Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review
Abstract
1. Introduction
2. Results and Discussion
2.1. General Findings
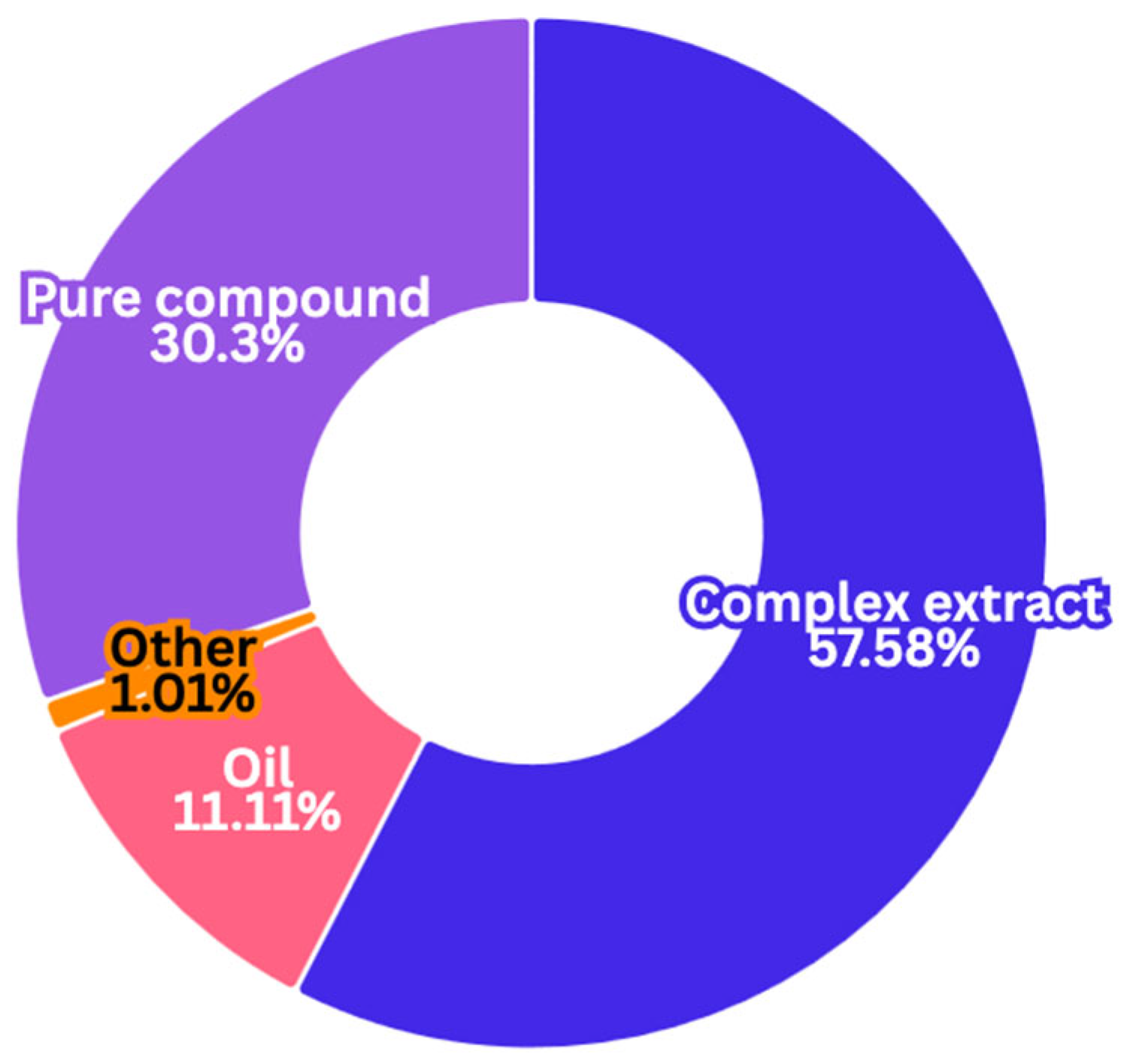
2.1.1. Natural Sample Type
Marine Origin
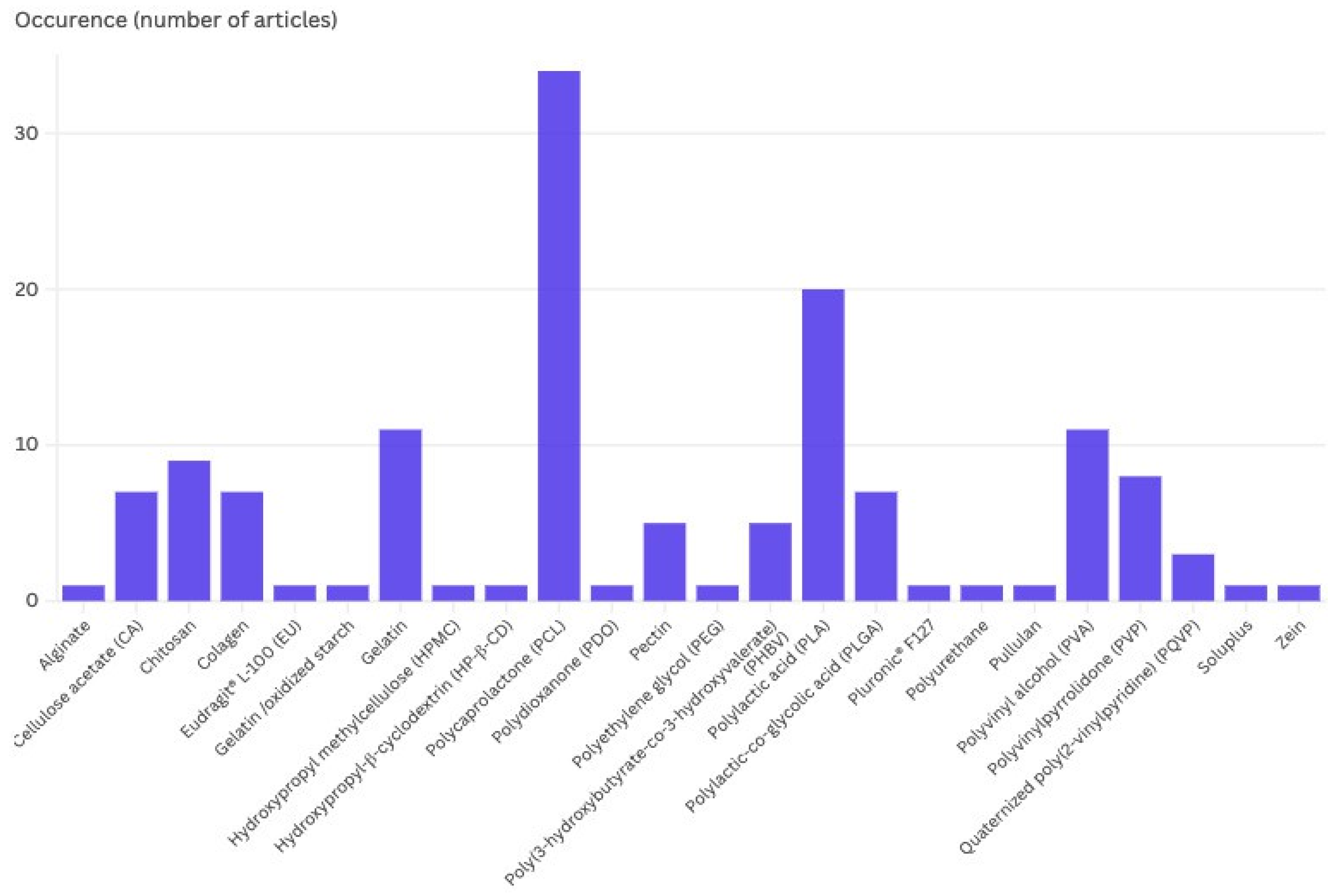
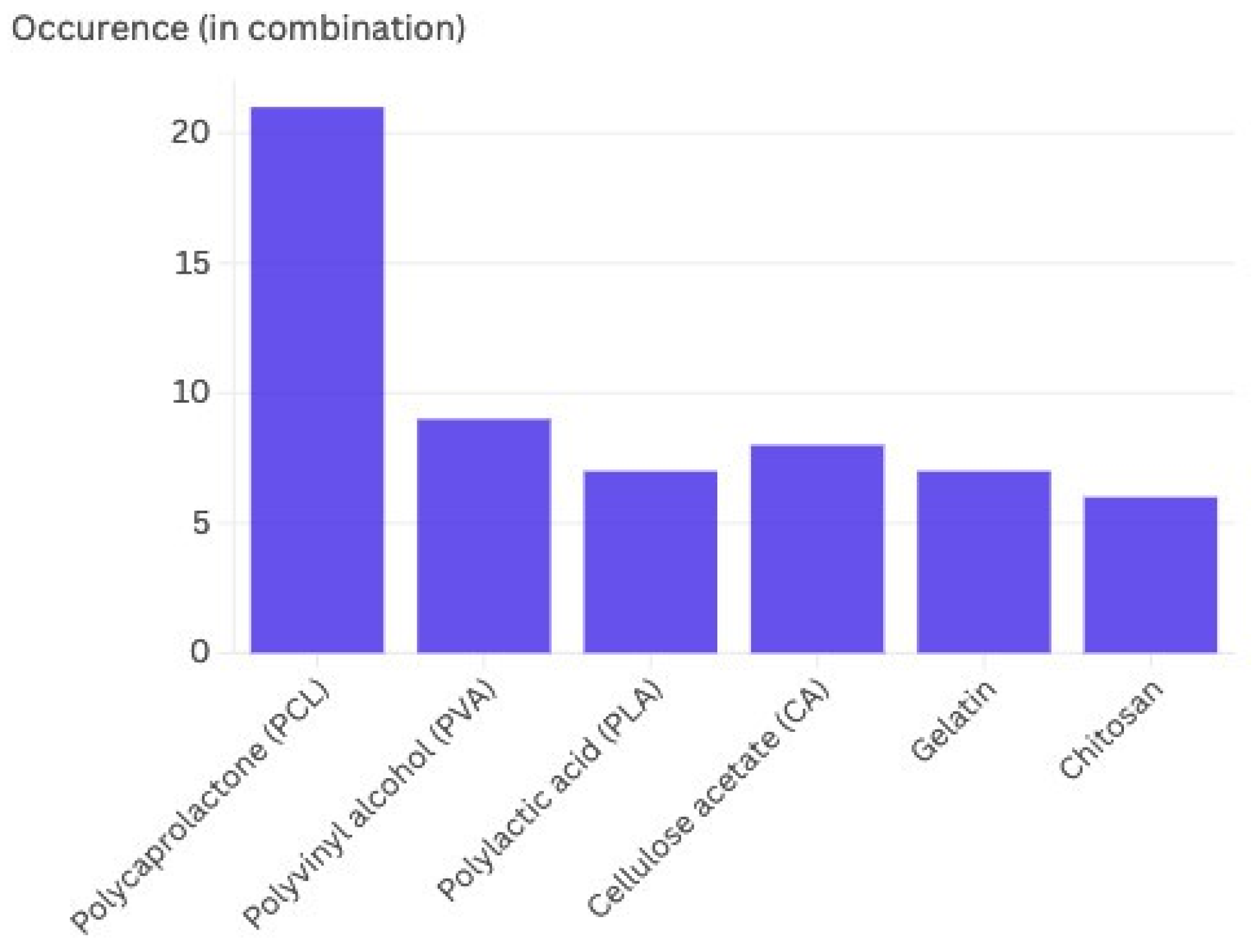
2.1.2. Polymers
2.1.3. Effects of Various Parameters on Electrospinning
Solvents Used in Electrospinning
Other Parameters
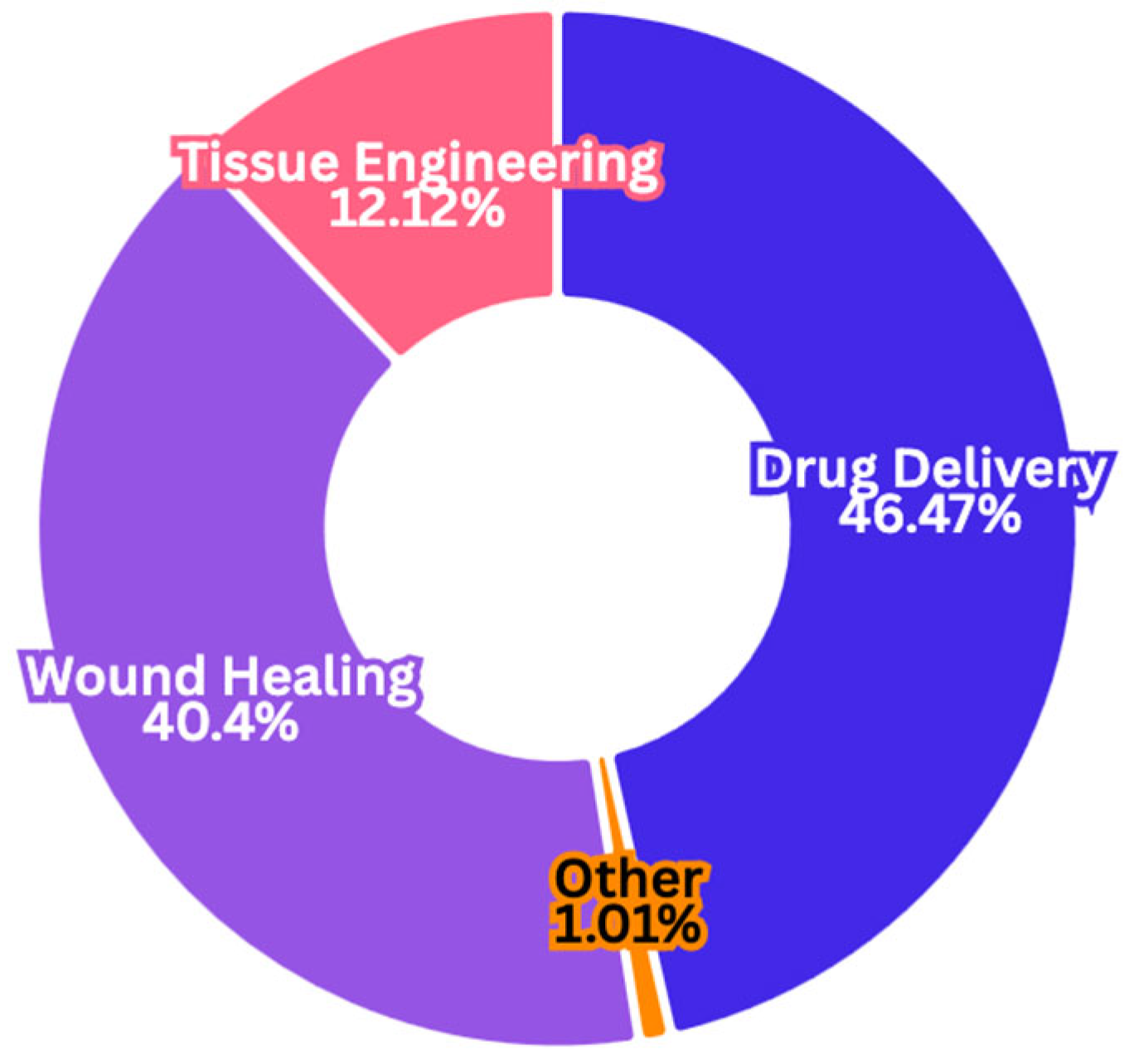
2.1.4. Pharmacological Applications
2.2. Specifc Findings
2.2.1. Drug Delivery
Controlled Release
Cancer Therapy
Other Findings in Drug Delivery
2.2.2. Wound Healing Dressing and Skin Tissue Regeneration
Applications in Wound Healing
Scaffolds for Chronic Wound Management
Antimicrobial Properties
Antioxidant Properties
2.2.3. Tissue Engineering
2.2.4. Miscellaneous Applications
2.2.5. Bioavailability of Natural Compounds in Nanofibers
2.2.6. Challenges in Electrospinning: Standardization and Scalability
3. Potential of Electrospinning for Cosmetic Applications
4. Marine Natural Compound Exploration
5. Materials and Methods
5.1. Selection and Exclusion Criteria
5.2. Databases
5.2.1. Keywords
5.2.2. Selection of Articles and Application of Inclusion and Exclusion Criteria
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, M.A.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Si, Y.; Han, Y.; Wu, T.; Iqbal, M.I.; Fei, B.; Li, R.K.Y.; Hu, J.; Qu, J. Recent Progress in Protective Membranes Fabricated via Electrospinning: Advanced Materials, Biomimetic Structures, and Functional Applications. Adv. Mater. 2022, 34, 2107938. [Google Scholar] [CrossRef]
- Dziemidowicz, K.; Sang, Q.; Wu, J.; Zhang, Z.; Zhou, F.; Lagaron, J.M.; Mo, X.; Parker, G.J.M.; Yu, D.G.; Zhu, L.M.; et al. Electrospinning for healthcare: Recent advancements. J. Mater. Chem. B 2021, 9, 939–951. [Google Scholar] [CrossRef]
- Han, W.H.; Wang, Q.Y.; Kang, Y.Y.; Shi, L.R.; Long, Y.; Zhou, X.; Hao, C.C. Cross-linking electrospinning. Nanoscale 2023, 15, 15513–15551. [Google Scholar] [CrossRef]
- Jouybar, A.; Seyedjafari, E.; Ardeshirylajimi, A.; Zandi-Karimi, A.; Feizi, N.; Khani, M.; Pousti, I. Enhanced Skin Regeneration by Herbal Extract-Coated Poly-L-Lactic Acid Nanofibrous Scaffold. Artif. Organs 2017, 41, E296–E307. [Google Scholar] [CrossRef]
- Luo, M.; Ming, Y.; Wang, L.; Li, Y.; Li, B.; Chen, J.; Shi, S. Local delivery of deep marine fungus-derived equisetin from polyvinylpyrrolidone (PVP) nanofibers for anti-MRSA activity. Chem. Eng. J. 2018, 350, 157–163. [Google Scholar] [CrossRef]
- Binotto, J.P.; Mendes, L.G.; de Gaspari Gaspi, F.O.; Esquisatto, M.A.M.; de Andrade, T.A.M.; Mendonça, F.A.S.; Santos, G.M.T. Poly(Lactic Acid) membrane and Sedum dendroideum extract favors the repair of burns in rats. Acta Cir. Bras. 2020, 35, e202000302. [Google Scholar] [CrossRef]
- Salles, T.H.C.; Volpe-Zanutto, F.; de Oliveira Sousa, I.M.; Machado, D.; Zanatta, A.C.; Vilegas, W.; Lancellotti, M.; Foglio, M.A.; D’ávila, M.A. Electrospun PCL-based nanofibers Arrabidaea chica Verlot—Pterodon pubescens Benth loaded: Synergic effect in fibroblast formation. Biomed. Mater. 2020, 15, 065001. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Moghadam, A.S.; Enderami, S.E.; Islami, M.; Kaabi, M.; Saburi, E.; Farshchi, A.D.; Soleimanifar, F.; Mansouri, V. Aloe Vera–Derived Gel-Blended PHBV Nanofibrous Scaffold for Bone Tissue Engineering. ASAIO J. 2020, 66, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.M.; Abu Hashim, I.I.; Meshali, M.M. Novel Clove Essential Oil Nanoemulgel Tailored by Taguchi’s Model and Scaffold-Based Nanofibers: Phytopharmaceuticals with Promising Potential as Cyclooxygenase-2 Inhibitors in External Inflammation. Int. J. Nanomed. 2020, 15, 2171–2195. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, P.; Alaei, M.; Khaksarihadad, M.; Ranjbar, M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J. Nanobiotechnol. 2020, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Barati, A.; Arjomandzadegan, M.; Vatankhah, E. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int. J. Biol. Macromol. 2020, 162, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Kikionis, S.; Papakyriakopoulou, P.; Mavrogiorgis, P.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Valsami, G.; Ioannou, E.; Roussis, V. Development of Novel Pharmaceutical Forms of the Marine Bioactive Pigment Echinochrome A Enabling Alternative Routes of Administration. Mar. Drugs 2023, 21, 250. [Google Scholar] [CrossRef]
- Kwak, H.W.; Kang, M.J.; Bae, J.H.; Hur, S.B.; Kim, I.-S.; Park, Y.H.; Lee, K.H. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int. J. Biol. Macromol. 2014, 63, 198–204. [Google Scholar] [CrossRef]
- Thamer, B.M.; Esmail, G.A.; Al-Dhabi, N.A.; Moydeen, M.; Arasu, M.V.; Al-Enizi, A.M.; El-Newehy, M.H. Fabrication of biohybrid electrospun nanofibers for the eradication of wound infection and drug-resistant pathogens. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 609, 125691. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, D.; Hsiao, B.S.; Chu, B.; Chen, W. Optimization and Characterization of Dextran Membranes Prepared by Electrospinning. Biomacromolecules 2004, 5, 326–333. [Google Scholar] [CrossRef]
- Ohgo, K.; Zhao, C.; Kobayashi, M.; Asakura, T. Preparation of non-woven nanofibers of Bombyx mori silk, Samia cynthia ricini silk and recombinant hybrid silk with electrospinning method. Polymer 2013, 44, 841–846. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, M.; Liu, X.; Wang, C.; Xu, N.; Xing, D. Multi-material electrospinning: From methods to biomedical applications. Mater. Today Bio 2023, 21, 100710. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Lee, C.-H.; Kan, C.-W.; Lu, X. Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials. Polymers 2024, 16, 154. [Google Scholar] [CrossRef]
- Nissi, J.S.; Vyaishnavi, S.; Sivaranjanee, R.; Sekar, M.P.; Sundaramurthi, D.; Vadivel, V. Development and characterization of Morinda tinctoria incorporated electrospun PHBV fiber mat for wound healing application. Macromol. Res. 2023, 31, 393–405. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: Physicochemical properties and release mechanism of α-mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Ulag, S.; Dena, A.S.A.; Sahin, A.; Grinholc, M.; Gunduz, O.; El-Sherbiny, I.; Megahed, M. Chitosan/Gold Hybrid Nanoparticles Enriched Electrospun PVA Nanofibrous Mats for the Topical Delivery of Punica granatum L. Extract: Synthesis, Characterization, Biocompatibility and Antibacterial Properties. Int. J. Nanomed. 2021, 16, 5133–5151. [Google Scholar] [CrossRef]
- Suwantong, O.; Pankongadisak, P.; Deachathai, S.; Supaphol, P. Electrospun poly(l-lactic acid) fiber mats containing crude Garcinia mangostana extracts for use as wound dressings. Polym. Bull. 2014, 71, 925–949. [Google Scholar] [CrossRef]
- Ravichandran, S.; Radhakrishnan, J.; Jayabal, P.; Venkatasubbu, G.D. Antibacterial screening studies of electrospun Polycaprolactone nano fibrous mat containing Clerodendrum phlomidis leaves extract. Appl. Surf. Sci. 2019, 484, 676–687. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, H.J.; Jang, J.Y.; Shin, H.S. Development of coaxial alginate-PCL nanofibrous dressing for controlled release of Spirulina extract. J. Biomater. Sci. Polym. Ed. 2018, 29, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Wang, L.; Zhang, Q.; Wang, Y.; Xia, X. Emulsion electrospun polylactic acid/Apocynum venetum nanocellulose nanofiber membranes with controlled sea buckthorn extract release as a drug delivery system. Text. Res. J. 2020, 91, 1046–1055. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun polylactide-based materials for curcumin release: Photostability, antimicrobial activity, and anticoagulant effect. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Teng, G.; Zhang, X.; Zhang, C.; Chen, L.; Sun, W.; Qiu, T.; Zhang, J. Lappaconitine trifluoroacetate contained polyvinyl alcohol nanofibrous membranes: Characterization, biological activities and transdermal application. Mater. Sci. Eng. C 2019, 108, 110515. [Google Scholar] [CrossRef]
- Mira, A.; Rubio-Camacho, M.; Alarcón, D.; Rodríguez-Cañas, E.; Fernández-Carvajal, A.; Falco, A.; Mallavia, R. L-Menthol-Loadable Electrospun Fibers of PMVEMA Anhydride for Topical Administration. Pharmaceutics 2021, 13, 1845. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Tapia-Hernández, J.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; de Jesús Ornelas-Paz, J.; Del-Toro-Sánchez, C.L.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials 2022, 12, 2303. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Andiappan, M.; Muthalagu, M. Preparation and characterization of Terminalia bellerica loaded PCL nanofibrous mats for biomedical applications. Mater. Today Proc. 2021, 45, 7247–7252. [Google Scholar] [CrossRef]
- Sugumaran, D.; Rathinam, R. Siddha drug incorporated electrospun nanofibrous mat with controlled drug release. Mater. Lett. 2021, 302, 130365. [Google Scholar] [CrossRef]
- Nam, S.; Lee, J.-J.; Lee, S.Y.; Jeong, J.Y.; Kang, W.-S.; Cho, H.-J. Angelica gigas Nakai extract-loaded fast-dissolving nanofiber based on poly(vinyl alcohol) and Soluplus for oral cancer therapy. Int. J. Pharm. 2017, 526, 225–234. [Google Scholar] [CrossRef]
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 2021, 67, 102984. [Google Scholar] [CrossRef]
- Kiliç, E.; Yakar, A.; Bayramgil, N.P. Preparation of electrospun polyurethane nanofiber mats for the release of doxorubicine. J. Mater. Sci. Mater. Med. 2018, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Radmansouri, M.; Bahmani, E.; Sarikhani, E.; Rahmani, K.; Sharifianjazi, F.; Irani, M. Doxorubicin hydrochloride–Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol. 2018, 116, 378–384. [Google Scholar] [CrossRef]
- Akpan, U.; Pellegrini, M.; Obayemi, J.; Ezenwafor, T.; Browl, D.; Ani, C.; Yiporo, D.; Salifu, A.; Dozie-Nwachukwu, S.; Odusanya, S.; et al. Prodigiosin-loaded electrospun nanofibers scaffold for localized treatment of triple negative breast cancer. Mater. Sci. Eng. C 2020, 114, 110976. [Google Scholar] [CrossRef]
- Akpan, U.M.; Pellegrini, M.; Salifu, A.A.; Obayemi, J.D.; Ezenwafor, T.; Browe, D.; Ani, C.J.; Danyuo, Y.; Dozie-Nwachukwu, S.; Odusanya, O.S.; et al. In vitro studies of Annona muricata L. extract-loaded electrospun scaffolds for localized treatment of breast cancer. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2041–2056. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Wen, Y.; Linhardt, R.J.; Zong, M.-H.; Zou, Y.-X.; Wu, H. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019, 10, 1816–1825. [Google Scholar] [CrossRef]
- Arbade, G.K.; Kumar, V.; Tripathi, V.; Menon, A.; Bose, S.; Patro, T.U. Emblica officinalis-loaded poly(ε-caprolactone) electrospun nanofiber scaffold as potential antibacterial and anticancer deployable patch. New J. Chem. 2019, 43, 7427–7440. [Google Scholar] [CrossRef]
- Kyritsi, A.; Kikionis, S.; Tagka, A.; Koliarakis, N.; Evangelatou, A.; Papagiannis, P.; Stratigos, A.; Karalis, V.; Dallas, P.; Vitsos, A.; et al. Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract. Cancers 2021, 13, 2596. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Vongsetskul, T.; Phurayar, P.; Chutimasakul, T.; Tuchinda, P.; Uamsiri, S.; Kumkate, S.; Pearngam, P.; Jitpibull, J.; Samphaongern, C.; Tangboriboonrat, P. Acanthus ebracteatus Vahl. extract-loaded cellulose acetate ultrafine fibers as a topical carrier for controlled-release applications. Polym. Bull. 2016, 73, 3319–3331. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Liang, H.; Zhang, J.; Yuan, H.; Zhao, D.; Wang, C. An antioxidative, green and safe nanofibers-based film containing pullulan, sodium hyaluronate and Ganoderma lucidum fermentation for enhanced skincare. Int. J. Biol. Macromol. 2023, 253, 127047. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilneia, S.; Dehkharghani, R.A.; Benisi, S.Z. Architecture of a dual biocompatible platform to immobilize genistin: Fabrication with physio-chemical and in vitro evaluation. Sci. Rep. 2023, 13, 22439. [Google Scholar] [CrossRef] [PubMed]
- Hani, N.M.; E Torkamani, A.; Azarian, M.H.; Mahmood, K.W.; Ngalim, S.H. Characterisation of electrospun gelatine nanofibres encapsulated with Moringa oleifera bioactive extract. J. Sci. Food Agric. 2017, 97, 3348–3358. [Google Scholar] [CrossRef]
- Spasova, M.; Stoyanova, N.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A. Facile preparation of novel antioxidant fibrous material based on natural plant extract from Portulaca oleracea and polylactide by electrospinning for biomedical applications. Polym. Int. 2022, 71, 689–696. [Google Scholar] [CrossRef]
- Seyhan, S.A.; DEMIREL, A.B.; Cesur, S.; Alkaya, D.B. Production, characterization, and antioxidant activity evaluation of Rheum Ribes L. extract-loaded PLA/PEG nanofibers. J. Res. Pharm. 2023, 27, 146–156. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Tihminlioglu, F. A novel bilayer zein/MMT nanocomposite incorporated with H. perforatum oil for wound healing. J. Mater. Sci. Mater. Med. 2020, 31, 1–19. [Google Scholar] [CrossRef]
- Mirbehbahani, F.S.; Hejazi, F.; Najmoddin, N.; Asefnejad, A. Artemisia annua L. as a promising medicinal plant for powerful wound healing applications. Prog. Biomater. 2020, 9, 139–151. [Google Scholar] [CrossRef]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef]
- Isoglu, I.A.; Koc, N. Centella asiatica Extract Containing Bilayered Electrospun Wound Dressing. Fibers Polym. 2020, 21, 1453–1465. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, M.; Li, Y.; Li, K.; Chen, S.; Zhao, W.; Wu, S.; Han, Y. Electrospun Chitosan–Polyvinyl Alcohol Nanofiber Dressings Loaded with Bioactive Ursolic Acid Promoting Diabetic Wound Healing. Nanomaterials 2022, 12, 2933. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.C.; Anaya-Mancipe, J.M.; da Silva de Barros, A.O.; Santos-Oliveira, R.; Dias, M.L.; da Silva Moreira Thiré, R.M. Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing. Molecules 2022, 27, 5351. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.; Zakeri-Milani, P.; Ghorbani, M. Designing and Evaluating pH-Responsive Electrospun Eudragit® L-100/Hydroxypropyl Methyl Cellulose Composite Mats for Release of Propolis as a Novel Wound Dressing. J. Polym. Environ. 2023, 31, 3215–3229. [Google Scholar] [CrossRef]
- Behyari, M.; Imani, R.; Keshvari, H. Evaluation of Silk Fibroin Nanofibrous Dressing Incorporating Niosomal Propolis, for Potential Use in Wound Healing. Fibers Polym. 2021, 22, 2090–2101. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Demirhan, R.; Sahin, A.; Yilmaz, B.K.; Aksu, B.; Sengor, M.; Ficai, D.; Titu, A.M.; Ficai, A.; et al. Propolis-Based Nanofiber Patches to Repair Corneal Microbial Keratitis. Molecules 2021, 26, 2577. [Google Scholar] [CrossRef]
- Khan, A.K.; Kaleem, S.; Pervaiz, F.; Sherazi, T.A.; Khan, S.A.; Khan, F.A.; Jamshaid, T.; Umar, M.I.; Hassan, W.; Ijaz, M.; et al. Antibacterial and wound healing potential of electrospun PVA/MMT nanofibers containing root extract of Berberis lycium. J. Drug Deliv. Sci. Technol. 2023, 79, 103987. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidoniummajus L. Incorporated Emulsion Electrospun PCL/PVA_PEC Nanofibrous Meshes for Antibacterial Wound Dressing Applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Salami, M.S.; Bahrami, G.; Arkan, E.; Izadi, Z.; Miraghaee, S.; Samadian, H. Co-electrospun nanofibrous mats loaded with bitter gourd (Momordica charantia) extract as the wound dressing materials: In vitro and in vivo study. BMC Complement. Med. Ther. 2021, 21, 111. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef]
- Gao, S.; Xiong, X.; Xie, H.; Zha, X.; Li, P.; Kong, F.; Fan, Y.; Meng, S.; Yuan, J.; Jiang, Q. Periplaneta americana extract incorporated silk fibroin nanofiber mat: A potential antioxidant dressing for wound healing. Process. Biochem. 2023, 134, 218–231. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surfaces B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef]
- Jung, S.-M.; Kim, D.S.; Ju, J.H.; Shin, H.S. Assessment of Spirulina-PCL nanofiber for the regeneration of dermal fibroblast layers. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 27–33. [Google Scholar] [CrossRef]
- Sadia, M.; Mohd Zaki, M.A.; Jaganathan, S.K.; Md Shakhih, M.F.; Kamarozaman, A.S.; Ab’lah, N.; Saidin, S. Blending of Moringa oleifera into Biodegradable Polycaprolactone/Silver Electrospun Membrane for Hemocompatibility Improvement. Arab. J. Sci. Eng. 2023, 48, 7323–7336. [Google Scholar] [CrossRef]
- Nejati-Koshki, K.; Pilehvar-Soltanahmadi, Y.; Alizadeh, E.; Ebrahimi-Kalan, A.; Mortazavi, Y.; Zarghami, N. Development of Emu oil-loaded PCL/collagen bioactive nanofibers for proliferation and stemness preservation of human adipose-derived stem cells: Possible application in regenerative medicine. Drug Dev. Ind. Pharm. 2017, 43, 1978–1988. [Google Scholar] [CrossRef]
- Asghari, F.; Faradonbeh, D.R.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef]
- Mouthuy, P.-A.; Škoc, M.S.; Gašparović, A.Č.; Milković, L.; Carr, A.J.; Žarković, N. Investigating the use of curcumin-loaded electrospun filaments for soft tissue repair applications. Int. J. Nanomed. 2017, 12, 3977–3991. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; He, L.; Chen, N.; Ramakrishna, S.; So, K.-F.; Mo, X. Lycium barbarum polysaccharide encapsulated Poly lactic-co-glycolic acid Nanofibers: Cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci. Rep. 2018, 8, 8669. [Google Scholar] [CrossRef]
- Eldurini, S.; El-Hady, B.M.A.; Shafaa, M.W.; Gad, A.A.M.; Tolba, E. A multicompartment vascular implant of electrospun wintergreen oil/polycaprolactone fibers coated with poly(ethylene oxide). Biomed. J. 2020, 44, 589–597. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Sutera, A.; Botta, L.; Fontana, R.M.; Gallo, G. Plasma modified PLA electrospun membranes for actinorhodin production intensification in Streptomyces coelicolor immobilized-cell cultivations. Colloids Surfaces B Biointerfaces 2017, 157, 233–241. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Hodaei, H.; Esmaeili, Z.; Erfani, Y.; Esnaashari, S.S.; Geravand, M.; Adabi, M. Preparation of biocompatible Zein/Gelatin/Chitosan/PVA based nanofibers loaded with vitamin E-TPGS via dual-opposite electrospinning method. Sci. Rep. 2024, 14, 23796. [Google Scholar] [CrossRef]
- Duan, X.; Chen, H.-L.; Guo, C. Polymeric Nanofibers for Drug Delivery Applications: A Recent Review. J. Mater. Sci. Mater. Med. 2022, 33, 78. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract. Pharmaceutics 2020, 12, 770. [Google Scholar] [CrossRef]
- Maduna, L.; Patnaik, A. Challenges Associated with the Production of Nanofibers. Processes 2024, 12, 2100. [Google Scholar] [CrossRef]
- Kannan, B.; Cha, H.; Hosie, I.C. Electrospinning—Commercial applications, challenges and opportunities. In Nano-Size Polymers: Preparation, Properties, Applications; Springer: Cham, Switzerland, 2016; pp. 309–342. [Google Scholar] [CrossRef]
- Hameed, A.; Rehman, T.U.; Rehan, Z.A.; Noreen, R.; Iqbal, S.; Batool, S.; Qayyum, M.A.; Ahmed, T.; Farooq, T. Development of polymeric nanofibers blended with extract of neem (Azadirachta indica), for potential biomedical applications. Front. Mater. 2022, 9, 1042304. [Google Scholar] [CrossRef]




| Type of Polymers | Examples | Source |
|---|---|---|
| Polysaccharides | Alginate | Cell wall of brown algae |
| Cellulose acetate | The primary source of cellulose acetate is plant cellulose, extracted from natural fibers like cotton and wood pulp | |
| Chitosan | Chitosan is a biopolymer derived from chitin, which is found in the exoskeletons of crustaceans like shrimp and crabs | |
| Starch | Starch is a polysaccharide made up of glucose units; it is the main form of energy storage in plants and is found in foods like potatoes, rice, and corn | |
| Pullulan | Pullulan is obtained by fermenting starch using the fungus Aureobasidium pullulans (Kaulf. ex Spreng.) Nees and Hornsch | |
| Protein | Collagen | Collagen is obtained from the connective tissue of animals, such as cattle, pigs, and fish, extracted from skin, bones, and cartilage |
| Gelatin | Gelatin is a protein obtained by the partial hydrolysis of collagen, which is found in animal connective tissues, such as skin, bones, and cartilage | |
| Silk Fibroin | Protein derived from the silk produced by silkworms, primarily Bombyx mori; it is the structural component of silk fibers, known for its strength, durability, and biocompatibility | |
| Zein | Zein is a storage protein found in corn, known for its solubility in alcohol and ability to form hydrophobic films |
| Category | Type of Analysis | Analysis |
|---|---|---|
| Physicochemical Analysis | Morphological Analysis | Scanning Electron Microscopy (SEM): Provides high-resolution images of the nanofiber surface, assessing fiber diameter, uniformity, and morphology (e.g., smooth, porous, beaded). Transmission Electron Microscopy (TEM): Offers higher resolution for imaging internal structures of nanofibers, including the distribution of encapsulated compounds within the matrix. |
| Polymer Characterization | Fourier Transform Infrared Spectroscopy (FTIR): Identifies chemical bonds within the polymer and detects interactions between the polymer and the encapsulated compound. X-Ray Diffraction (XRD): Determines the crystallinity of the polymer and any changes due to the presence of the bioactive compound. | |
| Encapsulation Efficiency | Spectrophotometry or Chromatography (e.g., HPLC): Quantifies the amount of bioactive compound successfully loaded into the nanofibers. | |
| Release Kinetics | In Vitro Release Assay: Measures the release of the bioactive compound from the nanofibers over time, in relevant media, at physiological conditions. | |
| Surface Area and Porosity | Brunauer–Emmett–Teller (BET) Analysis: Measures the surface area and pore size distribution of the nanofibers. | |
| Mechanical Properties | Tensile Testing: Evaluates the mechanical strength and elasticity of nanofiber mats. | |
| Biological Analysis | In Vitro Biological Evaluation | Cytotoxicity Assays (e.g., MTT, Alamar Blue): Evaluates the biocompatibility of the nanofibers and the encapsulated compound by measuring cell viability or proliferation. Cellular Uptake Studies: Measures the uptake of the bioactive compound or nanofibers by cells. Functional Assays: Tests the biological activity of the encapsulated compound. |
| In Vivo Biological Evaluation | Pharmacokinetic Studies (PK): Evaluates the absorption, distribution, metabolism, and excretion (ADME) of the bioactive compound in animal models. Pharmacodynamic Studies (PD): Evaluates the therapeutic efficacy of the encapsulated compound by measuring relevant biomarkers or clinical outcomes. Histological Analysis: Examines tissue sections after in vivo administration. | |
| Safety Studies | Toxicity Studies: Evaluates potential adverse effects of the nanofibers and encapsulated compounds in animal models. Immunogenicity Studies: Evaluates immune responses of the nanofiber system. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, R.F.; Jimenez, P.C. Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review. Drugs Drug Candidates 2025, 4, 8. https://doi.org/10.3390/ddc4010008
Franco RF, Jimenez PC. Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review. Drugs and Drug Candidates. 2025; 4(1):8. https://doi.org/10.3390/ddc4010008
Chicago/Turabian StyleFranco, Rayssa F., and Paula C. Jimenez. 2025. "Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review" Drugs and Drug Candidates 4, no. 1: 8. https://doi.org/10.3390/ddc4010008
APA StyleFranco, R. F., & Jimenez, P. C. (2025). Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review. Drugs and Drug Candidates, 4(1), 8. https://doi.org/10.3390/ddc4010008







