Epigenetic Alterations in Cancer: The Therapeutic Potential of Epigenetic Drugs in Cancer Therapy
Abstract
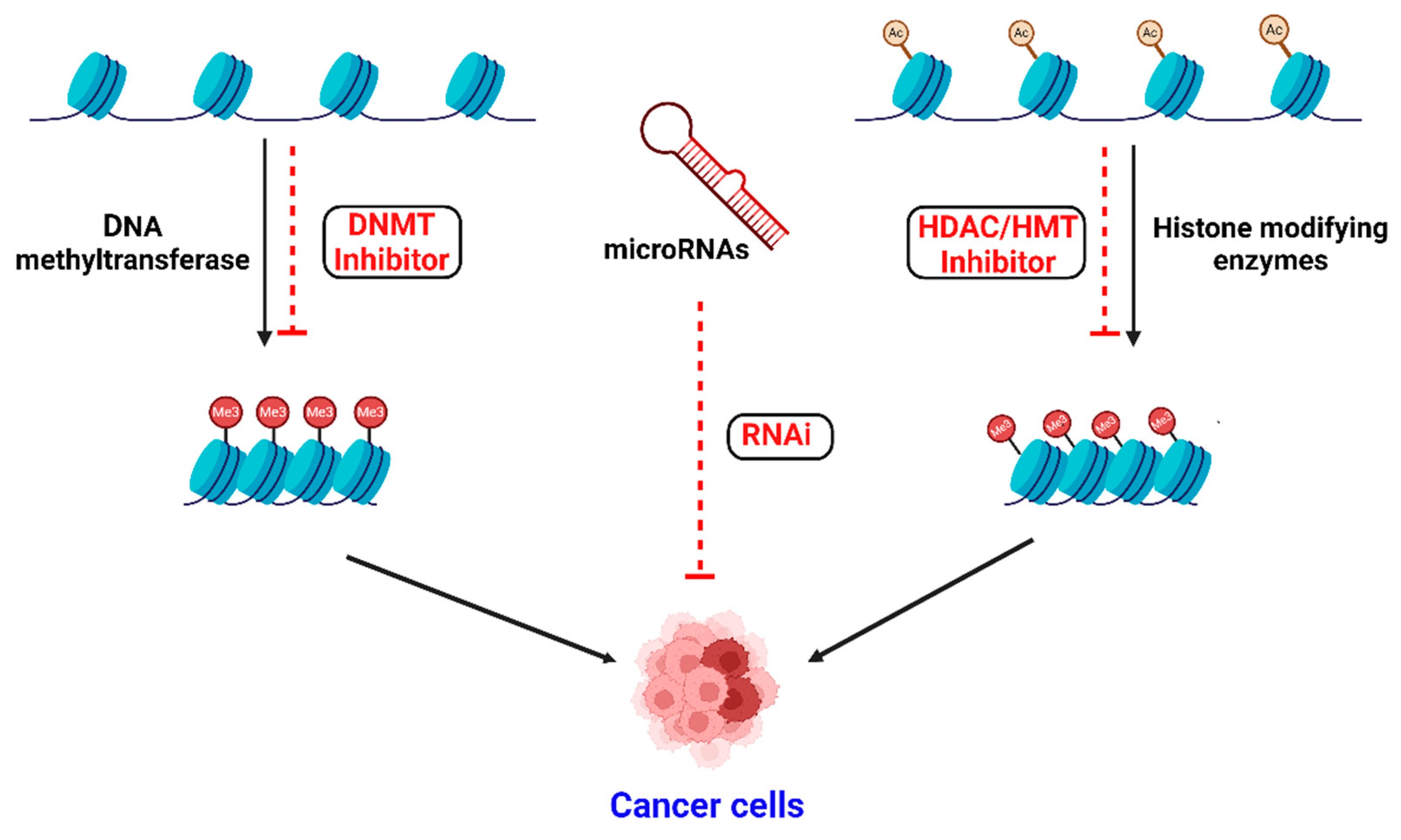
1. Introduction
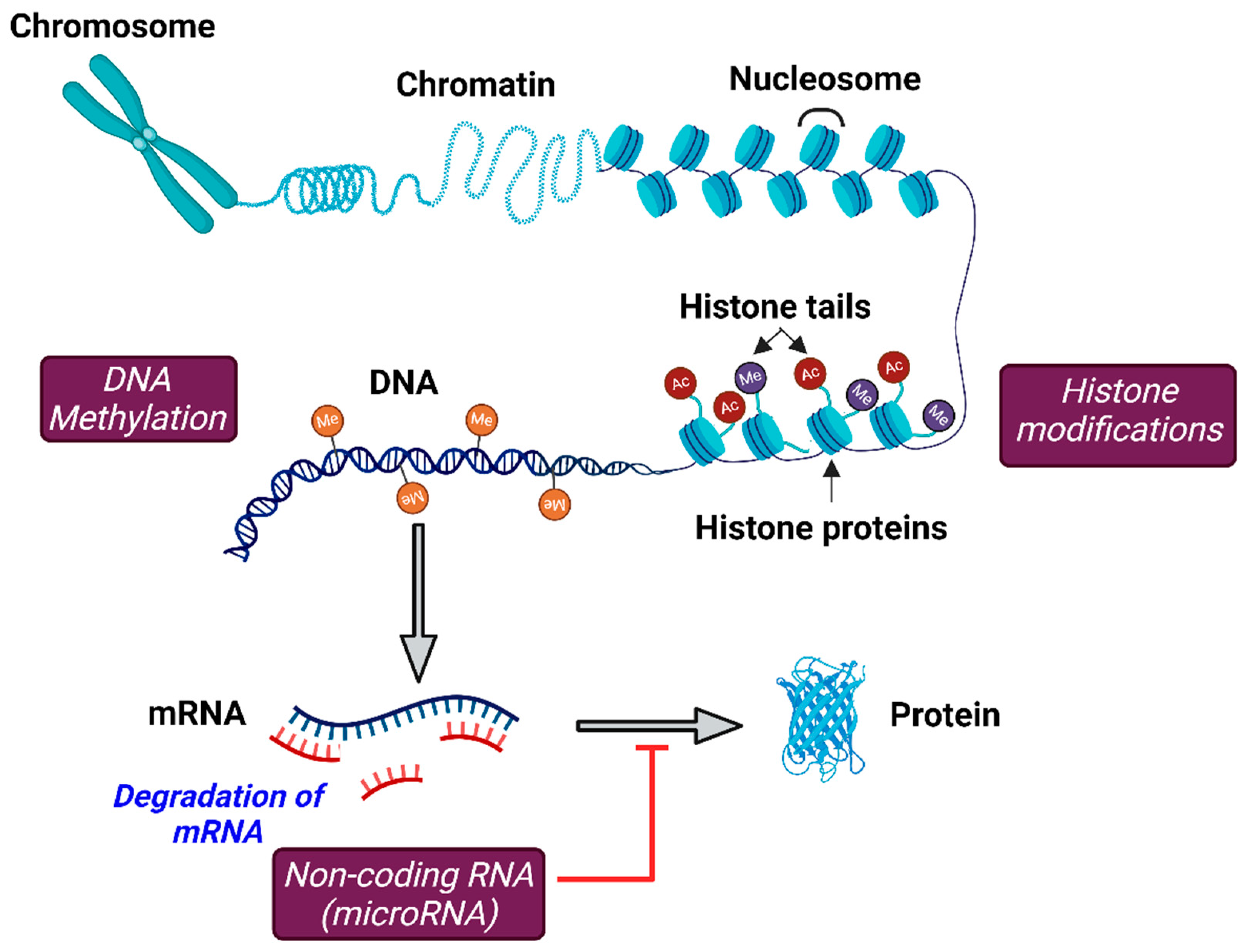
2. Epigenetic Modifications
3. Epigenetic Modifications in Cancer
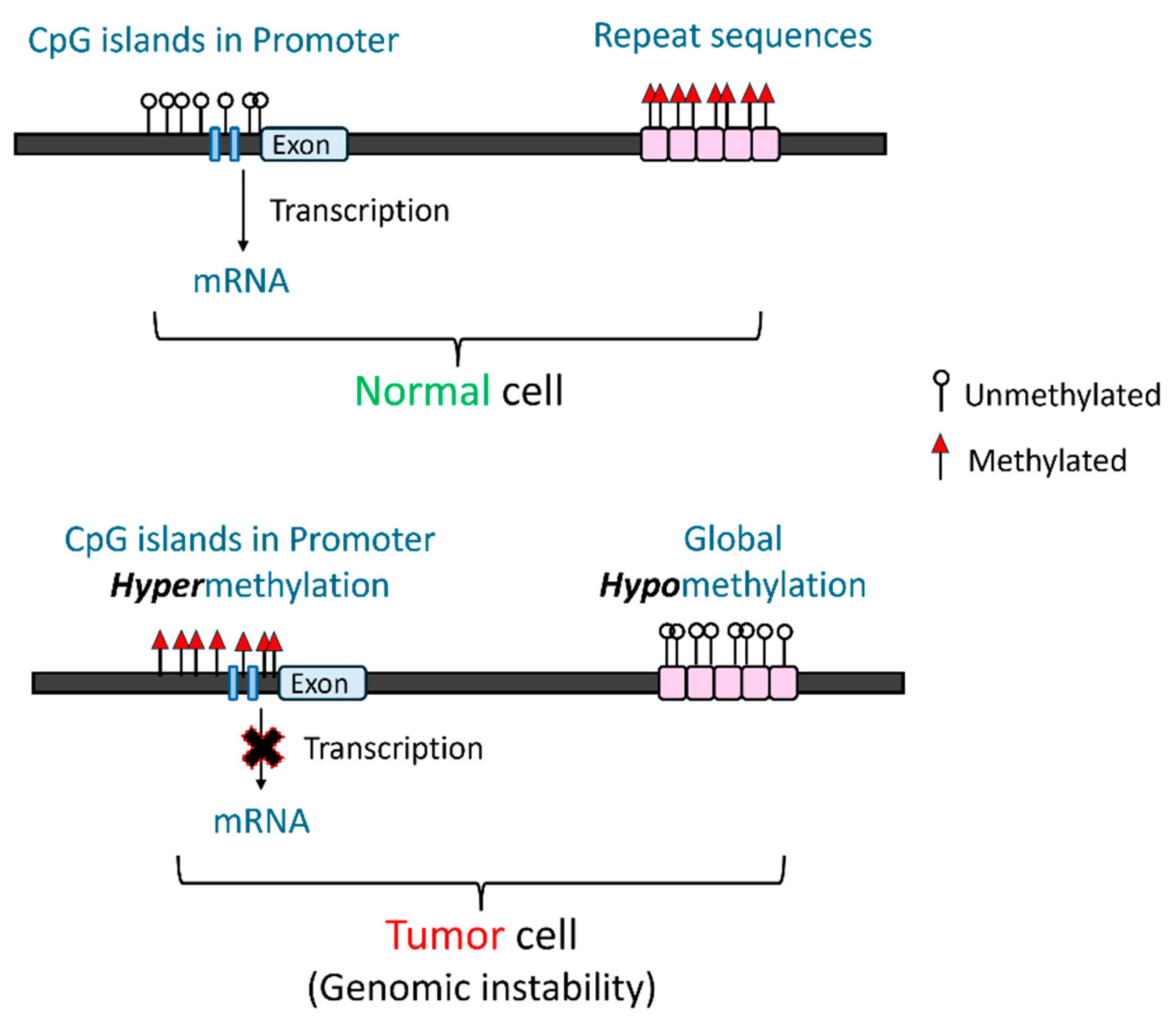
3.1. DNA Methylation
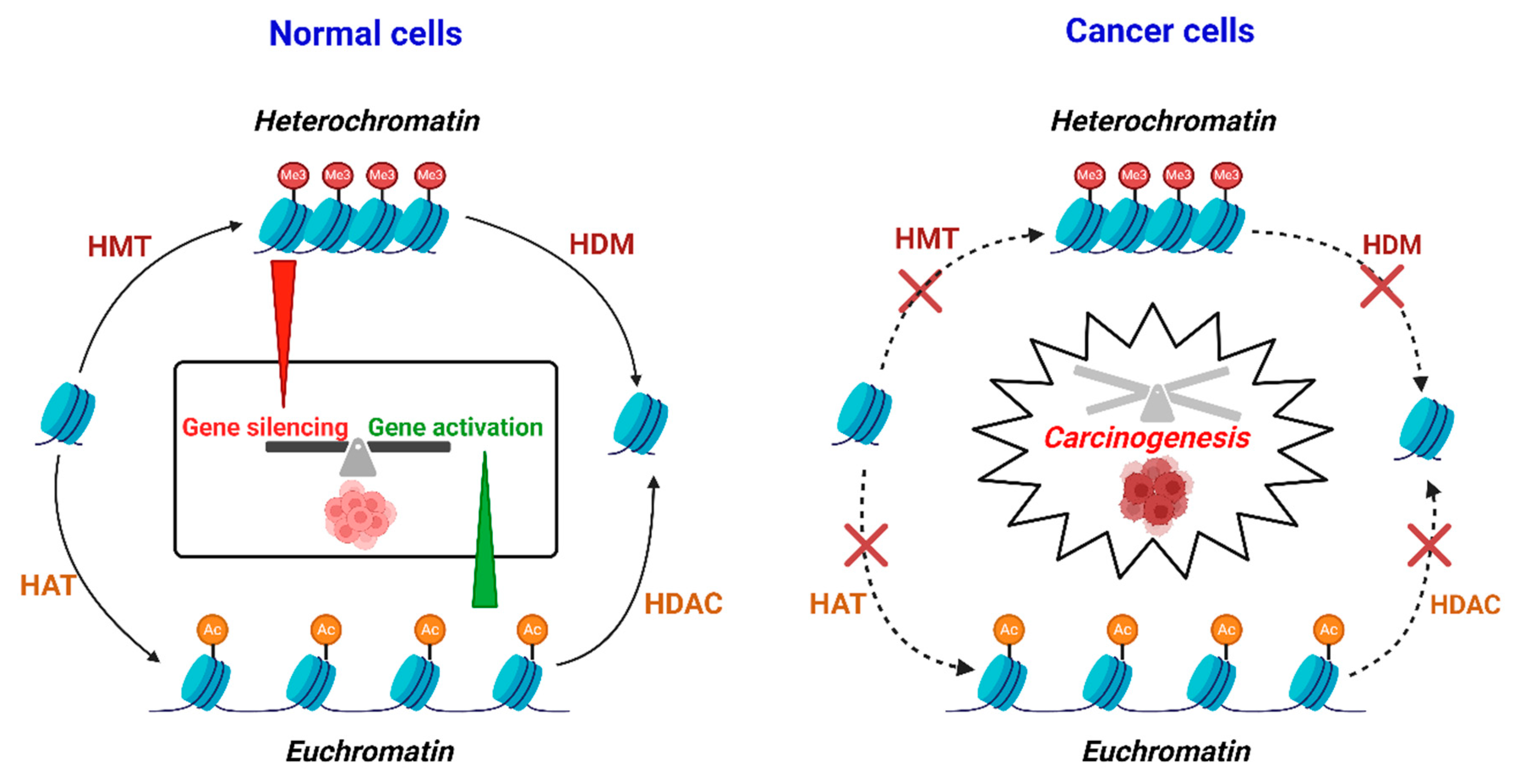
3.2. Histone Modifications
3.3. Non-Coding RNAs
3.3.1. MicroRNAs
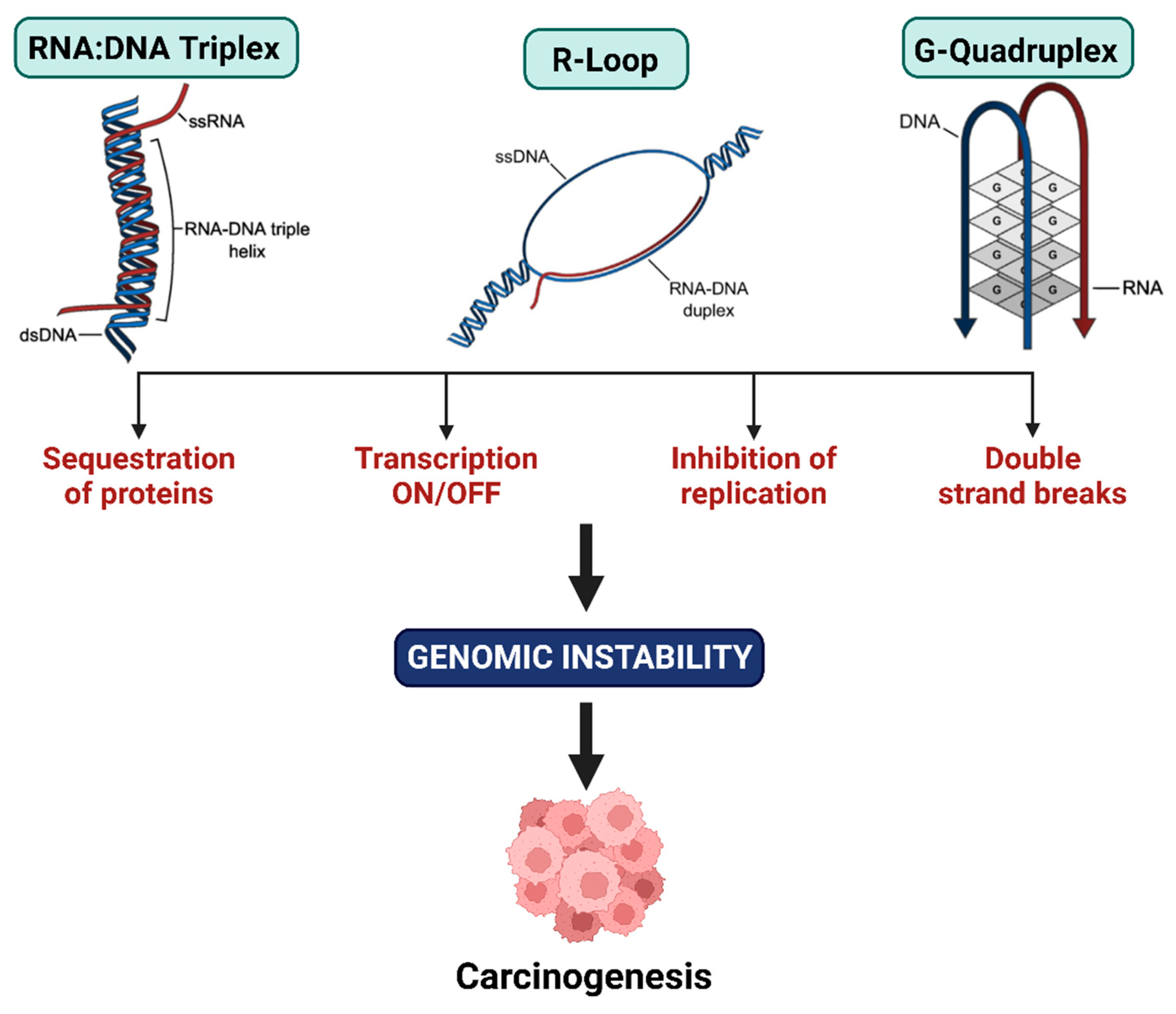
3.3.2. Long Non-Coding RNA
4. Epigenetic Drugs
5. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Szyf, M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 243–263. [Google Scholar] [CrossRef]
- Grewal, S.I.S. The molecular basis of heterochromatin assembly and epigenetic inheritance. Mol. Cell 2023, 83, 1767–1785. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, N. Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am. J. Cancer Res. 2020, 10, 1954–1978. [Google Scholar]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.P.; Barbash, O.; Creasy, C.L. Targeting epigenetic modifications in cancer therapy: Erasing the roadmap to cancer. Nat. Med. 2019, 25, 403–418. [Google Scholar] [CrossRef]
- Zhang, F.L.; Li, D.Q. Targeting Chromatin-Remodeling Factors in Cancer Cells: Promising Molecules in Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 2815. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting transcription factors in cancer—From undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Miranda Furtado, C.L.; Dos Santos Luciano, M.C.; Silva Santos, R.D.; Furtado, G.P.; Moraes, M.O.; Pessoa, C. Epidrugs: Targeting epigenetic marks in cancer treatment. Epigenetics 2019, 14, 1164–1176. [Google Scholar] [CrossRef]
- Li, G.; Reinberg, D. Chromatin higher-order structures and gene regulation. Curr. Opin. Genet. Dev. 2011, 21, 175–186. [Google Scholar] [CrossRef]
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gagnidze, K.; Pfaff, D.W. Epigenetic Mechanisms: DNA Methylation and Histone Protein Modification. In Neuroscience in the 21st Century: From Basic to Clinical; Pfaff, D.W., Volkow, N.D., Rubenstein, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2677–2716. [Google Scholar]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Al Aboud, N.M.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Farooq, Z.; Shah, A.; Tauseef, M.; Rather, R.A.; Anwar, M. Evolution of Epigenome as the Blueprint for Carcinogenesis. In Epigenetics to Optogenetics-A New Paradigm in the Study of Biology; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 2022, 83, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Szyf, M. DNA methylation signatures for breast cancer classification and prognosis. Genome Med. 2012, 4, 26. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-targeted drugs: Current paradigms and future challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W. Chapter 5—DNA Methylation Alterations in Human Cancers. In Epigenetics in Human Disease, 2nd ed.; Tollefsbol, T.O., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 109–139. [Google Scholar]
- Ramsahoye, B.H.; Biniszkiewicz, D.; Lyko, F.; Clark, V.; Bird, A.P.; Jaenisch, R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl. Acad. Sci. USA 2000, 97, 5237–5242. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Batra, R.N.; Lifshitz, A.; Vidakovic, A.T.; Chin, S.-F.; Sati-Batra, A.; Sammut, S.-J.; Provenzano, E.; Ali, H.R.; Dariush, A.; Bruna, A.; et al. DNA methylation landscapes of 1538 breast cancers reveal a replication-linked clock, epigenomic instability and cis-regulation. Nat. Commun. 2021, 12, 5406. [Google Scholar] [CrossRef]
- Xiao, M.; Liang, X.; Yan, Z.; Chen, J.; Zhu, Y.; Xie, Y.; Li, Y.; Li, X.; Gao, Q.; Feng, F.; et al. A DNA-Methylation-Driven Genes Based Prognostic Signature Reveals Immune Microenvironment in Pancreatic Cancer. Front. Immunol. 2022, 13, 803962. [Google Scholar]
- Hoang PH, L.M. DNA Methylation in Lung Cancer: Mechanisms and Associations with Histological Subtypes, Molecular Alterations, and Major Epidemiological Factors. Cancers 2022, 14, 961. [Google Scholar] [CrossRef]
- Shi, J.-F.; Li, X.-J.; Si, X.-X.; Li, A.-D.; Ding, H.-J.; Han, X.; Sun, Y.-J. ERα positively regulated DNMT1 expression by binding to the gene promoter region in human breast cancer MCF-7 cells. Biochem. Biophys. Res. Commun. 2012, 427, 47–53. [Google Scholar] [PubMed]
- Qu, Y.; Dang, S.; Hou, P. Gene methylation in gastric cancer. Clin. Chim. Acta 2013, 424, 53–65. [Google Scholar] [PubMed]
- Kientz, C.; Prieur, F.; Clemenson, A.; Joly, M.-O.; Stachowicz, M.-L.; Auclair, J.; Attignon, V.; Schiappa, R.; Wang, Q. MLH1 promoter hypermethylation: Are you absolutely sure about the absence of MLH1 germline mutation? About a new case. Fam. Cancer 2020, 19, 11–14. [Google Scholar] [CrossRef]
- Pistore, C.; Giannoni, E.; Colangelo, T.; Rizzo, F.; Magnani, E.; Muccillo, L.; Giurato, G.; Mancini, M.; Rizzo, S.; Riccardi, M.; et al. DNA methylation variations are required for epithelial-to-mesenchymal transition induced by cancer-associated fibroblasts in prostate cancer cells. Oncogene 2017, 36, 5551–5566. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef]
- Morrison, O.; Thakur, J. Molecular Complexes at Euchromatin, Heterochromatin and Centromeric Chromatin. Int. J. Mol. Sci. 2021, 22, 6922. [Google Scholar] [CrossRef]
- Audia, J.E.; Campbell, R.M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Rao, S.M.; Zhu, Y.-J. Identification of the MLL2 complex as a coactivator for estrogen receptor α. J. Biol. Chem. 2006, 281, 15714–15720. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sharma, A.; Dhar, S.S.; Lee, S.-H.; Gu, B.; Chan, C.-H.; Lin, H.-K.; Lee, M.G. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res. 2014, 74, 1705–1717. [Google Scholar]
- Larsson, C.; Cordeddu, L.; Siggens, L.; Pandzic, T.; Kundu, S.; He, L.; Ali, M.A.; Pristovšek, N.; Hartman, K.; Ekwall, K. Restoration of KMT2C/MLL3 in human colorectal cancer cells reinforces genome-wide H3K4me1 profiles and influences cell growth and gene expression. Clin. Epigenetics 2020, 12, 74. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, S.; Cai, L. Epigenetic (De)regulation in Prostate Cancer. In Epigenetics in Oncology; Chen, J., Wang, G.G., Lu, J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 321–360. [Google Scholar]
- Strepkos, D.; Markouli, M.; Klonou, A.; Papavassiliou, A.G.; Piperi, C. Histone methyltransferase SETDB1: A common denominator of tumorigenesis with therapeutic potential. Cancer Res. 2021, 81, 525–534. [Google Scholar]
- Vieira, F.Q.; Costa-Pinheiro, P.; Ramalho-Carvalho, J.; Pereira, A.; Menezes, F.D.; Antunes, L.; Carneiro, I.; Oliveira, J.; Henrique, R.; Jeronimo, C. Deregulated expression of selected histone methylases and demethylases in prostate carcinoma. Endocr.-Relat. Cancer 2014, 21, 51–61. [Google Scholar]
- Sanese, P.; Fasano, C.; Lepore Signorile, M.; De Marco, K.; Forte, G.; Disciglio, V.; Grossi, V.; Simone, C. Methyltransferases in cancer drug resistance: Unlocking the potential of targeting SMYD3 to sensitize cancer cells. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189203. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Shi, M.; Tian, H.; Li, J.; Zhu, K.; Guo, Y.; Mu, Y.; Geng, J.; Li, Z. A Positive Feedback Loop of lncRNA HOXD-AS2 and SMYD3 Facilitates Hepatocellular Carcinoma Progression via the MEK/ERK Pathway. J. Hepatocell. Carcinoma 2023, 10, 1237–1256. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsumoto, A.; Hieda, M.; Shinchi, Y.; Ogihara, E.; Hamada, M.; Nishioka, Y.; Kimura, H.; Yoshidome, K.; Tsujimoto, M.; et al. Loss of histone H4K20 trimethylation predicts poor prognosis in breast cancer and is associated with invasive activity. Breast Cancer Res. 2014, 16, R66. [Google Scholar] [CrossRef] [PubMed]
- Di Martile, M.; Del Bufalo, D.; Trisciuoglio, D. The multifaceted role of lysine acetylation in cancer: Prognostic biomarker and therapeutic target. Oncotarget 2016, 7, 55789. [Google Scholar] [PubMed]
- Iyer, A.; Fairlie, D.P.; Brown, L. Lysine acetylation in obesity, diabetes and metabolic disease. Immunol. Cell Biol. 2012, 90, 39–46. [Google Scholar]
- Pasqualucci, L.; Dominguez-Sola, D.; Chiarenza, A.; Fabbri, G.; Grunn, A.; Trifonov, V.; Kasper, L.H.; Lerach, S.; Tang, H.; Ma, J. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011, 471, 189–195. [Google Scholar]
- Trisciuoglio, D.; Di Martile, M.; Del Bufalo, D. Emerging Role of Histone Acetyltransferase in Stem Cells and Cancer. Stem Cells Int. 2018, 2018, 8908751. [Google Scholar] [CrossRef]
- Pastore, A.; Jurinovic, V.; Kridel, R.; Hoster, E.; Staiger, A.M.; Szczepanowski, M.; Pott, C.; Kopp, N.; Murakami, M.; Horn, H. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015, 16, 1111–1122. [Google Scholar]
- Qian, M.; Zhang, H.; Kham, S.K.-Y.; Liu, S.; Jiang, C.; Zhao, X.; Lu, Y.; Goodings, C.; Lin, T.-N.; Zhang, R. Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res. 2017, 27, 185–195. [Google Scholar] [PubMed]
- Sun, X.-J.; Man, N.; Tan, Y.; Nimer, S.D.; Wang, L. The role of histone acetyltransferases in normal and malignant hematopoiesis. Front. Oncol. 2015, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Zhao, J.; Meng, Z.; Wu, H.; Wang, B.; Wu, H.; Jin, X. Overexpressed histone acetyltransferase 1 regulates cancer immunity by increasing programmed death-ligand 1 expression in pancreatic cancer. J. Exp. Clin. Cancer Res. 2019, 38, 47. [Google Scholar]
- Wang, W.; Zhao, M.; Cui, L.; Ren, Y.; Zhang, J.; Chen, J.; Jia, L.; Zhang, J.; Yang, J.; Chen, G.; et al. Characterization of a novel HDAC/RXR/HtrA1 signaling axis as a novel target to overcome cisplatin resistance in human non-small cell lung cancer. Mol. Cancer 2020, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Ammazzalorso, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Amoroso, R. HDAC Inhibitors for the Therapy of Triple Negative Breast Cancer. Pharmaceuticals 2022, 15, 667. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Zhu, H.-Y.; Zhou, B.-Y.; Chu, Y.; Huo, J.-R.; Tan, Y.-Y.; Liu, D.-L. Histone deacetylase 6 is overexpressed and promotes tumor growth of colon cancer through regulation of the MAPK/ERK signal pathway. OncoTargets Ther. 2019, 12, 2409–2419. [Google Scholar] [CrossRef]
- Cao, L.L.; Yue, Z.; Liu, L.; Pei, L.; Yin, Y.; Qin, L.; Zhao, J.; Liu, H.; Wang, H.; Jia, M. The expression of histone deacetylase HDAC1 correlates with the progression and prognosis of gastrointestinal malignancy. Oncotarget 2017, 8, 39241–39253. [Google Scholar] [CrossRef]
- Phimmachanh, M.; Han, J.Z.; O’Donnell, Y.E.; Latham, S.L.; Croucher, D.R. Histone deacetylases and histone deacetylase inhibitors in neuroblastoma. Front. Cell Dev. Biol. 2020, 8, 578770. [Google Scholar]
- Liang, T.; Wang, F.; Elhassan, R.M.; Cheng, Y.; Tang, X.; Chen, W.; Fang, H.; Hou, X. Targeting histone deacetylases for cancer therapy: Trends and challenges. Acta Pharm. Sin. B 2023, 13, 2425–2463. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Q. Histone deacetylase inhibitors and cell death. Cell. Mol. Life Sci. 2014, 71, 3885–3901. [Google Scholar]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.B.; Nadkarni, K.S.; Barik, G.K.; Tat, S.S.; Sahay, O.; Santra, M.K. Histone ubiquitination: Role in genome integrity and chromatin organization. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2024, 1867, 195044. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, J.; Zhan, Q.; Zhu, Y.; Chen, H.; Deng, X.; Hou, Z.; Shen, B.; Chen, Y.; Peng, C. H2AK119Ub1 and H3K27Me3 in molecular staging for survival prediction of patients with pancreatic ductal adenocarcinoma. Oncotarget 2014, 5, 10421–10433. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Guo, T.; Wang, Y.; Yang, J. Decreased H2B monoubiquitination and overexpression of ubiquitin-specific protease enzyme 22 in malignant colon carcinoma. Hum. Pathol. 2015, 46, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Prenzel, T.; Begus-Nahrmann, Y.; Kramer, F.; Hennion, M.; Hsu, C.; Gorsler, T.; Hintermair, C.; Eick, D.; Kremmer, E.; Simons, M. Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Res. 2011, 71, 5739–5753. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Yang, J.-L.; Wang, Y.-P.; Lou, J.-Y.; Chen, J.; Liu, C.; Guo, L.-D. Decreased histone H2B monoubiquitination in malignant gastric carcinoma. World J. Gastroenterol. WJG 2013, 19, 8099. [Google Scholar]
- Senft, D.; Qi, J.; Ronai, Z.e.A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 2018, 18, 69–88. [Google Scholar] [CrossRef]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef]
- Ding, X.; Sun, J.; Wang, L.; Li, G.; Shen, Y.; Zhou, X.; Chen, W. Overexpression of SENP5 in oral squamous cell carcinoma and its association with differentiation. Oncol. Rep. 2008, 20, 1041–1045. [Google Scholar]
- Katayama, A.; Ogino, T.; Bandoh, N.; Takahara, M.; Kishibe, K.; Nonaka, S.; Harabuchi, Y. Overexpression of small ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous cell carcinoma: Possible involvement in tumor proliferation and prognosis. Int. J. Oncol. 2007, 31, 517–524. [Google Scholar] [CrossRef]
- Shiio, Y.; Eisenman, R.N. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA 2003, 100, 13225–13230. [Google Scholar] [CrossRef] [PubMed]
- Bogachek, M.V.; De Andrade, J.P.; Weigel, R.J. Regulation of Epithelial–Mesenchymal Transition through SUMOylation of Transcription Factors. Cancer Res. 2015, 75, 11–15. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhao, M. N6-methyladenosine modification and post-translational modification of epithelial-mesenchymal transition in colorectal cancer. Discov. Oncol. 2024, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Samson, W.K.; Ji, H. Decoding noncoding RNA: Da Vinci redux? Circ. Res. 2013, 113, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as therapeutic targets in cardiovascular disease. J. Clin. Investig. 2022, 132, 11. [Google Scholar] [CrossRef]
- Abdallah, H.Y.; Faisal, S.; Tawfik, N.Z.; Soliman, N.H.; Kishk, R.M.; Ellawindy, A. Expression signature of immune-related MicroRNAs in autoimmune skin disease: Psoriasis and vitiligo insights. Mol. Diagn. Ther. 2023, 27, 405–423. [Google Scholar]
- Renaudineau, Y.; Berindan-Neagoe, I.; Stanciu, L.A. role of macrophage MicroRNAs in inflammatory diseases and cancer. Front. Immunol. 2021, 12, 764525. [Google Scholar]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The Oncogenic Role of miR-155 in Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef]
- Braga, T.V.; Evangelista, F.C.G.; Gomes, L.C.; Araújo, S.S.d.S.; Carvalho, M.d.G.; Sabino, A.d.P. Evaluation of MiR-15a and MiR-16-1 as prognostic biomarkers in chronic lymphocytic leukemia. Biomed. Pharmacother. 2017, 92, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Y.; Li, J.; Zhu, Q. Small Non-Coding RNAs in Human Cancer. Genes 2022, 13, 2072. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, Y.; Sun, X.; Xu, T.; Cheng, X.; Qin, Y. miR-221/222 promote tumor growth and suppress apoptosis by targeting lncRNA GAS5 in breast cancer. Biosci. Rep. 2019, 39, BSR20181859. [Google Scholar]
- Sheedy, P.; Medarova, Z. The fundamental role of miR-10b in metastatic cancer. Am. J. Cancer Res. 2018, 8, 1674. [Google Scholar]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef]
- Li, X.J.; Ren, Z.J.; Tang, J.H. MicroRNA-34a: A potential therapeutic target in human cancer. Cell Death Dis. 2014, 5, e1327. [Google Scholar] [CrossRef]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef]
- Greifenstein, A.A.; Jo, S.; Bierhoff, H. RNA:DNA triple helices: From peculiar structures to pervasive chromatin regulators. Essays Biochem. 2021, 65, 731–740. [Google Scholar] [CrossRef]
- Forrest, M.E.; Khalil, A.M. Review: Regulation of the cancer epigenome by long non-coding RNAs. Cancer Lett. 2017, 407, 106–112. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadid, Q.; Yang, Y. R-loop: An emerging regulator of chromatin dynamics. Acta Biochim. Et Biophys. Sin. 2016, 48, 623–631. [Google Scholar] [CrossRef]
- Allison, D.F.; Wang, G.G. R-loops: Formation, function, and relevance to cell stress. Cell Stress 2019, 3, 38–46. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, T.; Zhou, W.; Li, J.; Li, X.; Wang, Q.; Jin, X.; Yin, J.; Chen, L.; Zhang, Y. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat. Commun. 2020, 11, 1000. [Google Scholar]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018, 11, 63. [Google Scholar] [CrossRef]
- Huo, Y.; Li, Q.; Wang, X.; Jiao, X.; Zheng, J.; Li, Z.; Pan, X. MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2. Oncotarget 2017, 8, 46993. [Google Scholar] [PubMed]
- Wang, W.; Zhu, Y.; Li, S.; Chen, X.; Jiang, G.; Shen, Z.; Qiao, Y.; Wang, L.; Zheng, P.; Zhang, Y. Long noncoding RNA MALAT1 promotes malignant development of esophageal squamous cell carcinoma by targeting β-catenin via Ezh2. Oncotarget 2016, 7, 25668–25682. [Google Scholar] [CrossRef]
- Xing, C.; Sun, S.-g.; Yue, Z.-Q.; Bai, F. Role of lncRNA LUCAT1 in cancer. Biomed. Pharmacother. 2021, 134, 111158. [Google Scholar] [CrossRef]
- Ma, C.; Shi, X.; Zhu, Q.; Li, Q.; Liu, Y.; Yao, Y.; Song, Y. The growth arrest-specific transcript 5 (GAS5): A pivotal tumor suppressor long noncoding RNA in human cancers. Tumor Biol. 2016, 37, 1437–1444. [Google Scholar] [CrossRef]
- Elsakrmy, N.; Cui, H. R-Loops and R-Loop-Binding Proteins in Cancer Progression and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 64. [Google Scholar] [CrossRef]
- Li, D.; Shao, F.; Li, X.; Yu, Q.; Wu, R.; Wang, J.; Wang, Z.; Wusiman, D.; Ye, L.; Guo, Y.; et al. Advancements and challenges of R-loops in cancers: Biological insights and future directions. Cancer Lett. 2025, 610, 217359. [Google Scholar] [CrossRef]
- Kim, S.; Shin, W.H.; Kang, Y.; Kim, H.; Lee, J.Y. Direct visualization of replication and R-loop collision using single-molecule imaging. Nucleic Acids Res. 2024, 52, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hatchi, E.; Skourti-Stathaki, K.; Ventz, S.; Pinello, L.; Yen, A.; Kamieniarz-Gdula, K.; Dimitrov, S.; Pathania, S.; McKinney, K.M.; Eaton, M.L.; et al. BRCA1 Recruitment to Transcriptional Pause Sites Is Required for R-Loop-Driven DNA Damage Repair. Mol. Cell 2015, 57, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Dumas, L.; Herviou, P.; Dassi, E.; Cammas, A.; Millevoi, S. G-Quadruplexes in RNA biology: Recent advances and future directions. Trends Biochem. Sci. 2021, 46, 270–283. [Google Scholar]
- Zenkov, R.G.; Kirsanov, K.I.; Ogloblina, A.M.; Vlasova, O.A.; Naberezhnov, D.S.; Karpechenko, N.Y.; Fetisov, T.I.; Lesovaya, E.A.; Belitsky, G.A.; Dolinnaya, N.G.; et al. Effects of G-Quadruplex-Binding Plant Secondary Metabolites on c-MYC Expression. Int. J. Mol. Sci. 2022, 23, 9209. [Google Scholar] [CrossRef]
- Wanrooij, P.H.; Uhler, J.P.; Simonsson, T.; Falkenberg, M.; Gustafsson, C.M. G-quadruplex structures in RNA stimulate mitochondrial transcription termination and primer formation. Proc. Natl. Acad. Sci. USA 2010, 107, 16072–16077. [Google Scholar]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Moye, A.L.; Porter, K.C.; Cohen, S.B.; Phan, T.; Zyner, K.G.; Sasaki, N.; Lovrecz, G.O.; Beck, J.L.; Bryan, T.M. Telomeric G-quadruplexes are a substrate and site of localization for human telomerase. Nat. Commun. 2015, 6, 7643. [Google Scholar]
- Bahls, B.; Aljnadi, I.M.; Emídio, R.; Mendes, E.; Paulo, A. G-Quadruplexes in c-MYC Promoter as Targets for Cancer Therapy. Biomedicines 2023, 11, 969. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.; Zhou, X. G-Quadruplexes in Neurobiology and Virology: Functional Roles and Potential Therapeutic Approaches. JACS Au 2021, 1, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Ma, X.; Wu, Y.; Zhang, W. G-quadruplex stabilization via small-molecules as a potential anti-cancer strategy. Biomed. Pharmacother. 2021, 139, 111550. [Google Scholar] [CrossRef]
- Figueiredo, J.; Mergny, J.-L.; Cruz, C. G-quadruplex ligands in cancer therapy: Progress, challenges, and clinical perspectives. Life Sci. 2024, 340, 122481. [Google Scholar] [CrossRef]
- Huppert, J.L.; Balasubramanian, S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2006, 35, 406–413. [Google Scholar] [CrossRef]
- Gomez, D.; Guédin, A.; Mergny, J.-L.; Salles, B.; Riou, J.-F.; Teulade-Fichou, M.-P.; Calsou, P. A G-quadruplex structure within the 5′-UTR of TRF2 mRNA represses translation in human cells. Nucleic Acids Res. 2010, 38, 7187–7198. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.T.; Vallur, A.C.; Eddy, J.; Maizels, N. G quadruplexes are genomewide targets of transcriptional helicases XPB and XPD. Nat. Chem. Biol. 2014, 10, 313–318. [Google Scholar] [CrossRef]
- Coan, M.; Haefliger, S.; Ounzain, S.; Johnson, R. Targeting and engineering long non-coding RNAs for cancer therapy. Nat. Rev. Genet. 2024, 25, 578–595. [Google Scholar] [CrossRef]
- Cao, J.; Yan, Q. Cancer Epigenetics, Tumor Immunity, and Immunotherapy. Trends Cancer 2020, 6, 580–592. [Google Scholar] [CrossRef]
- Dawson, M.A. The cancer epigenome: Concepts, challenges, and therapeutic opportunities. Science 2017, 355, 1147–1152. [Google Scholar]
- Roulois, D.; Yau, H.L.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 2015, 162, 961–973. [Google Scholar]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563. e519. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212576s000lbl.pdf (accessed on 28 December 2024).
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; McCloskey, J.; Griffiths, E.A.; Yee, K.W.L.; Zeidan, A.M.; Al-Kali, A.; Deeg, H.J.; Patel, P.A.; Sabloff, M.; Keating, M.M.; et al. Oral decitabine-cedazuridine versus intravenous decitabine for myelodysplastic syndromes and chronic myelomonocytic leukaemia (ASCERTAIN): A registrational, randomised, crossover, pharmacokinetics, phase 3 study. Lancet Haematol. 2024, 11, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Bataller, A.; Montalban-Bravo, G.; Bazinet, A.; Alvarado, Y.; Chien, K.; Venugopal, S.; Ishizawa, J.; Hammond, D.; Swaminathan, M.; Sasaki, K.; et al. Oral decitabine plus cedazuridine and venetoclax in patients with higher-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: A single-centre, phase 1/2 study. Lancet Haematol. 2024, 11, e186–e195. [Google Scholar] [CrossRef]
- Kantarjian, H.; Oki, Y.; Garcia-Manero, G.; Huang, X.; O’Brien, S.; Cortes, J.; Faderl, S.; Bueso-Ramos, C.; Ravandi, F.; Estrov, Z.; et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2006, 109, 52–57. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, J.; Hong, W.; Li, L.; Wu, M. Inhibition of cancer cell proliferation by 5-fluoro-2′-deoxycytidine, a DNA methylation inhibitor, through activation of DNA damage response pathway. SpringerPlus 2012, 1, 65. [Google Scholar] [CrossRef][Green Version]
- O’Connell, C.L.; Baer, M.R.; Ørskov, A.D.; Saini, S.K.; Duong, V.H.; Kropf, P.; Hansen, J.W.; Tsao-Wei, D.; Jang, H.S.; Emadi, A. Safety, outcomes, and T-cell characteristics in patients with relapsed or refractory MDS or CMML treated with atezolizumab in combination with guadecitabine. Clin. Cancer Res. 2022, 28, 5306–5316. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Roboz, G.; Walsh, K.; Kantarjian, H.; Ritchie, E.; Kropf, P.; O’Connell, C.; Tibes, R.; Lunin, S.; Rosenblat, T. Guadecitabine (SGI-110) in patients with intermediate or high-risk myelodysplastic syndromes: Phase 2 results from a multicentre, open-label, randomised, phase 1/2 trial. Lancet Haematol. 2019, 6, e317–e327. [Google Scholar] [CrossRef]
- Chung, W.; Kelly, A.D.; Kropf, P.; Fung, H.; Jelinek, J.; Su, X.Y.; Roboz, G.J.; Kantarjian, H.M.; Azab, M.; Issa, J.-P.J. Genomic and epigenomic predictors of response to guadecitabine in relapsed/refractory acute myelogenous leukemia. Clin. Epigenetics 2019, 11, 106. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Roboz, G.J.; Kropf, P.L.; Yee, K.W.; O’Connell, C.L.; Tibes, R.; Walsh, K.J.; Podoltsev, N.A.; Griffiths, E.A.; Jabbour, E. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: Phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 2017, 18, 1317–1326. [Google Scholar] [PubMed]
- Noviello, T.M.R.; Di Giacomo, A.M.; Caruso, F.P.; Covre, A.; Mortarini, R.; Scala, G.; Costa, M.C.; Coral, S.; Fridman, W.H.; Sautès-Fridman, C.; et al. Guadecitabine plus ipilimumab in unresectable melanoma: Five-year follow-up and integrated multi-omic analysis in the phase 1b NIBIT-M4 trial. Nat. Commun. 2023, 14, 5914. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.; Fazal, Z.; Singh, R.; Bikorimana, E.; Adra, N.; Hanna, N.H.; Einhorn, L.H.; Perkins, S.M.; Sandusky, G.E.; Christensen, B.C.; et al. A phase 1 study of combined guadecitabine and cisplatin in platinum refractory germ cell cancer. Cancer Med. 2021, 10, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Prebet, T.; Goldberg, A.D.; Jurcic, J.G.; Khaled, S.; Dail, M.; Feng, Y.; Green, C.; Li, C.; Ma, C.; Medeiros, B.C. A phase 1b study of atezolizumab in combination with guadecitabine for the treatment of acute myeloid leukemia. Leuk. Lymphoma 2022, 63, 2180–2188. [Google Scholar]
- Papadatos-Pastos, D.; Yuan, W.; Pal, A.; Crespo, M.; Ferreira, A.; Gurel, B.; Prout, T.; Ameratunga, M.; Chénard-Poirier, M.; Curcean, A.; et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. Cancer 2022, 10, e004495. [Google Scholar] [CrossRef]
- Bever, K.M.; Thomas, D.L., 2nd; Zhang, J.; Diaz Rivera, E.A.; Rosner, G.L.; Zhu, Q.; Nauroth, J.M.; Christmas, B.; Thompson, E.D.; Anders, R.A.; et al. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin. Epigenetics 2021, 13, 25. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Covre, A.; Finotello, F.; Rieder, D.; Danielli, R.; Sigalotti, L.; Giannarelli, D.; Petitprez, F.; Lacroix, L.; Valente, M. Guadecitabine plus ipilimumab in unresectable melanoma: The NIBIT-M4 clinical trial. Clin. Cancer Res. 2019, 25, 7351–7362. [Google Scholar] [CrossRef]
- Wei, C.X.; Mamdani, H.; Gentzler, R.; Kalra, M.; Perkins, S.; Althouse, S.; Jalal, S.I. A brief report of a phase II trial evaluating efficacy and safety of hypomethylating agent guadecitabine in combination with carboplatin in extensive stage small cell lung cancer. Clin. Lung Cancer 2023, 24, 347–352. [Google Scholar] [CrossRef]
- Jang, H.J.; Hostetter, G.; Macfarlane, A.W.; Madaj, Z.; Ross, E.A.; Hinoue, T.; Kulchycki, J.R.; Burgos, R.S.; Tafseer, M.; Alpaugh, R.K. A phase II trial of guadecitabine plus atezolizumab in metastatic urothelial carcinoma progressing after initial immune checkpoint inhibitor therapy. Clin. Cancer Res. 2023, 29, 2052–2065. [Google Scholar]
- Chen, S.; Xie, P.; Cowan, M.; Huang, H.; Cardenas, H.; Keathley, R.; Tanner, E.J.; Fleming, G.F.; Moroney, J.W.; Pant, A.; et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J Clin Investig 2022, 132, 14. [Google Scholar] [CrossRef]
- Sheikh, T.N.; Chen, X.; Xu, X.; McGuire, J.T.; Ingham, M.; Lu, C.; Schwartz, G.K. Growth inhibition and induction of innate immune signaling of chondrosarcomas with epigenetic inhibitors. Mol. Cancer Ther. 2021, 20, 2362–2371. [Google Scholar] [PubMed]
- Kaminskas, E.; Farrell, A.T.; Wang, Y.-C.; Sridhara, R.; Pazdur, R. FDA drug approval summary: Azacitidine (5-azacytidine, Vidaza™) for injectable suspension. Oncologist 2005, 10, 176–182. [Google Scholar]
- Winquist, E.; Knox, J.; Ayoub, J.-P.; Wood, L.; Wainman, N.; Reid, G.K.; Pearce, L.; Shah, A.; Eisenhauer, E. Phase II trial of DNA methyltransferase 1 inhibition with the antisense oligonucleotide MG98 in patients with metastatic renal carcinoma: A National Cancer Institute of Canada Clinical Trials Group investigational new drug study. Investig. New Drugs 2006, 24, 159–167. [Google Scholar]
- Stewart, D.; Donehower, R.; Eisenhauer, E.; Wainman, N.; Shah, A.; Bonfils, C.; MacLeod, A.; Besterman, J.; Reid, G. A phase I pharmacokinetic and pharmacodynamic study of the DNA methyltransferase 1 inhibitor MG98 administered twice weekly. Ann. Oncol. 2003, 14, 766–774. [Google Scholar]
- Candelaria, M.; Gallardo-Rincón, D.; Arce, C.; Cetina, L.; Aguilar-Ponce, J.L.; Arrieta, O.; Gonzalez-Fierro, A.; Chavez-Blanco, A.; de La Cruz-Hernandez, E.; Camargo, M. A phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Ann. Oncol. 2007, 18, 1529–1538. [Google Scholar] [PubMed]
- Liu, Y.C.; Su, C.W.; Ko, P.S.; Lee, R.C.; Liu, C.J.; Huang, Y.H.; Gau, J.P.; Liu, J.H. A clinical trial with valproic acid and hydralazine in combination with gemcitabine and cisplatin followed by doxorubicin and dacarbazine for advanced hepatocellular carcinoma. Asia Pac. J. Clin. Oncol. 2022, 18, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.; Pérez-Plasencia, C.; González-Fierro, A.; de la Cruz-Hernández, E.; Revilla-Vázquez, A.; Chávez-Blanco, A.; Trejo-Becerril, C.; Pérez-Cárdenas, E.; Taja-Chayeb, L.; Bargallo, E. A proof-of-principle study of epigenetic therapy added to neoadjuvant doxorubicin cyclophosphamide for locally advanced breast cancer. PLoS ONE 2006, 1, e98. [Google Scholar]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velázquez-Martínez, C.A. Resveratrol-salicylate derivatives as selective DNMT3 inhibitors and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Zhang, C.; Zhao, B.; Jiao, A. DNMTs inhibitor SGI-1027 induces apoptosis in Huh7 human hepatocellular carcinoma cells. Oncol. Lett. 2018, 16, 5799–5806. [Google Scholar] [CrossRef]
- Fagan, R.L.; Cryderman, D.E.; Kopelovich, L.; Wallrath, L.L.; Brenner, C. Laccaic Acid A Is a Direct, DNA-competitive Inhibitor of DNA Methyltransferase 1*. J. Biol. Chem. 2013, 288, 23858–23867. [Google Scholar] [CrossRef] [PubMed]
- Brueckner, B.; Garcia Boy, R.; Siedlecki, P.; Musch, T.; Kliem, H.C.; Zielenkiewicz, P.; Suhai, S.; Wiessler, M.; Lyko, F. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005, 65, 6305–6311. [Google Scholar]
- Kuck, D.; Caulfield, T.; Lyko, F.; Medina-Franco, J.L. Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells. Mol. Cancer Ther. 2010, 9, 3015–3023. [Google Scholar]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef]
- Lee, H.Z.; Kwitkowski, V.E.; Del Valle, P.L.; Ricci, M.S.; Saber, H.; Habtemariam, B.A.; Bullock, J.; Bloomquist, E.; Li Shen, Y.; Chen, X.H.; et al. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clin. Cancer Res. 2015, 21, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Petrich, A.; Nabhan, C. Use of class I histone deacetylase inhibitor romidepsin in combination regimens. Leuk. Lymphoma 2016, 57, 1755–1765. [Google Scholar]
- Abaza, Y.M.; Kadia, T.M.; Jabbour, E.J.; Konopleva, M.Y.; Borthakur, G.; Ferrajoli, A.; Estrov, Z.; Wierda, W.G.; Alfonso, A.; Chong, T.H. Phase 1 dose escalation multicenter trial of pracinostat alone and in combination with azacitidine in patients with advanced hematologic malignancies. Cancer 2017, 123, 4851–4859. [Google Scholar] [PubMed]
- Yong, W.; Goh, B.; Soo, R.; Toh, H.; Ethirajulu, K.; Wood, J.; Novotny-Diermayr, V.; Lee, S.; Yeo, W.; Chan, D. Phase I and pharmacodynamic study of an orally administered novel inhibitor of histone deacetylases, SB939, in patients with refractory solid malignancies. Ann. Oncol. 2011, 22, 2516–2522. [Google Scholar]
- Razak, A.; Hotte, S.; Siu, L.; Chen, E.; Hirte, H.; Powers, J.; Walsh, W.; Stayner, L.; Laughlin, A.; Novotny-Diermayr, V. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2011, 104, 756–762. [Google Scholar]
- Yalniz, F.F.; Berdeja, J.G.; Maris, M.B.; Lyons, R.M.; Reeves Jr, J.A.; Essell, J.H.; Patel, P.; Sekeres, M.; Hughes, A.; Mappa, S. A phase II study of addition of pracinostat to a hypomethylating agent in patients with myelodysplastic syndromes who have not responded to previous hypomethylating agent therapy. Br. J. Haematol. 2020, 188, 404–412. [Google Scholar]
- Garcia-Manero, G.; Montalban-Bravo, G.; Berdeja, J.G.; Abaza, Y.; Jabbour, E.; Essell, J.; Lyons, R.M.; Ravandi, F.; Maris, M.; Heller, B. Phase 2, randomized, double-blind study of pracinostat in combination with azacitidine in patients with untreated, higher-risk myelodysplastic syndromes. Cancer 2017, 123, 994–1002. [Google Scholar] [PubMed]
- Quintás-Cardama, A.; Kantarjian, H.; Estrov, Z.; Borthakur, G.; Cortes, J.; Verstovsek, S. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk. Res. 2012, 36, 1124–1127. [Google Scholar]
- Chu, Q.-C.; Nielsen, T.; Alcindor, T.; Gupta, A.; Endo, M.; Goytain, A.; Xu, H.; Verma, S.; Tozer, R.; Knowling, M. A phase II study of SB939, a novel pan-histone deacetylase inhibitor, in patients with translocation-associated recurrent/metastatic sarcomas—NCIC-CTG IND 200. Ann. Oncol. 2015, 26, 973–981. [Google Scholar] [PubMed]
- Garcia-Manero, G.; Kazmierczak, M.; Wierzbowska, A.; Fong, C.Y.; Keng, M.K.; Ballinari, G.; Scarci, F.; Adès, L. Pracinostat combined with azacitidine in newly diagnosed adult acute myeloid leukemia (AML) patients unfit for standard induction chemotherapy: PRIMULA phase III study. Leuk. Res. 2024, 140, 107480. [Google Scholar] [CrossRef]
- Singh, A.; Patel, V.K.; Jain, D.K.; Patel, P.; Rajak, H. Panobinostat as Pan-deacetylase Inhibitor for the Treatment of Pancreatic Cancer: Recent Progress and Future Prospects. Oncol. Ther. 2016, 4, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Eleutherakis-Papaiakovou, E.; Kanellias, N.; Kastritis, E.; Gavriatopoulou, M.; Terpos, E.; Dimopoulos, M.A. Efficacy of Panobinostat for the Treatment of Multiple Myeloma. J. Oncol. 2020, 2020, 7131802. [Google Scholar] [CrossRef]
- Ikeda, M.; Ohno, I.; Ueno, H.; Mitsunaga, S.; Hashimoto, Y.; Okusaka, T.; Kondo, S.; Sasaki, M.; Sakamoto, Y.; Takahashi, H. Phase I study of resminostat, an HDAC inhibitor, combined with S-1 in patients with pre-treated biliary tract or pancreatic cancer. Investig. New Drugs 2019, 37, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Walewski, J.; Paszkiewicz-Kozik, E.; Borsaru, G.; Hellmann, A.; Janikova, A.; Warszewska, A.; Mais, A.; Ammendola, A.; Herz, T.; Krauss, B. Resminostat in patients with relapsed or refractory Hodgkin lymphoma: Results of the phase II SAPHIRE study. Leuk. Lymphoma 2019, 60, 675–684. [Google Scholar] [CrossRef]
- Bitzer, M.; Horger, M.; Giannini, E.G.; Ganten, T.M.; Wörns, M.A.; Siveke, J.T.; Dollinger, M.M.; Gerken, G.; Scheulen, M.E.; Wege, H. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma–the SHELTER study. J. Hepatol. 2016, 65, 280–288. [Google Scholar]
- Tambo, Y.; Hosomi, Y.; Sakai, H.; Nogami, N.; Atagi, S.; Sasaki, Y.; Kato, T.; Takahashi, T.; Seto, T.; Maemondo, M. Phase I/II study of docetaxel combined with resminostat, an oral hydroxamic acid HDAC inhibitor, for advanced non-small cell lung cancer in patients previously treated with platinum-based chemotherapy. Investig. New Drugs 2017, 35, 217–226. [Google Scholar]
- Streubel, G.; Schrepfer, S.; Kallus, H.; Parnitzke, U.; Wulff, T.; Hermann, F.; Borgmann, M.; Hamm, S. Histone deacetylase inhibitor resminostat in combination with sorafenib counteracts platelet-mediated pro-tumoral effects in hepatocellular carcinoma. Sci. Rep. 2021, 11, 9587. [Google Scholar] [CrossRef]
- Venugopal, B.; Baird, R.; Kristeleit, R.S.; Plummer, R.; Cowan, R.; Stewart, A.; Fourneau, N.; Hellemans, P.; Elsayed, Y.; Mcclue, S. A phase I study of quisinostat (JNJ-26481585), an oral hydroxamate histone deacetylase inhibitor with evidence of target modulation and antitumor activity, in patients with advanced solid tumors. Clin. Cancer Res. 2013, 19, 4262–4272. [Google Scholar] [PubMed]
- Moreau, P.; Facon, T.; Touzeau, C.; Benboubker, L.; Delain, M.; Badamo-Dotzis, J.; Phelps, C.; Doty, C.; Smit, H.; Fourneau, N. Quisinostat, bortezomib, and dexamethasone combination therapy for relapsed multiple myeloma. Leuk. Lymphoma 2016, 57, 1546–1559. [Google Scholar] [PubMed]
- Child, F.; Ortiz-Romero, P.; Alvarez, R.; Bagot, M.; Stadler, R.; Weichenthal, M.; Alves, R.; Quaglino, P.; Beylot-Barry, M.; Cowan, R. Phase II multicentre trial of oral quisinostat, a histone deacetylase inhibitor, in patients with previously treated stage IB–IVA mycosis fungoides/Sézary syndrome. Br. J. Dermatol. 2016, 175, 80–88. [Google Scholar]
- Booth, S.W.; Eyre, T.A.; Whittaker, J.; Campo, L.; Wang, L.M.; Soilleux, E.; Royston, D.; Rees, G.; Kesavan, M.; Hildyard, C. A Phase 2a cohort expansion study to assess the safety, tolerability, and preliminary efficacy of CXD101 in patients with advanced solid-organ cancer expressing HR23B or lymphoma. BMC Cancer 2021, 21, 851. [Google Scholar]
- Saunders, M.P.; Graham, J.; Cunningham, D.; Plummer, R.; Church, D.; Kerr, R.; Cook, S.; Zheng, S.; La Thangue, N.; Kerr, D. CXD101 and nivolumab in patients with metastatic microsatellite-stable colorectal cancer (CAROSELL): A multicentre, open-label, single-arm, phase II trial. ESMO Open 2022, 7, 100594. [Google Scholar]
- Aggarwal, R.; Thomas, S.; Pawlowska, N.; Bartelink, I.; Grabowsky, J.; Jahan, T.; Cripps, A.; Harb, A.; Leng, J.; Reinert, A. Inhibiting histone deacetylase as a means to reverse resistance to angiogenesis inhibitors: Phase I study of abexinostat plus pazopanib in advanced solid tumor malignancies. J. Clin. Oncol. 2017, 35, 1231–1239. [Google Scholar]
- Evens, A.M.; Balasubramanian, S.; Vose, J.M.; Harb, W.; Gordon, L.I.; Langdon, R.; Sprague, J.; Mani, C.; Yue, J.; Luan, Y. A phase I/II multicenter, open-label study of the oral histone deacetylase inhibitor abexinostat in relapsed/refractory lymphoma. Clin. Cancer Res. 2016, 22, 1059–1066. [Google Scholar]
- Choy, E.; Flamand, Y.; Balasubramanian, S.; Butrynski, J.E.; Harmon, D.C.; George, S.; Cote, G.M.; Wagner, A.J.; Morgan, J.A.; Mani, C. Phase 1 study of oral abexinostat, a histone deacetylase inhibitor, in combination with doxorubicin in patients with metastatic sarcoma. Cancer 2015, 121, 1223–1230. [Google Scholar]
- Morschhauser, F.; Terriou, L.; Coiffier, B.; Bachy, E.; Varga, A.; Kloos, I.; Lelièvre, H.; Sarry, A.-L.; Depil, S.; Ribrag, V. Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Investig. New Drugs 2015, 33, 423–431. [Google Scholar]
- Ribrag, V.; Kim, W.S.; Bouabdallah, R.; Lim, S.T.; Coiffier, B.; Illes, A.; Lemieux, B.; Dyer, M.J.; Offner, F.; Felloussi, Z. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: Results of a phase II study. Haematologica 2017, 102, 903. [Google Scholar] [PubMed]
- Xu, Y.; Zhang, P.; Liu, Y. Chidamide tablets: HDAC inhibition to treat lymphoma. Drugs Today 2017, 53, 167–176. [Google Scholar] [CrossRef]
- Collier, K.A.; Valencia, H.; Newton, H.; Hade, E.M.; Sborov, D.W.; Cavaliere, R.; Poi, M.; Phelps, M.A.; Liva, S.G.; Coss, C.C. A phase 1 trial of the histone deacetylase inhibitor AR-42 in patients with neurofibromatosis type 2-associated tumors and advanced solid malignancies. Cancer Chemother. Pharmacol. 2021, 87, 599–611. [Google Scholar] [PubMed]
- Liva, S.G.; Coss, C.C.; Wang, J.; Blum, W.; Klisovic, R.; Bhatnagar, B.; Walsh, K.; Geyer, S.; Zhao, Q.; Garzon, R. Phase I study of AR-42 and decitabine in acute myeloid leukemia. Leuk. Lymphoma 2020, 61, 1484–1492. [Google Scholar] [CrossRef]
- Lin, J.; Elkon, J.; Ricart, B.; Palmer, E.; Zevallos-Delgado, C.; Noonepalle, S.; Burgess, B.; Siegel, R.; Ma, Y.; Villagra, A. Phase I study of entinostat in combination with enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. Oncologist 2021, 26, e2136–e2142. [Google Scholar]
- Gojo, I.; Jiemjit, A.; Trepel, J.B.; Sparreboom, A.; Figg, W.D.; Rollins, S.; Tidwell, M.L.; Greer, J.; Chung, E.J.; Lee, M.-J. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood 2007, 109, 2781–2790. [Google Scholar]
- Kummar, S.; Gutierrez, M.; Gardner, E.R.; Donovan, E.; Hwang, K.; Chung, E.J.; Lee, M.-J.; Maynard, K.; Kalnitskiy, M.; Chen, A. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin. Cancer Res. 2007, 13, 5411–5417. [Google Scholar]
- Iwata, H.; Nakamura, R.; Masuda, N.; Yamashita, T.; Yamamoto, Y.; Kobayashi, K.; Tsurutani, J.; Iwasa, T.; Yonemori, K.; Tamura, K. Efficacy and exploratory biomarker analysis of entinostat plus exemestane in advanced or recurrent breast cancer: Phase II randomized controlled trial. Jpn. J. Clin. Oncol. 2023, 53, 4–15. [Google Scholar]
- Hellmann, M.D.; Jänne, P.A.; Opyrchal, M.; Hafez, N.; Raez, L.E.; Gabrilovich, D.I.; Wang, F.; Trepel, J.B.; Lee, M.-J.; Yuno, A. Entinostat plus pembrolizumab in patients with metastatic NSCLC previously treated with anti–PD-(L) 1 therapy. Clin. Cancer Res. 2021, 27, 1019–1028. [Google Scholar]
- Xu, B.; Zhang, Q.; Hu, X.; Li, Q.; Sun, T.; Li, W.; Ouyang, Q.; Wang, J.; Tong, Z.; Yan, M. Entinostat, a class I selective histone deacetylase inhibitor, plus exemestane for Chinese patients with hormone receptor-positive advanced breast cancer: A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Acta Pharm. Sin. B 2023, 13, 2250–2258. [Google Scholar]
- Bauer, T.M.; Besse, B.; Martinez-Marti, A.; Trigo, J.M.; Moreno, V.; Garrido, P.; Ferron-Brady, G.; Wu, Y.; Park, J.; Collingwood, T.; et al. Phase I, Open-Label, Dose-Escalation Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of GSK2879552 in Relapsed/Refractory SCLC. J. Thorac. Oncol. 2019, 14, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, T.; Yu, B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct Target Ther. 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Wass, M.; Göllner, S.; Besenbeck, B.; Schlenk, R.F.; Mundmann, P.; Göthert, J.R.; Noppeney, R.; Schliemann, C.; Mikesch, J.-H.; Lenz, G. A proof of concept phase I/II pilot trial of LSD1 inhibition by tranylcypromine combined with ATRA in refractory/relapsed AML patients not eligible for intensive therapy. Leukemia 2021, 35, 701–711. [Google Scholar] [PubMed]
- Ribrag, V.; Iglesias, L.; De Braud, F.; Ma, B.; Yokota, T.; Zander, T.; Spreafico, A.; Subbiah, V.; Illert, A.L.; Tan, D.; et al. A first-in-human phase 1/2 dose-escalation study of MAK683 (EED inhibitor) in patients with advanced malignancies. Eur. J. Cancer 2025, 216, 115122. [Google Scholar] [CrossRef]
- Waters, N.J.; Daigle, S.R.; Rehlaender, B.N.; Basavapathruni, A.; Campbell, C.T.; Jensen, T.B.; Truitt, B.F.; Olhava, E.J.; Pollock, R.M.; Stickland, K.A.; et al. Exploring drug delivery for the DOT1L inhibitor pinometostat (EPZ-5676): Subcutaneous administration as an alternative to continuous IV infusion, in the pursuit of an epigenetic target. J. Control. Release 2015, 220, 758–765. [Google Scholar] [CrossRef]
- Watts, J.; Minden, M.D.; Bachiashvili, K.; Brunner, A.M.; Abedin, S.; Crossman, T.; Zajac, M.; Moroz, V.; Egger, J.L.; Tarkar, A.; et al. Phase I/II study of the clinical activity and safety of GSK3326595 in patients with myeloid neoplasms. Ther. Adv. Hematol. 2024, 15, 20406207241275376. [Google Scholar] [CrossRef]
- Gajer, J.M.; Furdas, S.D.; Gründer, A.; Gothwal, M.; Heinicke, U.; Keller, K.; Colland, F.; Fulda, S.; Pahl, H.L.; Fichtner, I.; et al. Histone acetyltransferase inhibitors block neuroblastoma cell growth in vivo. Oncogenesis 2015, 4, e137. [Google Scholar] [CrossRef]
- Michaelides, M.R.; Kluge, A.; Patane, M.; Van Drie, J.H.; Wang, C.; Hansen, T.M.; Risi, R.M.; Mantei, R.; Hertel, C.; Karukurichi, K.; et al. Discovery of Spiro Oxazolidinediones as Selective, Orally Bioavailable Inhibitors of p300/CBP Histone Acetyltransferases. ACS Med. Chem. Lett. 2018, 9, 28–33. [Google Scholar] [CrossRef]
- Lu, W.; Xiong, H.; Chen, Y.; Wang, C.; Zhang, H.; Xu, P.; Han, J.; Xiao, S.; Ding, H.; Chen, Z.; et al. Discovery and biological evaluation of thiobarbituric derivatives as potent p300/CBP inhibitors. Bioorganic Med. Chem. 2018, 26, 5397–5407. [Google Scholar] [CrossRef]
- Oike, T.; Komachi, M.; Ogiwara, H.; Amornwichet, N.; Saitoh, Y.; Torikai, K.; Kubo, N.; Nakano, T.; Kohno, T. C646, a selective small molecule inhibitor of histone acetyltransferase p300, radiosensitizes lung cancer cells by enhancing mitotic catastrophe. Radiother. Oncol. 2014, 111, 222–227. [Google Scholar] [CrossRef]
- Milite, C.; Feoli, A.; Sasaki, K.; La Pietra, V.; Balzano, A.L.; Marinelli, L.; Mai, A.; Novellino, E.; Castellano, S.; Tosco, A. A novel cell-permeable, selective, and noncompetitive inhibitor of KAT3 histone acetyltransferases from a combined molecular pruning/classical isosterism approach. J. Med. Chem. 2015, 58, 2779–2798. [Google Scholar] [PubMed]
- Lasko, L.M.; Jakob, C.G.; Edalji, R.P.; Qiu, W.; Montgomery, D.; Digiammarino, E.L.; Hansen, T.M.; Risi, R.M.; Frey, R.; Manaves, V. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 2017, 550, 128–132. [Google Scholar] [PubMed]
- Zeng, X.; Sigoillot, F.; Gaur, S.; Choi, S.; Pfaff, K.L.; Oh, D.-C.; Hathaway, N.; Dimova, N.; Cuny, G.D.; King, R.W. Pharmacologic Inhibition of the Anaphase-Promoting Complex Induces A Spindle Checkpoint-Dependent Mitotic Arrest in the Absence of Spindle Damage. Cancer Cell 2010, 18, 382–395. [Google Scholar] [CrossRef]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.-J.; Zhao, C.; Glenn, K.; Wen, Y. Discovery of RG7112: A small-molecule MDM2 inhibitor in clinical development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [PubMed]
- Montesinos, P.; Beckermann, B.M.; Catalani, O.; Esteve, J.; Gamel, K.; Konopleva, M.Y.; Martinelli, G.; Monnet, A.; Papayannidis, C.; Park, A.; et al. MIRROS: A randomized, placebo-controlled, Phase III trial of cytarabine ± idasanutlin in relapsed or refractory acute myeloid leukemia. Future Oncol. 2020, 16, 807–815. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z.; Rew, Y.; Gribble, M.; Bartberger, M.D.; Beck, H.P.; Canon, J.; Chen, A.; Chen, X.; Chow, D.; et al. Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J. Med. Chem. 2014, 57, 1454–1472. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.; Clarke, S.; Lee, A.; Brahmbhatt, H.; Macdiarmid, J.; Pattison, S.; Leslie, F.; Huynh, Y.; et al. P1.02—MesomiR 1: A Phase I study of TargomiRs in patients with refractory malignant pleural mesothelioma (MPM) and lung cancer (NSCLC). Ann. Oncol. 2015, 26, ii16. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar]
- Kotecki, N.; Opdam, F.; Robbrecht, D.; Strijbos, M.; Kroon, K.; Janicot, M.; Yahyanejad, S.; Telford, B.; van den Bosch, M.; Alemdehy, F. Phase I/Ib study with INT-1B3, a novel LNP-formulated micro-RNA (miR-193a-3p mimic) therapeutic for patients with advanced solid cancer. J. Clin. Oncol. 2021, 39, TPS2666. [Google Scholar]
- Abplanalp, W.T.; Fischer, A.; John, D.; Zeiher, A.M.; Gosgnach, W.; Darville, H.; Montgomery, R.; Pestano, L.; Allée, G.; Paty, I. Efficiency and target derepression of anti-miR-92a: Results of a first in human study. Nucleic Acid Ther. 2020, 30, 335–345. [Google Scholar]
- Romano, G.; Acunzo, M.; Nana-Sinkam, P. microRNAs as Novel Therapeutics in Cancer. Cancers 2021, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, L.; Zhang, G.; Shan, A.; Ye, C.; Liang, B.; Sun, J.; Liao, X.; Zhu, C.; Chen, Y.; et al. MicroRNAs target the Wnt/β-catenin signaling pathway to regulate epithelial-mesenchymal transition in cancer (Review). Oncol. Rep. 2020, 44, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Medarova, Z. 383P Clinical experience with TTX-MC138: A first-in-class therapy against metastatic cancer. Ann. Oncol. 2024, 35, S379. [Google Scholar] [CrossRef]
- Gong, N.; Teng, X.; Li, J.; Liang, X.-J. Antisense oligonucleotide-conjugated nanostructure-targeting lncRNA MALAT1 inhibits cancer metastasis. ACS Appl. Mater. Interfaces 2018, 11, 37–42. [Google Scholar]
- Amodio, N.; Stamato, M.A.; Juli, G.; Morelli, E.; Fulciniti, M.; Manzoni, M.; Taiana, E.; Agnelli, L.; Cantafio, M.E.G.; Romeo, E. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia 2018, 32, 1948–1957. [Google Scholar]
- Liang, H.; Peng, J. LncRNA HOTAIR promotes proliferation, invasion and migration in NSCLC cells via the CCL22 signaling pathway. PLoS ONE 2022, 17, e0263997. [Google Scholar]
- Suzuki, M.M.; Iijima, K.; Ogami, K.; Shinjo, K.; Murofushi, Y.; Xie, J.; Wang, X.; Kitano, Y.; Mamiya, A.; Kibe, Y. TUG1-mediated R-loop resolution at microsatellite loci as a prerequisite for cancer cell proliferation. Nat. Commun. 2023, 14, 4521. [Google Scholar]
- Tasaki, Y.; Suzuki, M.; Katsushima, K.; Shinjo, K.; Iijima, K.; Murofushi, Y.; Naiki-Ito, A.; Hayashi, K.; Qiu, C.; Takahashi, A. Cancer-specific targeting of taurine-upregulated gene 1 enhances the effects of chemotherapy in pancreatic cancer. Cancer Res. 2021, 81, 1654–1666. [Google Scholar]
- Li, M.; Ding, X.; Zhang, Y.; Li, X.; Zhou, H.; Yang, L.; Li, Y.; Yang, P.; Zhang, X.; Hu, J. Antisense oligonucleotides targeting lncRNA AC104041. 1 induces antitumor activity through Wnt2B/β-catenin pathway in head and neck squamous cell carcinomas. Cell Death Dis. 2020, 11, 672. [Google Scholar]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Gallo Cantafio, M.E. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia 2020, 34, 234–244. [Google Scholar]
- Zhu, Z.; Du, S.; Yin, K.; Ai, S.; Yu, M.; Liu, Y.; Shen, Y.; Liu, M.; Jiao, R.; Chen, X. Knockdown long noncoding RNA nuclear paraspeckle assembly transcript 1 suppresses colorectal cancer through modulating miR-193a-3p/KRAS. Cancer Med. 2019, 8, 261–275. [Google Scholar] [CrossRef]
- Esposito, R.; Polidori, T.; Meise, D.F.; Pulido-Quetglas, C.; Chouvardas, P.; Forster, S.; Schaerer, P.; Kobel, A.; Schlatter, J.; Kerkhof, E. Multi-hallmark long noncoding RNA maps reveal non-small cell lung cancer vulnerabilities. Cell Genom. 2022, 2, 100171. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Huang, Y.; Zhang, B.; Song, Y.; Yang, L.; Chen, Q.; Wang, Z.; Wang, Y.; He, Q.; Yang, W. Targeting LncRNA LLNLR-299G3. 1 with antisense oligonucleotide inhibits malignancy of esophageal squamous cell carcinoma cells in vitro and in vivo. Oncol. Res. 2023, 31, 463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Hou, W.; Ding, X.; Wang, W. Long non-coding RNA MALAT1 promotes cell proliferation, migration and invasion by targeting miR-590-3p in osteosarcoma. Exp. Ther. Med. 2022, 24, 672. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, W.; Zhao, M.; Li, S.; Jin, W.; Wang, K. Oncogenic role of lncRNA CRNDE in acute promyelocytic leukemia and NPM1-mutant acute myeloid leukemia. Cell Death Discov. 2020, 6, 121. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Lin, J.; Xiao, J.; Tian, Y. lncRNA CCAT1 promotes bladder cancer cell proliferation, migration and invasion. Int. Braz. J. Urol. 2019, 45, 549–559. [Google Scholar] [CrossRef]
- Fang, H.; Liu, H.-M.; Wu, W.-H.; Liu, H.; Pan, Y.; Li, W.-J. Upregulation of long noncoding RNA CCAT1-L promotes epithelial–mesenchymal transition in gastric adenocarcinoma. OncoTargets Ther. 2018, 11, 5647–5655. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Feng, Y.; He, X.; Wang, L. Evaluation of antimetastatic effect of lncRNA-ATB siRNA delivered using ultrasound-targeted microbubble destruction. DNA Cell Biol. 2016, 35, 393–397. [Google Scholar] [CrossRef]
- Aguilar, R.; Spencer, K.B.; Kesner, B.; Rizvi, N.F.; Badmalia, M.D.; Mrozowich, T.; Mortison, J.D.; Rivera, C.; Smith, G.F.; Burchard, J. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature 2022, 604, 160–166. [Google Scholar] [CrossRef]
- Abulwerdi, F.A.; Xu, W.; Ageeli, A.A.; Yonkunas, M.J.; Arun, G.; Nam, H.; Schneekloth Jr, J.S.; Dayie, T.K.; Spector, D.; Baird, N. Selective small-molecule targeting of a triple helix encoded by the long noncoding RNA, MALAT1. ACS Chem. Biol. 2019, 14, 223–235. [Google Scholar] [PubMed]
- Rossi, A.; Zacchi, F.; Reni, A.; Rota, M.; Palmerio, S.; Menis, J.; Zivi, A.; Milleri, S.; Milella, M. Progresses and Pitfalls of Epigenetics in Solid Tumors Clinical Trials. Int. J. Mol. Sci. 2024, 25, 11740. [Google Scholar] [CrossRef] [PubMed]
- Morel, D.; Jeffery, D.; Aspeslagh, S.; Almouzni, G.; Postel-Vinay, S. Combining epigenetic drugs with other therapies for solid tumours—Past lessons and future promise. Nat. Rev. Clin. Oncol. 2020, 17, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Feehley, T.; O’Donnell, C.W.; Mendlein, J.; Karande, M.; McCauley, T. Drugging the epigenome in the age of precision medicine. Clin. Epigenetics 2023, 15, 6. [Google Scholar] [CrossRef]

| Modification | Epi-Drug | Target | Cancer | Clinical Phase | Other Drug(s)/Intervention(s) | Outcome(s)/Mechanism | Brand Name/Trial ID/Reference(s) |
|---|---|---|---|---|---|---|---|
| DNMT | Decitabine (5-aza-2′-deoxycytidine) | DNMT1 | MDS, CML | FDA approved, 2020 | In combination with cedazuridine | Prolonged overall survival | INQOVI, Astex Pharmaceuticals, Inc. [118] NCT02103478 [119] NCT03306264 [120] |
| Decitabine | DNMT1 | High risk MDS, CML | Phase 1/2 study | In combination with cedazuridine and venetoclax | Higher response rate in short period of time; displays tolerable toxicity and satisfactory activity | NCT04655755 [121] | |
| Decitabine | DNMT1 | MDS | Phase 3 | - | Higher overall response rate, prolonged survival; well-tolerated dosage, with a manageable toxicity profile | [122] | |
| 5-fluoro-2′-deoxycytidine | DNMT1 | Breast cancer and other solid tumors | Phase 2 | - | Arrest cell cycle at G2/M phase; activates p53 signaling and DNA damage response pathway; upregulates tumor suppressor genes | [123] | |
| Guadecitabine (SGI-110) | DNMT1 | AML, MDS, CMML | Phase 1/2 | - | Well-tolerated with clinical and biological activity | NCT01261312 [124,125,126,127,128] | |
| Guadecitabine | DNMT1 | AML, MDS, CMML | Phase 1/2 | In combination with Atezolizumab | Acceptable tolerance and clinically active | NCT02935361 [124] | |
| Guadecitabine | DNMT1 | Platinum refractory germ cell cancer | Phase 1 | In combination with Cisplatin | Exhibits good tolerance and demonstrates clinical activity | NCT02429466 [129] | |
| Guadecitabine | DNMT1 | AML | Phase 1b | In combination with Atezolizumab | Limited clinical activity and an overall unfavorable benefit–risk profile at tested doses | NCT02892318 [130] | |
| Guadecitabine | DNMT1 | Solid tumors (NSCLS) | Phase 1 | In combination with Pembrolizumab | Tolerable, with biological and anticancer activity | NCT02998567 [131] | |
| Guadecitabine | DNMT1 | Colorectal cancer | Phase 1 | In combination with Cy/GVAX (cyclophosphamide with GM-CSF secreting colon vaccine) | Tolerable, but no significant immunologic activity | NCT01966289 [132] | |
| Guadecitabine | DNMT1 | Melanoma | Phase 1b | In combination with ipilimumab (anti-CTLA-4 antibody) | Safe and tolerable, with initial signs of clinical and immunologic activity | NCT0260843 [128,133] | |
| Guadecitabine | DNMT1 | SCLC | Phase 2 | In combination with carboplatin | Exhibits good efficacy along with possible adverse events | NCT03913455 [134] | |
| Guadecitabine | DNMT1 | Urothelial carcinoma | Phase 2 | In combination with atezolizumab | Possible prolonged patient survival | NCT03179943 [135] | |
| Guadecitabine | DNMT1 | Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Phase 2 | In combination with Pembrolizumab | Exhibits clinical activity and possibly activates antitumor immunity | NCT02901899 [136] | |
| Guadecitabine | DNMT1 | Chondrosarcomas | Phase 2 | In combination with Belinostat or ASTX727 | Active trial; not recruiting | NCT04340843 [137] | |
| 5-Azacitidine | DNMT1 | MDS, AML, CMML | FDA approved, 2004; EMEA approved 2009 | - | Satisfactory safety profile and provides clinical benefit | Vidaza®; Celgene Corp., Summit, NJ, USA [138] | |
| MG98 | DNMT1 | Renal carcinoma | Phase 2 (terminated) | - | Exhibits no anti-cancer activity | [139] | |
| MG98 | DNMT1 | Solid tumors | Phase 1 | - | Shows early evidence of clinical activity with good tolerance | NCT00003890 [140] | |
| Hydralazine | DNMT1/3A/3B | Refractory solid tumors | Phase 2 | In combination with Magnesium valproate | Shows clinical benefits with potential to overcome resistance to chemotherapy | NCT00404508 [141] | |
| Hydralazine | DNMT1/3A/3B | Hepatocellular carcinoma | Phase 2 | In combination with valproic acid | Exhibits manageable toxicity and clinical efficacy | TPVGH97-07-07 [142] | |
| Hydralazine | DNMT1/3A/3B | Breast cancer | Phase 2 (terminated) | In combination with Magnesium valproate | Well-tolerated and appears to increase the efficacy of chemotherapy | NCT00395655 [143] | |
| Hydralazine | DNMT1/3A/3B | Cervical cancer | Phase 3 | In combination with Magnesium valproate; placebo-controlled | Unknown | NCT00532818 (Unpublished) | |
| Hydralazine | DNMT1/3A/3B | Ovarian cancer | Phase 3 | In combination with Magnesium valproate; placebo-controlled | Unknown | NCT00533299 (Unpublished) | |
| Epigallocatechin-3-gallate (EGCG) | DNMT | Colon cancer (HT-29 cells), esophageal cancer (KYSE 150 cells), and prostate cancer (PC3 cells) | Preclinical phase | - | Reactivation of some methylation-silenced genes | [144] | |
| Resveratrol-salicylate derivatives | DNMT3 | Colon cancer (HT-29), liver cancer (HepG2) and breast cancer (SK-BR-3) | Preclinical phase | - | Unknown | [145] | |
| SGI-1027 (4-anilinoquinoline) | DNMT1/3A/3B | Hepatocellular carcinoma (Huh7) | Preclinical phase | - | Reactivates silenced tumor suppressor genes and induces apoptosis in cancer cells | [146] | |
| Laccaic acid A (LCA) | DNMT1 | Breast cancer (MCF-7) | Preclinical phase | - | Reactivates the expression of silenced tumor suppressor genes | [147] | |
| RG-108 | DNMT1 | Colorectal cancer (HCT116) and acute lymphoblastic leukemia (NALM-6) | Preclinical phase | - | Reactivates key tumor suppressor genes | [148] | |
| Nanaomycin A | DNMT3B | Lung cancer (A549), leukemia (HL60), and colorectal cancer (HCT116) | Preclinical phase | - | Reactivates silenced tumor suppressor genes | [149] |
| Modification | Epi-drug | Target | Cancer | Clinical Phase | Other drug(s)/Intervention(s) | Outcome(s)/Mechanism | Brand Name/Trial ID/Reference(s) |
|---|---|---|---|---|---|---|---|
| HDAC | Vorinostat (suberoylanilide hydroxamic acid) | Pan HDAC | Cutaneous T-cell lymphoma (CTCL) | FDA approved 2006 | - | Shows clinical benefit | Zolinza®; Merck & Co., Inc., Whitehouse Station, NJ [150] |
| Belinostat | Pan HDAC | Peripheral T cell lymphoma (PTCL) | FDA approved 2006 | - | Shows clinical benefit | Beleodaq; Spectrum Pharmaceuticals, Inc. [151] | |
| Romidepsin | HDAC I | T-cell lymphoma (both CTCL and PTCL) | FDA approved | - | Shows clinical benefit | Istodax, Celgene Corporation [152] | |
| Pracinostat (SB939) | HDAC I/II/IV | Leukemia, solid tumors | Phase 1 | - | Unknown | NCT01184274 (Unpublished) | |
| Pracinostat | HDAC I/II/IV | Solid tumors, MDS, hematologic malignancies | Phase 1 | Alone or in combination with azacitidine | Safe, with modest single-agent activity | NCT00741234 [153] | |
| Pracinostat | HDAC I/II/IV | Solid malignancies | Phase 1 | - | Well-tolerated and show inhibitory effects | SCS-PN0022 [154] | |
| Pracinostat | HDAC I/II/IV | Solid tumors | Phase 1 | - | Shows good tolerability | [155] | |
| Pracinostat | HDAC I/II/IV | MDS | Phase 2 | In combination with azacitidine and decitabine | Exhibits improved efficacy and tolerance at reduced doses | NCT01993641 [156] | |
| Pracinostat | HDAC I/II/IV | MDS | Phase 2 | In combination with azacitidine | Fails to improve outcomes with high rate of treatment discontinuation | NCT01873703 [157] | |
| Pracinostat | HDAC I/II/IV | Myelofibrosis | Phase 2 | - | Exhibits modest tolerability and clinical activity | NCT01200498 [158] | |
| Pracinostat | HDAC I/II/IV | Translocation-associated sarcoma (TAS) | Phase 2 | - | Stopped prematurely due to prolonged unavailability of pracinostat | NCT01112384 [159] | |
| Pracinostat | HDAC I/II/IV | AML | Phase 3 | In combination with azacitidine | Terminated due to lack of clinical response | NCT03151408 [160] | |
| Panobinostat (LBH-589) | HDAC I/II/III/IV | Multiple myeloma | FDA and EMA approved | In combination with bortezomib and dexamethasone | Potential inhibitory activity | Farydac® (Novartis) [161,162] | |
| Resminostat | HDAC I/IIb/IV | Biliary tract or pancreatic cancer | Phase 1 | In combination with chemotherapy (S1) | Well-tolerated | JapicCTI-152864 [163] | |
| Resminostat | HDAC I/IIb/IV | CTCL, MF | Phase 1 | - | Unknown | NCT04955340 (Unpublished) | |
| Resminostat | HDAC I/IIb/IV | Hodgkin lymphoma | Phase 2 | - | Exhibits acceptable tolerance and clinical activity | NCT01037478 [164] | |
| Resminostat | HDAC I/IIb/IV | Hepatocellular carcinoma | Phase 1/2 | In combination with sorafenib | Display early signs of efficacy and well-tolerated | NCT00943449 [165] | |
| Resminostat | HDAC I/IIb/IV | NSCLC | Phase 1/2 | In combination with docetaxel | Fails to improve progression-free survival and increases toxicity | JapicCTI-132123 [166] | |
| Resminostat | HDAC I/IIb/IV | Hepatocellular carcinoma cells | Preclinical phase | In combination with sorafenib | Blocked platelet-induced hepatocellular carcinoma cell invasion | [167] | |
| Quisinostat | HDAC I/IIb/IV | Solid Malignancies and Lymphoma | Phase 1 | - | Intermittent schedules show better tolerance than continuous schedules | NCT00677105 [168] | |
| Quisinostat | HDAC I/IIb/IV | Multiple myeloma | Phase 1 | In combination with dexamethasone and bortezonib | Shows clinical efficacy and safe tolerance | NCT01464112 [169] | |
| Quisinostat | HDAC I/IIb/IV | CTCL | Phase 2 | - | Shows an acceptable safety profile | NCT01486277 [170] | |
| CDX101 | HDAC I | Lymphoma or advanced solid organ cancers | Phase 2a | - | Shows acceptable tolerance with clinical efficacy | NCT01977638 [171] | |
| CDX101 | HDAC I | Colorectal carcinoma | Phase 2 | In combination with nivolumab | Well tolerated and efficacious | NCT03993626 [172] | |
| Abexinostat | Pan HDAC | NSCLC, melanoma, urothelial carcinoma, squamous cell carcinoma of head and neck | Phase 1 | In combination with pembrolizumab | Unknown | NCT03590054 (Unpublished) | |
| Abexinostat | Pan HDAC | Multiple myeloma, Hodgkin, and non-Hodgkin lymphoma | Phase 1 | - | Unknown | NCT01149668 (Unpublished) | |
| Abexinostat | Pan HDAC | Solid tumors | Phase 1 | In combination with pazopanib | Shows good tolerability and anticancer effects | NCT01543763 [173] | |
| Abexinostat | Pan HDAC | Follicular lymphoma | Phase 2 | - | Unknown | NCT03934567 NCT03600441 (Unpublished) | |
| Abexinostat | Pan HDAC | Non-Hodgkin lymphoma | Phase 1/2 | - | Unknown | NCT04024696 (Unpublished) | |
| Abexinostat | Pan HDAC | Follicular lymphoma or mantle cell lymphoma. | Phase 1/2 | - | Well-tolerated and exhibits significant clinical activity | NCT00724984 [174] | |
| Abexinostat | Pan HDAC | Sarcoma | Phase 1/2 | In combination with doxorubicin and GCSF | Shows manageable toxicity and clinical response | NCT01027910 [175] | |
| Abexinostat | Pan HDAC | CML, Hodgkin and non-Hodgkin lymphoma | Phase 1/2 | - | Exhibits manageable toxicity and partial clinical response | EudraCT 2009-013691-47 [176,177] | |
| Chidamide (Epidaza) | HDAC I | Peripheral T-cell lymphoma | Approved in China | - | Exhibits clinical benefit | [178] | |
| AR42 | Pan HDAC | Neurofibromatosis type 2-associated tumors and advanced solid malignancies | Phase 1 | - | Safe and well-tolerated | NCT01129193 [179] | |
| AR42 | Pan HDAC | AML | Phase 1 | In combination with decitabine | Exhibits multi-organ failure as severe adverse effect | NCT01798901 [180] | |
| AR42 | Pan HDAC | Multiple myeloma | Phase 1 | In combination with pomalidomide | Unknown | NCT02569320 (Unpublished) | |
| AR42 | Pan HDAC | Neurofibromatosis type 2 | Phase 2/3 (recruiting) | Placebo-controlled | Unknown | NCT05130866 (Unpublished) | |
| Entinostat | HDAC I | Castration-resistant prostate cancer | Phase 1 | In combination with enzalutamid | Shows acceptable safety profile | NCT03829930 [181] | |
| Entinostat | HDAC I | Acute leukemias | Phase 1 | - | Effective inhibition of HDAC in vivo | NCT00015925 [182] | |
| Entinostat | HDAC I | Solid tumors and lymphoid malignancies | Phase 1 | - | Shows good tolerability at the tested doses | NCT00020579 [183] | |
| Entinostat | HDAC I | Breast cancer | Phase 2 | In combination with exemestane; placebo-controlled | Shows acceptable safety | NCT03291886 [184] | |
| Entinostat | HDAC I | NSCLC | Phase 1/2 | In combination with pembrolizumab | Exhibits clinical benefit | NCT02437136 [185] | |
| Entinostat | HDAC I | HR-positive breast cancer | Phase 3 | In combination with exemestane; placebo-controlled | Unknown | NCT03538171 [186] | |
| HDM | GSK2879552 | LSD 1 | AML | Phase 1 (terminated) | Alone or in combination with All-Trans Retinoic Acid (ATRA) | Exhibits toxicity and adverse effect | N CT02177812 (Unpublished) |
| GSK2879552 | LSD 1 | SCLC | Phase 1 (terminated) | - | Shows many adverse effects with poor disease control | NCT02034123 [187] | |
| INCB059872 | LSD1 | Solid Tumors and Hematologic Malignancy | Phase 1/2 (terminated) | Alone or in combination with ATRA, azacitidine, and nivolumab | Unknown | NCT02712905 [188] | |
| Tranylcypromine | LSD1 | AML | Phase 1/2 | In combination with ATRA | Exhibits clinical response with acceptable toxicity | NCT02261779 [189] | |
| HMT | Tazemetostat | EZH2 | Epithelioid sarcoma (ES), follicular lymphoma | FDA approved 2020 | - | Exhibits clinical benefit | TAZVERIK, Epizyme, Inc. |
| MAK683 | EED/PRC2 | Diffuse large B-cell lymphoma (DLBCL) and epithelioid sarcoma (ES) | Phase 1/2 | - | Well-tolerated with observed clinical activity | NCT02900651 [190] | |
| Pinometostat (EPZ-5676) | DOT1L | Hematologic malignancies, leukemia | Phase 1/2 | - | Shows inhibition of tumor growth | NCT02141828, NCT01684150 [191] | |
| GSK3326595 | PRMT5 | MDS, CMML, AML | Phase 1/2 | - | Exhibits limited clinical activity | NCT03614728 [192] | |
| HAT | PU139 and PU141 | Pan HAT | Neuroblastoma | Preclinical phase | - | PU139 blocks the HATs Gcn5, p300/CBP-associated factor (PCAF), CREB (cAMP response element-binding) protein (CBP) and p300, whereas PU141 is selective toward CBP and p300; Blocks growth of SK-N-SH neuroblastoma xenografts in mice | [193] |
| Spiro Oxazolidinediones derivatives | EP300/CBP histone acetyltransferase | Lung squamous cell carcinoma cell line LK2-xenografted mouse model | Preclinical phase | - | Inhibits acetylation of H3K27 in the human lung cancer cell line LK2 | [194] | |
| DCH36_06 (thiobarbituric acid derivative) | p300/CBP | Leukemia cell lines | Preclinical phase | - | Shows anti-tumor activity in leukemia xenograft | [195] | |
| C646 and its derivatives | p300 | NSCLC (A549, H460 and H157 cells) | Preclinical phase | - | Radio sensitization of NSCLC cells by enhancing mitotic catastrophe through the abrogation of G2 checkpoint maintenance | [196] | |
| EML425 | KAT3 (p300/CBP) | Leukemia (U937 cells) | Preclinical phase | - | Cell cycle arrest in G0/G1 phase | [197] | |
| A-485 | p300/CBP | Hematological malignancies, androgen receptor-positive prostate cancer | Preclinical phase | - | Shows potent anti-tumor activity | [198] | |
| Ubiquitin ligase | Tosyl-L-arginine methyl ester (TAME) | Anaphase-promoting complex (APC) | - | Preclinical stage | - | Induces mitotic arrest and cell death (xenopus cell extract) | [199] |
| Nutlin, RG7112 (RO5045337) | MDM2 | Advanced Solid Tumors and hematologic neoplasms | Phase 1 | - | Inhibits p53-MDM2 interaction; induces cell cycle arrest, apoptosis, and inhibition or regression of human tumor xenografts | NCT00559533, NCT00623870 [200] | |
| Idasanutlin, RG7388 (RO5503781) | MDM2 | AML | Phase 3 | In combination with cytarabine; placebo-controlled | Exhibits good clinical response with acceptable toxicity | NCT02545283 [201] | |
| AMG 232 | MDM2 | Breast carcinoma, malignant solid tumor, multiple myeloma | Phase 1 | - | Exhibit adverse events, dose limiting toxicities, and clinically significant changes in vital signs | NCT01723020 [202] | |
| SAR405838 | MDM2 | Solid tumors | Phase 1 | In combination with pimasertib | Unknown | NCT01985191 (Unpublished) |
| Modification | Epi-Drug | Target | Cancer | Clinical Phase | Other Drug(s)/Intervention(s) | Outcome(s)/Mechanism | Brand Name/Trial ID/Reference(s) |
|---|---|---|---|---|---|---|---|
| miRNA | MRG-106 (Cobomarsen) | miR-155 | CTCL, mycosis fungoides (MF) subtype | Phase 2 (terminated) | Vorinostat | Unknown | NCT03713320 (Unpublished) |
| MRG-106 | miR-155 | Mycosis Fungoides MF, CLL, DLBCL or ATLL | Phase 1 | - | Unknown | NCT02580552 (Unpublished) | |
| MRG-106 | miR-155 | CTCL, MF subtype | Phase 1 (Terminated) | - | Unknown | NCT03837457 (Unpublished) | |
| TargomiRs | miR-16 | Malignant pleural mesothelioma (MPM) and NSCLC | Phase 1 | - | Well-tolerated with early signs of clinical activity | NCT02369198 [203] | |
| MRX34 | miR-34a | Liver cancer, SCLC, lymphoma, melanoma, MM, RCC, NSLCL | Phase 1 (Terminated) | - | Exhibits serious adverse effects | NCT01829971 [204] | |
| INT-1B3 | miR-193a-3p | Advanced solid tumors | Phase 1 | - | Potential clinical benefit | NCT04675996 [205] | |
| MRG-110 | miR-92a-3p | Healthy adults | Phase 1 | Placebo-controlled | De-repression miR-92a targets | NCT03494712 [206] | |
| MRG-201 | miR-29b | Keloid | Phase 2 | Placebo-controlled | Exhibits clinical response and manageable adverse effects | NCT03601052 (Unpublished) | |
| RGLS5579 | miR-10b | Glioblastoma multiforme | Preclinical phase | - | Improved survival in animal model | [207,208] | |
| TTX-MC138 | miR-10b | Metastatic breast cancer | Preclinical phase | - | Shows early signs of clinical activity | [209] |
| Drug Module | Target | Cancer | Reference |
|---|---|---|---|
| Antisense oligonucleotides (ASOs) | MALAT1 | Lung cancer, breast cancer, multiple myeloma | [210,211] |
| HOTAIR | Lung cancer | [212] | |
| TUG1 | Glioma, glioblastoma, pancreatic cancer | [213,214] | |
| AC104041.1 | Head and neck cancer | [215] | |
| NEAT1 | Neuroblastoma, multiple myeloma, colorectal cancer | [216,217] | |
| GCAWKR, CHiLL1 | Lung cancer | [218] | |
| LLNLR-299G3.1 | Esophageal cancer | [219] | |
| siRNAs | HOTAIR | Breast cancer | [220] |
| MALAT1 | Lung, cervical, esophageal, and colorectal cancer, osteosarcoma, glioblastoma and lymphoma | [112,221] | |
| CRNDE | Acute promyelocytic leukemia | [222] | |
| CCAT1 | Gastric, bladder and colorectal cancer | [223,224] | |
| lncRNA-ATB | Liver cancer | [225] | |
| Small molecules | XIST | Breast cancer | [226] |
| MALAT1 | Breast cancer | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, P. Epigenetic Alterations in Cancer: The Therapeutic Potential of Epigenetic Drugs in Cancer Therapy. Drugs Drug Candidates 2025, 4, 15. https://doi.org/10.3390/ddc4020015
Gupta P. Epigenetic Alterations in Cancer: The Therapeutic Potential of Epigenetic Drugs in Cancer Therapy. Drugs and Drug Candidates. 2025; 4(2):15. https://doi.org/10.3390/ddc4020015
Chicago/Turabian StyleGupta, Preeti. 2025. "Epigenetic Alterations in Cancer: The Therapeutic Potential of Epigenetic Drugs in Cancer Therapy" Drugs and Drug Candidates 4, no. 2: 15. https://doi.org/10.3390/ddc4020015
APA StyleGupta, P. (2025). Epigenetic Alterations in Cancer: The Therapeutic Potential of Epigenetic Drugs in Cancer Therapy. Drugs and Drug Candidates, 4(2), 15. https://doi.org/10.3390/ddc4020015






