Leflunomide Applicability in Rheumatoid Arthritis: Drug Delivery Challenges and Emerging Formulation Strategies
Abstract
1. Introduction
2. Overview of Rheumatoid Arthritis (RA)
2.1. Pathophysiology and Current Treatment Landscape
2.2. Rationale for Reviewing Leflunomide’s Utility
2.3. Mechanism of Action of Leflunomide
3. Pharmacokinetics of Leflunomide
3.1. Absorption, Metabolism, and Elimination
3.2. Conversion to Active Metabolite (Teriflunomide)
3.3. Drug Interactions and Bioavailability Considerations
4. Challenges in LEF Delivery to RA Patients
4.1. Safety and Tolerability Issues
4.1.1. Hepatotoxicity and Monitoring Requirements
4.1.2. Gastrointestinal (GI) and Hematological Effects
4.1.3. Skin Rash and Wound Healing
4.1.4. Bone Marrow Suppression
4.1.5. Hypertension
4.1.6. Weight Loss and Diabetes
4.1.7. Teratogenicity
4.2. Patient Counseling
4.3. Restricted Use in Specific Subject Populations
4.4. Differing Clinical Responses
4.5. Relative Efficiency Compared to Other DMARDs
4.6. Slow Onset and Prolonged Retention
4.7. Further Research Requirements
5. Emerging Drug Delivery and Formulation Strategies to Overcome LEF Challenges
5.1. Novel Formulations and Drug Delivery Systems
5.1.1. Nanocarrier-Based Drug Delivery Systems
Nanoparticles
5.1.2. Lipid-Based Formulations
5.1.3. Micelle-Based Systems
5.1.4. Transdermal and Topical Formulations
5.1.5. Hybrid and Multifunctional Systems
5.1.6. Hydrogel and Injectable Depot Systems
5.2. Stimuli Responsive-Based Drug Delivery Systems (SRDDSs)
5.3. Photothermal and Photodynamic Therapy
5.4. Surface Functionalization of Nanocarriers for LEF Delivery
5.5. Personalized LEF Therapy
6. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACPAs | Anti-citrullinated protein antibodies |
| AIA | Adjuvant induced arthritis |
| Anti-CCP | Anti-cyclic citrullinated peptide |
| APC | Antigen-presenting cells |
| ATP | adenosine triphosphate |
| BMI | Body mass index |
| CBC | Complete blood count |
| CD 28 and 80 | Cluster of differentiation 28 and 80 |
| CHS | Chondroitin sulfate |
| CRP | C-reactive protein |
| CS | Chitosan |
| CUR | Curcumin |
| DDS(s) | Drug delivery systems |
| DHODH | Dihydro orotate dehydrogenase |
| DMARD(s) | Disease modifying antirheumatic drugs |
| DNA | Deoxyribonucleic acid |
| EMA | European medicines agency |
| ESR | Erythrocytes sedimentation rate |
| FA | Folic acid |
| GIT | Gastrointestinal tract |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GTBA-NP-L | Gold-thiol-beaded albumin nanoparticles |
| HA | Hyaluronic acid |
| IA | Intraarticular administration |
| ICG | Indocyanine green dye |
| IgG | Immunoglobin G |
| IgM | Immunoglobin M |
| IL-6 | Interleukin-6 |
| INF-α | Interferon-α |
| LEF | Leflunomide |
| LFT | Liver function test |
| mAbs | Monoclonal antibodies |
| M-CSF | Macrophage colony stimulating factor |
| MHC | Major histocompatibility complex |
| MMPs | Matrix metalloproteinases |
| NE | Nano-emulsion |
| NIR | Near-infrared |
| NLCs | nanostructured lipid crystals |
| NPs | Nanoparticles |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OC | Osteoclast |
| PCL | Poly ε-caprolactone |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PLGA | poly lactic-co-glycolic acid |
| PNP(s) | Polymeric nanoparticles |
| Pre-OC | Pre-osteoclast |
| PTT | Photothermal therapy |
| QbD | Quality by design |
| RA | Rheumatoid arthritis |
| RANK-L | Receptor activator of nuclear factor kappa-b ligand. |
| RF | Rheumatoid factor |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SGOT | Serum glutamic oxaloacetic transaminase |
| SGPT | Serum glutamic pyruvic transaminase |
| SLNs | Solid lipid nanoparticles |
| SMEDDS | Self-micro emulsifying drug delivery systems |
| SNEDDS | Self-nano emulsifying drug delivery systems |
| TCR | T-cell receptor |
| TH-1 and 17 | T helper cell-1 and 17 |
| TNF-α | Tumor necrosis factor-α |
| TRAE | Treatment-related adverse events |
| UMP | Uridine-5′-monophosphate |
| USFDA | U.S. Food and Drug Administration |
| Β-GP | β glycerophosphate |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Tateiwa, D.; Yoshikawa, H.; Kaito, T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 2019, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Osiri, M.; Shea, B.; Welch, V.; Suarez-Almazor, M.E.; Strand, V.; Tugwell, P.; Wells, G.A. Leflunomide for the Treatment of Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2009, 1, CD002047. [Google Scholar] [CrossRef] [PubMed]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and Environmental Risk Factors for Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 1, 3–18. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Padjen, I.; Crnogaj, M.R.; Anić, B. Conventional Disease-Modifying Agents in Rheumatoid Arthritis–A Review of Their Current Use and Role in Treatment Algorithms. Reumatologia 2020, 58, 390–400. [Google Scholar] [CrossRef]
- Findeisen, K.E.; Sewell, J.; Ostor, A.J.K. Biological Therapies for Rheumatoid Arthritis: An Overview for the Clinician. Biologics 2021, 15, 343–352. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The Role of Cyclooxygenases in Inflammation, Cancer, and Development. Oncogene. 2000, 18, 7908–7916. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.S.; Silakari, O. Tumor Necrosis Factor Alpha Converting Enzyme: An Encouraging Target for Various Inflammatory Disorders. Chem. Biol. Drug Des. 2010, 75, 415–443. [Google Scholar] [CrossRef]
- Schett, G.; Dayer, J.M.; Manger, B. Interleukin-1 Function and Role in Rheumatic Disease. Nat. Rev. Rheumatol. 2015, 12, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef] [PubMed]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The Forgotten Key Players in Rheumatoid Arthritis: IL-8 and IL-17-Unmet Needs and Therapeutic Perspectives. Front Med. 2023, 10, 956127. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Chu, C.Q.; Fionula, M.B.; Maini, R.N.; Feldmann, M. Immunoregulatory Role of Interleukin 10 in Rheumatoid Arthritis. J. Exp. Med. 1994, 179, 1517–1527. [Google Scholar] [CrossRef]
- Pope, R.M.; Shahrara, S. IL-12 Family Cytokines in the Pathogenesis and Treatment of Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2012, 9, 252–256. [Google Scholar] [CrossRef]
- Yang, X.K.; Xu, W.D.; Leng, R.X.; Liang, Y.; Liu, Y.Y.; Fang, X.Y.; Feng, C.C.; Li, R.; Cen, H.; Pan, H.F.; et al. Therapeutic Potential of IL-15 in Rheumatoid Arthritis. Hum. Immunol. 2015, 76, 812–818. [Google Scholar] [CrossRef]
- Volin, M.V.; Koch, A.E. Interleukin-18: A Mediator of Inflammation and Angiogenesis in Rheumatoid Arthritis. J. Interf. Cytokine Res. 2011, 31, 10. [Google Scholar] [CrossRef]
- Pulik, Ł.; Łęgosz, P.; Motyl, G. Matrix Metalloproteinases in Rheumatoid Arthritis and Osteoarthritis: A State of the Art Review. Reumatologia 2023, 61, 191–201. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Raj Sigdel, K.; Duan, L. Role of NLRP3 Inflammasome in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 931690. [Google Scholar] [CrossRef]
- Whang, J.A.; Chang, B.Y. Bruton’s Tyrosine Kinase Inhibitors for the Treatment of Rheumatoid Arthritis. Drug Discov. Today 2014, 19, 1200–1204. [Google Scholar] [CrossRef]
- Okamoto, H.; Kobayashi, A. Tyrosine Kinases in Rheumatoid Arthritis. J. Inflamm. 2011, 8, 21. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus Kinase-Targeting Therapies in Rheumatology: A Mechanisms-Based Approach. Nat. Rev. Rheumatol. 2022, 18, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Paolino, S.; Pizzorni, C. CTLA-4 Blockade in the Treatment of Rheumatoid Arthritis: An Update. Expert. Rev. Clin. Immunol. 2016, 12, 417–425. [Google Scholar] [CrossRef]
- Kremer, J.M. What I Would Like to Know About Leflunomide. Available online: https://www.jrheum.org/content/jrheum/31/6/1029.full.pdf (accessed on 22 March 2025).
- Rozman, B. Clinical Pharmacokinetics of Leflunomide. Clin. Pharmacokinet. 2002, 41, 421–430. [Google Scholar] [CrossRef]
- Jones, P.B.B.; White, D.H.N. Reappraisal of the Clinical Use of Leflunomide in Rheumatoid Arthritis and Psoriatic Arthritis. Open Access Rheumatol. 2010, 2, 53. [Google Scholar] [CrossRef]
- USFDA Label; CDER ARAVA® Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/020905s033lbl.pdf (accessed on 18 July 2025).
- Arava®, European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arava (accessed on 18 July 2025).
- Leflunomide Tablets, 20MG, 30 TABLET (BOTTLE). Available online: https://www.apotex.com/products/us/detail.asp?m=67802 (accessed on 18 July 2025).
- Leflunomide Tablets, 10MG, 30 TABLET (BOTTLE). Available online: https://www.apotex.com/products/us/detail.asp?m=67801 (accessed on 18 July 2025).
- Rumalef-Zydus Healthcare Limited. Available online: https://www.zydushealthcare.com/brands/?brand=ruma (accessed on 18 July 2025).
- Torrent Pharmaceuticals | Pioneering Global Healthcare Solutions. Available online: https://www.torrentpharma.com/pi/IN/products/ (accessed on 20 July 2025).
- Leflunomide Tablets USP. Available online: https://www.lupin.com/US/product/leflunomide-tablets-usp (accessed on 20 July 2025).
- India Products-Sun Pharmaceutical Industries Ltd. Available online: https://sunpharma.com/india-products/ (accessed on 20 July 2025).
- Leflunomide Teva. Available online: https://www.ema.europa.eu/en/documents/product-information/leflunomide-teva-epar-product-information_en.pdf (accessed on 20 July 2025).
- Mylan-Leflunomide-Uses, Side Effects, Interactions-MedBroadcast.Com. Available online: https://medbroadcast.com/drug/getdrug/mylan-leflunomide (accessed on 20 July 2025).
- SANDOZ LEFLUNOMIDE. Available online: https://sandoz-ca.cms.sandoz.com/sites/default/files/Media%20Documents/PDF%20236.pdf (accessed on 20 July 2025).
- Leflunomide Ratiopharm | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/leflunomide-ratiopharm#authorisation-details (accessed on 20 July 2025).
- Herrmann, M.L.; Schleyerbach, R.; Kirschbaum, B.J. Leflunomide: An Immunomodulatory Drug for the Treatment of Rheumatoid Arthritis and Other Autoimmune Diseases. Int. Immunopharmacol. 2000, 47, 273–289. [Google Scholar] [CrossRef]
- Lucien, J.; Dias, V.C.; LeGatt, D.F.; Yatscoff, R.W. Blood Distribution and Single–Dose Pharmacokinetics of Leflunomide. Ther. Drug Monit. 1995, 17, 454–459. [Google Scholar] [CrossRef]
- Breedveld, F.C.; Dayer, J.M. Leflunomide: Mode of Action in the Treatment of Rheumatoid Arthritis. Ann. Rheum. Dis. 2000, 59, 841–849. [Google Scholar] [CrossRef]
- Cada, D.J.; Demaris, K.; Levien, T.L.; Baker, D.E. Teriflunomide. Hosp. Pharm. 2013, 8, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and Its Mechanism of Action in Multiple Sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Rakhila, H.; Rozek, T.; Hopkins, A.; Proudman, S.; Cleland, L.; James, M.; Wiese, M. Quantitation of Total and Free Teriflunomide (A77 1726) in Human Plasma by LC–MS/MS. J. Pharm. Biomed. Anal. 2011, 55, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Teriflunomide | C12H9F3N2O2 | CID 54684141–PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Teriflunomide (accessed on 21 July 2025).
- Leflunomide | C12H9F3N2O2 | CID 3899–PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/leflunomide (accessed on 21 July 2025).
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2022 Update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Leflunomide for Adults in Rheumatology Shared Care Guideline V1.0. 2024. Available online: https://doclibrary-rcht.cornwall.nhs.uk/DocumentsLibrary/RoyalCornwallHospitalsTrust/Clinical/Pharmacy/LeflunomideForAdultsInRheumatologySharedCareGuideline.pdf (accessed on 21 July 2025).
- Teschner, S.; Burst, V. Leflunomide: A Drug with a Potential beyond Rheumatology. Immunotherapy 2010, 2, 637–650. [Google Scholar] [CrossRef]
- Drosos Alexandros, A. Methotrexate Intolerance in Elderly Patients with Rheumatoid Arthritis: What Are the Alternatives? Drugs Aging 2003, 20, 723–736. [Google Scholar] [CrossRef]
- Deshmukh, R. Rheumatoid Arthritis: Pathophysiology, Current Therapeutic Strategies and Recent Advances in Targeted Drug Delivery System. Mater. Today Commun. 2023, 35, 105877. [Google Scholar] [CrossRef]
- Tieri, P.; Zhou, X.Y.; Zhu, L.; Nardini, C. Multi-Omic Landscape of Rheumatoid Arthritis: Re-Evaluation of Drug Adverse Effects. Front. Cell Dev. Biol. 2014, 2, 59. [Google Scholar] [CrossRef]
- Loke, Y.K.; Price, D.; Herxheimer, A. Systematic Reviews of Adverse Effects: Framework for a Structured Approach. BMC Med. Res. Methodol. 2007, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, P.J.; DeBroe, S.; McBride, A.; Milne, R. Leflunomide and Rheumatoid Arthritis: A Systematic Review of Effectiveness, Safety and Cost Implications. J. Clin. Pharm. Ther. 2000, 25, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kalden, J.R.; Schattenkirchner, M.; Sörensen, H.; Emery, P.; Deighton, C.; Rozman, B.; Breedveld, F. The Efficacy and Safety of Leflunomide in Patients with Active Rheumatoid Arthritis: A Five-Year Followup Study. Arthritis Rheum. 2003, 48, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.A.; Islahudin, F.; Shamsuddin, A.F.; Shahri, N.S. A Study on Leflunomide-Induced Liver Injury in Rheumatoid Arthritis Patients. Res. J. Pharm. Technol. 2013, 6, 556–561. [Google Scholar]
- Liu, Y.; Zhang, X. Leflunomide-Induced Acute Liver Failure: A Case Report. J. Med. Coll. PLA 2010, 25, 62–64. [Google Scholar] [CrossRef]
- Nandan, A.; Haritha, J.; Maniscalco, D.; Gill, R.; Puri, P.; Rodriguez, V.; Syed, H. Hepatotoxicity from Disease-Modifying Anti-Rheumatic Drugs (DMARDs) and Association with Metabolic Syndrome in Rheumatoid Arthritis. Cureus 2025, 17, e78626. [Google Scholar] [CrossRef]
- Craig, E.; Cappelli, L.C. Gastrointestinal and Hepatic Disease in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2018, 44, 89. [Google Scholar] [CrossRef]
- Arava® Medsafe. Available online: https://www.medsafe.govt.nz/Profs/Datasheet/a/aravatab.pdf (accessed on 24 April 2025).
- Agrawal, S.; Sharma, A. Dual Mycobacterial Infection in the Setting of Leflunomide Treatment for Rheumatoid Arthritis. Ann. Rheum. Dis. 2007, 66, 277. [Google Scholar] [CrossRef]
- Panoulas, V.F.; John, H.; Kitas, G.D. Six-Step Management of Hypertension in Patients with Rheumatoid Arthritis. Future Rheumatol. 2008, 3, 21–35. Available online: https://www.openaccessjournals.com/articles/sixstep-management-of-hypertension-in-patients-with-rheumatoid-arthritis.pdf (accessed on 20 July 2025). [CrossRef]
- Mladenovic, V.; Domljan, Z.; Rozman, B.; Jajic, I.; Mihajlovic, D.; Dordevic, J.; Popovic, M.; Dimitrijevic, M.; Zivkovic, M.; Campion, G.; et al. Safety and Effectiveness of Leflunomide in the Treatment of Patients with Active Rheumatoid Arthritis. Arthritis Rheum. 1995, 38, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Coblyn, J.S.; Shadick, N.; Helfgott, S. Leflunomide-Associated Weight Loss in Rheumatoid Arthritis. Arthritis Rheum. 2001, 44, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Kanamori, S.; Hirashiba, M.; Hishikawa, A.; Muranaka, R.-I.; Kaneto, M.; Nakamura, K.; Kato, I. Teratogenicity Study of the Dihydroorotate-Dehydrogenase Inhibitor and Protein Tyrosine Kinase Inhibitor Leflunomide in Mice. Reprod. Toxicol. 2007, 24, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Schattenkirchner, M. The Use of Leflunomide in the Treatment of Rheumatoid Arthritis: An Experimental and Clinical Review. Immunopharmacology 2000, 47, 291–298. [Google Scholar] [CrossRef]
- Voshaar, M.J.H.; Nota, I.; Van De Laar, M.A.F.J.; Van Den Bemt, B.J.F. Patient-Centred Care in Established Rheumatoid Arthritis. Best. Pr. Res. Clin. Rheumatol. 2015, 29, 643–663. [Google Scholar] [CrossRef]
- Nguyen, M.; Kabir, M.; Ravaud, P. Short-Term Efficacy and Safety of Leflunomide in the Treatment of Active Rheumatoid Arthritis in Everyday Clinical Use: Open-Label, Prospective Study. Clin. Drug Investig. 2004, 24, 103–112. [Google Scholar] [CrossRef]
- Alivernini, S.; Mazzotta, D.; Zoli, A.; Ferraccioli, G. Leflunomide Treatment in Elderly Patients with Rheumatoid or Psoriatic Arthritis: Retrospective Analysis of Safety and Adherence to Treatment. Drugs Aging 2009, 26, 395–402. [Google Scholar] [CrossRef]
- Lewis, M.J.; Barnes, M.R.; Blighe, K.; Goldmann, K.; Rana, S.; Hackney, J.A.; Ramamoorthi, N.; John, C.R.; Watson, D.S.; Kummerfeld, S.K.; et al. Molecular Portraits of Early Rheumatoid Arthritis Identify Clinical and Treatment Response Phenotypes. Cell Rep. 2019, 28, 2455–2470.e5. [Google Scholar] [CrossRef]
- Alfaro-Lara, R.; Espinosa-Ortega, H.F.; Arce-Salinas, C.A. Systematic Review and Meta-Analysis of the Efficacy and Safety of Leflunomide and Methotrexate in the Treatment of Rheumatoid Arthritis. Reumatol. Clín. 2019, 15, 133–139. [Google Scholar] [CrossRef]
- Tonima, A.; Sachchidanand, P.; Sagnika, T.; Bhabagrahi, R. Leflunomide versus Methotrexate and Hydroxychloroquine Combination Therapy in Rheumatoid Arthritis: A Prospective Study on Effectiveness and Quality of Life. Int. J. Pharm. Clin. Res. 2023, 15, 882–888. [Google Scholar] [CrossRef]
- Belani, P.J.; Kavadichanda, C.G.; Negi, V.S. Comparison between Leflunomide and Sulfasalazine Based Triple Therapy in Methotrexate Refractory Rheumatoid Arthritis: An Open-Label, Non-Inferiority Randomized Controlled Trial. Rheumatol. Int. 2022, 42, 771–782. [Google Scholar] [CrossRef]
- Peng, K.; Chan, S.C.W.; Wang, Y.; Cheng, F.W.T.; Yeung, W.W.Y.; Jiao, Y.; Chan, E.W.Y.; Wong, I.C.K.; Lau, C.S.; Li, X. Cost-Effectiveness of Biosimilars vs Leflunomide in Patients with Rheumatoid Arthritis. JAMA Netw. Open. 2024, 7, e2418800. [Google Scholar] [CrossRef]
- Verma, A.-I.E.O.D.-M.A.-R.D.; Easwari, I.S. Anti-Inflammatory Effect of Disease-Modifying Anti-Rheumatic Drug, Leflunomide: A Review. Int. J. Pharm. Sci. Res. 2022, 13, 1000–1007. [Google Scholar] [CrossRef]
- Shareef, A.; Ullah, N.; Sajid, I.; Mehmood, A.; Shah, S.U.; Khan, S.; Alanzi, A.R.; Niyazi, S.; Alharbi, H.A.; Shah, K.U. Leflunomide Loaded Transferosomes Based Hydrogel against Rheumatoid Arthritis: In Vitro and In Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2025, 109, 106962. [Google Scholar] [CrossRef]
- Singh, E.; Osmani, R.A.M.; Banerjee, R.; Abu Lila, A.S.; Moin, A.; Almansour, K.; Arab, H.H.; Alotaibi, H.F.; Khafagy, E.S. Poly ε-Carpolactone Nanoparticles for Sustained Intra-Articular Immune Modulation in Adjuvant-Induced Arthritis Rodent Model. Pharmaceutics 2022, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.; ur Rehman, A.; Gul, R.; Chaudhery, I.; Shah, K.U.; Ahmed, N. Folate Decorated Chitosan-Chondroitin Sulfate Nanoparticles Loaded Hydrogel for Targeting Macrophages against Rheumatoid Arthritis. Carbohydr. Polym. 2024, 327, 121683. [Google Scholar] [CrossRef] [PubMed]
- Zewail, M.; Nafee, N.; Helmy, M.W.; Boraie, N. Synergistic and Receptor-Mediated Targeting of Arthritic Joints via Intra-Articular Injectable Smart Hydrogels Containing Leflunomide-Loaded Lipid Nanocarriers. Drug Deliv. Transl. Res. 2021, 11, 2496–2519. [Google Scholar] [CrossRef]
- Krishnan, Y.; Mukundan, S.; Akhil, S.; Gupta, S.; Viswanad, V. Enhanced Lymphatic Uptake of Leflunomide Loaded Nanolipid Carrier via Chylomicron Formation for the Treatment of Rheumatoid Arthritis. Adv. Pharm. Bull. 2018, 8, 257–265. [Google Scholar] [CrossRef]
- Abd-El-Azim, H.; Abbas, H.; El Sayed, N.S.; Fayez, A.M.; Zewail, M. Non-Invasive Management of Rheumatoid Arthritis Using Hollow Microneedles as a Tool for Transdermal Delivery of Teriflunomide Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2023, 644, 123334. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef]
- Zewail, M.B.; El-Gizawy, S.A.; Asaad, G.F.; Shabana, M.E.; El-Dakroury, W.A. Chitosan Coated Clove Oil-Based Nanoemulsion: An Attractive Option for Oral Delivery of Leflunomide in Rheumatoid Arthritis. Int. J. Pharm. 2023, 643, 123224. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Gad, H.A.; El Sayed, N.S.; Rashed, L.A.; Khattab, M.A.; Noor, A.O.; Zewail, M. Development and Evaluation of Novel Leflunomide SPION Bioemulsomes for the Intra-Articular Treatment of Arthritis. Pharmaceutics 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Nashaat, D.; Elsabahy, M.; Hassanein, K.M.A.; El-Gindy, G.A.; Ibrahim, E.H. Development and in Vivo Evaluation of Therapeutic Phytosomes for Alleviation of Rheumatoid Arthritis. Int. J. Pharm. 2023, 644, 123332. [Google Scholar] [CrossRef]
- Verma, V. A Novel Approach of Leflunomide Nanoemulgel for Topical Drug Delivery System. Int. J. Pharm. Investig. 2022, 12, 199–204. [Google Scholar] [CrossRef]
- Yun, L.; Shang, H.; Gu, H.; Zhang, N. Polymeric Micelles for the Treatment of Rheumatoid Arthritis. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 219–238. [Google Scholar] [CrossRef]
- Shewaiter, M.A.; Hammady, T.M.; El-Gindy, A.; Hammadi, S.H.; Gad, S. Formulation and Characterization of Leflunomide/Diclofenac Sodium Microemulsion Base-Gel for the Transdermal Treatment of Inflammatory Joint Diseases. J. Drug Deliv. Sci. Technol. 2021, 61, 102110. [Google Scholar] [CrossRef]
- Jurca, T.; Józsa, L.; Suciu, R.; Pallag, A.; Marian, E.; Bácskay, I.; Mureşan, M.; Stan, R.L.; Cevei, M.; Cioară, F.; et al. Formulation of Topical Dosage Forms Containing Synthetic and Natural Anti-Inflammatory Agents for the Treatment of Rheumatoid Arthritis. Molecules 2021, 26, 24. [Google Scholar] [CrossRef]
- Bae, J.; Park, J.W. Topical Delivery of Leflunomide for Rheumatoid Arthritis Treatment: Evaluation of Local Tissue Deposition of Teriflunomide and Its Anti-Inflammatory Effects in an Arthritis Rat Model. Drug Dev. Ind. Pharm. 2016, 42, 254–262. [Google Scholar] [CrossRef]
- Jain, N.; Daniel, K. Formulation and Evaluation of Topical Leflunomide Hydrogel for Rheumatoid Arthritis. Int. J. Pharm. Res. Appl. 2023, 8, 2249–7781. Available online: https://ijprajournal.com/issue_dcp/Formulation%20and%20Evaluation%20of%20Topical%20Leflunomide%20Hydrogel%20for%20Rheumatoid%20Arthritis.pdf (accessed on 20 July 2025).
- Kalashnikova, I.; Chung, S.J.; Nafiujjaman, M.; Hill, M.L.; Siziba, M.E.; Contag, C.H.; Kim, T. Ceria-Based Nanotheranostic Agent for Rheumatoid Arthritis. Theranostics 2020, 10, 11863–11880. [Google Scholar] [CrossRef]
- Zewail, M.; Nafee, N.; Boraie, N. Intra-Articular Dual Drug Delivery for Synergistic Rheumatoid Arthritis Treatment. J. Pharm. Sci. 2021, 110, 2808–2822. [Google Scholar] [CrossRef] [PubMed]
- Alhelal, H.M.; Mehta, S.; Kadian, V.; Kakkar, V.; Tanwar, H.; Rao, R.; Aldhubiab, B.; Sreeharsha, N.; Shinu, P.; Nair, A.B. Solid Lipid Nanoparticles Embedded Hydrogels as a Promising Carrier for Retarding Irritation of Leflunomide. Gels 2023, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gowda, B.H.J.; Gade, S.; Pandya, A.K.; Thakur, R.R.S. Injectable Depot-Forming Hydrogels for Long-Acting Drug Delivery. In Hydrogels in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 241–272. [Google Scholar] [CrossRef]
- Gadeval, A.; Anup, N.; Pawar, B.; Mule, S.; Otavi, S.; Sahu, R.; Kumar Tekade, R. Gold-Thiol-Beaded Albumin Nanoparticles for Chemo-Combined Pulsatile Plasmonic Laser Therapy of Rheumatoid Arthritis in Rat Model. Int. J. Pharm. 2024, 667, 124882. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Goh, M.C.; Lan, J.; Du, M.; Chen, Z. Synergy of Dissolving Microneedles and Ultrasound to Enhance Transdermal Delivery for Rheumatoid Arthritis. Drug Deliv. Transl. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Huang, P.-F.; Ma, M.; Wu, H.; Niu, W.-M.; Liu, L.-L.; Tong, J.-B. Curcumin Delivered by ROS-Responsive Ibuprofen Prodrug Based on Hyaluronic Acid Effectively Suppressed the Rheumatoid Arthritis. J. Drug Deliv. Sci. Technol. 2025, 108, 106868. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, W.; Cao, J. Treatment of Rheumatoid Arthritis by Phototherapy: Advances and Perspectives. Nanoscale 2021, 13, 14591–14608. [Google Scholar] [CrossRef]
- Gadeval, A.; Chaudhari, S.; Bollampally, S.P.; Polaka, S.; Kalyane, D.; Sengupta, P.; Kalia, K.; Tekade, R.K. Integrated Nanomaterials for Non-Invasive Photothermal Therapy of Rheumatoid Arthritis. Drug Discov. Today 2021, 26, 2315–2328. [Google Scholar] [CrossRef]
- Mi, L.; Gao, J.; Liu, Y.; Zhang, N.; Zhao, M.; Wang, S.; Xu, K. Photodynamic Therapy for Arthritis: A Promising Therapeutic Strategy. Rheumatol. Autoimmun. 2023, 3, 205–219. [Google Scholar] [CrossRef]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface Modification of Lipid-Based Nanocarriers: A Potential Approach to Enhance Targeted Drug Delivery. ACS Omega 2023, 8, 74–86. [Google Scholar] [CrossRef]
- Zewail, M. Leflunomide Nanocarriers: A New Prospect of Therapeutic Applications. J. Microencapsul. 2024, 41, 715–738. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface Modification of Nano-Drug Delivery Systems for Enhancing Antibiotic Delivery and Activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1758. [Google Scholar] [CrossRef]
- Xiao, R.; Zhou, G.; Wen, Y.; Ye, J.; Li, X.; Wang, X. Recent Advances on Stimuli-Responsive Biopolymer-Based Nanocomposites for Drug Delivery. Compos. B Eng. 2023, 266, 111018. [Google Scholar] [CrossRef]
- Zewail, M.; Nafee, N.; Helmy, M.W.; Boraie, N. Coated Nanostructured Lipid Carriers Targeting the Joints–An Effective and Safe Approach for the Oral Management of Rheumatoid Arthritis. Int. J. Pharm. 2019, 567, 118447. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Rana, D.; Benival, D.; Garkhal, K. Comprehensive Insights into Glioblastoma Multiforme: Drug Delivery Challenges and Multimodal Treatment Strategies. Ther. Deliv. 2024, 16, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D. PEGylation in Pharmaceutical Development: Current Status and Emerging Trends in Macromolecular and Immunotherapeutic Drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef]
- Zewail, M.B.; Asaad, G.F.; Shabana, M.E.; Elbokhomy, A.S.; Elbadry, A.M.M.; Riad, P.Y.; Salama, G.A.; El-Dakroury, W.A. PEGylated Lipid Polymeric Nanoparticles for Management of Rheumatoid Arthritis. J. Drug Deliv. Sci. Technol. 2024, 101, 106242. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated Liposomes: Immunological Responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Gan, J.; Huang, D.; Che, J.; Zhao, Y.; Sun, L. Biomimetic Nanoparticles with Cell-Membrane Camouflage for Rheumatoid Arthritis. Matter 2024, 7, 794–825. [Google Scholar] [CrossRef]
- Zhou, R.; Xue, S.; Cheng, Y.; Chen, Y.; Wang, Y.; Xing, J.; Liu, H.; Xu, Y.; Lin, Y.; Pei, Z.; et al. Macrophage Membrane-Camouflaged Biomimetic Nanoparticles for Rheumatoid Arthritis Treatment via Modulating Macrophage Polarization. J. Nanobiotechnol. 2024, 22, 578. [Google Scholar] [CrossRef]
- Han, H.; Bártolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic Platelet Membrane-Coated Nanoparticles for Targeted Therapy. Eur. J. Pharm. Biopharm. 2022, 172, 1–15. [Google Scholar] [CrossRef]
- Yang, N.; Li, M.; Wu, L.; Song, Y.; Yu, S.; Wan, Y.; Cheng, W.; Yang, B.; Mou, X.; Yu, H.; et al. Peptide-Anchored Neutrophil Membrane-Coated Biomimetic Nanodrug for Targeted Treatment of Rheumatoid Arthritis. J. Nanobiotechnol. 2023, 21, 13. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Zheng, X.; Jia, M.; Mei, Z.; Wang, Y.; Zhang, Z.; Zhou, M.; Li, C. M2-Type Exosomes Nanoparticles for Rheumatoid Arthritis Therapy via Macrophage Re-Polarization. J. Control. Release 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Gu, C.; Yang, S.; Liu, X.; Jin, Y.; Yu, Y.; Lu, L. A Biomimetic Adipocyte Mesenchymal Stem Cell Membrane-Encapsulated Drug Delivery System for the Treatment of Rheumatoid Arthritis. Nano Res. 2023, 16, 11401–11410. [Google Scholar] [CrossRef]
- Khokhar, M.; Dey, S.; Tomo, S.; Jaremko, M.; Emwas, A.H.; Pandey, R.K. Unveiling Novel Drug Targets and Emerging Therapies for Rheumatoid Arthritis: A Comprehensive Review. ACS Pharmacol. Transl. Sci. 2024, 7, 1664–1693. [Google Scholar] [CrossRef]
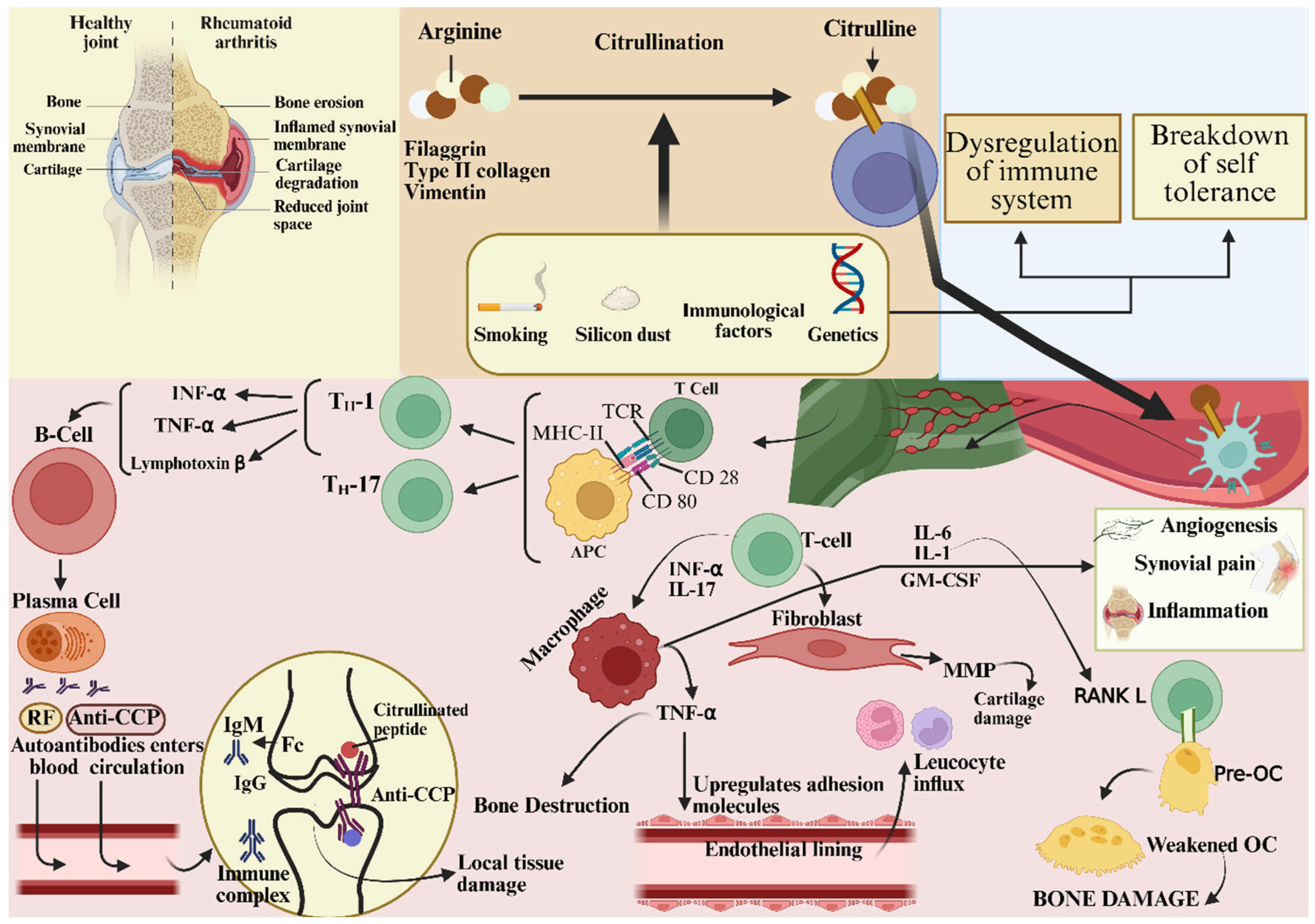
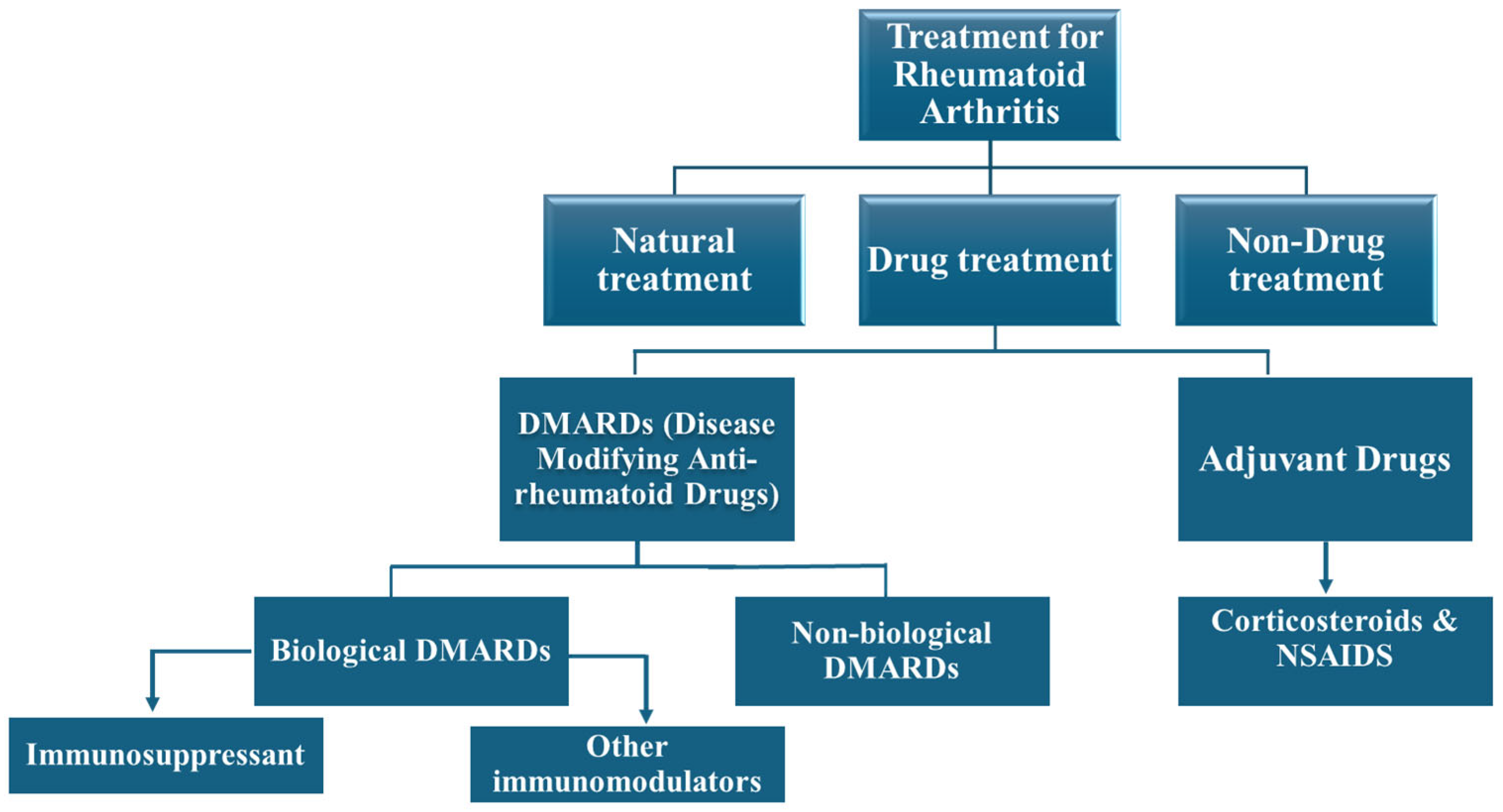
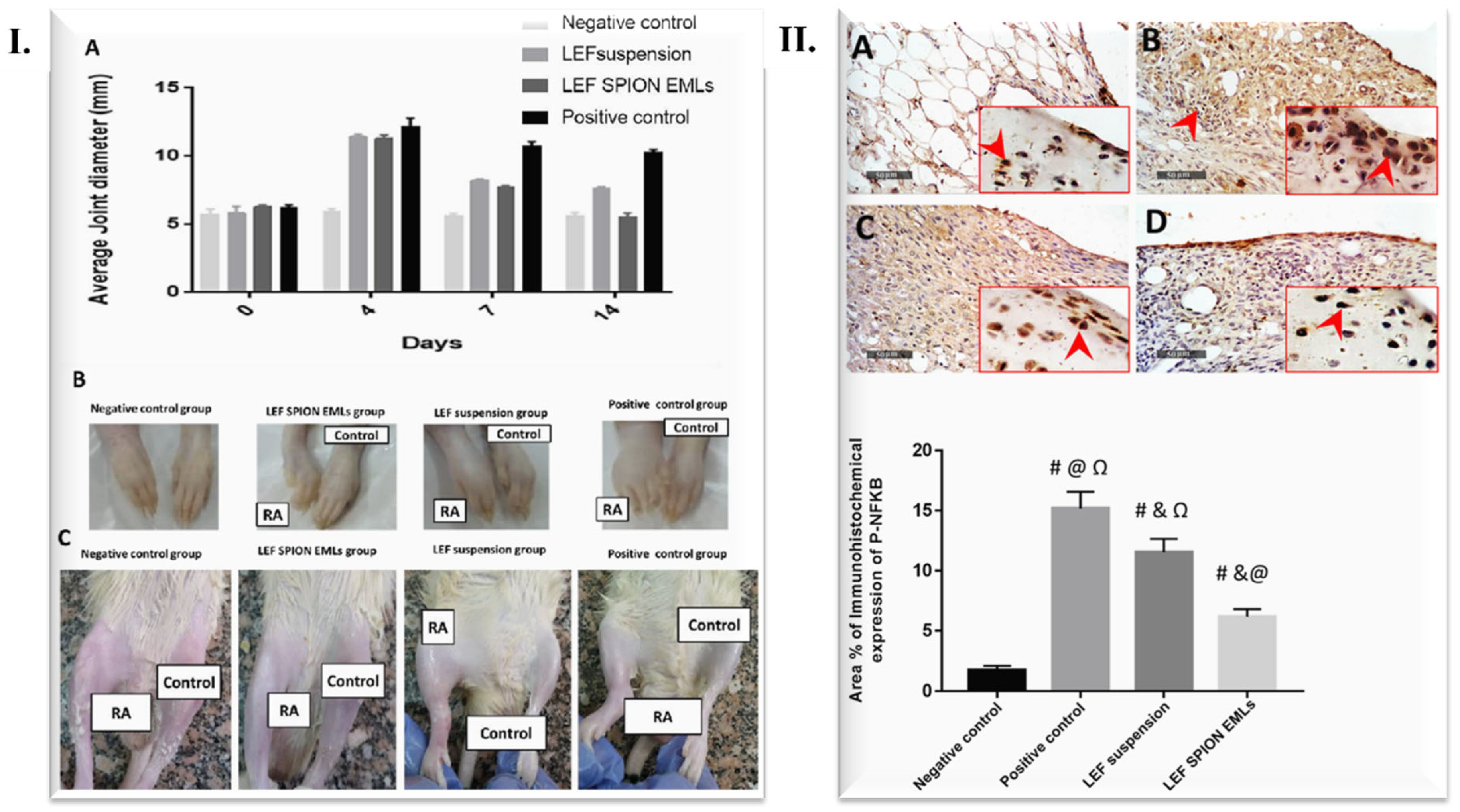
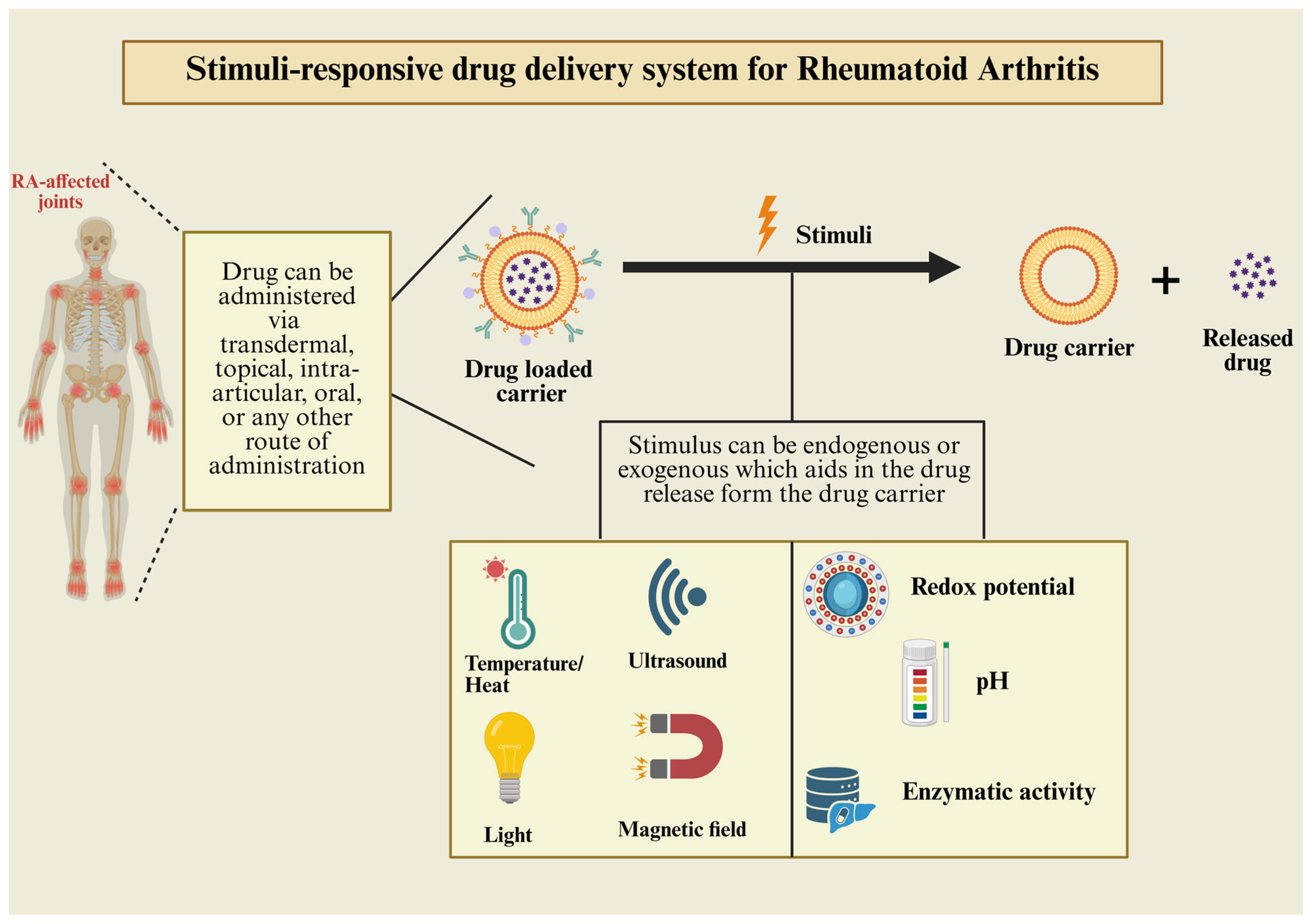
| Mechanistic Molecule/Enzymes | Role in RA Development | Reference |
|---|---|---|
| Cyclooxygenases 1 and 2 (COX 1 and 2) | Produce prostanoids involved in inflammatory and physiological processes | [11] |
| Tumor necrosis factor (TNF α and TNF- β) | Activation of macrophage, chondrocytes, endothelial cells, synovial fibroblasts, and osteophytes, resulting in cell division and elevated MMP and adhesion molecule overregulation | [12] |
| TNF-α converting enzyme (TACE) | Converts the membrane bound form of TNF-α into its soluble form | [13] |
| Interleukin-1 | Pro-inflammatory initiator of various inflammatory factors like MMPs, eicosanoids, and inducible nitric oxide synthase | [14] |
| Interleukin-1β | Cartilage degradation and expression of pro-inflammatory chemokine receptors, thus facilitating the recruitment and retention of inflammatory cells in RA synovium | [14] |
| Interleukin-6 | Production of autoantibodies by stimulating B-cell differentiation and activating auto-reactive T-cells and promotes bone reabsorption | [15] |
| Interleukin-8 | Encourages the harmful stimulation of immune and stromal cells in the tendons, blood vessels, lungs, and synovial membrane of RA, resulting in extra-articular issues | [16] |
| Interleukin-10 | Significant immunoregulatory element that controls the production of monocytes and, in certain situations, T cell cytokines in the cytokine network of RA | [17] |
| Interleukin-12 | Enhances Th1 responses, raises inflammation, and encourages joint damages, which contributes to the advancement of RA | [18] |
| Interleukin-15 | Onset of serious inflammatory arthritis | [19] |
| Interleukin-17 | Stimulates neutrophil infiltration, cartilage destruction, and chronic inflammation | [16] |
| Interleukin-18 | Induces RA synovial fibroblasts to emit chemokines, endothelial cell adhesion proteins are upregulated, and monocytes, lymphocytes, and neutrophils directly function as chemo-attractants to induce leukocyte extravasation. | [20] |
| Matrix metalloproteinases (MMPs) | Group of 25 zinc- and calcium-dependent proteinases, having a role in breaking down the extracellular matrix leading to bone and cartilage destruction | [21] |
| NOD-like receptor family pyrin domain-containing 3 (NLRP-3) | Stimulates the release of pro-inflammatory cytokines, which in turn causes synovial inflammation, joint damage, and autoimmune response. | [22] |
| TLRs (toll-like receptors) | Promotes the release of pro-inflammatory cytokines leading to a reduction in autoimmunity, increases joint degradation, and synovial inflammation. | [22] |
| Bruton’s tyrosine kinase (BTK) | Overexpression of BTK can lead to abnormal B cells proliferation | [23] |
| Spleen tyrosine kinase (SYK) | Synovial inflammation, immune cell signaling, and joint degeneration | [24] |
| Phosphatidylinositol 3-kinases (PI3K) | Upregulation of PI3K causes abnormal cell growth, overexpressed cell survival, and intracellular trafficking | [25] |
| Janus kinase (JAK)-Signal transducer and activator of transcription (STAT) | Inflammation, cytokine signaling, and immune cell stimulation | [26] |
| Phosphodiesterase-4 (PDE-4) | PDE-4 degrades cAMPs/cGMPs, which control the activity of multiple immune cells | [27] |
| Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) | Suppression of autoimmunity and excessive T cell proliferation | [28] |
| Manufacturer | Brand Name | Common Dosage Strengths | 100 mg Strength Availability (Used as Loading Dose) | Region | Reference |
|---|---|---|---|---|---|
| Sanofi-Aventis | Arava® | 10 mg, 20 mg | Yes | Global/USA/EU | [33] |
| Apotex Inc. | Leflunomide Apotex® | 10 mg, 20 mg | Not available | Canada/Australia | [34,35] |
| Zydus Cadila | Lefumide®, Rumalef ® | 10 mg, 20 mg | Yes | India/UK | [36] |
| Torrent Pharmaceuticals | Lefra® | 10 mg, 20 mg | Yes | India | [37] |
| Lupin Pharmaceuticals | Lefno® | 10 mg, 20 mg | Yes | India | [38] |
| Sun Pharmaceuticals | Cleft® | 10 mg, 20 mg | No | India/USA | [39] |
| Teva Pharmaceuticals | Leflunomide-Teva® | 10 mg, 20 mg | Yes (select markets only) | EU/UK | [40] |
| Mylan/Viatris | Leflunomide Mylan® | 10 mg, 20 mg | Yes | Europe/Australia/UK | [41] |
| Sandoz | Leflunomide Sandoz® | 10 mg, 20 mg | Yes (limited availability) | Europe | [42] |
| Ratiopharm GmbH | Leflunomide Ratiopharm | 10 mg, 20 mg | Yes | Europe/UK | [43] |
| Adverse/Side Effect | Prevention | Management |
|---|---|---|
| Hepatotoxicity |
|
|
| Gastrointestinal complications (e.g., diarrhea) |
|
|
| Skin rash and allergic reactions |
|
|
| Teratogenicity |
|
|
| Alopecia |
|
|
| Respiratory infections |
|
|
| Hypertension |
|
|
| LEF Comparison with | Relative Efficacy | Therapeutic Remarks | Reference |
|---|---|---|---|
| Methotrexate (MTX) | Comparable (MTX is slightly higher for long-term structural results) | Similar symptomatic alleviation and physical function. Long-term tolerance was higher for MTX. Leflunomide has slow onset of action. | [76] |
| Hydroxychloroquine (HCQ) | LEF has greater efficacy than HCQ | HCQ used in combination regimens or for mild RA. LEF is more efficacious than HCQ. | [77] |
| Sulfasalazine (SSZ) | SSZ have lesser efficacy than LEF | Leflunomide-based triple therapy is not less effective than sulfasalazine-based therapy in methotrexate-refractory RA, and it has a similar safety profile. | [78] |
| Biological DMARDs (bDMARD) | bDMARDs have higher efficacy than LEF | In general, bDMARDs are more successful in treating moderate-to-severe RA. Biosimilars provide a more affordable option with higher quality-adjusted life years than leflunomide. | [79] |
| Stimuli | Stimuli Source | Research Instance | In Vitro and In Vivo Outcomes | Reference |
|---|---|---|---|---|
| pH | Low pH aids in swelling and degradation of polymer matrix | LEF-loaded chitosan and chondroitin sulfate NPs fabricated with folic acid (FA) and NPs further loaded in carbopol hydrogel for transdermal delivery | In vivo evaluation showed the deposition of LEF-FA-NPs in inflamed joints with minimum adverse effects | [83] |
| Ultrasound | Drug release takes place when ultrasound assistance applied externally | Biocompatible drug microneedles (DMNs) coated with hyaluronic acid (HA) embedded with ultrasound-responsive NPs to increase drug penetration | Synergistic drug effects appeared in collagen-induced arthritis model in rats in vivo | [102] |
| Receptor mediated | Upregulated CD44 receptors active targeting to CD44 receptors overexpressed in the articular tissue | A receptor-mediated synergistic hydrogel loaded with LEF-nanocarriers for delivery through intra-articular (IA) administration in RA | In vivo IA administration in arthritis-induced rodent model showed speedy recovery after HA-conjugated nanostructured lipid carrier (NLC) injection | [84] |
| Redox | Raised levels of reactive oxygen species (ROS) causing inflammation acts as stimuli for NPs | Ibuprofen (IBF)- and curcumin (CUR)-loaded HA-fabricated NPs for RA | Combination of CUR and IBF decreased the pro-inflammatory factors, ROS concentration, and COX-2 in vitro. Alas, dual drug-loaded HA-NPs alleviated foot tumefaction and lowered the expression of proinflammatory mediators in the RA-model mice in vivo | [103] |
| External magnetic field | An external magnetic field applied on inflamed joint after IA injection | LEF-loaded emulsomes (EMLs) loaded with supramagnetic nanoparticles (SPIONs) for IA administration in RA | In vivo evaluation depicted deposition of EMLs in the intra-articular cavity upon administration, providing sustained release and amelioration of inflammation in joints | [89] |
| Temperature | Local irradiation of the plasmonic laser produces thermal effect to promote drug release from NPs | Gold–thiol-beaded albumin nanoparticles (GTBA-NP-L) loaded with LEF for chemo-combined pulsatile plasmonic laser treatment for RA | Both in vitro and in vivo, GTBA-NP-L treatment demonstrated reduced inflammation and decreased pro-inflammatory cytokines | [101] |
| Coating Membrane Origin in Biomimetic DDS | Benefits in RA Therapeutics | Supporting Instance | Reference |
|---|---|---|---|
| Macrophage | Inherently targets inflamed joints; neutralizes cytokines | Macrophage membrane-camouflaged biomimetic nanoparticles for RA treatment via modulating macrophage polarization | [118] |
| Platelets | Interacts with endothelial damage at inflamed joints | Biomimetic platelet membrane-coated nanoparticles for targeted therapy | [119] |
| Neutrophils | Migrates to inflamed synovia; inflammatory tropism | Peptide-anchored neutrophil membrane-coated biomimetic nanodrug for targeted treatment of RA | [120] |
| Exosomes | Natural nanocarriers with communication signals | M2-type exosome nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization | [121] |
| Stem cells | Biocompatible, with regenerative cues | A biomimetic adipocyte mesenchymal stem cell membrane-encapsulated drug delivery system for the treatment of rheumatoid arthritis | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, A.; Garkhal, K. Leflunomide Applicability in Rheumatoid Arthritis: Drug Delivery Challenges and Emerging Formulation Strategies. Drugs Drug Candidates 2025, 4, 36. https://doi.org/10.3390/ddc4030036
Dhiman A, Garkhal K. Leflunomide Applicability in Rheumatoid Arthritis: Drug Delivery Challenges and Emerging Formulation Strategies. Drugs and Drug Candidates. 2025; 4(3):36. https://doi.org/10.3390/ddc4030036
Chicago/Turabian StyleDhiman, Ashish, and Kalpna Garkhal. 2025. "Leflunomide Applicability in Rheumatoid Arthritis: Drug Delivery Challenges and Emerging Formulation Strategies" Drugs and Drug Candidates 4, no. 3: 36. https://doi.org/10.3390/ddc4030036
APA StyleDhiman, A., & Garkhal, K. (2025). Leflunomide Applicability in Rheumatoid Arthritis: Drug Delivery Challenges and Emerging Formulation Strategies. Drugs and Drug Candidates, 4(3), 36. https://doi.org/10.3390/ddc4030036








