Antimicrobial and Anti-Inflammatory Potentials of Silver Tungstate Nanoparticles, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs
Abstract
1. Introduction
2. Results
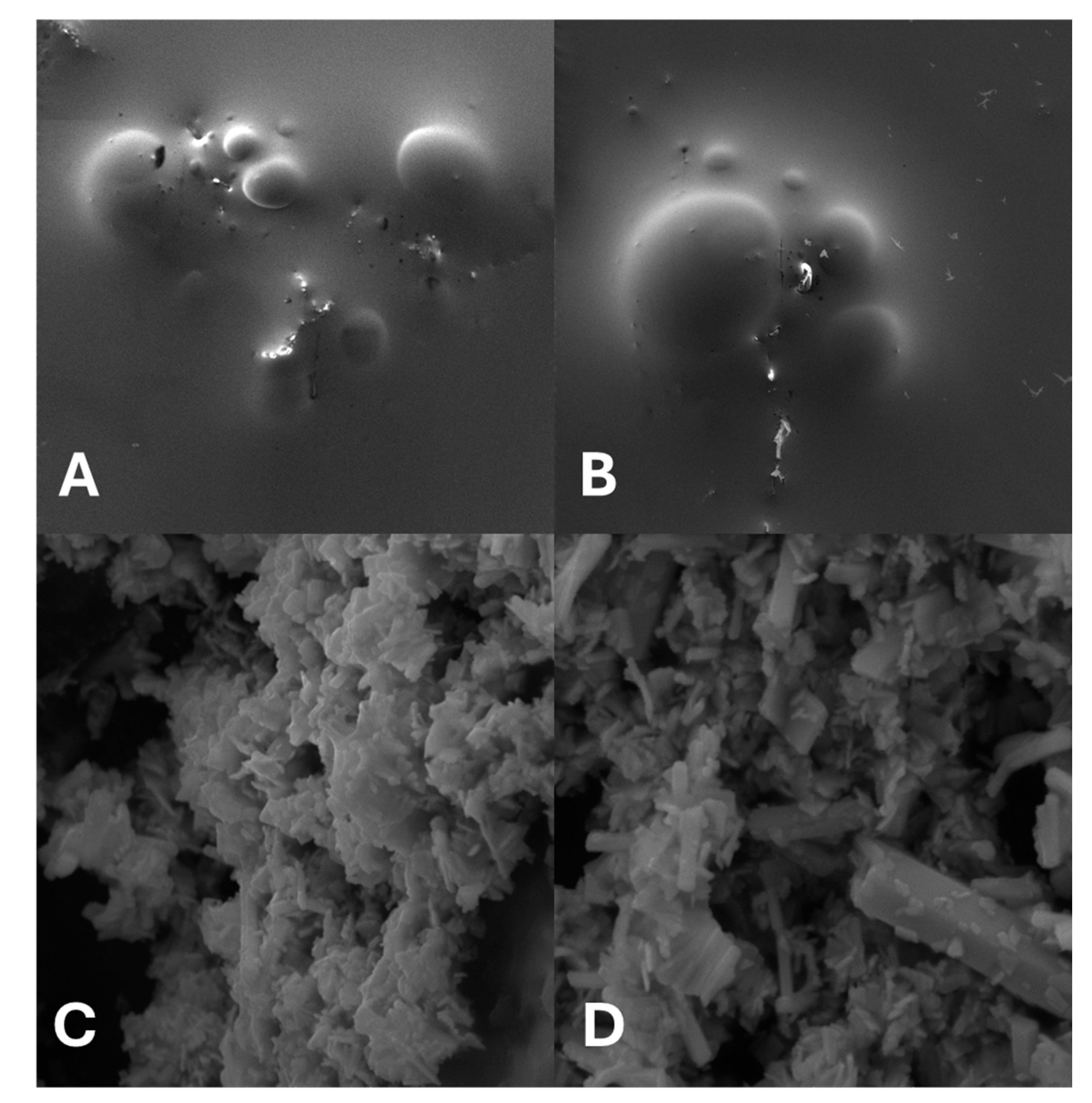
2.1. Structural, Optical and Morphological Study of the Nanoparticles
2.2. Antimicrobial Activity of STN
2.3. Cytotoxicity and Anti-Inflammatory Potential of STN
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Silver Tungstate Nanoparticles (STN)
4.2. Cytotoxicity Assay
4.3. Bacterial Isolates
4.4. Minimal Inhibitory Concentration (MIC) Assay
4.5. Drug Interactions Assay
4.6. Anti-Inflammatory Potential of STN
4.7. Scanning Electron Microscopy (SEM)
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRAD | bacterial resistance to antimicrobial drugs |
| BSA | bovine serum albumin |
| CFU | colony forming units |
| FIC | fractional inhibitory concentration |
| FICi | fractional inhibitory concentration index |
| MIC | minimal inhibitory concentration |
| STN | silver tungstate nanoparticles |
| XRD | X-ray diffraction |
References
- Huang, A.Q. Power semiconductor devices for smart grid and renewable energy systems. In Power Electronics in Renewable Energy Systems and Smart Grid: Technology and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 85–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, D.; Li, Q.; Zhang, R.; Udrea, F.; Wang, H. Wide-bandgap semiconductors and power electronics as pathways to carbon neutrality. Nat. Rev. Electr. Eng. 2025, 2, 155–172. [Google Scholar] [CrossRef]
- Maximenko, S.I.; Moore, J.E.; Affouda, C.A.; Jenkins, P.P. Optimal Semiconductors for 3H and 63Ni Betavoltaics. Sci. Rep. 2019, 9, 10892. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Vardeny, Z.V.; Beard, M.C. Control of light, spin and charge with chiral metal halide semiconductors. Nat. Rev. Chem. 2022, 6, 470–485. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Manfredi, G.; Mete, M.; Colombo, E.; Bramini, M.; Di Marco, S.; Shmal, D.; Mantero, G.; Dipalo, M.; Rocchi, A.; et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020, 15, 698–708. [Google Scholar] [CrossRef]
- Tan, Y.; Xiong, M.; Liu, Q.; Yin, Y.; Yin, X.; Liao, S.; Wang, Y.; Hu, L.; Zhang, X.-B. Precisely controlling the cellular internalization of DNA-decorated semiconductor polymer nanoparticles for drug delivery. RSC Adv. 2022, 12, 31173–31179. [Google Scholar] [CrossRef] [PubMed]
- Farsi, M.; Nezamzadeh-Ejhieh, A. A coupled Cobalt(II) oxide-Silver Tungstate nano-photocatalyst: Moderate characterization and evaluation of the photocatalysis kinetics towards methylene blue in aqueous solution. Polyhedron 2022, 219, 115823. [Google Scholar] [CrossRef]
- Rakshitha, R.; Gurupadayya, B.; Devi, S.H.K.; Pallavi, N. Coprecipitation aided synthesis of bimetallic silver tungstate: A response surface simulation of sunlight-driven photocatalytic removal of 2,4-dichlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 59433–59443. [Google Scholar] [CrossRef]
- de Abreu, C.B.; Gebara, R.C.; dos Reis, L.L.; Rocha, G.S.; Alho, L.d.O.G.; Alvarenga, L.M.; Virtuoso, L.S.; Assis, M.; Mansano, A.d.S.; Longo, E.; et al. Toxicity of α-Ag2WO4 microcrystals to freshwater microalga Raphidocelis subcapitata at cellular and population levels. Chemosphere 2022, 288 Pt 2, 132536. [Google Scholar] [CrossRef]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar]
- Dias-Souza, M.V.; Perpétuo, A.A.; dos Santos, G.S.; Machado, L.F.C.; dos Santos, R.M. Natural products in drug discovery: Meeting the urgency for new antimicrobials for human and veterinary use. AIMS Mol. Sci. 2023, 10, 11–21. [Google Scholar] [CrossRef]
- Macedo, N.G.; Gouveia, A.F.; Roca, R.A.; Assis, M.; Gracia, L.; Andrés, J.; Leite, E.R.; Longo, E. Surfactant-mediated morphology and photocatalytic activity of α-Ag2WO4 material. J. Phys. Chem. C 2018, 122, 8667–8679. [Google Scholar] [CrossRef]
- Isupov, V.A. ferroelectric and ferroelastic phase transitions in molybdates and tungstates of monovalent and bivalent elements. Ferroelectrics 2005, 322, 83–114. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Almeida, M.A.P.; Avansi, W.; Tranquilin, R.L.; Longo, E.; Batista, N.C.; Mastelaro, V.R.; Li, M.S. Cluster coordination and photoluminescence properties of α-Ag2WO4 microcrystals. Inorg. Chem. 2012, 51, 10675–10687. [Google Scholar] [CrossRef]
- Alvarez-Roca, R.; Gouveia, A.F.; de Foggi, C.C.; Lemos, P.S.; Gracia, L.; da Silva, L.F.; Vergani, C.E.; San-Miguel, M.; Longo, E.; Andrés, J. Selective synthesis of α-, β-, and γ-Ag2WO4 polymorphs: Promising platforms for photocatalytic and antibacterial materials. Inorg. Chem. 2021, 61, 1245–1258. [Google Scholar] [CrossRef]
- Goldmann, D.; Rajan, S.; Udayakumar, K. Preventing and Controlling Global Antimicrobial Resistance—Implementing a Whole-System Approach. N. Engl. J. Med. 2024, 391, 681–685. [Google Scholar] [CrossRef]
- Kokilavani, S.; Al-Farraj, S.A.; Thomas, A.M.; El-Serehy, H.A.; Raju, L.L.; Khan, S.S. Enhanced visible light driven photocatalytic and antibacterial activities of Ag2WO4 decorated ZnS nanocomposite. Ceram. Int. 2021, 47, 12997–13006. [Google Scholar] [CrossRef]
- Longo, E.; Volanti, D.P.; Longo, V.M.; Gracia, L.; Nogueira, I.C.; Almeida, M.A.P.; Pinheiro, A.N.; Ferrer, M.M.; Cavalcante, L.S.; Andrés, J. Toward an understanding of the growth of Ag filaments on α-Ag2WO4 and their photoluminescent properties: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 1229–1239. [Google Scholar] [CrossRef]
- Foggi, C.C.; De Oliveira, R.C.; Fabbro, M.T.; Vergani, C.E.; Andres, J.; Longo, E.; Machado, A.L. Tuning the morphological, optical, and antimicrobial properties of α-Ag2WO4 microcrystals using different solvents. Cryst. Growth Des. 2017, 17, 6239–6246. [Google Scholar] [CrossRef]
- Bastos, I.S.; Nobre, F.X.; da Silva, E.R.; Orlandi, P.P.; Lima, D.C.; da Cunha Mendes, O.; Manzato, L.; Pereira, M.L.R.D.; Leyet, Y.; Couceiro, P.R.C.; et al. Silver tungstate microcrystals and their performance over several clinical multidrug resistant microorganisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129132. [Google Scholar] [CrossRef]
- Foggi, C.C.; Fabbro, M.T.; Santos, L.P.; de Santana, Y.V.; Vergani, C.E.; Machado, A.L.; Cordoncillo, E.; Andrés, J.; Longo, E. Synthesis and evaluation of α-Ag2WO4 as novel antifungal agent. Chem. Phys. Lett. 2017, 674, 125–129. [Google Scholar] [CrossRef]
- Nobre, F.X.; Bastos, I.S.; Fontenelle, R.O.d.S.; Júnior, E.A.A.; Takeno, M.L.; Manzato, L.; de Matos, J.M.E.; Orlandi, P.P.; Mendes, J.d.F.S.; Brito, W.R.; et al. Antimicrobial properties of α-Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrason. Sonochemistry 2019, 58, 104620. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.F.C.; Marinho, J.Z.; de Lima, R.C.; Ceravolo, I.P.; Dias-Souza, M.V. Rapidly synthesized zinc oxide nanoparticles can increase the activity of antimicrobial drugs against clinical isolates of Pseudomonas aeruginosa and Escherichia coli. Acta Pharm. Sci. 2023, 61, 414. [Google Scholar] [CrossRef]
- Pimentel, B.N.A.d.S.; De Annunzio, S.R.; Assis, M.; Barbugli, P.A.; Longo, E.; Vergani, C.E. Biocompatibility and inflammatory response of silver tungstate, silver molybdate, and silver vanadate microcrystals. Front. Bioeng. Biotechnol. 2023, 11, 1215438. [Google Scholar] [CrossRef]
- Alcântara, L.K.S.; Machado, L.F.C.; Ceravolo, I.P.; Martins, R.; Souza, M.V.D. Antibiofilm effect of moringa oleifera leaf extract against staphylococcus aureus, cytotoxicity, biochemical aspects, anti-inflammatory potential, and interference on the activity of antimicrobial drugs. Fabad J. Pharm. Sci. 2023, 48, 241–254. [Google Scholar] [CrossRef]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepato-ma cells. Toxicol. Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Chávez, N.L.H.; de Avila, E.D.; Barbugli, P.A.; de Oliveira, R.C.; de Foggi, C.C.; Longo, E.; Vergani, C.E. Promising effects of silver tungstate microcrystals on fibroblast human cells and three dimensional collagen matrix models: A novel non-cytotoxic material to fight oral disease. Colloids Surf. B Biointerfaces 2018, 170, 505–513. [Google Scholar] [CrossRef]
- Dias-Souza, M.V.; dos Santos, R.M.; Cerávolo, I.P.; Cosenza, G.; Marçal, P.H.F.; Figueiredo, F.J.B. Euterpe oleracea pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb. Pathog. 2018, 114, 29–35. [Google Scholar] [CrossRef]
- CLSI Standard M07; CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Osman, M.E.; Abo-Elnasr, A.A.; Mohamed, E.T. Therapeutic potential activity of quercetin complexes against Streptococcus pneumoniae. Sci. Rep. 2024, 14, 12876. [Google Scholar] [CrossRef]


| Bacterial Species | MIC Values (μg/mL) | ||||
|---|---|---|---|---|---|
| STN | Azithromycin | Gentamycin | Sulfamethoxazole | Clindamycin | |
| S. aureus | 128 | NT | NT | 16 | 8 |
| P. aeruginosa | NE | 8 | 8 | NT | NT |
| E. coli | 256 | 8 | 8 | NT | NT |
| Parameter | Sulfamethoxazole | Clindamycin | STN |
|---|---|---|---|
| MIC alone | 16 μg/mL | 8 μg/mL | 128 μg/mL |
| MIC in combination | 0.5 μg/mL | 2 μg/mL | 16 μg/mL α 32 μg/mL β |
| FIC | 0.03125 | 0.25 | 0.125 γ 0.25 δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, W.d.S.; Marinho, J.Z.; Ceravolo, I.P.; Nascimento, L.L.; Patrocínio, A.O.d.T.; Dias-Souza, M.V. Antimicrobial and Anti-Inflammatory Potentials of Silver Tungstate Nanoparticles, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs. Drugs Drug Candidates 2025, 4, 30. https://doi.org/10.3390/ddc4030030
Leal WdS, Marinho JZ, Ceravolo IP, Nascimento LL, Patrocínio AOdT, Dias-Souza MV. Antimicrobial and Anti-Inflammatory Potentials of Silver Tungstate Nanoparticles, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs. Drugs and Drug Candidates. 2025; 4(3):30. https://doi.org/10.3390/ddc4030030
Chicago/Turabian StyleLeal, Washington de Souza, Juliane Zacour Marinho, Isabela Penna Ceravolo, Lucas Leão Nascimento, Antonio Otávio de Toledo Patrocínio, and Marcus Vinícius Dias-Souza. 2025. "Antimicrobial and Anti-Inflammatory Potentials of Silver Tungstate Nanoparticles, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs" Drugs and Drug Candidates 4, no. 3: 30. https://doi.org/10.3390/ddc4030030
APA StyleLeal, W. d. S., Marinho, J. Z., Ceravolo, I. P., Nascimento, L. L., Patrocínio, A. O. d. T., & Dias-Souza, M. V. (2025). Antimicrobial and Anti-Inflammatory Potentials of Silver Tungstate Nanoparticles, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs. Drugs and Drug Candidates, 4(3), 30. https://doi.org/10.3390/ddc4030030







