem. 2021, 364, 130420. [Google Scholar]
- Fatima, I.; Sahar, A.; Tariq, A.; Naz, T.; Usman, M. Exploring the role of licorice and its derivatives in cell signaling pathway NF-κB and MAPK. J. Nutr. Metab. 2024, 2024, 9988167. [Google Scholar]
- Wang, Q.; Song, G.C.; Weng, F.Y.; Zou, B.; Jin, J.Y.; Yan, D.M.; Tan, B.; Zhao, J.; Li, Y.; Qiu, F.R. Hepatoprotective effects of glycyrrhetinic acid on lithocholic acid-induced cholestatic liver injury through choleretic and anti-inflammatory mechanisms. Front. Pharmacol. 2022, 13, 881231. [Google Scholar]
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A review of the antiviral activities of glycyrrhizic acid, glycyrrhetinic acid and glycyrrhetinic acid monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sheng, Z.; Xiao, J.; Li, Y.; Huang, J.; Jia, J.; Zeng, X.; Li, L. Advances in the roles of glycyrrhizic acid in cancer therapy. Front. Pharmacol. 2023, 14, 1265172. [Google Scholar] [PubMed]
- Sakamoto, R.; Okano, M.; Takena, H.; Ohtsuki, K. Inhibitory effect of glycyrrhizin on the phosphorylation and DNA-binding abilities of high mobility group proteins 1 and 2 in vitro. Biol. Pharm. Bull. 2001, 24, 906–911. [Google Scholar] [CrossRef]
- Kato, T.; Horie, N.; Hashimoto, K.; Satoh, K.; Shimoyama, T.; Kaneko, T.; Kusama, K.; Sakagami, H. Bimodal effect of glycyrrhizin on macrophage nitric oxide and prostaglandin E2 production. In Vivo 2008, 22, 583–586. [Google Scholar]
- Wang, H.-L.; Li, Y.-X.; Niu, Y.-T.; Zheng, J.; Wu, J.; Shi, G.-J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.-Q. Observing anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in mice. Inflammation 2015, 38, 2269–2278. [Google Scholar]
- Okimasu, E.; Moromizato, Y.; Watanabe, S. Inhibition of phospholipase A2 and platelet aggregation by glycyrrhizin, an anti-inflammation drug. Acta Med. Okayama 1983, 37, 385–391. [Google Scholar]
- Takei, M.; Kobayashi, M.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Glycyrrhizin inhibits the manifestations of anti-inflammatory responses that appear in association with systemic inflammatory response syndrome (SIRS)-like reactions. Cytokine 2006, 35, 295–301. [Google Scholar]
- Ozaki, Y.; Ono, K. Antiinflammatory effect of glycyrrhizin topically applied to the cotton pellet by granuloma porch method in rats. Nat. Med. 2002, 56, 261–263. [Google Scholar]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-Y.; Zhao, F.; Deng, S.-X.; Zhu, H.-C.; Gong, Y.; Wang, W. Glycyrrhizin affects monocyte migration and apoptosis by blocking HMGB1 signaling. Mol. Med. Rep. 2018, 17, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Fang, Y.; Li, X.; Shen, L.; Cao, T.; Zhu, H. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit. Care 2013, 17, R44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Fang, Y.; Deng, S.; Li, X.; Zhou, Y.; Gong, Y.; Zhu, H.; Wang, W. Glycyrrhizin protects rats from sepsis by blocking HMGB1 signaling. Biomed Res. Int. 2017, 2017, 971964. [Google Scholar] [CrossRef]
- Wu, C.-X.; He, L.-X.; Guo, H.; Tian, X.-X.; Liu, Q.; Sun, H. Inhibition effect of glycyrrhizin in lipopolysaccharide-induced high-mobility group box 1 releasing and expression from RAW264.7 cells. Shock 2015, 43, 412–421. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, H.J.; Chang, K.C. Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int. Immunopharmacol. 2015, 26, 112–118. [Google Scholar] [CrossRef]
- Li, C.; Peng, S.; Liu, X.; Han, C.; Wang, X.; Jin, T.; Liu, S.; Wang, W.; Xie, X.; He, X.; et al. Glycyrrhizin, a direct HMGB1 antagonist, ameliorates inflammatory infiltration in a model of autoimmune thyroiditis via inhibition of TLR2-HMGB1 signaling. Thyroid 2017, 27, 722–731. [Google Scholar] [CrossRef]
- Ohuchi, K.; Kamada, Y.; Levine, L.; Tsurufuji, S. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med. 1981, 7, 457–463. [Google Scholar] [CrossRef]
- Akamatsu, H.; Komura, J.; Asada, Y.; Niwa, Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991, 57, 119–121. [Google Scholar] [CrossRef]
- Yoshida, T.; Tsuda, Y.; Takeuchi, D.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Glycyrrhizin inhibits neutrophil-associated generation of alternatively activated macrophages. Cytokine 2006, 33, 317–322. [Google Scholar] [CrossRef]
- Schröfelbauer, B.; Raffetseder, J.; Hauner, M.; Wolkerstorfer, A.; Ernst, W.; Szolar, O.H.J. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signaling. Biochem. J. 2009, 421, 473–482. [Google Scholar] [CrossRef]
- Xu, X.; Gong, L.; Wang, B.; Wu, Y.; Wang, Y.; Mei, X.; Xu, H.; Tang, L.; Liu, R.; Zeng, Z.; et al. Glycyrrhizin attenuates Salmonella enterica serovar typhimurium infection: New insights into its protective mechanism. Front. Immunol. 2018, 9, 2321. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-Q.; Wang, X.-Y.; Gong, F.-T.; Feng, M.; Bai, J.-J.; Zhang, R.-R.; Dang, X.-Q. Oral treatment with glycyrrhizin inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization after traumatic spinal cord injury. Brain Res. Bull. 2020, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Menegazzi, M.; Mazzon, E.; Crisafulli, C.; Di Paola, R.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin reduces secondary inflammatory process after spinal cord compression injury in mice. Shock 2009, 31, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Takaoka, K. Use of glycyrrhizin in prevention of tissue damage caused by ischemia-reperfusion in rabbit hind limbs. J. Orthop. Sci. 2006, 11, 375–379. [Google Scholar] [CrossRef]
- Xiao, R.-F.; Deng, Z.-L.; Lu, W.-Z.; Chen, F. Effect of compound glycyrrhizin on ischemia/reperfusion injury in the skeletal muscle of rabbits. J. Clin. Rehabil. Tissue Eng. Res. 2009, 13, 3843–3847. [Google Scholar]
- Chen, K.; Yang, R.; Shen, F.Q.; Zhu, H.L. Advances in Pharmacological Activities and Mechanisms of Glycyrrhizic Acid. Curr. Med. Chem. 2020, 27, 6219–6243. [Google Scholar] [CrossRef]
- Ieong, C.; Sun, H.; Wang, Q.; Ma, J. Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates inflammative effect after acute subarachnoid hemorrhage in rat model. J. Clin. Neurosci. 2018, 47, 278–284. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.-I.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc. Biol. 2012, 91, 967–976. [Google Scholar] [CrossRef]
- Liu, J.; Ma, B.; Hao, G.; Su, D.D.; Wang, T.; Ding, Z.; Guo, X. Glycyrrhizin inhibits lps-induced inflammatory responses in goat ruminal epithelial cells in vitro. BMC Mol. Cell Biol. 2023, 24, 28. [Google Scholar] [CrossRef]
- Shen, L.; Cui, Z.; Lin, Y.; Wang, S.; Zheng, D.; Tan, Q. Anti-inflammative effect of glycyrrhizin on rat thermal injury via inhibition of high-mobility group box 1 protein. Burns 2015, 41, 372–378. [Google Scholar] [CrossRef]
- Ueki, R.; Liu, L.; Kashiwagi, S.; Kaneki, M.; Khan, M.A.S.; Hirose, M.; Tompkins, R.G.; Martyn, J.A.J.; Yasuhara, S. Role of elevated fibrinogen in burn-induced mitochondrial dysfunction: Protective effects of glycyrrhizin. Shock 2016, 46, 382–389. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, E.; Wei, Z.; Liang, D.; Wang, W.; Wang, T.; Guo, M.; Zhang, N.; Yang, Z. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014, 281, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lv, H.; Shi, B.-H.; Hou, X.; Xu, X. Inhibition of IL-6 and IL-8 production in LPS-stimulated human gingival fibroblasts by glycyrrhizin via activating LXRα. Microb. Pathog. 2017, 110, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, E.; Wei, Z.; Song, X.; Liu, Z.; Wang, T.; Wang, W.; Zhang, N.; Liu, G.; Yang, Z. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-κB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhong, J.-Y.; Gao, E.-N.; Yang, H. Effects of combination of glycyrrhizin acid, ligustrazine and puerarin on LPS-induced cytokines expression in macrophage. Zhongguo Zhongyao Zazhi 2015, 40, 4068–4074. [Google Scholar] [PubMed]
- Zhang, X.-M.; Hu, X.; Ou, J.-Y.; Chen, S.-S.; Nie, L.-H.; Gao, L.; Zhu, L.-L. Glycyrrhizin ameliorates radiation enteritis in mice accompanied by the regulation of the HMGB1/TLR4 pathway. Evid.-Based Complement. Altern. Med. 2020, 2020, 8653783. [Google Scholar] [CrossRef]
- Zhou, X.-R.; Wang, X.-Y.; Sun, Y.-M.; Zhang, C.; Liu, K.J.; Zhang, F.-Y.; Xiang, B. Glycyrrhizin protects submandibular gland against radiation damage by enhancing antioxidant defense and preserving mitochondrial homeostasis. Antioxid. Redox Signal. 2024, 41, 723–743. [Google Scholar] [CrossRef]
- Wang, X.-R.; Hao, H.-G.; Chu, L. Glycyrrhizin inhibits LPS-induced inflammatory mediator production in endometrial epithelial cells. Microb. Pathog. 2017, 109, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Asano, Y.; Taniguchi, T.; Nakamura, K.; Saigusa, R.; Miura, S.; Toyama, T.; Takahashi, T.; Ichimura, Y.; Yoshizaki, A.; et al. Glycyrrhizin ameliorates fibrosis, vasculopathy, and inflammation in animal models of systemic sclerosis. J. Investig. Dermatol. 2017, 137, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, X.; Xing, J.; Han, K.; Sun, Y. Glycyrrhizin potentially suppresses the inflammatory response in preeclampsia rat model. Pregnancy Hypertens. 2021, 23, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, W.; Chen, W.; Zhu, J.; Sun, Q. Influence of compound glycyrrhizin on NF-κB and STAT3 signal transduction pathway in murine experimental colitis. Chin. J. Gastroenterol. 2011, 16, 86–89. [Google Scholar]
- Kudo, T.; Okamura, S.; Zhang, Y.; Masuo, T.; Mori, M. Topical application of glycyrrhizin preparation ameliorates experimentally induced colitis in rats. World J. Gastroenterol. 2011, 17, 2223–2228. [Google Scholar] [CrossRef]
- Xu, J.; Du, Y.; Lu, G.; Yang, Y.; Zhang, S.; Zhou, C.; Lü, F. Effect of compound glycyrrhizin on intestinal inflammation in rats with TNBS-induced experimental colitis. Chin. J. Gastroenterol. 2013, 18, 221–224. [Google Scholar]
- Chen, X.; Fang, D.; Li, L.; Chen, L.; Li, Q.; Gong, F.; Fang, M. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017, 65, 666–680. [Google Scholar] [CrossRef]
- Sethuraman, S.N.; Swaminathan, S.; Nelson, S.B.; Palaninathan, P.S.; Gopalan, T.K.; Velayudham, P. Modulation of PPARγ and TNFα by emu oil and glycyrrhizin in ulcerative colitis. Inflammopharmacology 2015, 23, 47–56. [Google Scholar] [CrossRef]
- Pisanty, S.; Azaz, E.; Segal, R. Glycyrrhizin as a vehicle for the application of triamcynolone in the treatment of recurrent aphthous stomatitis. Pharm. Acta Helv. 1984, 59, 341–344. [Google Scholar]
- Matsushima, Y.; Baba, T. An antigranulomatous effect of glycyrrhizin. J. Exp. Pathol. 1992, 6, 25–30. [Google Scholar] [CrossRef]
- Ohnishi, M.; Katsuki, H.; Fukutomi, C.; Takahashi, M.; Motomura, M.; Fukunaga, M.; Matsuoka, Y.; Isohama, Y.; Izumi, Y.; Kume, T.; et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology 2011, 61, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Youn, Y.C.; Kim, Y.K.; Hong, K.M.; Lee, C.S. Glycyrrhizin prevents 7-ketocholesterol toxicity against differentiated PC12 cells by suppressing mitochondrial membrane permeability change. Neurochem. Res. 2009, 34, 1433–1442. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Haredy, S.A.; Niazy, R.M.; Linhardt, R.J.; Warda, M. Dose-dependent neuroprotective effect of oriental phyto-derived glycyrrhizin on experimental neuroterminal norepinephrine depletion in a rat brain model. Chem. Biol. Interact. 2019, 308, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Egashira, T.; Takayama, F.; Wada, Y.; Yamanaka, Y.; Oda, S. Effects of glycyrrhizin on lipid peroxidation in rat brain following transient middle cerebral artery occlusion. Jpn. Pharmacol. Ther. 1994, 22, 19–27. [Google Scholar]
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE 2014, 9, e89450. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Weng, Z.; Zhou, T.; Feng, T.; Lin, Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014, 1582, 176–186. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Zhao, B.T.; Woo, M.H.; Jung, H.A.; Choi, J.S. Comparative study of selective in vitro and in silico BACE1 inhibitory potential of glycyrrhizin together with its metabolites, 18α- and 18β-glycyrrhetinic acid, isolated from Hizikia fusiformis. Arch. Pharm. Res. 2018, 41, 409–418. [Google Scholar] [CrossRef]
- Xiong, X.; Gu, L.; Wang, Y.; Luo, Y.; Zhang, H.; Lee, J.; Krams, S.; Zhu, S.; Zhao, H. Glycyrrhizin protects against focal cerebral ischemia via inhibition of T cell activity and HMGB1-mediated mechanisms. J. Neuroinflamm. 2016, 13, 241. [Google Scholar] [CrossRef]
- Chen, H.; Guan, B.; Wang, B.; Pu, H.; Bai, X.; Chen, X.; Liu, J.; Li, C.; Qiu, J.; Yang, D.; et al. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl. Stroke Res. 2020, 11, 967–982. [Google Scholar] [CrossRef]
- Mu, S.-W.; Dang, Y.; Fan, Y.-C.; Zhang, H.; Zhang, J.-H.; Wang, W.; Wang, S.-S.; Gu, J.-J. Effect of HMGB1 and RAGE on brain injury and the protective mechanism of glycyrrhizin in intracranial-sinus occlusion followed by mechanical thrombectomy recanalization. Int. J. Mol. Med. 2019, 44, 813–822. [Google Scholar] [CrossRef]
- Xiangjin, G.; Jin, X.; Banyou, M.; Gong, C.; Peiyuan, G.; Dong, W.; Weixing, H. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin. J. Traumatol. Engl. Ed. 2014, 17, 1–7. [Google Scholar]
- Okuma, Y.; Liu, K.; Wake, H.; Liu, R.; Nishimura, Y.; Hui, Z.; Teshigawara, K.; Haruma, J.; Yamamoto, Y.; Yamamoto, H.; et al. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction. Neuropharmacology 2014, 85, 18–26. [Google Scholar] [CrossRef]
- Guo, J.; Yang, C.X.; Yang, J.J.; Yao, Y. Glycyrrhizic acid ameliorates cognitive impairment in a rat model of vascular dementia associated with oxidative damage and inhibition of voltage-gated sodium channels. CNS Neurol. Disord. Drug Targets 2016, 15, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wu, G.; Jiang, Z. Glycyrrhizin treatment facilitates extinction of conditioned fear responses after a single prolonged stress exposure in rats. Cell. Physiol. Biochem. 2018, 45, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Shi, L.; Jiang, Z.; Lin, Z. Glycyrrhizin treatment ameliorates post-traumatic stress disorder-like behaviours and restores circadian oscillation of intracranial serotonin. Clin. Exp. Pharmacol. Physiol. 2020, 47, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-X.; Li, J.-R.; Mao, C.-Y.; Yin, W.-T.; Jiang, R.-H. Glycyrrhizin improves p75NTR-associated sciatic nerve regeneration in a BALB/c mouse model. Exp. Ther. Med. 2014, 7, 1141–1146. [Google Scholar] [CrossRef]
- Santoro, M.; Maetzler, W.; Stathakos, P.; Martin, H.L.; Hobert, M.A.; Rattay, T.W.; Gasser, T.; Forrester, J.V.; Berg, D.; Tracey, K.J.; et al. In-vivo evidence that high mobility group box 1 exerts deleterious effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and Parkinson’s disease which can be attenuated by glycyrrhizin. Neurobiol. Dis. 2016, 91, 59–68. [Google Scholar] [CrossRef]
- Ren, C.A.; Li, Y.X.; Cui, J.Y.; Sheng, Z.X.; Ran, X.H.; Wang, B.H.; Zhang, M.H. Efficacy of glycyrrhizin combined with cyclosporine in the treatment of non-severe aplastic anemia. Chin. Med. J. 2013, 126, 2083–2086. [Google Scholar]
- Kim, Y.J.; Lee, C.S. Glycyrrhizin attenuates MPTP neurotoxicity in mouse and MPP⁺-induced cell death in PC12 cells. Korean J. Physiol. Pharmacol. 2008, 12, 65–71. [Google Scholar]
- Boshra, S.A.; El-Haddad, A.E. The protective effects of MPLC isolated glycyrrhizin and mangiferin against brain toxicity in rats. Med. Chem. Res. 2018, 27, 1449–1459. [Google Scholar] [CrossRef]
- González-Reyes, S.; Santillán-Cigales, J.J.; Jiménez-Osorio, A.S.; Pedraza-Chaverri, J.; Guevara-Guzmán, R. Glycyrrhizin ameliorates oxidative stress and inflammation in hippocampus and olfactory bulb in lithium/pilocarpine-induced status epilepticus in rats. Epilepsy Res. 2016, 126, 126–133. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wang, L.; Zhang, B.; Gao, F.; Yang, C.-M. Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects in rats after lithium-pilocarpine-induced status epilepticus. J. Pharm. Pharmacol. 2019, 71, 390–399. [Google Scholar] [CrossRef]
- Luo, L.; Jin, Y.; Kim, I.-D.; Lee, J.-K. Glycyrrhizin suppresses HMGB1 inductions in the hippocampus and subsequent accumulation in serum of a kainic acid-induced seizure mouse model. Cell. Mol. Neurobiol. 2014, 34, 987–997. [Google Scholar] [CrossRef]
- Wei, L.; Ou, S.; Meng, Y.; Sun, L.; Zhang, L.; Lu, Y.; Wu, Y. Glycyrrhizin as a potential disease-modifying therapy for epilepsy: Insights into targeting pyroptosis to exert neuroprotective and anticonvulsant effects. Front. Pharmacol. 2025, 15, 1530735. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Khan, S.U.; Othman, I.; Shaikh, M.F. Naturally occurring HMGB1 inhibitor, glycyrrhizin, modulates chronic seizures-induced memory dysfunction in zebrafish model. ACS Chem. Neurosci. 2021, 12, 3288–3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, X.; Zhang, J.; Zhao, Y.; Li, S.; Tan, L.; Gao, J.; Fang, X.; Luo, A. Glycyrrhizin attenuates isoflurane-induced cognitive deficits in neonatal rats via its anti-inflammatory activity. Neuroscience 2016, 316, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Lee, J.-W.; Shim, B.; Lee, C.-Y.; Choi, S.; Kang, C.; Sohn, N.-W.; Shin, J.-W. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules 2013, 18, 15788–15803. [Google Scholar] [CrossRef]
- Kong, Z.-H.; Chen, X.; Hua, H.-P.; Liang, L.; Liu, L.-J. The oral pretreatment of glycyrrhizin prevents surgery-induced cognitive impairment in aged mice by reducing neuroinflammation and Alzheimer’s-related pathology via HMGB1 inhibition. J. Mol. Neurosci. 2017, 63, 385–395. [Google Scholar] [CrossRef]
- Sun, Q.; Li, L.; Li, J.; Li, S.-Y.; Zhang, Y.; Chen, X.-S.; Liu, S.-S.; Hua, Z.-Y. Glycyrrhizin alleviates brain injury in necrotizing enterocolitis model mice by suppressing HMGB1/TLR4 pathway. Int. Immunopharmacol. 2025, 150, 114294. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, M.; Zhang, L.; Zhang, H.; Xu, Z.; Xu, Z. Glycyrrhizin regulates the HMGB1/P38MAPK signalling pathway in status epilepticus. Mol. Med. Rep. 2023, 27, 45. [Google Scholar] [CrossRef]
- Chang, C.-Z.; Wu, S.-C.; Kwan, A.-L. Glycyrrhizin attenuates toll like receptor-2, -4 and experimental vasospasm in a rat model. J. Immunol. Res. 2014, 2014, 740549. [Google Scholar] [CrossRef]
- Li, Y.; Sun, F.; Jing, Z.; Wang, X.; Hua, X.; Wan, L. Glycyrrhizic acid exerts anti-inflammatory effect to improve cerebral vasospasm secondary to subarachnoid hemorrhage in a rat model. Neurol. Res. 2017, 39, 727–732. [Google Scholar] [CrossRef]
- Patidar, G.; Shaikh, A. Antistress potential of glycyrrhizin in chronic immobilization stress. Biomed. Pharmacol. J. 2012, 5, 273–283. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J. Pharmacol. 2005, 37, 390–394. [Google Scholar] [CrossRef]
- Kimura, M.; Kimura, I.; Nojima, H. Depolarizing neuromuscular blocking action induced by electropharmacological coupling in the combined effect of paeoniflorin and glycyrrhizin. Jpn. J. Pharmacol. 1985, 37, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K.; Kimura, I.; Miyahara, K.; Kimura, M. Complementary effects of paeoniflorin and glycyrrhizin on intracellular Ca2+ mobilization in the nerve-stimulated skeletal muscle of mice. Jpn. J. Pharmacol. 1995, 69, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, T.; Yanagi, K.; Itoyama, Y. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. 2001, 45, 51–54. [Google Scholar]
- Li, J.; Shi, J.; Sun, Y.; Zheng, F. Glycyrrhizin, a potential drug for autoimmune encephalomyelitis by inhibiting high-mobility group box 1. DNA Cell Biol. 2018, 37, 941–946. [Google Scholar] [CrossRef]
- Fujieda, M.; Hamada, F.; Nomura, I.; Morita, H.; Wakiguchi, H.; Kurashige, T.; Ogura, H. Subacute sclerosing panencephalitis: Clinical improvement after treatment with intrathecal interleukin-2 and intravenous high dose glycyrrhizin. Jpn. J. Clin. Immunol. 1992, 15, 201–207. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, X.; Liu, S.; Yu, J.; Wu, S.; Hei, M. Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting ferroptosis and neuroinflammation in neonatal rats via the HMGB1/GPX4 pathway. Oxid. Med. Cell. Longev. 2022, 2022, 8438528. [Google Scholar] [CrossRef]
- Yu, Z.; Ohtaki, Y.; Kai, K.; Sasano, T.; Shimauchi, H.; Yokochi, T.; Takada, H.; Sugawara, S.; Kumagai, K.; Endo, Y. Critical roles of platelets in lipopolysaccharide-induced lethality: Effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome. Int. Immunopharmacol. 2005, 5, 571–580. [Google Scholar] [CrossRef]
- Gu, J.; Ran, X.; Deng, J.; Zhang, A.; Peng, G.; Du, J.; Wen, D.; Jiang, B.; Xia, F. Glycyrrhizin alleviates sepsis-induced acute respiratory distress syndrome via suppressing of HMGB1/TLR9 pathways and neutrophils extracellular traps formation. Int. Immunopharmacol. 2022, 108, 108730. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Ogura, M.; Fujimoto, E.; Shono, S.; Okuda, E. Effects and cost of glycyrrhizin in the treatment of upper respiratory tract infections in members of the Japanese maritime self-defense force: Preliminary report of a prospective, randomized, double-blind, controlled, parallel-group, alternate-day treatment assignment clinical trial. Curr. Ther. Res. Clin. Exp. 2004, 65, 26–33. [Google Scholar]
- Zhang, J.-F.; Li, C.-Q.; Mo, J.-J.; Peng, W.; Chen, W.; Su, S.-B. Effects of glycyrrhizin on the expressions of glucocorticoid receptor and NF-κB in lung of rat with acute lung injury. Chin. J. Emerg. Med. 2010, 19, 245–249. [Google Scholar]
- Ni, Y.-F.; Kuai, J.-K.; Lu, Z.-F.; Yang, G.-D.; Fu, H.-Y.; Wang, J.; Tian, F.; Yan, X.-L.; Zhao, Y.-C.; Wang, Y.-J.; et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J. Surg. Res. 2011, 165, e29–e35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, S.H.; Kim, J.Y.; Lee, W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J. Thorac. Dis. 2019, 11, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Hu, Z.; Li, Z.; Fu, Z. Protective effect of compound glycyrrhizin on rat lung injury induced by lipopolysaccharide. Med. J. Wuhan Univ. 2012, 33, 627–634. [Google Scholar]
- Seo, E.-H.; Song, G.-Y.; Kwak, B.O.; Oh, C.-S.; Lee, S.H.; Kim, S.-H. Effects of glycyrrhizin on the differentiation of myeloid cells of the heart and lungs in lipopolysaccharide-induced septic mice. Shock 2017, 48, 371–376. [Google Scholar] [CrossRef]
- Wang, J.; Ren, C.; Bi, W.; Batu, W. Glycyrrhizin mitigates acute lung injury by inhibiting the NLRP3 inflammasome in vitro and in vivo. J. Ethnopharmacol. 2023, 303, 115948. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Z.; Tian, J.; Liu, T.; Zhou, H. Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway to reduce lipopolysaccharide-induced acute lung injury by inhibiting TLR2. J. Cell. Physiol. 2019, 234, 4597–4607. [Google Scholar] [CrossRef]
- Yao, L.; Sun, T. Glycyrrhizin administration ameliorates Streptococcus aureus-induced acute lung injury. Int. Immunopharmacol. 2019, 70, 504–511. [Google Scholar] [CrossRef]
- Fei, L.; Jifeng, F.; Tiantian, W.; Yi, H.; Linghui, P. Glycyrrhizin ameliorates ischemia-reperfusion lung injury through downregulation of TLR2 signaling cascade in alveolar macrophages. Front. Pharmacol. 2017, 8, 389. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, Q.; Xu, C.; Li, M.; Li, H.; Yi, P.-Q.; Xu, F.-F.; Cao, L.; Chen, J.-Y. Glycyrrhizin mitigates radiation-induced acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway. J. Cell. Mol. Med. 2020, 24, 214–226. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, Z.; Li, Y.; Wang, Y.; Wan, Y.; Chen, X.; Xu, Y.; Ge, L.; Li, H. Glycyrrhizin alleviates radiation-induced lung injury by regulating the NLRP3 inflammasome through endoplasmic reticulum stress. Toxicol. Res. 2024, 13, tfae009. [Google Scholar] [CrossRef]
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008, 58, 22–31. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Y.; Wang, B.; Liu, L.; Chen, Y.; Chen, C.; Feng, L.; Chen, J.; Dong, N. Glycyrrhizin protects against particulate matter-induced lung injury via regulation of endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis through the Nrf2/HO-1/NQO1 signaling pathway. Int. Immunopharmacol. 2023, 120, 110371. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Bagriyanik, A.; Uzuner, N. Glycyrrhizin and long-term histopathologic changes in a murine model of asthma. Curr. Ther. Res. Clin. Exp. 2011, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhou, X. Glycyrrhizin inhibits human neutrophil elastase-induced MUC5AC overproduction in human bronchial epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014, 39, 252–257. [Google Scholar] [PubMed]
- Lee, H.J.; Lee, S.Y.; Bae, H.S.; Kim, J.-H.; Chang, G.T.; Seok, J.H.; Lee, C.J. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine 2011, 18, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhou, X.-D.; Kolosov, V.P.; Perelman, J.M. Effects of glycyrrhizin on airway mucus hypersecretion induced by interleukin-13 in rats. Natl. Med. J. China 2013, 93, 2225–2229. [Google Scholar]
- Nishimoto, Y.; Hisatsune, A.; Katsuki, H.; Miyata, T.; Yokomizo, K.; Isohama, Y. Glycyrrhizin attenuates mucus production by inhibition of MUC5AC mRNA expression in vivo and in vitro. J. Pharmacol. Sci. 2010, 113, 76–83. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Komasawa, N.; Miyazaki, S.; Seno, H.; Minami, T. Glycyrrhizin administration for inhibition of mucus production during one-lung ventilation. J. Clin. Anesth. 2014, 26, 584. [Google Scholar] [CrossRef]
- Fu, J.-L.; Wang, Y.-R.; Zhou, Y.; Liu, P. Upregulation effect of glycyrrhizin on secretory component expression in Caco-2 cells. Afr. J. Pharm. Pharmacol. 2011, 5, 1567–1572. [Google Scholar] [CrossRef]
- Takei, H.; Baba, Y.; Hisatsune, A.; Katsuki, H.; Miyata, T.; Yokomizo, K.; Isohama, Y. Glycyrrhizin inhibits interleukin-8 production and nuclear factor-κB activity in lung epithelial cells, but not through glucocorticoid receptors. J. Pharmacol. Sci. 2008, 106, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Hou, Y.; Yang, Y.; Bai, G. Protective effects of glycyrrhizin against β2-adrenergic receptor agonist-induced receptor internalization and cell apoptosis. Biol. Pharm. Bull. 2011, 34, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Sun, J.; You, W.; Wei, Y.; Tian, H.; Jiang, S. Glycyrrhizin suppresses epithelial–mesenchymal transition by inhibiting high-mobility group box 1 via the TGF-β1/Smad2/3 pathway in lung epithelial cells. PeerJ 2020, 8, e8514. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, Y.J.; Han, E.S. Glycyrrhizin protection against 3-morpholinosydnonimine-induced mitochondrial dysfunction and cell death in lung epithelial cells. Life Sci. 2007, 80, 1759–1767. [Google Scholar] [CrossRef]
- Cavone, L.; Cuppari, C.; Manti, S.; Grasso, L.; Arrigo, T.; Calamai, L.; Salpietro, C.; Chiarugi, A. Increase in the level of proinflammatory cytokine HMGB1 in nasal fluids of patients with rhinitis and its sequestration by glycyrrhizin induces eosinophil cell death. Clin. Exp. Otorhinolaryngol. 2015, 8, 123–128. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhou, A.-G. Evaluation of the immunity activity of glycyrrhizin in AR mice. Molecules 2012, 17, 716–727. [Google Scholar] [CrossRef]
- Li, H.; Guo, D.; Zhang, L.; Feng, X. Glycyrrhizin attenuates histamine-mediated muc5ac upregulation, inflammatory cytokine production, and aquaporin 5 downregulation through suppressing the NF-κB pathway in human nasal epithelial cells. Chem. Biol. Interact. 2018, 285, 21–26. [Google Scholar] [CrossRef]
- Parisella, M.L.; Angelone, T.; Gattuso, A.; Cerra, M.C.; Pellegrino, D. Glycyrrhizin and glycyrrhetinic acid directly modulate rat cardiac performance. J. Nutr. Biochem. 2012, 23, 69–75. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Y.; Li, C.; Yang, S.; Yu, J. Mechanism of glycyrrhizin in the treatment of chicken embryo allantoic cavity artery vasospasm. Int. J. Clin. Exp. Med. 2017, 10, 10079–10090. [Google Scholar]
- Wu, P.; Zhang, Y.; Liu, Y.; Wang, X.; Guo, Z.; Zhang, Y.; Liang, X.; Lai, W. Effects of glycyrrhizin on production of vascular aldosterone and corticosterone. Horm. Res. 1999, 51, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-S.; Kim, D.-H.; Lee, Y.J.; Lee, S.-E.; Kang, W.J.; Chang, H.-J.; Shin, J.-S. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 2014, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-W.; Luo, C.-Y.; Wang, X.-A.; Zhou, T.; Zheng, X.-X.; Zhang, Z.-Q.; Yu, B.; Zhang, J.; Tong, X.-H. Glycyrrhizin, a high-mobility group box 1 inhibitor, improves lipid metabolism and suppresses vascular inflammation in Apolipoprotein E knockout mice. J. Vasc. Res. 2019, 55, 365–377. [Google Scholar] [CrossRef]
- Pun, C.K.; Huang, H.-C.; Chang, C.-C.; Chuang, C.-L.; Yen, C.-H.; Hsu, S.-J.; Lee, F.-Y.; Hou, M.-C.; Huang, Y.-H. Glycyrrhizin attenuates portal hypertension and collateral shunting via inhibition of extrahepatic angiogenesis in cirrhotic rats. Int. J. Mol. Sci. 2021, 22, 7662. [Google Scholar] [CrossRef]
- Liu, S.; Hu, R.; Du, J.; Li, Y.; Li, X. Glycyrrhizin ameliorates vascular endothelial cell senescence by inhibiting HMGB1 in HFD/STZ-induced diabetic rats and human umbilical vein endothelial cells. Eur. J. Pharmacol. 2022, 931, 175196. [Google Scholar] [CrossRef]
- Mauricio, I.; Francischetti, B.; Monteiro, R.Q.; Guimarães, J.A. Identification of glycyrrhizin as a thrombin inhibitor. Biochem. Biophys. Res. Commun. 1997, 235, 259–263. [Google Scholar] [CrossRef]
- Mendes-Silva, W.; Assafim, M.; Ruta, B.; Monteiro, R.Q.; Guimarães, J.A.; Zingali, R.B. Antithrombotic effect of glycyrrhizin, a plant-derived thrombin inhibitor. Thromb. Res. 2003, 112, 93–98. [Google Scholar] [CrossRef]
- Nakata, N.; Kira, Y.; Yabunaka, Y.; Takaoka, K. Prevention of venous thrombosis by preoperative glycyrrhizin infusion in a rat model. J. Orthop. Sci. 2008, 13, 456–462. [Google Scholar] [CrossRef]
- Zhai, C.-L.; Zhang, M.-Q.; Zhang, Y.; Xu, H.-X.; Wang, J.-M.; An, G.-P.; Wang, Y.-Y.; Li, L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol. Sin. 2012, 33, 1477–1487. [Google Scholar] [CrossRef]
- Cai, X.; Wang, X.; Li, J.; Chen, S. Protective effect of glycyrrhizin on myocardial ischemia/reperfusion injury-induced oxidative stress, inducible nitric oxide synthase and inflammatory reactions through high-mobility group box 1 and mitogen-activated protein kinase expression. Exp. Ther. Med. 2017, 14, 1219–1226. [Google Scholar] [CrossRef]
- Boissady, E.; Daou, Y.A.Z.; Faucher, E.; Kohlhauer, M.; Lidouren, F.; Hedjaj, C.E.; Chateau-Joubert, S.; Hocini, H.; Hue, S.; Ghaleh, B.; et al. High-mobility group box 1–signaling inhibition with glycyrrhizin prevents cerebral t-cell infiltration after cardiac arrest. J. Am. Heart Assoc. 2023, 12, e027749. [Google Scholar] [CrossRef]
- Gendy, A.M.; El-Sadek, H.M.; Amin, M.M.; Ahmed, K.A.; El-Sayed, M.K.; El-Haddad, A.E.; Soubh, A. Glycyrrhizin prevents 3-nitropropionic acid-induced neurotoxicity by downregulating HMGB1/TLR4/NF-κB p65 signaling, and attenuating oxidative stress, inflammation, and apoptosis in rats. Life Sci. 2023, 314, 121317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, B.; Peng, W.; Xu, Z. Protective effect of glycyrrhizin on coronary microembolization-induced myocardial dysfunction in rats. Pharmacol. Res. Perspect. 2021, 9, e00714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, Y.; Zhang, Z. Glycyrrhizin administration ameliorates coxsackievirus B3-induced myocarditis in mice. Am. J. Med. Sci. 2012, 344, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhu, Y.; Deng, Y.; Zhang, S.; Zhang, Q.; Zhao, B.; Li, G. Glycyrrhizin improved autophagy flux via HMGB1-dependent Akt/mTOR signaling pathway to prevent doxorubicin-induced cardiotoxicity. Toxicology 2020, 441, 152508. [Google Scholar] [CrossRef]
- Nose, M.; Ito, M.; Kamimura, K.; Shimizu, M.; Ogihara, Y. A comparison of the antihepatotoxic activity between glycyrrhizin and glycyrrhetinic acid. Planta Med. 1994, 60, 136–139. [Google Scholar] [CrossRef]
- Shiki, Y.; Shirai, K.; Saito, Y.; Yoshida, S.; Mori, Y.; Wakashin, M. Effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J. Gastroenterol. Hepatol. 1992, 7, 12–16. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Lin, L.-J.; Kao, S.-T.; Lo, H.-Y.; Chou, S.-T.; Ho, T.-Y. Glycyrrhizin, silymarin, and ursodeoxycholic acid regulate a common hepatoprotective pathway in HepG2 cells. Phytomedicine 2015, 22, 768–777. [Google Scholar] [CrossRef]
- Morita, A.; Omoya, Y.; Ito, R.; Ishibashi, Y.; Hiramoto, K.; Ohnishi, S.; Yoshikawa, N.; Kawanishi, S. Glycyrrhizin and its derivatives promote hepatic differentiation via sweet receptor, Wnt, and Notch signaling. Biochem. Biophys. Rep. 2021, 28, 101181. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.; Yang, J.; Liu, X.; Davey, A.K.; Wang, J. Effects of glycyrrhizin on biliary transport and hepatic levels of glutathione in rats. Biopharm. Drug Dispos. 2012, 33, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Katoh, H.; Tsutsui, H.; Yamamoto, S.; Morisawa, S. Protection of liver cells from experimentally induced liver cell injury by glycyrrhizin. Gastroenterol. Jpn. 1985, 20, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-T.; Li, J.; Wang, F.-P.; Li, L.; Wang, J.-Y.; Jiang, W. Glycyrrhizin regulates CD4+ T cell response during liver fibrogenesis via JNK, ERK and PI3K/AKT pathway. Int. Immunopharmacol. 2012, 14, 410–421. [Google Scholar] [CrossRef]
- Kimura, M.; Moro, T.; Motegi, H.; Maruyama, H.; Sekine, M.; Okamoto, H.; Inoue, H.; Sato, T.; Ogihara, M. In vivo glycyrrhizin accelerates liver regeneration and rapidly lowers serum transaminase activities in 70% partially hepatectomized rats. Eur. J. Pharmacol. 2008, 579, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Qiao, H.; Meng, F.; Sun, X. Glycyrrhizin attenuates endotoxin-induced acute liver injury after partial hepatectomy in rats. Braz. J. Med. Biol. Res. 2007, 40, 1637–1646. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, S.-W.; Kim, Y.S.; Kang, S.S.; Kim, J.A.; Lee, S.H.; Lee, S.-M. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol. Pharm. Bull. 2007, 30, 1898–1904. [Google Scholar] [CrossRef]
- Moro, T.; Shimoyama, Y.; Kushida, M.; Hong, Y.Y.; Nakao, S.; Higashiyama, R.; Sugioka, Y.; Inoue, H.; Okazaki, I.; Inagaki, Y. Glycyrrhizin and its metabolite inhibit Smad3-mediated type I collagen gene transcription and suppress experimental murine liver fibrosis. Life Sci. 2008, 83, 531–539. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, W.-H.; Zong, L.; Xu, M.-Y.; Lu, L.-G. 18α-Glycyrrhizin induces apoptosis and suppresses activation of rat hepatic stellate cells. Med. Sci. Monit. 2012, 18, BR24–BR32. [Google Scholar] [CrossRef]
- Qu, Y.; Zong, L.; Xu, M.; Dong, Y.; Lu, L. Effects of 18α-glycyrrhizin on TGF-β1/Smad signaling pathway in rats with carbon tetrachloride-induced liver fibrosis. Int. J. Clin. Exp. Pathol. 2015, 8, 1292–1301. [Google Scholar]
- Zhao, J.; Wan, X.-Y.; Luo, M.; Chen, T.-S.; He, P. Antifibrotic effects of glycyrrhizin and matrine in vitro and in vivo. Biomed. Prev. Nutr. 2012, 2, 132–137. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Abulhamd, A.T.; Hamad, A.M.; Alanazi, A.H.; Ali, R.; Alqasoumi, S.I. Evaluation of the hepatoprotective effect of combination between hinokiflavone and Glycyrrhizin against CCl4 induced toxicity in rats. Saudi Pharm. J. 2018, 26, 496–503. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Saleem, S.; Ansari, S.A.; Iqbal, J.; Asif, M.; Kamal, M.A.; Al-Qahtani, M.H.; Karim, S. Assessment of circulating biochemical markers in mice receiving cinnamon and glycyrrhizin under carbon tetrachloride induced hepatic injury. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 105–111. [Google Scholar] [CrossRef]
- Shibayama, Y. Prevention of hepatotoxic responses to chemicals by glycyrrhizin in rats. Exp. Mol. Pathol. 1989, 51, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Shiga, J.; Kataoka, M.; Genmma, M.; Tamura, H. Effect of glycyrrhizin on fulminant hepatitis and carcinogenesis of long evans cinnamon (LEC) rat. Acta Hepatol. Jpn. 1999, 40, 491–499. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Guo, J.-S.; Li, H.; Liu, S.-L.; Zern, M.A. Inhibitory effect of glycyrrhizin on NF-κB binding activity in CCl4 plus ethanol-induced liver cirrhosis in rats. Liver 1998, 18, 180–185. [Google Scholar] [CrossRef]
- Zou, L.-Y.; Wu, T.; Cui, L. Effect of glycyrrhizin on CCl4-induced liver cirrhosis and bone loss in mice. Chin. Pharmacol. Bull. 2002, 18, 437–441. [Google Scholar]
- Zhai, D.; Zhao, Y.; Chen, X.; Guo, J.; He, H.; Yu, Q.; Yang, J.; Davey, A.K.; Wang, J. Protective effect of glycyrrhizin, glycyrrhetic acid and matrine on acute cholestasis induced by α-naphthyl isothiocyanate in rats. Planta Med. 2007, 73, 128–133. [Google Scholar] [CrossRef]
- Chigurupati, H.; Auddy, B.; Biyani, M.; Stohs, S.J. Hepatoprotective effects of a proprietary glycyrrhizin product during alcohol consumption: A randomized, double-blind, placebo-controlled, crossover study. Phytother. Res. 2016, 30, 1943–1953. [Google Scholar] [CrossRef]
- Chigurupati, H.; Auddy, B.; Biyani, M.; Chakrabarti, S.; Pandit, S.; Biswas, T.K.; Mondal, T.; Stohs, S.J. Antioxidant and DNA protective effects of NTX, a proprietary glycyrrhizin/D-mannitol product, in association with alcohol consumption: A randomized, placebo-controlled, double-blind, crossover study. J. Funct. Foods 2017, 34, 28–35. [Google Scholar] [CrossRef]
- Lin, G.; Nnane, I.P.; Cheng, T.-Y. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon 1999, 37, 1259–1270. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, D.; Chen, X.; He, H.; Lu, Q.; Yu, Q. Protective effect of glycyrrhizin and matrine on acute vanishing bile duct syndrome induced by alpha-naphthylisothiocyanate in rats. Hepatol. Res. 2007, 37, 143–151. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Z.-Z.; Meng, R.; Cao, Y.-F.; Tanaka, N.; Krausz, K.W.; Gonzalez, F.J. Glycyrrhizin and glycyrrhetinic acid inhibits alpha-naphthyl isothiocyanate-induced liver injury and bile acid cycle disruption. Toxicology 2017, 386, 133–142. [Google Scholar] [CrossRef]
- Tsai, J.-J.; Kuo, H.-C.; Lee, K.-F.; Tsai, T.-H. Glycyrrhizin represses total parenteral nutrition-associated acute liver injury in rats by suppressing endoplasmic reticulum stress. Int. J. Mol. Sci. 2013, 14, 12563–12580. [Google Scholar] [CrossRef]
- Karimani, A.; Heidarpour, M.; Moghaddam Jafari, A. Protective effects of glycyrrhizin on sub-chronic diazinon-induced biochemical, hematological alterations and oxidative stress indices in male Wistar rats. Drug Chem. Toxicol. 2019, 42, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, S.M.; Rady, F.M.; Shehata, F.E.; Helal, A.D.; El-Sayed, A.E.; Mohamed, F.F. Diminution of aflatoxicosis in rabbits by addition of glycyrrhizin in their polluted rations. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 581–593. [Google Scholar]
- Wan, X.-Y.; Luo, M.; Li, X.-D.; He, P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem. Biol. Interact. 2009, 181, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, Y.-S.; Jiang, Y.; Peng, Y.-F.; Sun, Z.; Dai, X.-N.; Cao, Q.-T.; Sun, Y.-M.; Han, J.-C.; Gao, Y.-J. Targeted metabolomic study indicating glycyrrhizin’s protection against acetaminophen-induced liver damage through reversing fatty acid metabolism. Phytother. Res. 2014, 28, 933–936. [Google Scholar] [CrossRef]
- Chen, A.; Duan, D.; Dong, R.; Yin, J.; Yang, T.; Tan, K.; Chen, Y.; Lu, J.; Du, X. Glycyrrhizin (GL) can strongly reverse the acetaminophen (APAP)-induced disrupted homeostasis of bile acids. Lat. Am. J. Pharm. 2014, 33, 511–514. [Google Scholar]
- Dang, X.-L.; Yang, L.-F.; Shi, L.; Li, L.-F.; He, P.; Chen, J.; Zheng, B.-J.; Yang, P.; Wen, A.-D. Post-treatment with glycyrrhizin can attenuate hepatic mitochondrial damage induced by acetaminophen in mice. Exp. Biol. Med. 2021, 246, 1219–1227. [Google Scholar] [CrossRef]
- Yan, T.; Wang, H.; Zhao, M.; Yagai, T.; Chai, Y.; Krausz, K.W.; Xie, C.; Cheng, X.; Zhang, J.; Che, Y.; et al. Glycyrrhizin protects against acetaminophen-induced acute liver injury via alleviating tumor necrosis factor α-mediated apoptosis. Drug Metab. Dispos. 2016, 44, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ikeda, T.; Wake, K.; Sato, T.; Sato, T.; Inoue, H. Glycyrrhizin prevents of lipopolysaccharide/D-galactosamine-induced liver injury through down-regulation of matrix metalloproteinase-9 in mice. J. Pharm. Pharmacol. 2008, 60, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Abe, K.; Ikeda, T.; Matsushita, T.; Wake, K.; Sato, T.; Inoue, H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and D-galactosamine-induced mouse liver injury. Eur. J. Pharmacol. 2007, 576, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Ma, Z.-Y.; Li, J.-H.; Li, R.-D.; Tao, Y.-F.; Zhang, Q.-B.; Wang, Z.-X. Glycyrrhizin improves inflammation and apoptosis via suppressing HMGB1 and PI3K/mTOR pathway in lipopolysaccharide-induced acute liver injury. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7122–7130. [Google Scholar]
- Kuroda, N.; Inoue, K.; Ikeda, T.; Hara, Y.; Wake, K.; Sato, T. Apoptotic response through a high mobility group box 1 protein-dependent mechanism in LPS/GalN-induced mouse liver failure and glycyrrhizin-mediated inhibition. PLoS ONE 2014, 9, e92884. [Google Scholar] [CrossRef]
- Ikeda, T.; Abe, K.; Kuroda, N.; Kida, Y.; Inoue, H.; Wake, K.; Morito, M.; Sato, T. The inhibition of apoptosis by glycyrrhizin in hepatic injury induced by injection of lipopolysaccharide/D-galactosamine in mice. Arch. Histol. Cytol. 2008, 71, 163–178. [Google Scholar] [CrossRef]
- Gwak, G.-Y.; Moon, T.G.; Lee, D.H.; Yoo, B.C. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J. Gastroenterol. 2012, 18, 679–684. [Google Scholar] [CrossRef]
- Nagai, T.; Egashira, T.; Kudo, Y.; Yamanaka, Y.; Shimada, T. Attenuation of dysfunction in the ischemia-reperfused liver by glycyrrhizin. Jpn. J. Pharmacol. 1992, 58, 209–218. [Google Scholar] [CrossRef]
- Nagai, T.; Egashira, T.; Yamanaka, Y.; Kohno, M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch. Environ. Contam. Toxicol. 1991, 20, 432–436. [Google Scholar] [CrossRef]
- Mabuchi, A.; Wake, K.; Marlini, M.; Watanabe, H.; Wheatley, A.M. Protection by glycyrrhizin against warm ischemia-reperfusion induced cellular injury and derangement of the microcirculatory blood flow in the rat liver. Microcirculation 2009, 16, 364–376. [Google Scholar] [CrossRef]
- Ogiku, M.; Kono, H.; Hara, M.; Tsuchiya, M.; Fujii, H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J. Pharmacol. Exp. Ther. 2011, 339, 93–98. [Google Scholar] [CrossRef]
- Su, S.; Wu, J.; Gong, T.; He, K.; Feng, C.; Zhang, M.; Li, B.; Xia, X. Inhibition of high mobility group box 1-toll-like receptor-4 signaling by glycyrrhizin contributes to the attenuation of cold ischemic injury of liver in a rat model. Transplant. Proc. 2016, 48, 191–198. [Google Scholar] [CrossRef]
- Hua, S.; Ma, M.; Fei, X.; Zhang, Y.; Gong, F.; Fang, M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated Kupffer cells pyroptosis. Int. Immunopharmacol. 2019, 68, 145–155. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, R.; Cao, Y.; Yang, W.; Zhang, Z.; Zhou, Q.; Ren, J.; Shi, X.; Gao, Y.; Guo, X. Glycyrrhizin attenuates myocardial ischemia reperfusion injury by suppressing inflammation, oxidative stress, and ferroptosis via the HMGB1-TLR4-GPX4 pathway. Exp. Cell Res. 2024, 435, 113912. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-Z.; Lou, Y.-J. Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol. Sin. 2003, 24, 771–777. [Google Scholar] [PubMed]
- Kou, X.; Zhu, J.; Xie, X.; Hao, M.; Zhao, Y. The protective effect of glycyrrhizin on hepatic ischemia-reperfusion injury in rats and possible related signal pathway. Iran. J. Basic Med. Sci. 2020, 23, 1232–1238. [Google Scholar] [PubMed]
- Yan, T.; Wang, H.; Cao, L.; Wang, Q.; Takahashi, S.; Yagai, T.; Li, G.; Krausz, K.W.; Wang, G.; Gonzalez, F.J.; et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab. Dispos. 2018, 46, 1310–1319. [Google Scholar] [CrossRef]
- Bagheri, H.; Yaghmaei, P.; Modaresi, M.; Sabbaghian, M.; Ebrahim-Habibi, A. Glycyrrhizin improves fatty liver symptoms, increases adiponectin and reduces UCP2 expression in Wistar rats. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Zhou, J.-M.; Ma, Z.-C.; Li, H.; Liang, Q.-D.; Tan, H.-L.; Xiao, C.-R.; Zhang, B.-L.; Gao, Y. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: A possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem. Biol. Interact. 2012, 200, 11–20. [Google Scholar] [CrossRef]
- Han, J.-C.; Yu, J.; Gao, Y.-J. Lipidomics investigation of reversal effect of glycyrrhizin (GL) towards lithocholic acid (LCA)-induced alteration of phospholipid profiles. Pharm. Biol. 2014, 52, 1624–1628. [Google Scholar] [CrossRef]
- Cao, Z.-X.; Zhao, Z.-F.; Zhao, X.-F. Effect of compound glycyrrhizin injection on liver function and cellular immunity of children with infectious mononucleosis complicated liver impairment. Chin. J. Integr. Med. 2006, 12, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Numazaki, K. Glycyrrhizin therapy for liver dysfunction associated with cytomegalovirus infection in immunocompetent children. Antimicrob. Infect. Dis. Newsl. 1998, 17, 70–71. [Google Scholar] [CrossRef]
- Numazaki, K. Glycyrrhizin therapy for viral infections. Afr. J. Biotechnol. 2003, 2, 456–458. [Google Scholar] [CrossRef]
- Liang, S.B.; Hou, W.B.; Zheng, R.X.; Liang, C.H.; Yan, L.J.; Wang, H.N.; Cao, H.J.; Han, M.; Robinson, N.; Liu, J.P. Compound glycyrrhizin injection for improving liver function in children with acute icteric hepatitis: A systematic review and meta-analysis. Integr. Med. Res. 2022, 11, 100772. [Google Scholar] [CrossRef]
- Ashida, M.; Kojima, H.; Hamada, C. Inhibitory effect of glycyrrhizin on hepatitis A virus replication. Kanzo 1989, 30, 1740–1741. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohta, Y.; Takino, T. Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis. Double blind trial. Asian Med. J. 1983, 26, 423–438. [Google Scholar]
- Su, X.S.; Chen, H.M.; Wang, L.H.; Jiang, C.F.; Liu, J.H.; Zhao, M.Q.; Ma, X.H.; Zhao, Y.C.; Han, D.W. Clinical and laboratory observation on the effect of glycyrrhizin in acute and chronic viral hepatitis. J. Tradit. Chin. Med. 1984, 4, 127–132. [Google Scholar]
- Takahara, T.; Watanabe, A.; Shiraki, K. Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. J. Hepatol. 1994, 21, 601–609. [Google Scholar] [CrossRef]
- Li, Y.W.; Yang, H.Z.; Ke, Q.S.; Chen, W.; Chen, X.J. Effects of glycyrrhizin on the expression of hepatitis B virus and Toll like receptors 2,4 in HepG2.2.15 cells expressing low HBsAg. Zhong Yao Cai 2008, 31, 403–407. [Google Scholar]
- Sato, H.; Goto, W.; Yamamura, J.-I.; Kurokawa, M.; Kageyama, S.; Takahara, T.; Watanabe, A.; Shiraki, K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antivir. Res. 1996, 30, 171–177. [Google Scholar] [CrossRef]
- Lin, C.-C.; Wang, P.-H. Intravenous glycyrrhizin improved serum transaminases rapidly in a chronic hepatitis B patient with acute exacerbation. J. Formos. Med. Assoc. 2015, 114, 188–189. [Google Scholar]
- Hayashi, J.; Kajiyama, W.; Noguchi, A.; Nakashima, K.; Hirata, M.; Hayashi, S.; Kashiwagi, S. Glycyrrhizin withdrawal followed by human lymphoblastoid interferon in the treatment of chronic hepatitis B. Gastroenterol. Jpn. 1991, 26, 742–746. [Google Scholar] [CrossRef]
- Hayashi, J.; Kashiwagi, S.; Noguchi, A.; Ikematsu, H.; Tsuda, H.; Tsuji, Y.; Motomura, M. Combination therapy of glycyrrhizin withdrawal and human fibroblast interferon for chronic hepatitis B. Clin. Ther. 1989, 11, 161–169. [Google Scholar]
- Matsuo, K.; Takenaka, K.; Shimomura, H.; Fujii, N.; Shinagawa, K.; Kiura, K.; Harada, M. Lamivudine and glycyrrhizin for treatment of chemotherapy-induced hepatitis B virus (HBV) hepatitis in a chronic HBV carrier with non-Hodgkin lymphoma. Leuk. Lymphoma 2001, 41, 191–195. [Google Scholar] [PubMed]
- Okuno, T.; Arai, K.; Shindo, M. Efficacy of interferon combined glycyrrhizin therapy in patients with interferon-resistant chronic hepatitis C. Nippon Rinsho 1995, 53, 1022–1025. [Google Scholar] [PubMed]
- Anand, A.C.; Seth, A.K.; Nagpal, A.; Varma, P.P.; Gadela, S.R.; Baliga, K.V.; Dutta, V.; Chopra, G.S. Initial experience with ribavirin plus glycyrrhizin in renal allograft recipients with chronic hepatitis C. Indian J. Gastroenterol. 2004, 23, 226–227. [Google Scholar] [PubMed]
- Okuno, T.; Arai, K.; Shindo, M. Efficacy of interferon combined glycyrrhizin therapy in patients with chronic hepatitis C resistant to interferon therapy. Nippon Rinsho 1994, 52, 1823–1827. [Google Scholar]
- Abe, Y.; Ueda, T.; Kato, T.; Kohli, Y. Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C. Nippon Rinsho 1994, 52, 1817–1822. [Google Scholar]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef]
- Acharya, S.K.; Sreenivas, V.; Gupta, S.D.; Kumar, S.; Chawla, Y.K.; Tandon, A.; Habeeb, A.; Kar, P.; Chowdhury, A.; Choudhuri, G.; et al. Treatment of chronic hepatitis due to hepatitis C virus (CH-C) in India: A randomized controlled trial comparing daily interferon-alfa-2b and ribavirin with daily interferon-alfa-2b and glycyrrhizin—A multicenter study. J. Clin. Exp. Hepatol. 2012, 2, 10–18. [Google Scholar] [CrossRef]
- Itoh, H.; Nakata, H.; Hara, T.; Imoto, K.; Nakazawa, K.; Oka, H.; Shiotani, A.; Nishioka, S.; Matsumoto, M. Evaluation of combined therapy with interferon-β and glycyrrhizin for chronic active hepatitis C. Ther. Res. 1997, 18, 419–428. [Google Scholar]
- Kumada, H. Long-term treatment of chronic hepatitis C with glycyrrhizin [Stronger Neo-Minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology 2002, 62 (Suppl. S1), 94–100. [Google Scholar] [CrossRef]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, M.; Kumada, H. The long-term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar] [CrossRef]
- Ikeda, K.; Arase, Y.; Kobayashi, M.; Saitoh, S.; Someya, T.; Hosaka, T.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Suzuki, F.; et al. A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: A cohort study of 1249 patients. Dig. Dis. Sci. 2006, 51, 603–609. [Google Scholar] [CrossRef]
- Van Rossum, T.G.J.; Vulto, A.G.; Hop, W.C.J.; Brouwer, J.T.; Niesters, H.G.M.; Schalm, S.W. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: A double-blind, randomized, placebo-controlled phase I/II trial. J. Gastroenterol. Hepatol. 1999, 14, 1093–1099. [Google Scholar] [CrossRef]
- Van Rossum, T.G.J.; De Jong, F.H.; Hop, W.C.J.; Boomsma, F.; Schalm, S.W. ‘Pseudo-aldosteronism’ induced by intravenous glycyrrhizin treatment of chronic hepatitis C patients. J. Gastroenterol. Hepatol. 2001, 16, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, T.G.J.; Vulto, A.G.; Hop, W.C.J.; Schalm, S.W. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am. J. Gastroenterol. 2001, 96, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Orlent, H.; Hansen, B.E.; Willems, M.; Brouwer, J.T.; Huber, R.; Kullak-Ublick, G.A.; Gerken, G.; Zeuzem, S.; Nevens, F.; Tielemans, W.C.M.; et al. Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: A randomized phase II trial. J. Hepatol. 2006, 45, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Wedemeyer, H.; Singer, A.; Khomutjanskaja, N.; Dienes, H.P.; Roskams, T.; Goldin, R.; Hehnke, U.; Inoue, H.; Stauber, R.; et al. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: Biochemical and histological effects after 52 weeks. J. Viral Hepat. 2012, 19, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Kondou, T.; Fukuhara, A.; Tounou, S.; Mine, M.; Mataki, N.; Hanada, K.; Ozaka, M.; Mitani, K.; Nakaya, T.; et al. Efficacy of a glycyrrhizin suppository for the treatment of chronic hepatitis C: A pilot study. Hepatol. Res. 2003, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Kanemasa, K.; Fukumoto, K.; Yoshida, N.; Sakai, K. Utility of a little phlebotomy intermittently just before intravenous injection of glycyrrhizin for patients with chronic hepatitis C. Jpn. J. Gastroenterol. 2007, 104, 1044–1050. [Google Scholar]
- Akagi, K.; Murai, K.; Shikata, T.; Yamanaka, M.; Omae, T. In vitro effects of glycyrrhizin on the serum enzymes in chronic hepatitis patients. Rinsho Yakuri 1980, 11, 125–129. [Google Scholar] [CrossRef]
- Tandon, A.; Tandon, B.N.; Bhujwala, R.A. Treatment of subacute hepatitis with lamivudine and intravenous glycyrrhizin: A pilot study. Hepatol. Res. 2001, 20, 1–8. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Sakamoto, M.; Matsushita, M.; Fujita, T.; Nishioka, K. Glycyrrhizin inhibits the lytic pathway of complement—Possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol. Immunol. 2000, 44, 799–804. [Google Scholar] [CrossRef]
- Tandon, A.; Tandon, B.N.; Bhujwala, R.A. Clinical spectrum of acute sporadic hepatitis E and possible benefit of glycyrrhizin therapy. Hepatol. Res. 2002, 23, 55–61. [Google Scholar] [CrossRef]
- Akashi, K.; Shirahama, M.; Iwakiri, R.; Yoshimatsu, H.; Nagafuchi, S.; Hayashi, J.; Ishibashi, H. Drug-induced allergic hepatitis caused by glycyrrhizin, or extract of licorice root. Kanzo 1988, 29, 1633–1637. [Google Scholar] [CrossRef]
- Miyazawa, N.; Takahashi, H.; Yoshiike, Y.; Ogura, T.; Watanuki, Y.; Sato, M.; Kakemizu, N.; Yamakawa, Y.; Goto, H.; Odagiri, S. Effect of glycyrrhizin on anti-tuberculosis drug-induced hepatitis. Kekkaku 2003, 78, 15–19. [Google Scholar]
- Abe, M.; Akbar, F.; Hasebe, A.; Horiike, N.; Onji, M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J. Gastroenterol. 2003, 38, 962–967. [Google Scholar] [CrossRef]
- Yasui, S.; Fujiwara, K.; Tawada, A.; Fukuda, Y.; Nakano, M.; Yokosuka, O. Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Dig. Dis. Sci. 2011, 56, 3638–3647. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Qi, C.; Hua, S.; Fei, X.; Gong, F.; Fang, M. Glycyrrhizin alleviates Con A-induced hepatitis by differentially regulating the production of IL-17 and IL-25. Biomed. Pharmacother. 2019, 110, 692–699. [Google Scholar] [CrossRef]
- Okamoto, T. The protective effect of glycyrrhizin on anti-Fas antibody-induced hepatitis in mice. Eur. J. Pharmacol. 2000, 387, 229–232. [Google Scholar] [CrossRef]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J. Exp. Biol. 2013, 51, 129–138. [Google Scholar] [PubMed]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates metabolic syndrome-induced liver damage in experimental rat model. Mol. Cell. Biochem. 2015, 409, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sil, R.; Chakraborti, A.S. Oxidative inactivation of liver mitochondria in high fructose diet-induced metabolic syndrome in rats: Effect of glycyrrhizin treatment. Phytother. Res. 2016, 30, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-G.; Sohn, E.-J.; Mun, Y.-J.; Woo, W.-H.; Lee, H.-S. Glycyrrhizin ameliorates renal function defects in the early-phase of ischemia-induced acute renal failure. Phytother. Res. 2003, 17, 947–951. [Google Scholar] [CrossRef]
- Sohn, E.-J.; Kang, D.-G.; Lee, H.-S. Protective effects of glycyrrhizin on gentamicin-induced acute renal failure in rats. Pharmacol. Toxicol. 2003, 93, 116–122. [Google Scholar] [CrossRef]
- Nomiyama, K.; Nomiyama, H. Cadmium-induced renal dysfunction was improved by treating hepatic injury with glycyrrhizin. J. Trace Elem. Exp. Med. 1993, 6, 171–178. [Google Scholar]
- Li, L.; Zhang, Y.; Wang, Z.; Chen, X.; Fang, M. Glycyrrhizin attenuates renal inflammation in a mouse Con A-hepatitis model via the IL-25/M2 axis. Ren. Fail. 2024, 46, 2. [Google Scholar] [CrossRef]
- Wang, L.-N.; Yu, L.; Zhang, L.; Hao, Z.-H.; Zhao, D.; Zhang, Y.-X. Protective effects of glycyrrhizin on adriamycin nephropathy in rats. J. Chin. Integr. Med. 2006, 4, 413–417. [Google Scholar] [CrossRef]
- Yu, L.; Bi, X.; Zhu, G.; Han, Z.; Ye, Y.; Liang, Y.; Zhang, L.; Hao, Z.; Zeng, G.; He, H.; et al. Protective effect of glycyrrhizin on nephrotic syndrome induced by adriamycin in rats. Clin. Investig. Med. 2009, 32, E229–E238. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Hao, Z.-H.; Wang, L.-N.; Deng, Y.; Zhang, Y.-X. Renoprotective effects of glycyrrhizin on experimental glomerulosclerosis in rats. Chin. Tradit. Herb. Drugs 2010, 41, 250–255. [Google Scholar]
- Takeshita, K.; Susuki, C.; Itoh, S.; Tsuji, T. Preventive effect of α-tocopherol and glycyrrhizin against platelet-neutrophil complex formation induced by hemodialysis membranes. Int. J. Artif. Organs 2009, 32, 282–290. [Google Scholar] [CrossRef]
- Yang, D.-K.; Sun, Y.-F.; Liang, H.-J.; Shen, B.-S.; Qiao, H.-C. Treatment of hepatitis B virus-associated glomerulonephritis with compound glycyrrhizin and lamivudine: An analysis of 40 cases. World Chin. J. Dig. 2010, 18, 1380–1383. [Google Scholar] [CrossRef]
- Shibayama, K.; Ebihara, K.; Murohashi, M.; Makino, T. Treatment of idiopathic renal hematuria with combined administration of glycyrrhizin and antiplasmin. Acta Urol. Jpn. 1977, 23, 399–402. [Google Scholar]
- Yildirim, A.O.; Ince, M.; Eyi, Y.E.; Tuncer, S.K.; Kaldirim, U.; Eroglu, M.; Oztas, E.; Cayci, T.; Kilic, A.; Inal, V.; et al. The effects of glycyrrhizin on experimental acute pancreatitis in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2981–2987. [Google Scholar] [PubMed]
- Fakhari, S.; Abdolmohammadi, K.; Panahi, Y.; Nikkhoo, B.; Peirmohammadi, H.; Rahmani, M.R.; Moghadam, A.S.; Jalili, A. Glycyrrhizin attenuates tissue injury and reduces neutrophil accumulation in experimental acute pancreatitis. Int. J. Clin. Exp. Pathol. 2014, 7, 101–109. [Google Scholar]
- Pan, Y.-L. The effects of glycyrrhizin on acute pancreatitis in mice. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3943–3947. [Google Scholar]
- Zhang, R.; Asikaer, A.; Chen, Q.; Wang, F.; Lan, J.; Liu, Y.; Hu, L.; Zhao, H.; Duan, H. Network pharmacology and in vitro experimental verification unveil glycyrrhizin from Glycyrrhiza glabra alleviates acute pancreatitis via modulation of MAPK and STAT3 signaling pathways. BMC Complement. Med. Ther. 2024, 24, 58. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, W.; Shi, J.; Lai, F.; Luo, S.; Du, Y.; Wang, X.; Xiang, Y. Glycyrrhizin Ameliorates Cardiac Injury in Rats with Severe Acute Pancreatitis by Inhibiting Ferroptosis via the Keap1/Nrf2/HO-1 Pathway. Dig. Dis. Sci. 2024, 69, 2477–2487. [Google Scholar] [CrossRef]
- Xiang, K.; Cheng, L.; Luo, Z.; Ren, J.; Tian, F.; Tang, L.; Chen, T.; Dai, R. Glycyrrhizin suppresses the expressions of HMGB1 and relieves the severity of traumatic pancreatitis in rats. PLoS ONE 2014, 9, e115982. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Tanno, K. Effect of glycyrrhizin on the damage to the pancreas caused by DL-ethionin in rats; light microscopic observation. Pharmacometrics 1993, 46, 33–38. [Google Scholar]
- Wu, K.; Zhang, R.-L.; Wang, X.-P. The effects and mechanisms of glycyrrhizin on the TNBS-induced pancreatic fibrosis in rat. Chin. Pharmacol. Bull. 2003, 19, 1424–1427. [Google Scholar]
- Srikantam, S.; Arumugam, G. Glycyrrhizin modulates ER stress-induced UPR and concomitant mitochondrial dysfunction and activation of NF-κB in alcohol and cerulein-induced pancreatitis in rats. J. Appl. Pharm. Sci. 2021, 11, 130–140. [Google Scholar]
- Kageyama, Y.; Suzuki, H.; Saruta, T. Glycyrrhizin induces mineralocorticoid activity through alterations in cortisol metabolism in the human kidney. J. Endocrinol. 1992, 135, 147–152. [Google Scholar] [CrossRef]
- Takegoshi, T.; Takeuchi, N.; Imura, M. A case of pseudoaldosteronism induced by high doses of glycyrrhizin. Med. J. Mutual Aid Assoc. 1977, 26, 65–68+11. [Google Scholar]
- Yang, J.-P.; Ullah, A.; Su, Y.-N.; Otoo, A.; Adu-Gyamfi, E.A.; Feng, Q.; Wang, Y.-X.; Wang, M.-J.; Ding, Y.-B. Glycyrrhizin ameliorates impaired glucose metabolism and ovarian dysfunction in a polycystic ovary syndrome mouse model. Biol. Reprod. 2023, 109, 83–96. [Google Scholar] [CrossRef]
- Sekihata, K.; Akasaka, Y.; Hatta, A.; Inoue, H. Inhibitory effects of glycyrrhizin on scratching behavior in response to substance P, PAR-2 agonist and LTB4 in mice. Jpn. Pharmacol. Ther. 2015, 43, 1295–1303. [Google Scholar]
- Zhang, H.-Q.; Liu, F.; Sun, B.; Li, G.-H. Anti-allergic action of glycyrrhizin. Acta Pharmacol. Sin. 1986, 7, 175–177. [Google Scholar]
- Zhang, Y.; Shang, T. Observation of therapeutic effect of mizolastine combined with compound glycyrrhizin in chronic idiopathic urticarial. J. Clin. Dermatol. 2012, 41, 695–698. [Google Scholar]
- Yoshida, S.; Lee, J.O.; Nakamura, K.; Suzuki, S.; Hendon, D.N.; Kobayashi, M.; Suzuki, F. Effect of glycyrrhizin on pseudomonal skin infections in human-mouse chimeras. PLoS ONE 2014, 9, e83747. [Google Scholar] [CrossRef]
- Rossi, T.; Benassi, L.; Magnoni, C.; Ruberto, A.I.; Coppi, A.; Baggio, G. Effects of glycyrrhizin on UVB-irradiated melanoma cells. In Vivo 2005, 19, 319–322. [Google Scholar]
- Li, Y.; Yu, N.; Han, D.; Ding, Y.; Xu, Y. A first report of porphyria cutanea tarda successfully treated with glycyrrhizin. Dermatol. Ther. 2019, 32, e13014. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Z.; Zhang, F.-R. Glycyrrhizin combined with acitretin improve clinical symptom of psoriasis via reducing Th17 cell differentiation and related serum cytokine concentrations. Int. J. Clin. Exp. Med. 2015, 8, 16266–16272. [Google Scholar] [PubMed]
- Liu, H.; Lin, Y.-K.; Jiang, Z.-Y.; Wang, F.; Qin, X.; Li, W.-Y. Effects of compound glycyrrhizin on circulating Th17 cells and IL-22 levels in patients with psoriasis vulgaris. J. Clin. Dermatol. 2017, 46, 203–206. [Google Scholar]
- Yu, N.; Li, Y.; Ding, Y.; Shi, Y. Combination therapy with acitretin and glycyrrhizin in generalized pustular psoriasis with liver test abnormalities: A case series. Dermatol. Ther. 2020, 33, e13318. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, Y.; Tan, G.; Han, Y.; Tang, Z.; Xu, W.; Zeng, F.; Guo, Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c Mice and inhibits TNF-α-Induced ICAM-1 Expression via NF-κB/MAPK in HaCaT Cells. Cell. Physiol. Biochem. 2015, 35, 1335–1346. [Google Scholar] [CrossRef]
- Qiong, H.; Han, L.; Zhang, N.; Chen, H.; Yan, K.; Zhang, Z.; Ma, Y.; Xu, J. Glycyrrhizin improves the pathogenesis of psoriasis partially through IL-17A and the SIRT1-STAT3 axis. BMC Immunol. 2021, 22, 34. [Google Scholar] [CrossRef]
- Qi, S.-S.; Shi, W.-D.; Xu, F.; Sheng, Y.-Y.; Hu, R.-M.; Miao, Y.; Rui, W.-L.; Zhao, J.; Yang, Q.-P. The clinical efficacy and safety of oral compound glycyrrhizin in adult patients with mild-to-moderate active alopecia areata: A randomized controlled study. Eur. J. Integr. Med. 2019, 32, 100975. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, J.; Ma, J.; Qi, S.; Hu, R.; Yang, Q. Efficacy of compound betamethasone combined with compound glycyrrhizin in the treatment of severe active alopecia areata. Acta Med. Mediterr. 2020, 36, 347–351. [Google Scholar]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int. Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Ju, M.; Lai, W.; Lu, X.; Shi, H.; Shi, W.; Gu, H.; Li, L. A multicenter, randomized, double-blind, placebo-controlled study of compound glycyrrhizin capsules combined with a topical corticosteroid in adults with chronic eczema. Evid.-Based Complement. Altern. Med. 2020, 2020, 6127327. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Roh, H.; Jung, J.H.; Ahn, H.M.; Lee, J.H.; Yun, C.-O.; Lee, W.J. Antifibrotic effects of high-mobility group box 1 protein inhibitor (glycyrrhizin) on keloid fibroblasts and keloid spheroids through reduction of autophagy and induction of apoptosis. Int. J. Mol. Sci. 2019, 20, 4134. [Google Scholar] [CrossRef]
- Abe, H.; Ohya, N.; Yamamoto, K.F.; Shibuya, T.; Arichi, S.; Odashima, S. Effects of glycyrrhizin and glycyrrhetinic acid on growth and melanogenesis in cultured B16 melanoma cells. Eur. J. Cancer Clin. Oncol. 1987, 23, 1549–1555. [Google Scholar] [CrossRef]
- Jung, G.-D.; Yang, J.-Y.; Song, E.-S.; Park, J.-W. Stimulation of melanogenesis by glycyrrhizin in B16 melanoma cells. Exp. Mol. Med. 2001, 33, 131–135. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Park, E.; Kim, J.; Hong, S.; Park, J.; Park, S.; Lee, S.; et al. Glycyrrhizin induces melanogenesis by elevating a cAMP level in B16 melanoma cells. J. Investig. Dermatol. 2005, 124, 405–411. [Google Scholar] [CrossRef]
- Xu, Z.; Xing, X.; Zhang, C.; Chen, L.; Xiang, L.F. A pilot study of oral tranexamic acid and glycyrrhizin compound in the treatment of recalcitrant Riehl’s melanosis. J. Cosmet. Dermatol. 2019, 18, 286–292. [Google Scholar] [CrossRef]
- Wang, L.; Wen, X.; Hao, D.; Li, Y.; Du, D.; Jiang, X. Combination therapy with salicylic acid chemical peels, glycyrrhizin compound, and vitamin C for Riehl’s melanosis. J. Cosmet. Dermatol. 2020, 19, 1377–1380. [Google Scholar] [PubMed]
- Mou, K.H.; Han, D.; Liu, W.L.; Li, P. Combination therapy of orally administered glycyrrhizin and UVB improved active-stage generalized vitiligo. Braz. J. Med. Biol. Res. 2016, 49, e5354. [Google Scholar] [CrossRef] [PubMed]
- Mou, K.; Pan, W.; Han, D.; Wen, X.; Cao, F.; Miao, Y.; Li, P. Glycyrrhizin protects human melanocytes from H2O2-induced oxidative damage via the Nrf2-dependent induction of HO-1. Int. J. Mol. Med. 2019, 44, 253–261. [Google Scholar] [CrossRef]
- Li, L.; Ma, Q.; Li, H. Effect of vitiligo treatment using compound glycyrrhizin combined with fractional carbon dioxide laser and topical triamcinolone acetonide on serum interleukin-17 and tissue growth factor-β levels. J. Int. Med. Res. 2019, 47, 5623–5631. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Chen, Y.; Liu, J. Therapeutic effects of mesoderm introduction of compound glycyrrhizin injection on the treatment of rosacea. Skin Res. Technol. 2023, 29, e13328. [Google Scholar] [CrossRef]
- Chen, Y.; Han, W.; Li, S.; Nie, Y.; Chen, P.; Sun, J.; Chen, Y.; Li, L. Effects of mesotherapy introduction of compound glycyrrhizin injection on the treatment of moderate to severe acne. J. Cosmet. Dermatol. 2023, 22, 1973–1979. [Google Scholar] [CrossRef]
- Burillon, C.; Chiambaretta, F.; Pisella, P.-J. Efficacy and safety of glycyrrhizin 2.5% eye drops in the treatment of moderate dry eye disease: Results from a prospective, open-label pilot study. Clin. Ophthalmol. 2018, 12, 2629–2636. [Google Scholar] [CrossRef]
- Song, Z.; Gong, Y.; Liu, H.; Ren, Q.; Sun, X. Glycyrrhizin could reduce ocular hypertension induced by triamcinolone acetonide in rabbits. Mol. Vis. 2011, 17, 2056–2064. [Google Scholar] [PubMed]
- He, H.; Wei, D.; Liu, H.; Zhu, C.; Lu, Y.; Ke, Z.; Jiang, S.; Huang, J. Glycyrrhizin protects against sodium iodate-induced RPE and retinal injury through activation of AKT and Nrf2/HO-1 pathway. J. Cell. Mol. Med. 2019, 23, 3495–3504. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, Y.; Steinle, J.J. Glycyrrhizin protects IGFBP-3 knockout mice from retinal damage. Cytokine 2020, 125, 154856. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid. Based Complement. Altern. Med. 2018, 2018, 843810. [Google Scholar] [CrossRef]
- Wang, P.; Hao, P.; Chen, X.; Li, L.; Zhou, Y.; Zhang, X.; Zhu, L.; Ying, M.; Han, R.; Wang, L.; et al. Targeting HMGB1–NFκB axis and miR-21 by glycyrrhizin: Role in amelioration of corneal injury in a mouse model of alkali burn. Front. Pharmacol. 2022, 13, 841267. [Google Scholar] [CrossRef]
- Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Kharotia, S.; Hazlett, L.D. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5799–5809. [Google Scholar] [CrossRef]
- Somayajulu, M.; McClellan, S.A.; Farooq, S.M.; Pitchaikannu, A.; Xu, S.; Hazlett, L. Glycyrrhizin interacts with TLR4 and TLR9 to resolve P. aeruginosa keratitis. Pathogens 2022, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Vistisen, K.; Hazlett, L.D. Characterization of three ocular clinical isolates of P. aeruginosa: Viability, biofilm formation, adherence, infectivity, and effects of glycyrrhizin. Pathogens 2017, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. Topical glycyrrhizin is therapeutic for Pseudomonas aeruginosa keratitis. J. Ocul. Pharmacol. Ther. 2018, 34, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Magd, N.F.; El-Mesery, M.; El-Karef, A.; El-Shishtawy, M.M. Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating Nrf2 pathway. Life Sci. 2018, 193, 159–170. [Google Scholar] [CrossRef]
- Madhavadas, S.; Subramanian, S. Combination of spirulina with glycyrrhizin prevents cognitive dysfunction in aged obese rats. Indian J. Pharmacol. 2015, 47, 39–44. [Google Scholar] [CrossRef]
- Takii, H.; Kometani, T.; Nishimura, T.; Nakae, T.; Okada, S.; Fushiki, T. Antidiabetic effect of glycyrrhizin in genetically diabetic KK-Ay mice. Biol. Pharm. Bull. 2001, 24, 484–487. [Google Scholar] [CrossRef]
- Sen, S.; Roy, M.; Chakraborti, A.S. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J. Pharm. Pharmacol. 2011, 63, 287–296. [Google Scholar] [CrossRef]
- Thakur, V.; Nargis, S.; Gonzalez, M.; Pradhan, S.; Terreros, D.; Chattopadhyay, M. Role of glycyrrhizin in the reduction of inflammation in diabetic kidney disease. Nephron 2017, 137, 137–147. [Google Scholar] [CrossRef]
- Somayajulu, M.; McClellan, S.A.; Pitchaikannu, A.; Bessert, D.; Liu, L.; Steinle, J.; Hazlett, L.D. Effects of glycyrrhizin treatment on diabetic cornea. J. Ocul. Pharmacol. Ther. 2021, 37, 12–23. [Google Scholar] [CrossRef]
- Chen, F.; Song, J. Cardioprotective action of glycyrrhizin on diabetic rats with myocardial remodeling. J. Healthc. Eng. 2021, 2021, 6343677. [Google Scholar]
- Thakur, V.; Alcoreza, N.; Delgado, M.; Joddar, B.; Chattopadhyay, M. Cardioprotective effect of glycyrrhizin on myocardial remodeling in diabetic rats. Biomolecules 2021, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Loucas, B.D.; Suzuki, S.; Kobayashi, M.; Suzuki, F. Glycyrrhizin protects γ-irradiated mice from gut bacteria-associated infectious complications by improving miR-222-associated Gas5 RNA reduction in macrophages of the bacterial translocation site. J. Immunol. 2020, 204, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Akao, T. Effects of glycyrrhizin and glycyrrhetic acid on the growth, glycyrrhizin β-D-glucuronidase and 3β-hydroxysteroid dehydrogenase of human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kobashi, K. Glycyrrhizin stimulates growth of Eubacterium sp. strain GLH, a human intestinal anaerobe. Appl. Environ. Microbiol. 1988, 54, 2027–2030. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yoshida, T.; Isobe, K.; Rahman, S.M.J.; Nagase, F.; Ding, L.; Nakashima, I. Modulation by glycyrrhizin of the cell-surface expression of H-2 class I antigens on murine tumour cell lines and normal cell populations. Immunology 1990, 70, 405–410. [Google Scholar]
- Suzuki, F.; Schmitt, D.A.; Utsunomiya, T.; Pollard, R.B. Stimulation of host resistance against tumors by glycyrrhizin, an active component of licorice roots. In Vivo 1992, 6, 589–596. [Google Scholar]
- Madhiba, H.; Matsunaga, K. Augmented antiproliferative effect of tumor necrosis factor (TNF), lymphotoxin and glycyrrhizin in combined use with diethyldithiocarbamate on meth A tumor cells in vitro. Jpn. J. Exp. Med. 1990, 60, 67–71. [Google Scholar]
- Malagoli, M.; Castelli, M.; Baggio, A.; Cermelli, C.; Garuti, L.; Rossi, T. Effect of glycyrrhizin and its diastereoisomers on the growth of human tumour cells: Preliminary findings. Phytother. Res. 1998, 12 (Suppl. S1), S95–S97. [Google Scholar]
- Kobayashi, M.; Fujita, K.; Katakura, T.; Utsunomiya, T.; Pollard, R.B.; Suzuki, F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res. 2002, 22, 4053–4058. [Google Scholar]
- Hsiang, C.-Y.; Lai, I.-L.; Chao, D.-C.; Ho, T.-Y. Differential regulation of activator protein 1 activity by glycyrrhizin. Life Sci. 2002, 70, 1643–1656. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Cichoń, T.; Matuszczak, S.; Mitrus, I.; Lesiak, M.; Kobusińska, M.; Kamysz, W.; Jarosz, M.; Sieroń, A.; Szala, S. The role of glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Arch. Immunol. Ther. Exp. 2012, 60, 391–399. [Google Scholar] [CrossRef]
- Yasukawa, K.; Takido, M.; Takeuchi, M.; Nakagawa, S. Inhibitory effect of glycyrrhizin and caffeine on two-stage carcinogenesis in mice. Yakugaku Zasshi 1988, 108, 794–796. [Google Scholar] [CrossRef]
- Agarwal, R.; Wang, Z.Y.; Mukhtar, H. Inhibition of mouse skin tumor-initiating activity of DMBA by chronic oral feeding of glycyrrhizin in drinking water. Nutr. Cancer 1991, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Sultana, S. Glycyrrhizin exhibits potential chemopreventive activity on 12-O-tetradecanoyl phorbol-13-acetate-induced cutaneous oxidative stress and tumor promotion in Swiss albino mice. J. Enzyme Inhib. Med. Chem. 2007, 22, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Chu, Y.-L.; Jiang, Z.-B.; Chen, X.-M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell. Physiol. Biochem. 2014, 33, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.-P.; Wang, M.-J.; Zeng, X.; Chen, G.G.; Huang, R.-Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Chen, Y.; Liu, X.; Wang, J.; Qin, X.; Yuan, D.; Yu, T.; Chen, G.; Mi, Y.; et al. Glycyrrhizin suppresses the growth of human NSCLC cell line HCC827 by downregulating HMGB1 level. Biomed. Res. Int. 2018, 2018, 6916797. [Google Scholar] [CrossRef]
- Zhao, R.; Meng, Y.; Wang, Y.; Hou, C. Glycyrrhizin affects malignant biological behaviors of non-small cell lung cancer HCC827 and A549 cells via regulating miR-142/ZEB1 axis. Chin. J. Cancer Biother. 2019, 26, 1337–1344. [Google Scholar]
- Hiramoto, K.; Yamate, Y.; Goto, K.; Ohnishi, S.; Morita, A.; Yoshikawa, N.; Kawanishi, S. Glycyrrhizin ameliorates melanoma cell extravasation into mouse lungs by regulating signal transduction through HMGB1 and its receptors. J. Clin. Biochem. Nutr. 2021, 69, 52–60. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, Z.; Jiang, J.; Fu, W.; Hu, S. Glycyrrhizin enhances the antitumor activity of cisplatin in non-small cell lung cancer cells by influencing DNA damage and apoptosis. Oncol. Lett. 2025, 29, 207. [Google Scholar] [CrossRef]
- Shiota, G.; Harada, K.-I.; Ishida, M.; Tomie, Y.; Okubo, M.; Katayama, S.; Ito, I.; Kawasaki, H. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis 1999, 20, 59–63. [Google Scholar] [CrossRef]
- Paolini, M.; Barillari, J.; Broccoli, M.; Pozzetti, L.; Perocco, P.; Cantelli-Forti, G. Effect of liquorice and glycyrrhizin on rat liver carcinogen metabolizing enzymes. Cancer Lett. 1999, 145, 35–42. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Cheng, J.; Guo, J.; Li, W.-F.; Wei, H.-S. Glycyrrhizin down-regulates expression of tissue inhibitor of metalloproteinases-1. World Chin. J. Dig. 2005, 13, 2183–2187. [Google Scholar]
- Rahman, S.; Sultana, S. Chemopreventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity in Wistar rats. Chem. Biol. Interact. 2006, 160, 61–69. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Nakahashi, Y.; Hachimine, D.; Seki, T.; Okazaki, K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007, 31, 1465–1472. [Google Scholar] [CrossRef]
- Koike, K. Expression of junB is markedly stimulated by glycyrrhizin in a human hepatoma cell line. Oncol. Rep. 2011, 25, 609–617. [Google Scholar] [CrossRef]
- Yang, R.-F.; Zhang, F.-H.; Sun, S.-H.; Lin, L.-J.; Luan, Q.; Wu, Y. Metabolomics to analyze the therapy and adverse effect of glycyrrhizin capsules towards liver cancer. Lat. Am. J. Pharm. 2015, 34, 1677–1679. [Google Scholar]
- Zhang, X.; Yang, H.; Yue, S.; He, G.; Qu, S.; Zhang, Z.; Ma, B.; Ding, R.; Peng, W.; Zhang, H.; et al. The mTOR inhibition in concurrence with ERK1/2 activation is involved in excessive autophagy induced by glycyrrhizin in hepatocellular carcinoma. Cancer Med. 2017, 6, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-F.; Li, J.; Zhu, H. Glycyrrhizin mediates autophagy through the STAT3/survivin pathway to inhibit proliferation and angiogenesis in hepatoma cells. Cytotechnology 2025, 77, 149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Gao, L.-N.; Zhu, X.-N.; Zhang, Y.; Zhang, C.-N.; Xu, D.; Cui, Y.-L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef]
- Niwa, K.; Lian, Z.; Onogi, K.; Yun, W.U.; Tang, L.; Mori, H.; Tamaya, T. Preventive effects of glycyrrhizin on estrogen-related endometrial carcinogenesis in mice. Oncol. Rep. 2007, 17, 617–622. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, B.; Liang, Q.; Zhang, Y.; Cai, J.; Li, G. The selective effect of glycyrrhizin and glycyrrhetinic acid on topoisomerase IIα and apoptosis in combination with etoposide on triple negative breast cancer MDA-MB-231 cells. Eur. J. Pharmacol. 2017, 809, 87–97. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, X.; Ma, Q.; Zhao, W.; Jia, Y.; Dong, G.; Zhu, Y.; Jia, X.; Tong, Z. Glycyrrhizin inhibits the invasion and metastasis of breast cancer cells via upregulation of expressions of miR-200c and e-cadherin. Trop. J. Pharm. Res. 2020, 19, 1807–1813. [Google Scholar] [CrossRef]
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005, 16, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Zhu, Z.-F.; Gao, Y.; Huang, M.-H. Effect of glycyrrhizin on gastric cancer BGC-823 cell proliferation. World Chin. J. Dig. 2015, 23, 2868–2873. [Google Scholar] [CrossRef]
- Thiugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar]
- Chang, H.-Y.; Chen, S.-Y.; Wu, C.-H.; Lu, C.-C.; Yen, G.-C. Glycyrrhizin attenuates the process of epithelial-to-mesenchymal transition by modulating HMGB1-initiated novel signaling pathway in prostate cancer cells. J. Agric. Food Chem. 2019, 67, 3323–3332. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, F.; Khan, I.; Ansari, I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G0/G1 in HPV18+ human cervical cancer HeLa cell line. Biomed. Pharmacother. 2018, 97, 752–764. [Google Scholar] [CrossRef]
- Ahmad, A.; Tiwari, R.K.; Saeed, M.; Ahmad, I.; Ansari, I.A. Glycyrrhizin mediates downregulation of Notch pathway resulting in initiation of apoptosis and disruption in the cell cycle progression in cervical cancer cells. Nutr. Cancer 2022, 74, 622–639. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, S.; Wei, W. Glycyrrhizin impairs the progression and metastasis of cervical cancer through the protein kinase B/glycogen synthase kinase-3β/β-catenin signaling pathway. Curr. Top. Nutraceutical Res. 2024, 22, 895–900. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Sun, X.; Guo, X. Naturally occurring glycyrrhizin triterpene exerts anticancer effects on colorectal cancer cells via induction of apoptosis and autophagy and suppression of cell migration and invasion by targeting MMP-9 and MMP-2 expression. J. BUON 2020, 25, 188–193. [Google Scholar] [PubMed]
- Wang, G.; Hiramoto, K.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin attenuates carcinogenesis by inhibiting the inflammatory response in a murine model of colorectal cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, T.; Sun, S.; Wang, W.; Wu, Y.; Zhang, Q. Glycyrrhizin antagonizes the hypoxia-induced chemoresistance of osteosarcoma cells. Lat. Am. J. Pharm. 2016, 35, 1199–1205. [Google Scholar]
- Ge, S.; Lan, X.; Satoru, S. The inhibiting effect of glycyrrhizin on proliferation of the mice submandibular gland fibrosarcoma cell line in vitro. Zhonghua Kou Qiang Yi Xue Za Zhi 1998, 33, 341–343. [Google Scholar]
- Oh, C.; Kim, Y.; Eun, J.; Yokoyama, T.; Kato, M.; Nakashima, I. Induction of T lymphocyte apoptosis by treatment with glycyrrhizin. Am. J. Chin. Med. 1999, 27, 217–226. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Isobe, K.; Nagase, F.; Lwin, T.; Kato, M.; Hamaguchi, M.; Yokochi, T.; Nakashima, I. Glycyrrhizin as a promoter of the late signal transduction for interleukin-2 production by splenic lymphocytes. Immunology 1993, 79, 528–534. [Google Scholar]
- Zhang, Y.-H.; Kato, M.; Isobe, K.-I.; Hamaguchi, M.; Yokochi, T.; Nakashima, I. Dissociated control by glycyrrhizin of proliferation and IL-2 production of murine thymocytes. Cell. Immunol. 1995, 162, 97–104. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Isobe, K.-I.; Iwamoto, T.; Nakashima, I. Bidirectional control by glycyrrhizin of the growth response of lymphocytes stimulated through a receptor-bypassed pathway. Immunol. Lett. 1992, 32, 147–152. [Google Scholar] [CrossRef]
- Dai, J.H.; Iwatani, Y.; Ishida, T.; Terunuma, H.; Kasai, H.; Iwakula, Y.; Fujiwara, H.; Ito, M. Glycyrrhizin enhances interleukin-12 production in peritoneal macrophages. Immunology 2001, 103, 235–243. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Kobayashi, M.; Ito, M.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Glycyrrhizin restores the impaired IL-12 production in thermally injured mice. Cytokine 2001, 14, 49–55. [Google Scholar] [CrossRef]
- Luoxiu, Z.; Weiming, X.; DeJi, P.; Zhipei, T.; Meizheng, M.; Hongpi, Y. The effects of glycyrrhizin on cAMP and PGE2 contents of rat macrophages and some immunological functions. Acta Acad. Med. Shanghai 1988, 15, 101–106. [Google Scholar]
- Utsunomiya, T.; Kobayashi, M.; Ito, M.; Pollard, R.B.; Suzuki, F. Glycyrrhizin improves the resistance of MAIDS mice to opportunistic infection of Candida albicans through the modulation of MAIDS-associated type 2 T cell responses. Clin. Immunol. 2000, 95, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Schmitt, D.A.; Utsunomiya, T.; Pollard, R.B.; Suzuki, F. Inhibition of burn-associated suppressor cell generation by glycyrrhizin through the induction of contrasuppressor T cells. Immunol. Cell Biol. 1993, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, N.; Karimi, M.H.; Amirghofran, Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell. Immunol. 2012, 280, 44–49. [Google Scholar] [CrossRef]
- Noriaki, I.; Hiroshi, K.; Yasuhiro, H.; Kimio, Y.; Atsushi, I. Effects of glycyrrhizin and glycyrrhetinic acid on dexamethasone-induced changes in histamine synthesis of mouse mastocytoma P-815 cells and in histamine release from rat peritoneal mast cells. Biochem. Pharmacol. 1989, 38, 2521–2526. [Google Scholar] [CrossRef]
- Nakajima, N.; Utsunomiya, T.; Kobayashi, M.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. In vitro induction of anti-type 2 T cells by glycyrrhizin. Burns 1996, 22, 612–617. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Ikemoto, Y.; Arai, T.; Yamamoto, S.; Morisawa, S. Effects of glycyrrhizin on antibody production of PWM-stimulated lymphocytes in vitro. Jpn. J. Allergol. 1984, 33, 328–335. [Google Scholar]
- Jiang, R.-H.; Xu, J.-J.; Zhu, D.-C.; Li, J.-F.; Zhang, C.-X.; Lin, N.; Gao, W.-Y. Glycyrrhizin inhibits osteoarthritis development through suppressing the PI3K/AKT/NF-κB signaling pathway: In vivo and in vitro. Food Funct. 2020, 11, 2126–2136. [Google Scholar]
- Luo, Y.; Li, J.; Wang, B.; Zhang, Q.; Bian, Y.; Wang, R. Protective effect of glycyrrhizin on osteoarthritis cartilage degeneration and inflammation response in a rat model. J. Bioenerg. Biomembr. 2021, 53, 285–293. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Si, Z.; Yu, L.; Hou, L. Glycyrrhizin, an HMGB1 inhibitor, suppresses interleukin-1β-induced inflammatory responses in chondrocytes from patients with osteoarthritis. Cartilage 2021, 13 (Suppl. S2), 947S–955S. [Google Scholar] [CrossRef]
- Liu, X.; Zhuang, J.; Wang, D.; Lv, L.; Zhu, F.; Yao, A.; Xu, T. Glycyrrhizin suppresses inflammation and cell apoptosis by inhibition of HMGB1 via p38/p-JUK signaling pathway in attenuating intervertebral disc degeneration. Am. J. Transl. Res. 2019, 11, 5105–5113. [Google Scholar]
- Hu, Z.; Xiao, M.; Cai, H.; Li, W.; Fang, W.; Long, X. Glycyrrhizin regulates rat TMJOA progression by inhibiting the HMGB1-RAGE/TLR4-NF-κB/AKT pathway. J. Cell. Mol. Med. 2022, 26, 925–936. [Google Scholar] [CrossRef]
- Shafik, N.M.; El-Esawy, R.O.; Mohamed, D.A.; Deghidy, E.A.; El-Deeb, O.S. Regenerative effects of glycyrrhizin and/or platelet rich plasma on type-ii collagen induced arthritis: Targeting autophagy machinery markers, inflammation and oxidative stress. Arch. Biochem. Biophys. 2019, 675, 108095. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-C.; Wang, M.-J.; Chen, X.-M.; Yu, W.-L.; Chu, Y.-L.; He, X.-H.; Huang, R.-Y. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget 2016, 7, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, C.; Liu, Z.; Yang, D.; Chen, S.; Cha, A.; Wu, Z.; Lu, A. Therapeutic effect of combined triptolide and glycyrrhizin treatment on rats with collagen induced arthritis. Planta Med. 2007, 73, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, C.; Zhu, X.; Li, Y.; Yu, R.; Xu, W. Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-κB and MAPK and activating AMPK/Nrf2. Calcif. Tissue Int. 2018, 103, 324–337. [Google Scholar] [CrossRef]
- Yamada, C.; Ho, A.; Akkaoui, J.; Garcia, C.; Duarte, C.; Movila, A. Glycyrrhizin mitigates inflammatory bone loss and promotes expression of senescence-protective sirtuins in an aging mouse model of periprosthetic osteolysis. Biomed. Pharmacother. 2021, 138, 111503. [Google Scholar] [CrossRef]
- Tang, Y.; Lv, X.L.; Bao, Y.Z.; Wang, J.R. Glycyrrhizin improves bone metabolism in ovariectomized mice via inactivating NF-κB signaling. Climacteric 2021, 24, 253–260. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin use for multi-drug resistant Pseudomonas aeruginosa: In vitro and in vivo studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2978–2989. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshida, S.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. Pivotal advance: Glycyrrhizin restores the impaired production of β-defensins in tissues surrounding the burn area and improves the resistance of burn mice to Pseudomonas aeruginosa wound infection. J. Leukoc. Biol. 2010, 87, 35–41. [Google Scholar] [CrossRef]
- Sela, M.N.; Steinberg, D.; Segal, R. Inhibition of the activity of glucosyltransferase from Streptococcus mutans by glycyrrhizin. Oral Microbiol. Immunol. 1987, 2, 125–128. [Google Scholar] [CrossRef]
- Khan, U.; Karmakar, B.C.; Basak, P.; Paul, S.; Gope, A.; Sarkar, D.; Mukhopadhyay, A.K.; Dutta, S.; Bhattacharya, S. Glycyrrhizin, an inhibitor of HMGB1 induces autolysosomal degradation function and inhibits Helicobacter pylori infection. Mol. Med. 2023, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, N.J.; McClellan, S.A.; Somayajulu, M.; Pitchaikannu, A.; Bessert, D.; Peng, X.; Huitsing, K.; Stemmer, P.M.; Hazlett, L.D. Effects of glycyrrhizin on multi-drug resistant Pseudomonas aeruginosa. Pathogens 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, T.; Kobayashi, M.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Effects of glycyrrhizin, an active component of licorice roots, on Candida albicans infection in thermally injured mice. Clin. Exp. Immunol. 1999, 116, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.-J.; Wang, Y.-L.; Chen, J.; Lv, C.-Y.; Liu, W.-W.; Chen, L.; He, J.-Y.; Jiang, D.-Y.; Xia, X.-W.; et al. The in vitro antiviral mechanisms of stronger Neo-minophafen C, an established formulation of compound glycyrrhizin. Anti-Infect. Agents 2018, 16, 136–143. [Google Scholar] [CrossRef]
- Baltina, L.A.; Zarubaev, V.V.; Baltina, L.A.; Orshanskaya, I.A.; Fairushina, A.I.; Kiselev, O.I.; Yunusov, M.S. Glycyrrhizic acid derivatives as influenza A/H1N1 virus inhibitors. Bioorg Med Chem Lett. 2015, 25, 1742–1746. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Ravenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2002, 70, 229–236. [Google Scholar] [CrossRef]
- Ito, M.; Nakashima, H.; Baba, M.; Pauwels, R.; De Clercq, E.; Shigeta, S.; Yamamoto, N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)]. Antivir. Res. 1987, 7, 127–137. [Google Scholar] [CrossRef]
- Ito, M.; Sato, A.; Hirabayashi, K.; Tanabe, F.; Shigeta, S.; Baba, M.; De Clercq, E.; Nakashima, H.; Yamamoto, N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antivir. Res. 1988, 10, 289–298. [Google Scholar] [CrossRef]
- Nakashima, H.; Matsui, T.; Yoshida, O.; Isowa, Y.; Kido, Y.; Motoki, Y.; Ito, M.; Shigeta, S.; Mori, T.; Yamamoto, N. A new anti-human immunodeficiency virus substance, glycyrrhizin sulfate; endowment of glycyrrhizin with reverse transcriptase-inhibitory activity by chemical modification. Jpn. J. Cancer Res. 1987, 78, 767–771. [Google Scholar]
- Hattori, T.; Ikematsu, S.; Koito, A.; Matsushita, S.; Maeda, Y.; Hada, M.; Fujimaki, M.; Takatsuki, K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antivir. Res. 1989, 11, 255–261. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Furusawa, S.; Ito, M.; Shibata, S.; Baba, M.; Ito, M.; Shigeta, S.; Nakashima, H.; Yamamoto, N. Antiviral activities of glycyrrhizin and its modified compounds against herpes simplex virus type 1 and human immunodeficiency virus type 1 in vitro. Antivir. Res. 1991, 16, 365–376. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yasuoka, A.; Tachikawa, N.; Teruya, K.; Genka, I.; Yamaguchi, M.; Yasuoka, C.; Kikuchi, Y.; Aoki, M.; Oka, S. Mitigation of hepatocellular injury caused by HAART with glycyrrhizin compound in patients co-infected with HIV and HCV. Jpn. J. Infect. Dis. 1999, 52, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Kobayashi, M.; Li, X.-D.; Pollard, R.B.; Suzuki, F. Glycyrrhizin inhibits R5 HIV replication in peripheral blood monocytes treated with 1-methyladenosine. Pathobiology 2005, 72, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sakai, H.; Suzuki, S.; Sugai, K.; Akutsu, Y.; Ishikawa, M.; Seino, Y.; Ishida, N.; Uchida, T.; Kariyone, S.; et al. Effects of Glycyrrhizin (SNMC: Stronger Neo-Minophagen C®) in Hemophilia Patients with HIV Infection. Tohoku J. Exp. Med. 1989, 158, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Badam, L. Glycyrrhizin: An alternate drug for Pneumocystis carinii pneumonia in AIDS patients. J. Assoc. Physicians India 2002, 50, 287–288. [Google Scholar]
- Baba, M.; Shigeta, S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antivir. Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Kobayashi, M.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Glycyrrhizin (20β-carboxy-11-oxo-30-norolean-12-en-3β-yl-2-O-β-d-glucopyranuronosyl-α-d-glucopyranosiduronic acid) improves the resistance of thermally injured mice to opportunistic infection of herpes simplex virus type 1. Immunol. Lett. 1995, 44, 59–66. [Google Scholar] [CrossRef]
- Cermelli, C.; Portolani, M.; Colombar, B.; Castelli, M.; Baggio, G.; Bossa, R.; Galatulas, I.; Rossi, T. Activity of glycyrrhizin and its diastereoisomers against two new human herpesviruses: HHV-6 and HHV-7. Phytother. Res. 1996, 10 (Suppl. S1), S27–S28. [Google Scholar]
- Huang, W.; Chen, X.; Li, Q.; Li, P.; Zhao, G.; Xu, M.; Xie, P. Inhibition of intercellular adhesion in Herpes Simplex Virus infection by glycyrrhizin. Cell Biochem. Biophys. 2012, 62, 137–140. [Google Scholar] [CrossRef]
- Aikawa, Y.; Yoshiike, T.; Ogawa, H. Effect of glycyrrhizin on pain and HLA-DR antigen expression on CD8-positive cells in peripheral blood of herpes zoster patients in comparison with other antiviral agents. Skin Pharmacol. Physiol. 1990, 3, 268–271. [Google Scholar] [CrossRef]
- Numazaki, K.; Chiba, S.; Umetsu, M. Effect of glycyrrhizin in children with liver dysfunction associated with cytomegalovirus infection. Tohoku J. Exp. Med. 1994, 172, 147–153. [Google Scholar] [CrossRef]
- Noda, Y.; Satoh, S.; Arai, Y.; Sato, Y.; Kawauchi, K.; Matsuyama, S. High-dose glycyrrhizin and human immunoglobulin were effective for cytomegalovirus retinitis in a hemophiliac patient with human immunodeficiency virus. Jpn. J. Clin. Ophthalmol. 1994, 48, 1357–1362. [Google Scholar]
- Chen, X.-X.; Zhou, H.-X.; Qi, W.-B.; Ning, Z.-Y.; Ma, Y.-J.; Li, Y.-L.; Wang, G.-C.; Chen, J.-X. Antiviral effects of the combination of glycyrrhizin and ribavirin against influenza A H1N1 virus infection in vivo. Yaoxue Xuebao 2015, 50, 966–972. [Google Scholar]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Ogbomo, H.; Altenbrandt, B.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immunol. 2010, 199, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE 2011, 6, e19705. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob. Agents Chemother. 1997, 41, 551–556. [Google Scholar] [CrossRef]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H.J. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009, 83, 171–178. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef] [PubMed]
- Gowda, P.; Patrick, S.; Joshi, S.D.; Kumawat, R.K.; Sen, E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 2021, 142, 155496. [Google Scholar] [CrossRef] [PubMed]
- Tolah, A.M.; Altayeb, L.M.; Alandijany, T.A.; Dwivedi, V.D.; El-Kafrawy, S.A.; Azhar, E.I. Computational and in vitro experimental investigations reveal anti-viral activity of licorice and glycyrrhizin against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals 2021, 14, 1216. [Google Scholar] [CrossRef] [PubMed]
- He, M.-F.; Liang, J.-H.; Shen, Y.-N.; Zhang, J.-W.; Liu, Y.; Yang, K.-Y.; Liu, L.-C.; Wang, J.; Xie, Q.; Hu, C.; et al. Glycyrrhizin inhibits SARS-CoV-2 entry into cells by targeting ACE2. Life 2022, 12, 1706. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Mohamed, H.S.; Abd-ellatief, R.B.; Gomaa, M.A.; Hammam, D.S. Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: A randomized clinical trial. Inflammopharmacology 2022, 30, 477–486. [Google Scholar] [CrossRef]
- Harada, S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biomed. J. 2005, 392, 191–199. [Google Scholar] [CrossRef]
- Sato, H.; Kageyama, S.; Kurokawa, M.; Shiraki, K.; Sato, H.; Kageyama, S.; Yamamoto, H.; Aoki, E.; Kurokawa, M.; Aoki, E. Glycyrrhizin renders cells resistant to apoptosis induced by human and feline immunodeficiency virus. J. Tradit. Med. 2011, 28, 139–148. [Google Scholar]
- Badam, L. In vitro antiviral activity of indigenous glycyrrhizin, licorice and glycyrrhizic acid (Sigma) on Japanese encephalitis virus. J. Commun. Dis. 1997, 29, 91–99. [Google Scholar]
- Crance, J.M.; Scaramozzino, N.; Jouan, A.; Garin, D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: Antiviral compounds active against pathogenic flaviviruses. Antivir. Res. 2003, 58, 73–79. [Google Scholar] [CrossRef]
- Huan, C.-C.; Wang, H.-X.; Sheng, X.-X.; Wang, R.; Wang, X.; Mao, X. Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Arch. Virol. 2017, 162, 1467–1476. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Y.; Kang, Y.; Xu, W.; Jiang, L.; Guo, T.; Huan, C. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int. J. Mol. Sci. 2020, 21, 2961. [Google Scholar] [CrossRef]
- Duan, E.; Wang, D.; Fang, L.; Ma, J.; Luo, J.; Chen, H.; Li, K.; Xiao, S. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antivir. Res. 2015, 120, 122–125. [Google Scholar] [CrossRef]
- Ohtsuki, K.; Iahida, N. Inhibitory effect of glycyrrhizin on polypeptide phosphorylation by polypeptide-dependent protein kinase (kinase P) in vitro. Biochem. Biophys. Res. Commun. 1988, 157, 597–604. [Google Scholar] [CrossRef]
- Ahmad Bhat, S.; Islam Siddiqui, Z.; Ahmad Parray, Z.; Sultan, A.; Afroz, M.; Ali Azam, S.; Rahman Farooqui, S.; Naqui Kazim, S. Naturally occurring hmgb1 inhibitor delineating the anti-hepatitis B virus mechanism of glycyrrhizin via in vitro and in silico studies. J. Mol. Liq. 2022, 356, 119029. [Google Scholar] [CrossRef]
- Kumagai, A.; Nishino, K.; Shimomura, A.; Kin, T.; Yamamura, Y. Effect of glycyrrhizin on estrogen action. Endocr. Jpn. 1967, 14, 34–38. [Google Scholar]
- Ren, Q.; Jiang, X.; Paudel, Y.N.; Gao, X.; Gao, D.; Zhang, P.; Sheng, W.; Shang, X.; Liu, K.; Zhang, X.; et al. Co-treatment with natural hmgb1 inhibitor glycyrrhizin exerts neuroprotection and reverses Parkinson’s disease like pathology in zebrafish. J. Ethnopharmacol. 2022, 292, 115234. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Kimura, M.; Sawano, A.; Iwasaki, H.; Nakajima, Y.; Matano, S.; Kasai, M. Prevention of cancer chemotherapeutic agent-induced toxicity in postoperative breast cancer patients with glycyrrhizin (SNMC). Gan No Rinsho 1986, 32, 869–872. [Google Scholar] [PubMed]
- Kong, X.; Liang, B.; Liao, G.; Feng, Q.; Li, Y.; Yin, X.; Zhou, T. Clinical effect observation of compound glycyrrhizin on the prevention and cure of cytarabine syndromes. J. Leuk. Lymphoma 2018, 27, 529–532. [Google Scholar]
- Nguyen, N.N.; Lin, C.-Y.; Tsai, W.-L.; Huang, H.-Y.; Chen, C.-M.; Tung, Y.-T.; Chen, Y.-C. Natural sweetener glycyrrhizin protects against precocious puberty by modulating the gut microbiome. Life Sci. 2024, 350, 122789. [Google Scholar] [CrossRef]
- Steinberg, D.; Sgan-Cohen, H.D.; Stabholz, A.; Pizanty, S.; Segal, R.; Sela, M.N. The anticariogenic activity of glycyrrhizin: Preliminary clinical trials. Isr. J. Dent. Sci. 1989, 2, 153–157. [Google Scholar]
- Sun, Y.; Zhao, B.; Li, Z.; Wei, J. Beneficial effects of glycyrrhizin in chronic periodontitis through the inhibition of inflammatory response. Dose-Response 2020, 18, 1559325820952660. [Google Scholar] [CrossRef]
- Assafim, M.; Ferreira, M.S.; Frattani, F.S.; Guimarães, J.A.; Monteiro, R.Q.; Zingali, R.B. Counteracting effect of glycyrrhizin on the hemostatic abnormalities induced by Bothrops jararaca snake venom. Br. J. Pharmacol. 2006, 148, 807–813. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Yang, J. A semi-physiologically based pharmacokinetic pharmacodynamic model for glycyrrhizin-induced pseudoaldosteronism and prediction of the dose limit causing hypokalemia in a virtual elderly population. PLoS ONE 2014, 9, e114049. [Google Scholar] [CrossRef]
- Nishiyama, S.; Tarutani, M.; Isonokami, M.; Takijiri, C.; Yoshikawa, K. A case of pseudoaldosteronism with muscle weakness due to oral glycyrrhizin. Skin Res. 1991, 33, 543–548. [Google Scholar]
- Fujiwara, Y.; Kikkawa, R.; Nakata, K.; Kitamura, E.; Takama, T.; Shigeta, Y. Note: Hypokalemia and sodium retention in patients with diabetes and chronic hepatitis receiving insulin and glycyrrhizin. Endocr. Jpn. 1983, 30, 243–249. [Google Scholar] [CrossRef]
- Hoshiai, M.; Kanemoto, N.; Ide, M.; Kobayashi, T. A case of ventricular flutter and fibrillation due to glycyrrhizin-induced pseudoaldosteronism. Respir. Circ. 1980, 28, 169–172. [Google Scholar]
- Takabatake, H.; Hirai, T.; Shiotani, K. A case of glycyrrhizin-induced pseudoaldosteronism with congestive heart failure in hypertrophic cardiomyopathy. IRYO Jpn. J. Natl. Med. Serv. 1989, 43, 1331–1335. [Google Scholar]
- Shintani, S.; Murase, H.; Tsukagoshi, H.; Shiigai, T. Glycyrrhizin (licorice)-induced hypokalemic myopathy: Report of 2 cases and review of the literature. Eur. Neurol. 1992, 32, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Maruyama, T.; Maruyama, K.; Yanagawa, S.; Tako, K.; Yanagisawa, N. Myotonic and repetitive discharges in hypokalemic myopathy associated with glycyrrhizin-induced hypochloremia. J. Neurol. Sci. 1992, 107, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Zhow, J.; Ichinose, H.; Yamamoto, S.; Nakabayashi, K.-I.; Yamakage, M. A case of respiratory arrest and laryngeal edema resulting from glycyrrhizin-induced severe hypokalemic myopathy. Anesth. Resusc. 2011, 47, 65–68. [Google Scholar]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nakama, Y.; Maruhashi, T.; Kagawa, E.; Dai, K.; Aokage, T.; et al. Clinical profile of patients with symptomatic glycyrrhizin-induced hypokalemia. J. Am. Geriatr. Soc. 2008, 56, 1579–1581. [Google Scholar] [CrossRef]
- Rossi, T.; Fano, R.A.; Castelli, M.; Malagoli, M.; Ruberto, A.I.; Baggio, G.; Zennaro, R.; Migaldi, M.; Barbolini, G. Correlation between high intake of glycyrrhizin and myolysis of the papillary muscles: An experimental in vivo study. Pharmacol. Toxicol. 1999, 85, 221–222. [Google Scholar] [CrossRef]
- Sobotka, T.J.; Spaid, S.L.; Brodie, R.E.; Reed, G.F. Neurobehavioral toxicity of ammoniated glycyrrhizin, a licorice component, in rats. Neurobehav. Toxicol. Teratol. 1981, 3, 37–44. [Google Scholar]
- Paolini, M.; Pozzetti, L.; Sapone, A.; Cantelli-Forti, G. Effect of licorice and glycyrrhizin on murine liver CYP-dependent monooxygenases. Life Sci. 1998, 62, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshino, T.; Maki, Y.; Ishiuchi, K.; Namiki, T.; Ogawa-Ochiai, K.; Minamizawa, K.; Makino, T.; Nakamura, T.; Mimura, M.; et al. Identification of glycyrrhizin metabolites in humans and of a potential biomarker of liquorice-induced pseudoaldosteronism: A multi-centre cross-sectional study. Arch. Toxicol. 2019, 93, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G. Glycyrrhizin: Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2006, 50, 1087–1088. [Google Scholar] [CrossRef]
- Ishida, S.; Sakiya, Y.; Ichikawa, T.; Taira, Z. Dose-Dependent Pharmacokinetics of Glycyrrhizin in Rats. Chem. Pharm. Bull. 1992, 40, 1917–1920. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Liao, J.-F.; Shum, A.Y.; Chen, C.-F. Pharmacokinetics of glycyrrhizin after intravenous administration to rats. J. Pharm. Sci. 1992, 81, 961–963. [Google Scholar] [CrossRef]
- Shibata, N.; Shimokawa, T.; Jiang, Z.-Q.; Jeong, Y.-I.; Ohno, T.; Kimura, G.; Yoshikawa, Y.; Koga, K.; Murakami, M.; Takada, K. Characteristics of intestinal absorption and disposition of glycyrrhizin in mice. Biopharm. Drug Dispos. 2000, 21, 95–101. [Google Scholar] [CrossRef]
- Koga, K.; Tomoyama, M.; Ohyanagi, K.; Takada, K. Pharmacokinetics of glycyrrhizin in normal and albumin-deficient rats. Biopharm. Drug Dispos. 2008, 29, 373–381. [Google Scholar] [CrossRef]
- Zhong, Y.-M.; Wang, S.-J.; Zeng, J.; Huang, L.-H.; Cheng, X.-G.; Wang, G.-X.; Zang, L.-Q. Metabolism of glycyrrhizin and glycyrrhetinic acid in the in situ vascularly perfused rat intestine-liver model. Chin. Pharmacol. Bull. 2014, 30, 501–505. [Google Scholar]
- Kočevar Glavač, N.; Kreft, S. Excretion profile of glycyrrhizin metabolite in human urine. Food Chem. 2012, 131, 305–308. [Google Scholar] [CrossRef]
- Chen, M.-F.; Shimada, F.; Kato, H.; Yano, S.; Kanaoka, M. Effect of oral administration of glycyrrhizin on the pharmacokinetics of prednisolone. Endocr. Jpn. 1991, 38, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.-H.; He, Y.-J.; Chen, Y.; Fan, L.; Zhang, W.; Tan, Z.-R.; Huang, Y.-F.; Guo, D.; Hu, D.-L.; Wang, D.; et al. Effect of glycyrrhizin on the activity of CYP3A enzyme in humans. Eur. J. Clin. Pharmacol. 2010, 66, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.; Huang, X.; Su, Y.; Ji, J.; Su, Y.; Jiang, Z.; Zhang, L. Glycyrrhizin accelerates the metabolism of triptolide through induction of CYP3A in rats. J. Ethnopharmacol. 2014, 152, 358–363. [Google Scholar] [CrossRef]
- Imai, T.; Sakai, M.; Ohtake, H.; Azuma, H.; Otagiri, M. In vitro and in vivo evaluation of the enhancing activity of glycyrrhizin on the intestinal absorption of drugs. Pharm. Res. 1999, 16, 80–86. [Google Scholar] [CrossRef]
- He, R.; Xu, Y.; Peng, J.; Ma, T.; Li, J.; Gong, M. The effects of 18β-glycyrrhetinic acid and glycyrrhizin on intestinal absorption of paeoniflorin using the everted rat gut sac model. J. Nat. Med. 2017, 71, 198–207. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Lv, J. Effects of glycyrrhizin on the pharmacokinetics of paeoniflorin in rats and its potential mechanism. Pharm. Biol. 2019, 57, 550–554. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Wang, H.; Feng, L. Effects of glycyrrhizin on the pharmacokinetics of puerarin in rats. Xenobiotica 2018, 48, 1157–1163. [Google Scholar] [CrossRef]
- Wang, H.; Dong, L.; Qu, F.; He, H.; Sun, W.; Man, Y.; Jiang, H. Effects of glycyrrhizin on the pharmacokinetics of nobiletin in rats and its potential mechanism. Pharmaceuticals 2020, 58, 352–356. [Google Scholar] [CrossRef]
- Ao, Y.; Chen, J.; Yue, J.; Peng, R.-X. Effects of 18α-glycyrrhizin on the pharmacodynamics and pharmacokinetics of glibenclamide in alloxan-induced diabetic rats. Eur. J. Pharmacol. 2008, 587, 330–335. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, X.; Liang, F.; Tang, Y. Glycyrrhizin attenuates caspase-11-dependent immune responses and coagulopathy by targeting high mobility group box 1. Int. Immunopharmacol. 2022, 107, 108713. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yuan, L.-B.; Hu, L.; Wu, W.; Yin, L.; Hou, J.-L.; Liu, Y.-H.; Zhou, L.-S. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol. Sin. 2012, 33, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Cao, Z.; Liu, Y. Glycyrrhizin protects spinal cord and reduces inflammation in spinal cord ischemia-reperfusion injury. Int. J. Neurosci. 2013, 123, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Wakita, A.; Banno, S.; Ogawa, K.; Kanai, M.; Ueda, R. Non-insulin-dependent Diabetes Mellitus in an Elderly Patient with Hypoglycemic Attacks Induced by Glycyrrhizin Administration. J. Jpn. Diabetes Soc. 2000, 43, 687–693. [Google Scholar]
- Li, X.-L.; Zhou, A.-G.; Zhang, L.; Chen, W.-J. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int. J. Mol. Sci. 2011, 12, 905–916. [Google Scholar] [CrossRef]
- Li, J.; Fan, X.; Wang, Q. Hypertensive crisis with 2 target organ impairment induced by glycyrrhizin. Medicine 2018, 97, e0073. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef]
- Okamoto, T.; Kanda, T. Glycyrrhizin protects mice from concanavalin A-induced hepatitis without affecting cytokine expression. Int. J. Mol. Med. 1999, 4, 149–152. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Abe, K.; Wake, K.; Takata, M.; Hatta, A.; Sato, T.; Inoue, H. Hepatic protection by glycyrrhizin and inhibition of iNOS expression in concanavalin A-induced liver injury in mice. Inflamm. Res. 2009, 58, 593–599. [Google Scholar] [CrossRef]
- Miyaji, C.; Miyakawa, R.; Watanabe, H.; Kawamura, H.; Abo, T. Mechanisms underlying the activation of cytotoxic function mediated by hepatic lymphocytes following the administration of glycyrrhizin. Int. Immunopharmacol. 2002, 2, 1079–1086. [Google Scholar] [CrossRef]
- Ohta, W.; Iwamura, K. Treatment of non-A, non-B hepatitis with glycyrrhizin. Nippon Rinsho 1988, 46, 2681–2688. [Google Scholar]
- Van Rossum, T.G.J.; Vulto, A.G.; Hop, W.C.J.; Brouwer, J.T.; Schalm, S.W. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: A double-blind placebo-controlled randomized trial. Eur. J. Gastroenterol. Hepatol. 1998, 10, A89. [Google Scholar] [CrossRef]
- Veldt, B.J.; Hansen, B.E.; Ikeda, K.; Verhey, E.; Suzuki, H.; Schalm, S.W. Long-term clinical outcome and effect of glycyrrhizin in 1093 chronic hepatitis C patients with non-response or relapse to interferon. Scand. J. Gastroenterol. 2006, 41, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hayashi, N.; Katayama, K.; Hagiwara, H.; Kasahara, A.; Kashiwagi, T.; Yoshihara, H.; Fusamoto, H.; Kamada, T. Effect of glycyrrhizin on viral replication and quasispecies in patients with type C chronic hepatitis. Int. Hepatol. Commun. 1997, 6, 233–238. [Google Scholar] [CrossRef]
- Yoshino, T.; Shimada, S.; Homma, M.; Makino, T.; Mimura, M.; Watanabe, K. Clinical risk factors of licorice-induced pseudoaldosteronism based on glycyrrhizin-metabolite concentrations: A narrative review. Front. Nutr. 2021, 8, 719197. [Google Scholar] [CrossRef] [PubMed]
- Jitrangsri, K.; Kamata, K.; Akiba, M.; Yajiri, Y.; Ishibashi, M.; Tatsuzaki, J.; Ishikawa, T. Is 18α-glycyrrhizin a real natural product? Improved preparation of 18α-glycyrrhizin from 18β-glycyrrhizin as a positive standard for HPLC analysis of licorice extracts. J. Nat. Med. 2022, 76, 367–378. [Google Scholar] [CrossRef]
- Huo, X.; Meng, X.; Zhang, J.; Zhao, Y. Hepatoprotective effect of different combinations of 18α- and 18β-glycyrrhizic acid against CCl4-induced liver injury in rats. Biomed. Pharmacother. 2020, 122, 109354. [Google Scholar] [CrossRef]
- Tabuchi, M.; Imamura, S.; Kawakami, Z.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of 18β-glycyrrhetinic acid, a major metabolite of glycyrrhizin in glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell. Mol. Neurobiol. 2012, 32, 1139–1146. [Google Scholar] [CrossRef]
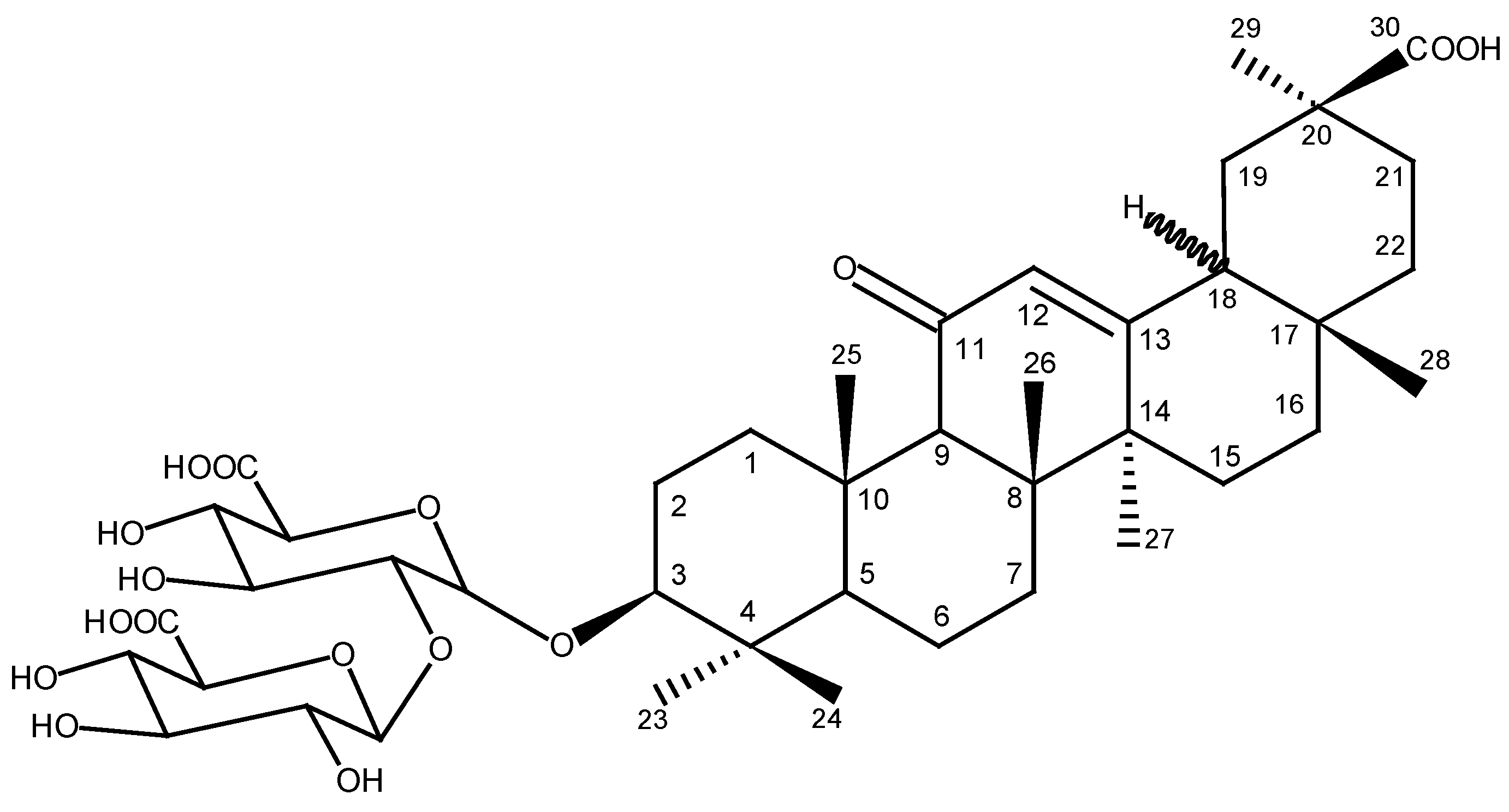
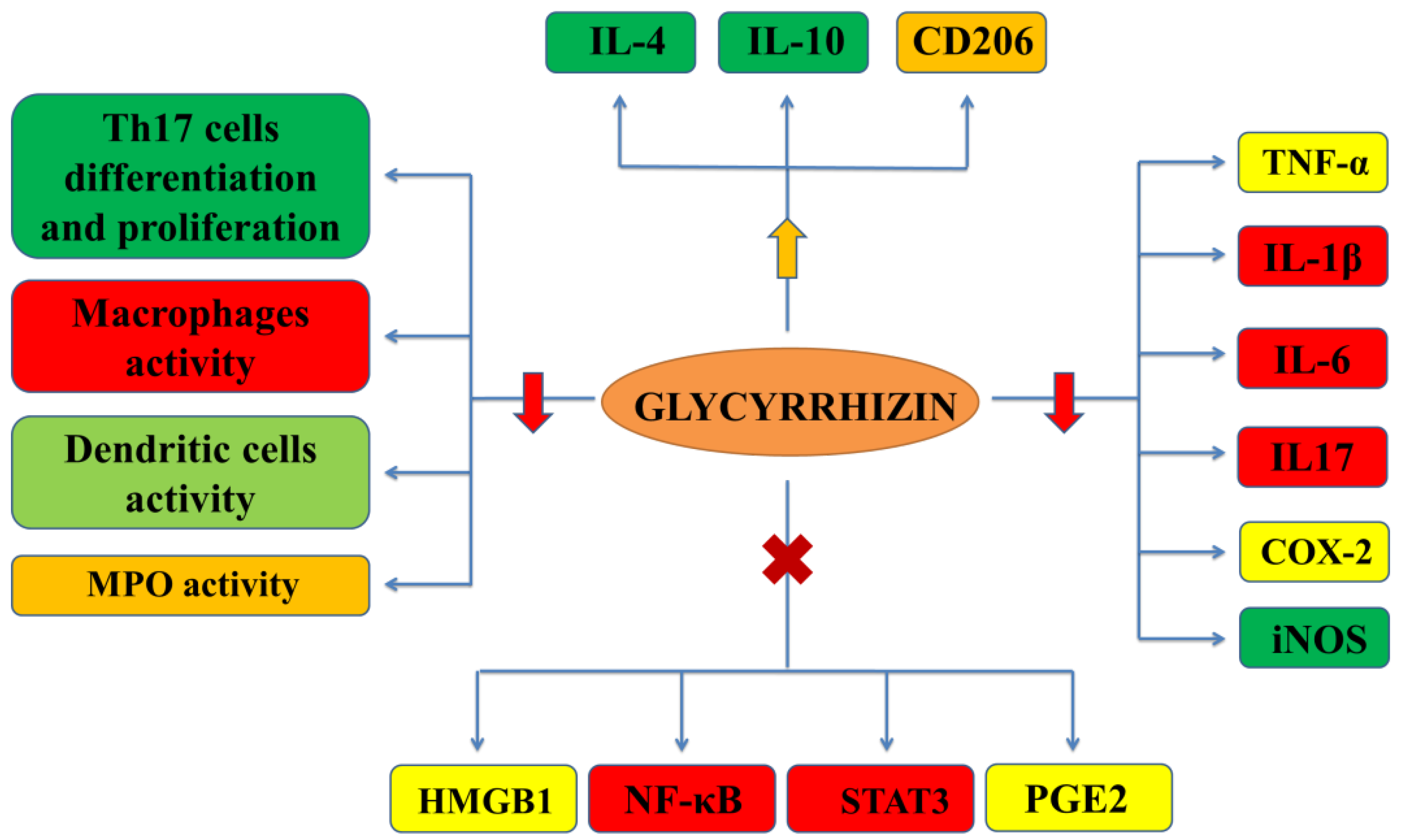

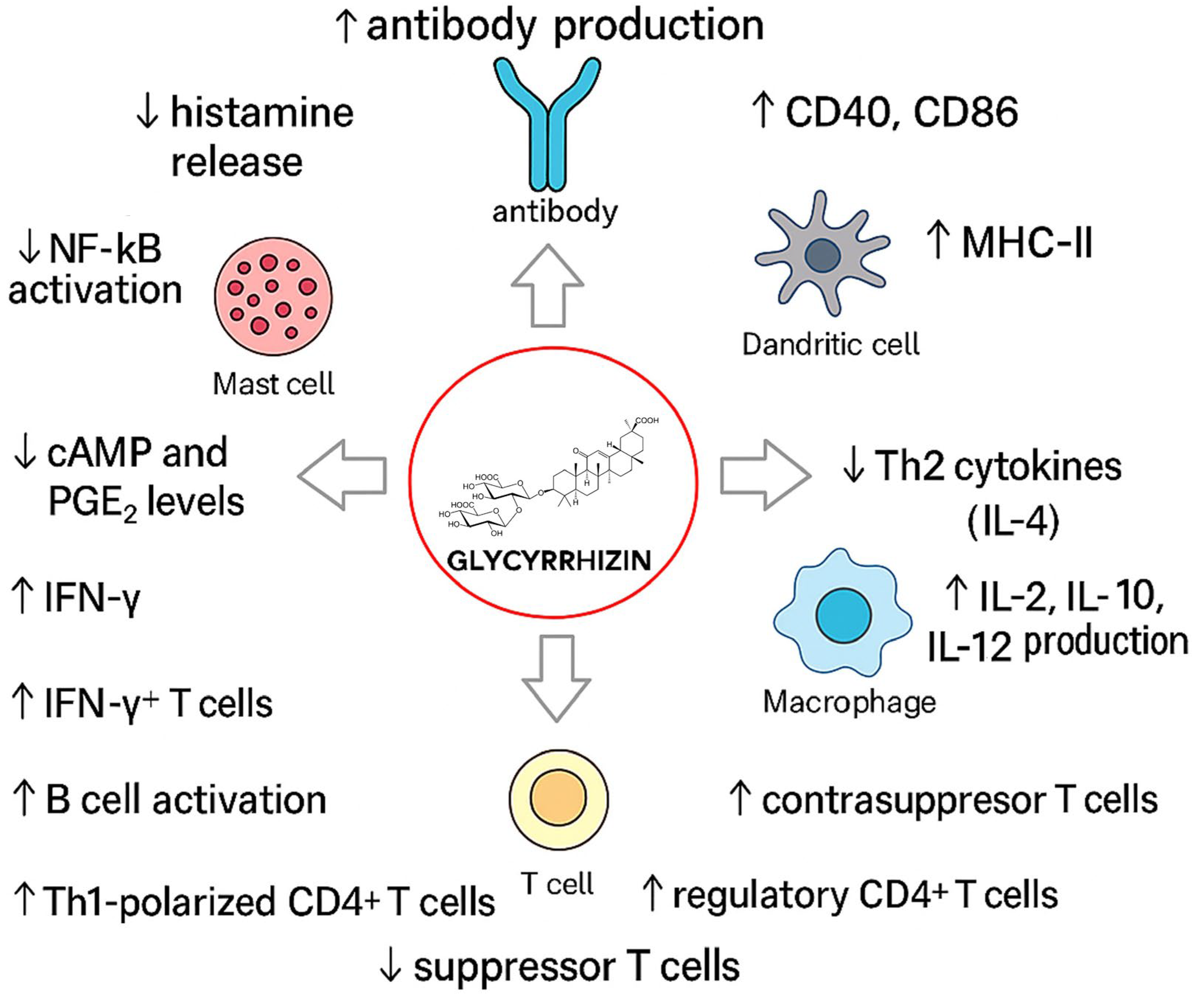
| Cancer Type | Model | Dose | Results | Mechanism | Reference |
|---|---|---|---|---|---|
| Skin Cancer | Sencar mice (in vivo) | Oral feeding of 100–250 mg/kg | Prolonged tumor latency, reduced tumor number, and inhibited [3H]DMBA–DNA binding. | Inhibits carcinogen metabolism and DNA adduct formation | [324] |
| Swiss albino mice (in vivo) | 2.0 and 4.0 mg/0.2 mL acetone/animal (topical) | Decreased lipid peroxidation, ODC activity, and DNA synthesis while restoring GSH and its enzymes, protecting against TPA-induced oxidative stress | Reduces oxidative stress, lipid peroxidation, and inflammation; restores GSH, lowers ODC | [325] | |
| Lung Cancer | A549 and NCI-H23 lung adenocarcinoma cell lines, and female nude mice for tumor formation (in vivo/in vitro) | 0.25–2.5 mM | Induced apoptosis via TxAS inhibition | Induces apoptosis; inhibits TxAS, STAT3, Survivin, HMGB1, JAK/STAT | [326] |
| Nude mice with human lung adenocarcinoma xenografts (in vivo) | GL (15, 45 and 135 mg/kg), cisplatin (2.5 mg/kg) | Co-treatment reduced TxAS and PCNA expression, improved liver/kidney function, and suppressed tumor growth via the TxA2 pathway. | Suppresses PCNA, TxA2 signaling | [327] | |
| Human NSCLC PDX mice model (in vivo) | 100 mg/kg, i.p. | Inhibited migration and invasion of lung cancer cells by suppressing HMGB1 levels; reduced JAK/STAT pathway activity. | Inhibits angiogenesis via HMGB1/RAGE, TLR4, NF-κB | [328] | |
| HCC827 and A549 non-small cell lung cancer (NSCLC) cells (in vitro) | 3 mmol/L | Upregulated miR-142, leading to downregulation of ZEB1 and inhibition of malignant behaviors in NSCLC cells. | Regulates miR-142/ZEB1 axis | [329] | |
| B16 melanoma metastasis in mice (in vivo) | 15 mg/kg orally, once every 2 days for 2 weeks | Reduced melanoma cell extravasation into lungs. | Modulates HMGB1/RAGE and TLR4 signaling; suppressed EMT and angiogenesis pathways. | [330] | |
| A549 cells (in vitro) | GL (0.25–8 mM); cisplatin (10–160 µM) | Reduced A549 cell viability; combined treatment reduced cell colony-forming ability, induced apoptosis and arrested the cell cycle at the G2 phase | Increases pro-apoptotic and DNA damage markers (Bax, cleaved-caspase-3, γH2AX, p-Chk1, p-p53); reduces anti-apoptotic and cell cycle proteins (Bcl-2, cyclin D1, CDK2, CDK4). | [331] | |
| Liver Cancer/Hepatocellular Carcinoma (HCC) | Diethylnitrosamine-treated BALB/c mice (in vivo) | 2 mg/kg, 3×/week for 2 weeks | Reduced the number of liver tumors and HCC incidence significantly compared to control. Liver function improved (AST, albumin) | Inhibits tumor formation and HCC development | [332] |
| Sprague-Dawley rats (in vivo) | 240–480 mg/kg | Induced liver enzyme activity; concern for cotoxicity and cocarcinogenic effects | Induces cytochrome P450 enzymes (CYP3A, CYP1A2, CYP2B1) in liver | [333] | |
| HepG2 (in vitro) | 1.0 mmol of GL for 48 h | Decreased the expression of TIMP-1, indicating anti-fibrotic and potential anticancer effects | Down-regulates TIMP-1 gene expression via inhibition of its promoter | [334] | |
| Rats treated with lead acetate (100 mg/kg, i.p.) (in vivo) | 150–300 mg/kg GL (oral) | Mitigated lead-induced oxidative stress, liver damage, and tumor promotion | Inhibits ODC activity, and DNA synthesis in liver | [335] | |
| Cisplatin-resistant Huh7 HCC cell line (in vitro) | GL (5 and 100 μg/mL), co-treatment with cisplatin (5 μg/mL) | Co-treatment reversed resistance and decreased cell viability, improving cisplatin retention in resistant cells | Modulates MRPs (MRP2, MRP3, MRP4, MRP5) and enhances cisplatin accumulation | [336] | |
| HepG2 (in vitro) | GL used in the form of Monoammonium GLate (10 mg/60 mm dish) | Increased junB gene expression, suggesting its potential as a tumor suppressor | Inhibits c-jun oncogene expression, stimulates junB gene | [337] | |
| 40 liver carcinoma patients treated with GL capsules (clinical) | 1 capsule/day (dose size not available) | Prevented elevated Asn levels in liver cancer patients, inhibited clopidogrel metabolism, and demonstrated mild toxicity | Normalizes Asn levels and alters clopidogrel metabolism | [338] | |
| HepG2 and MHCC97-H cells, xenograft mouse model | (1 and 2 mmol/L) | Induced autophagy and cytotoxicity, inhibited tumor growth in xenograft models | Induces autophagy, inhibits Akt/mTOR, and activates ERK1/2 signaling pathways | [339] | |
| HCC, HUVECs (in vitro) | <100 μM | Reduced cell proliferation, migration, and angiogenesis; decreased levels of p-STAT3, Survivin, LC3-I, P62, and VEGF-A | Inhibits of STAT3/Survivin pathway and induces autophagy; increases LC3-II and Beclin1, and reduces LC3-I, P62, and VEGF-A | [340] | |
| H22 tumor-bearing mice, RAW 264.7 macrophages (in vivo/in vitro) | DOX/GL-ALG nanogel particles (5.25, 21, 84 μg/mL), in vitro and 2.5 mg/kg, in vivo | Increased DOX bioavailability, reduced macrophage activation, and enhanced anticancer efficacy against HCC in vivo | Enhances targeting, reduces macrophage phagocytosis, and regulates apoptosis pathways | [341] | |
| Endometrial Cancer | Mice (Estradiol-induced carcinogenesis) (in vivo) | Glycyrrhizae radix and GL (0.0625%) | Decreased COX-2, IL-1α, and TNF-α; reduced adenocarcinoma incidence | Suppresses COX-2, IL-1α, TNF-α gene expression | [342] |
| Breast Cancer | MDA-MB-231 TNBC cells (in vitro) | GL (5–80 μM/L), Glycyrrhetinic acid (GA) | Induced apoptosis and enhanced etoposide-induced cytotoxicity | enhances TOPO 2A expression, sensitizes cells to etoposide, inducing apoptosis via MAPK and AKT pathways | [343] |
| MDA-MB-231 and BT549 cells (in vitro) | 5–40 μM | Decreased migration and invasion | Upregulates miR-200c and E-cadherin expression | [344] | |
| Gastric Cancer | KATO III, HL-60 (in vitro) | Not available | Growth inhibition observed, apoptosis induced, caspase involvement confirmed | Induces apoptosis via caspase activation and DNA fragmentation | [345] |
| BGC-823 (in vitro) | 40 μM/L | Decreased proliferation, migration, and adhesion; reduced expression of β-catenin, Bcl-2, CyclinD1, and survivin. | Modulates Wnt/β-catenin signaling, inhibits cell adhesion and migration | [346] | |
| Prostate Cancer | LNCaP, DU-145 (in vitro) | Not available | Inhibition of cell proliferation, induction of apoptosis | Induces apoptosis via caspase-independent pathways; causes DNA damage | [347] |
| DU145 (in vitro) | 25–200 μM | Reduced EMT markers, blocked migration and invasion. | Inhibits HMGB1-induced EMT | [348] | |
| Cervical Cancer | HeLa (in vitro) | 20–640 μM | Reduced cell viability, DNA fragmentation, ROS increase, apoptosis induction, and G0/G1 cell cycle arrest. | Induces ROS-dependent apoptosis, mitochondrial depolarization, and cell cycle arrest at G0/G1 phase | [349] |
| C33A (in vitro) | 10–100 μM | Induced apoptosis and G0/G1 cell cycle arrest. | Activates caspase, downregulates notch signaling, upregulates p21 | [350] | |
| End1/E6E7, C33a (in vitro) | 50 μmol/L | Decreased proliferation, migration, and invasion of C33a cells and increased apoptosis. | Modulates protein kinase B/glycogen synthase kinase-3β/β-catenin signaling pathway | [351] | |
| Colorectal Cancer | SW48, CCD-18Co (in vitro); azoxymethane/dextran sodium sulfate-induced mice (in vivo) | 12 μM/15 mg/kg/day orally (mice) | Inhibition of growth, apoptosis, autophagy, and reduced migration/invasion; inhibits the inflammatory response; inhibits DNA damage and cancer stem cell proliferation and dedifferentiation. | Targets MMP-9 and MMP-2 expression; inhibits HMGB1-TLR4-NF-κB signaling | [352,353] |
| Bone Cancer (Osteosarcoma) | U-O2S (in vitro) | 10–40 μM | Sensitized cells to doxorubicin, reversing drug resistance under hypoxia. | Antagonizes hypoxia-induced chemoresistance, inhibits HMGB1 signaling | [354] |
| Submandibular Gland Cancer | Mice submandibular gland fibrosarcoma cell line (in vitro) | 0.6 mg/mL and above | Inhibited cell proliferation above 600 mg/L. | Blocks G1 to S phase transition in the cell cycle | [355] |
| Virus | Model | Dose/Concentration | Key Findings | Mechanism of Action | References |
|---|---|---|---|---|---|
| HIV-1, R5 HIV | In vitro (MT-4, MOLT-4, PBM/MA); clinical (HIV+ patients) | 0.075–0.6 mM (in vitro); 400–1600 mg/day IV (clinical) | Inhibits HIV replication; reduces p24 antigen; improves CD4+ counts and liver function; suppresses PKC and CCR5 | Inhibition of protein kinase C; CCR5 suppression; β-chemokine induction; membrane stabilization | [390,391,392,393,394,395,396,397] |
| HSV-1, HSV-2, HHV-6/7, VZV | In vitro (Vero, HEp-2, rat CCEC, HEF cells); In vivo (burned/thermal injury mice) | 0.5–3.6 mM (in vitro); 10 mg/kg (mice) | Inhibits replication, cytopathic effects, and pain; enhances resistance in burns; synergizes with acyclovir | Inhibits viral gene expression and adhesion; immunomodulation; contrasuppressor T cell induction | [364,394,398,399,400,401,402,403] |
| CMV | In vitro (U-937, MRC-5 cells); clinical (HIV+ child) | 100 μg/mL (in vitro); 400 mg/day IV (clinical) | Suppresses CMV antigen expression; improves vision and reduced CMV recurrence in treated patient | Inhibits early viral gene expression; enhances host immunity | [404,405] |
| IFV (A, H1N1, H2N2, H5N1) | In vitro (A549, human lung cells); in vivo (BALB/c mice) | 25–100 μg/mL; 10–50 mg/kg (mice) | Inhibits viral entry and replication; 100% survival with ribavirin; reduces cytokine storm | Inhibits endocytosis; suppresses ROS-NFκB/JNK/p38; induces IFN-γ | [406,407,408,409,410] |
| SARS-CoV | In vitro (clinical isolates of CoV (FFM-1 and FFM-2) from patients) | 1000–4000 mg/L | Effective against SARS-CoV; blocked replication of the virus | Inhibits replication; ACE2 receptor interaction; cytokine modulation | [388] |
| SARS-CoV2 (S-RBD and Orf3a) | In vitro (Vero E6 cells; A549; NCI-H1299; BEAS-2B; AGS; A498); clinical (combination with boswellic acids 200 mg) | 0.004–4 mg/mL; 1 mM; 60 mg (clinical) | Effectively inhibits virus replication; mitigates viral proteins-induced lung cell pyroptosis and activation of macrophages; inhibits binding of spike protein to ACE2; reduces mortality and recovery time of COVID-19 patients | Blocks viral replication via inhibition of viral main protease Mpro; down-regulates ACE2 expression; reduces excessive release of pro-inflammatory cytokines (IL-1β, IL-6, IL-8) and ferritin; modulates cytokine responses and reduces systemic inflammation. | [411,412,413,414,415] |
| PV, MeV, CHPV | In vitro (Vero and HeLa cells) | 1.216 mM | Significant plaque reduction; broad antiviral activity | Inhibits viral adsorption and early replication | [398] |
| HTLV-I | In vitro (MT-2 cells) | ~0.6 mM | Inhibits viral production and syncytia formation | Suppression of viral fusion and protein synthesis | [416] |
| FIV | In vitro (FL-4 cells) | 0.15–0.6 mM | Reduces apoptosis in infected cells | Antioxidant and anti-apoptotic properties | [417] |
| Flaviviruses | In vitro (Vero cells, PS and HeLa) | 100–1000 μg/mL | Inhibits replication across various flaviviruses (DENV-1–4, JEV, WNV, USUV, LGTV, YFV and WESSV) | Broad-spectrum antiviral; likely via membrane fluidity alteration | [418,419] |
| PEDV | In vitro (Vero cells) | 0.1–0.8 mM | Inhibits viral entry and replication; reduces inflammation | Blocks HMGB1/TLR4-MAPK pathway | [420,421] |
| PRRSV | In vitro | 200–800 μM | Inhibits viral penetration phase | Prevents virus entry post-adsorption | [422] |
| VSV | In vitro (kinase assays) | 20 μM | Inhibits viral kinase-mediated phosphorylation | Inactivates viral kinase P | [423] |
| HBV | In vitro (HepG2 cells) | <400 µM | Inhibits secreted HBsAg and HBeAg; reduces replicative intermediates of HBV DNA and cccDNA | Acts as an HMGB1 inhibitor | [424] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semwal, D.K.; Kumar, A.; Semwal, R.B.; Dadhich, N.K.; Chauhan, A.; Kumar, V. Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms. Drugs Drug Candidates 2025, 4, 44. https://doi.org/10.3390/ddc4040044
Semwal DK, Kumar A, Semwal RB, Dadhich NK, Chauhan A, Kumar V. Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms. Drugs and Drug Candidates. 2025; 4(4):44. https://doi.org/10.3390/ddc4040044
Chicago/Turabian StyleSemwal, Deepak Kumar, Ankit Kumar, Ruchi Badoni Semwal, Nand Kishor Dadhich, Ashutosh Chauhan, and Vineet Kumar. 2025. "Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms" Drugs and Drug Candidates 4, no. 4: 44. https://doi.org/10.3390/ddc4040044
APA StyleSemwal, D. K., Kumar, A., Semwal, R. B., Dadhich, N. K., Chauhan, A., & Kumar, V. (2025). Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms. Drugs and Drug Candidates, 4(4), 44. https://doi.org/10.3390/ddc4040044








