1. Introduction
Lung cancer remains a formidable challenge for public health, persistently ranking as one of the leading causes of cancer-related deaths worldwide. With lung cancer accounting for around 18% of all cancer-related fatalities globally, lung cancer is a serious public health concern. With a five-year survival rate of about 21% for individuals with advanced-stage disease, it is the primary cause of cancer-related death in both men and women [
1]. The prognosis for individuals with lung cancer remains poor despite advancements in treatment modalities such as surgery, radiation, and chemotherapy. This is mostly because of the high rate of metastasis and the emergence of drug resistance [
2]. The mainstay of treating lung cancer, chemotherapy, frequently has serious side effects and a low success rate. This calls for the creation of more potent therapeutic approaches. One technique that shows promise for improving medicine absorption, reducing adverse effects, and producing synergistic effects is co-encapsulating multiple medicinal chemicals into a single delivery vehicle [
3].
Figure 1 shows various ways to overcome drug resistance in cancer.
Docetaxel (DTX), a well-established chemotherapeutic agent, is widely used in the treatment of various cancers, including lung cancer [
4]. It functions by stabilizing microtubules, thereby inhibiting cell division and inducing apoptosis [
5]. However, the clinical utility of DTX is limited by its poor solubility [
6], rapid clearance, and systemic toxicity [
7]. On the other hand, betulinic acid (BA), a naturally occurring pentacyclic triterpenoid, has garnered attention for its potent anticancer properties [
8]. It exhibits targeted cytotoxicity to cancer cells while sparing healthy cells, inducing apoptosis through many mechanisms, including mitochondrial dysfunction and activation of the intrinsic apoptotic pathway [
9]. Despite its potential, betulinic acid’s clinical application is hindered by its low aqueous solubility and bioavailability [
10].
The co-encapsulation of DTX and BA presents a novel strategy to overcome the limitations associated with their individual use. It is feasible to take advantage of the complimentary modes of action of both drugs by combining them into a single delivery method, which could result in increased anticancer activity and decreased toxicity. Because of this, the application of nanotechnology offers a valuable platform for improving the solubility, stability, and targeted distribution of medicines [
11]. Chemotherapeutics with better pharmacokinetic profiles and fewer adverse effects may be delivered by nanocarriers such as liposomes, polymeric nanoparticles, and lipid-based nanoparticles [
12].
Previously, the co-encapsulation of cisplatin, an antitumor agent, and fisetin, an antiangiogenic molecule, into liposomes showed promise in treating lung cancer. This approach enhances fisetin solubility and reduces cisplatin toxicity. Freeze-drying improved drug storage, maintaining efficacy for 30 days. In vitro studies on Lewis lung carcinoma cells showed strong synergy between co-loaded liposomes (CI = 0.7). In vivo, the co-encapsulated formulation effectively treated an ectopic murine model of Lewis lung carcinoma, potentially reducing cisplatin toxicity through co-encapsulation with fisetin [
13]. Another study showing the co-encapsulation of paclitaxel (PTX) and salinomycin (SAL) in lipid nanocapsules (LNCs) targets both bulk breast cancer cells (MCF-7) and cancer stem cells (bCSCs). LNCs, with nano-size (~90 nm) and high drug encapsulation efficiency, effectively release drugs and exhibit strong cytotoxicity. Co-loaded LNCs induced apoptosis in bCSCs, showing superior effects and reducing tumor growth, highlighting their potential for targeted breast cancer treatment [
14]. Another study demonstrated a drug delivery system using nanovesicles co-encapsulating interleukin-2 (IL-2) and doxorubicin (DOX), which effectively suppresses melanoma growth with minimal toxicity. Extending this approach, cytokine cocktails, including interferon-γ (IFN-γ), were developed to enhance immune responses and inhibit lung metastasis in triple-negative breast cancer [
15]. Another two studies highlight two strategies for co-encapsulation in cancer therapy. First, PLA-based nanoplatforms co-deliver salinomycin (SAL) and doxorubicin (DOX), targeting cancer stem cells and bulk tumors with reduced toxicity and improved efficacy. Second, niosomes co-encapsulating gemcitabine (GEM) and tocotrienols enhance the antiproliferative effects and cytotoxicity against pancreatic cancer cells. Both approaches demonstrate how combining drugs in nanocarriers can optimize treatment efficacy and reduce side effects, offering potential benefits for lung cancer therapy as well [
16,
17]. Based on these studies, it is clear that co-encapsulation is an effective strategy for enhancing lung cancer treatment outcomes.
In this study, we examine the co-encapsulation of BA and DTX via a delivery system based on poly-lactic-co-glycolic acid (PLGA) nanoparticles (NPs). Nanoencapsulation using PLGA, a biocompatible polymeric carrier, presents a promising approach. PLGA is a widely recognized biodegradable and biocompatible polymer that has been approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). PLGA nanoparticles and microparticles have been employed to encapsulate a range of therapeutics for purposes such as vaccination and treatments for cardiovascular diseases and cancer. Via the increased permeability and retention (EPR) effect, PLGA NPs are especially useful in binding medications with low solubility and promoting their extravasation via tumor vasculature [
18,
19].
This work aims to accomplish mainly two goals: first, to create and describe the co-encapsulated NPs in terms of their physicochemical characteristics, drug loading effectiveness, and release profiles; second, to assess the co-encapsulated formulation’s in vitro cytotoxicity and capacity to induce apoptosis against lung cancer cell lines. We hypothesize that the co-encapsulation of BA and DTX will synergistically boost their anticancer activity, leading to better therapeutic outcomes than if they were administered separately as BA triggers apoptosis and modifies the immunological response, whereas DTX stabilizes microtubules and prevents cancer cells from proliferating. It is anticipated that the nanoparticle formulation will reduce systemic toxicity, enhance both medicines’ solubility and bioavailability, and make it easier for them to be delivered to tumor locations together. When compared to administering each medication separately, co-encapsulation is anticipated to produce prolonged drug release, increased cellular absorption, and a more effective anticancer impact, which could potentially improve treatment outcomes and minimize adverse effects. With the use of nanotechnology’s special benefits, this research attempts to advance the creation of safer and more effective treatment alternatives for lung cancer patients.
In conclusion, by investigating a unique co-encapsulation strategy to improve the efficacy of currently available chemotherapeutic drugs, this research fills a key gap in the treatment of lung cancer. The results of this study may open the door to novel combination treatments that give people fighting this terrible illness better chances of survival and a higher standard of living.
3. Discussion
One of the significant challenges in the scientific community is developing safe and effective anticancer drugs. Nanotherapeutics can minimize the cytotoxic effects of these drugs on healthy cells. Recently, polymer-based nanoscale drug delivery systems (DDSs) have gained attention for their ability to maximize efficacy by minimizing drug degradation, reducing side effects through sustained release within a threshold concentration, and enabling targeted action due to their preferential uptake and retention in cancer cells [
24,
25].
In this study, combining DTX and BA in a single encapsulated formulation for lung cancer treatment can enhance therapeutic outcomes. DTX is a potent chemotherapeutic agent, while betulinic acid possesses anti-cancer and anti-inflammatory properties along with antioxidant properties. Encapsulation improves bioavailability, reduces toxicity, and ensures targeted delivery. This synergistic approach leverages the strengths of both compounds, potentially leading to more effective and less harmful lung cancer treatment [
6,
9,
10]. Lyophilized PLGA-based DTX and BA nanoencapsulations were obtained using the double-emulsification solvent evaporation techniques. Before loading the drug into the NPs, optimization was performed based on 2
3 designs using Design Expert version 10. Blank NPs were optimized based on the dependent variable (PVA concentration % (
w/
v) = A, sonication time (min) = B, and ultrasonic frequency (Hz) = C) to obtain the desired results of Y1 = size and Y2 = PDI. As per polynomial analysis and the 2FI process order, optimized values of A, B, and C were determined. Formulations with the drug were prepared using the optimized values of A, B, and C (0.1% PVA
w/
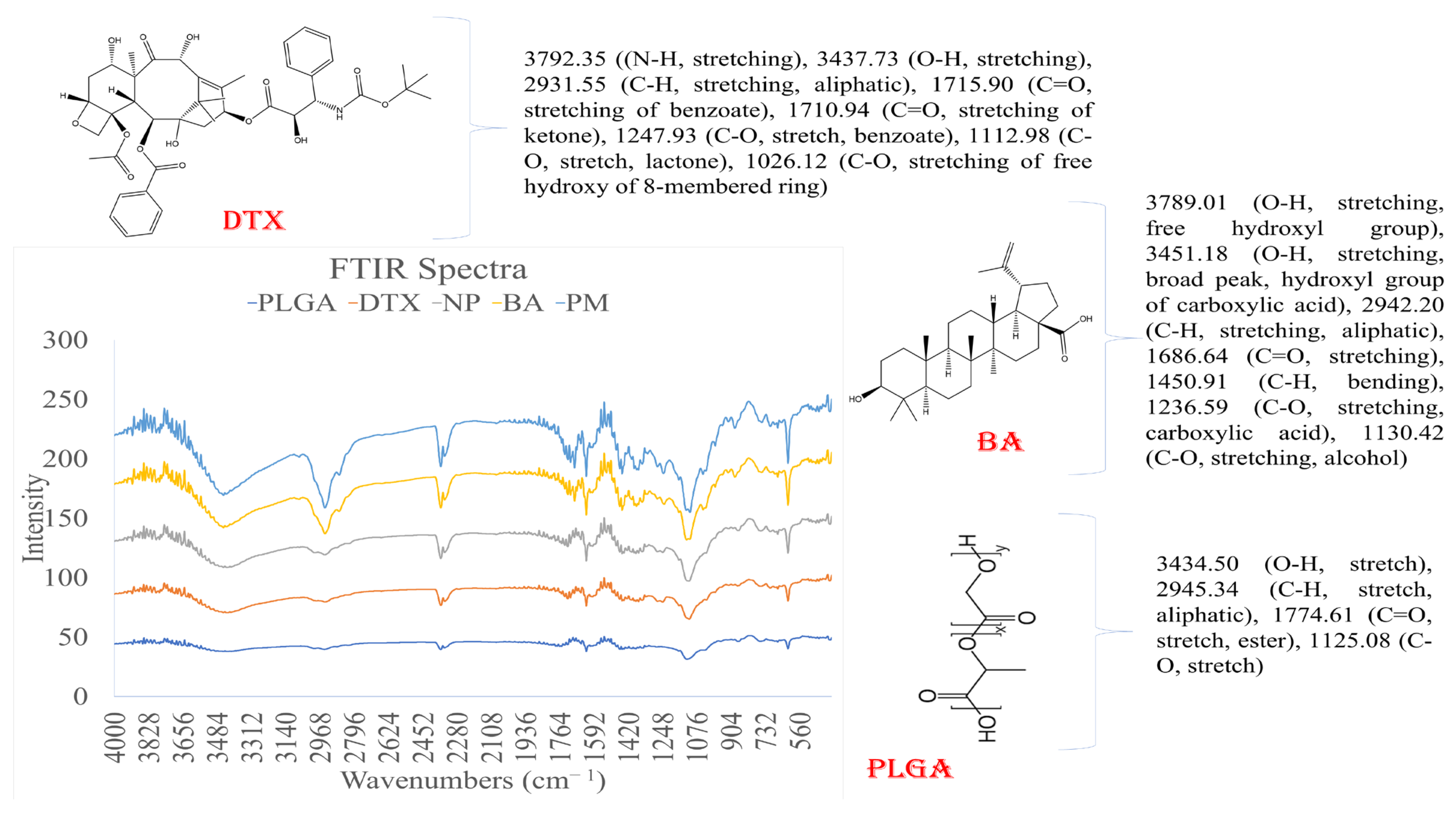
v, 11.45 min, and 50 Hz) for further studies. The slight shifting of non-characteristic peaks of DTX and BA in the drug-loaded formulation (DTX-BA-NPs) can be attributed to weak hydrogen bonds and electrical attractions, such as dipole moments and van der Waals forces. These interactions may prevent the crystallization of DTX and BA within the formulation, maintaining it in an amorphous state, which can lead to sustained drug release and prolonged bioavailability of DTX and BA from DTX-BA-NP. Nonetheless, the presence of all characteristic peaks of DTX-BA in DTX-BA-NP indicates that DTX and BA retain their chemical integrity within the formulation, essential for maintaining their biological activity upon release from the NPs.
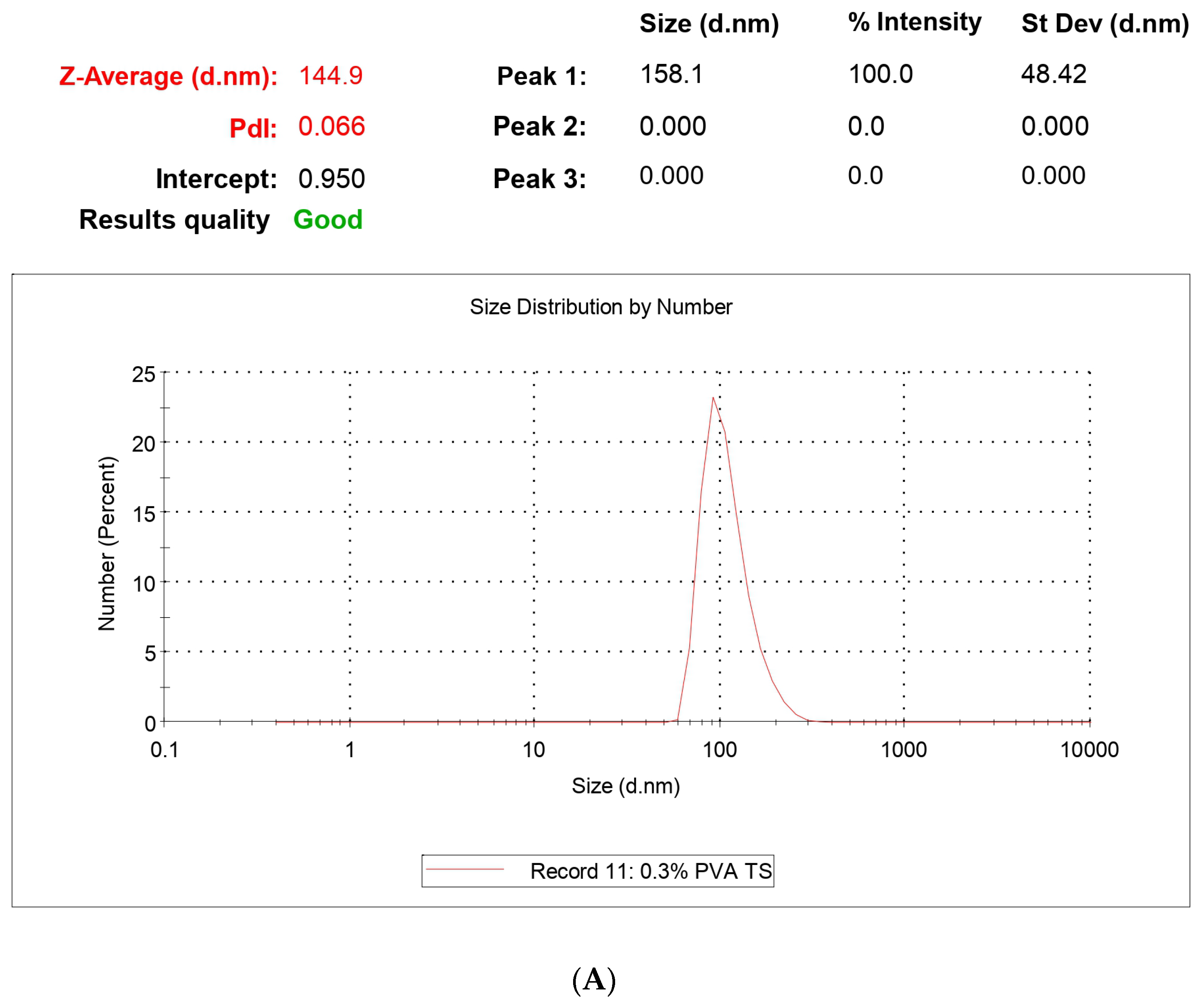
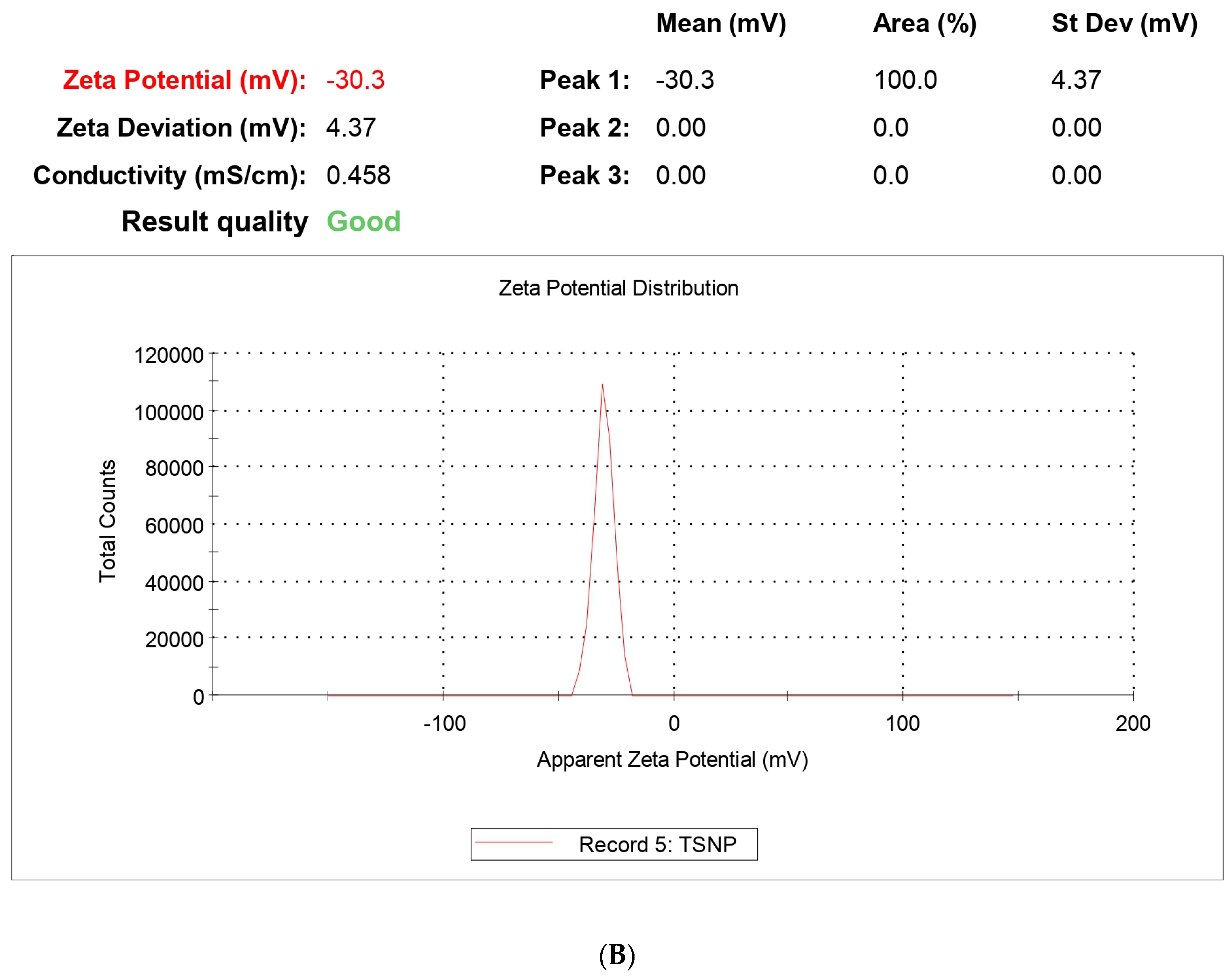
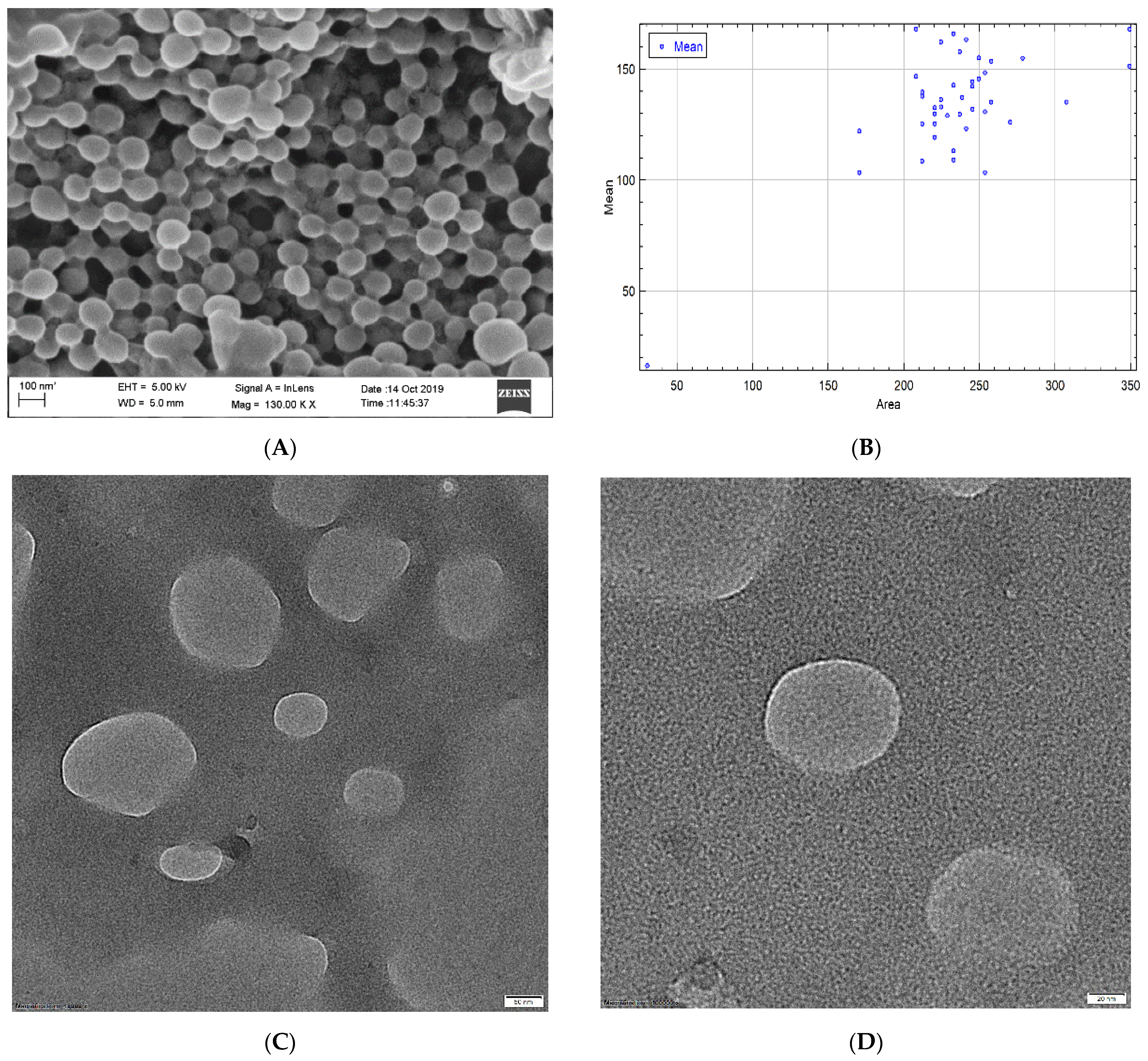
Surface morphology plays the most vital part in drug uptake by cells or organs due to the interaction with the biological membrane upon exposure to the biological system. Morphological analysis was performed using FESEM and HRTEM. The size of the NPs ranged between 59 and 220 nm, which fulfills the criteria for targeted nanoencapsulation. Morphological analysis was comparable to the size analysis with DLS as zeta size values ranged between 129 and 212 nm. The polydispersity index ranged from 0.011 to 0.357 for the NPs, confirming no aggregation in the formulation. PLGA contains the negative -COOH group and, for that, zeta potential has to have an average value of −27.8 ± 4.13 mv, which falls under the targeted −30 mv to +30 mv range. The potential values confirm that upon prolonged storage, NPs are prone to sedimentation and aggregation, so a lyophilized powder form is suitable for storage conditions and long-term stability. This study also confirms the stability of NPs with almost negligible changes in size and potential even after 3 months.
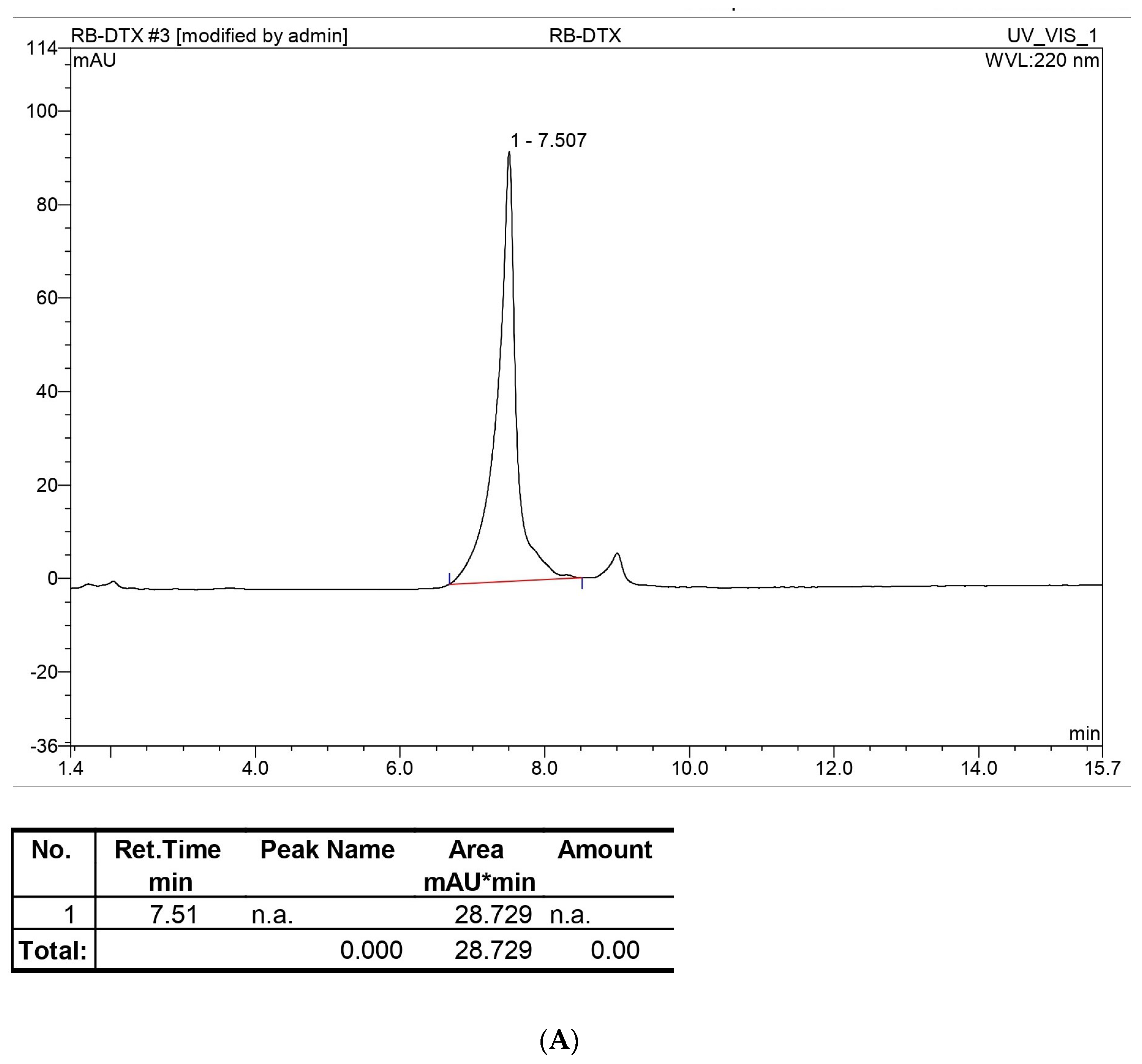
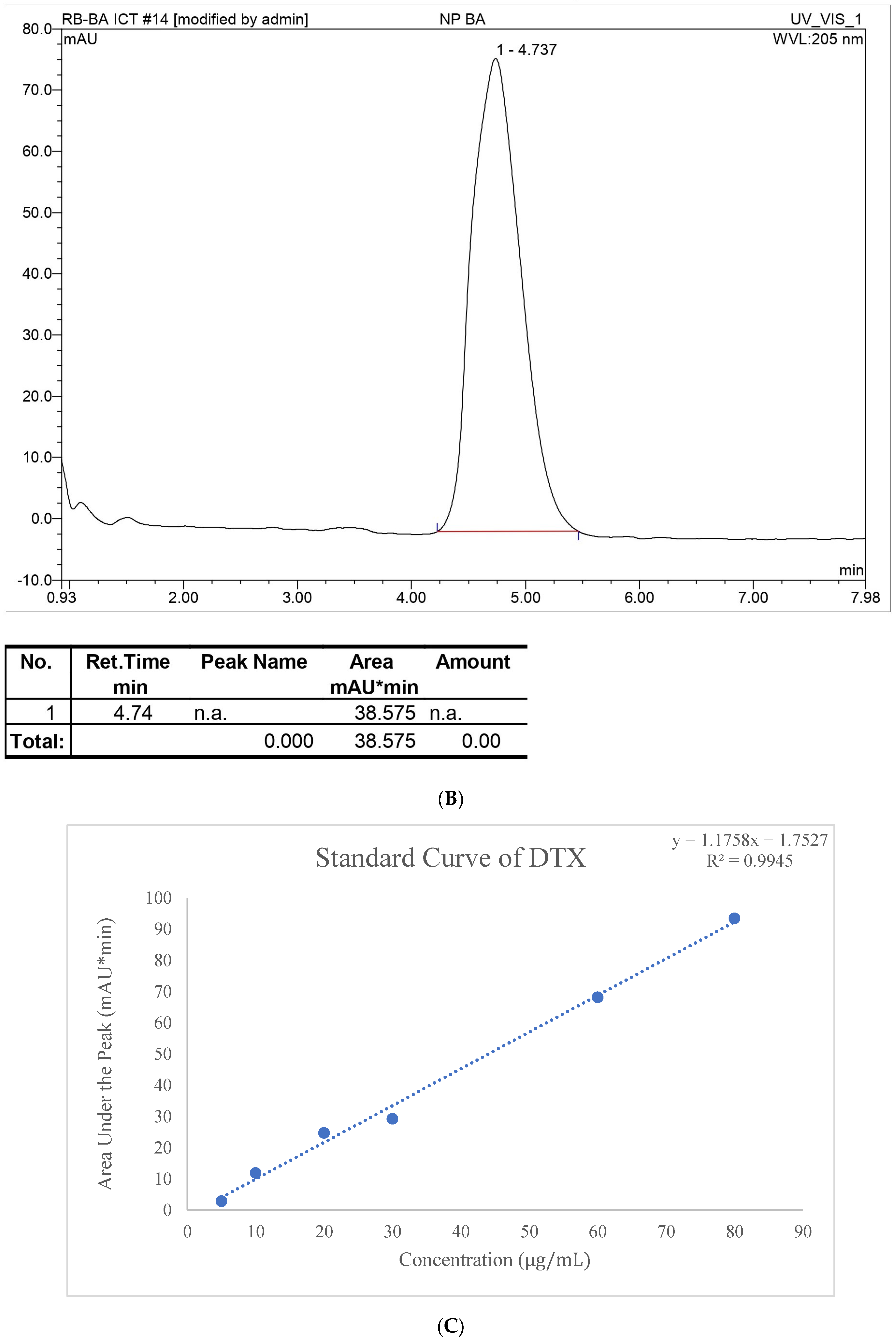
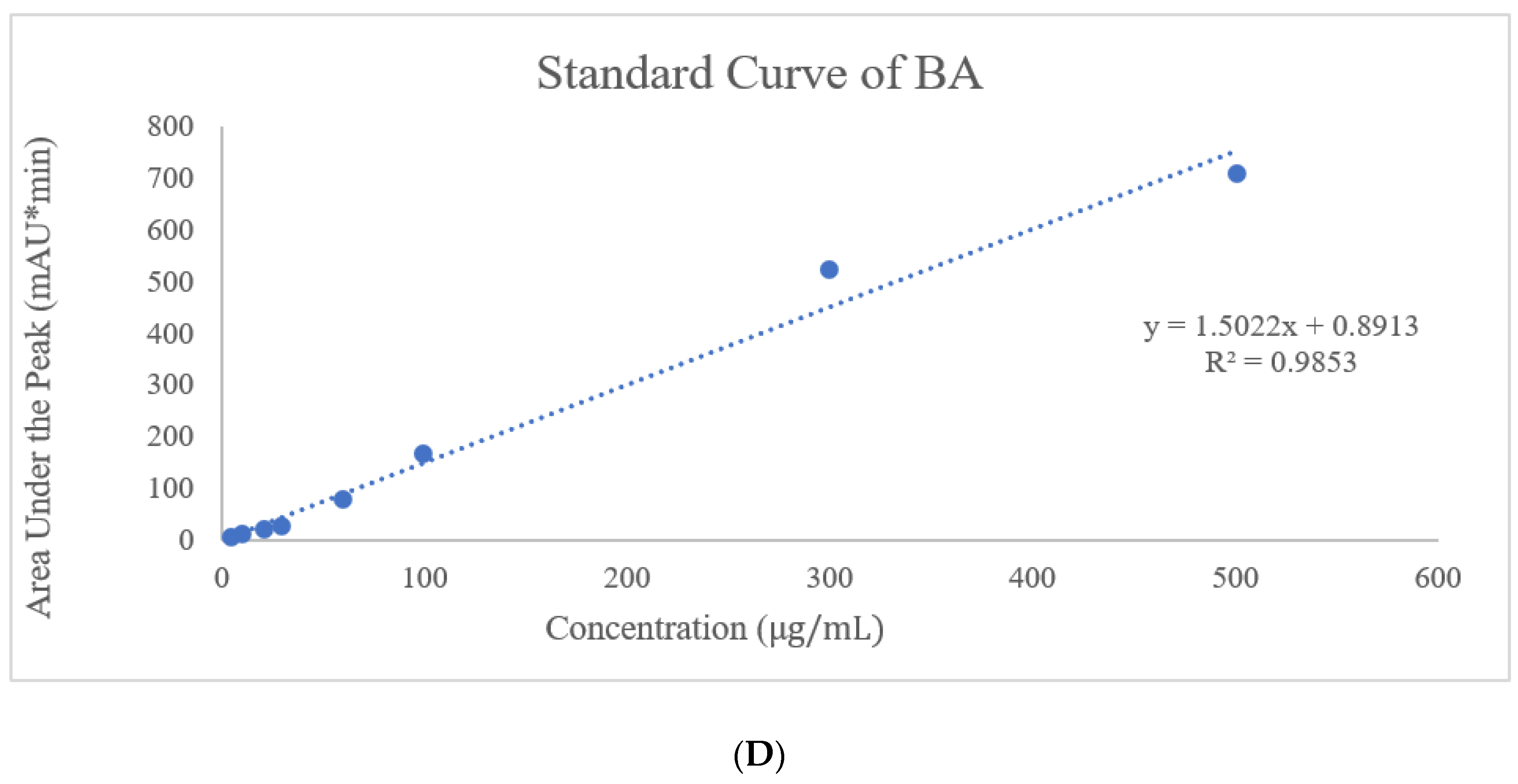
In the HPLC method, water and ACN were selected for the mobile phase at various ratios for the gradient system, and after further optimization, an isocratic system was developed at 45% ACN for DTX and 90% ACN for BA with a 1 mL/min flow rate. Along with the preparation of the standard curve with a range of 400, 200, 100, 80, 60, 40, 20, and 10 µg/mL, precession, accuracy, intraday, and interday analyses were performed. This method was used for entrapment and release behavior analysis, and those studies were perfectly suited for this method with LOD (Limit of detection) and LOQ (Limit of quantification) values of 3.072 µg/mL and 7.387 µg/mL, respectively, for DTX and 4.127 µg/mL and 7.527 µg/mL LOD and LOQ, respectively, for BA. The entrapment efficiency of DTX and BA was 67.12 ± 1.5% and 92. 32 ± 1.4%, respectively. The high entrapment of both drugs might be due to their lipophilic nature or the hydrogen bonding, hydrophobic interactions, and electrostatic interactions of the drugs [
26].
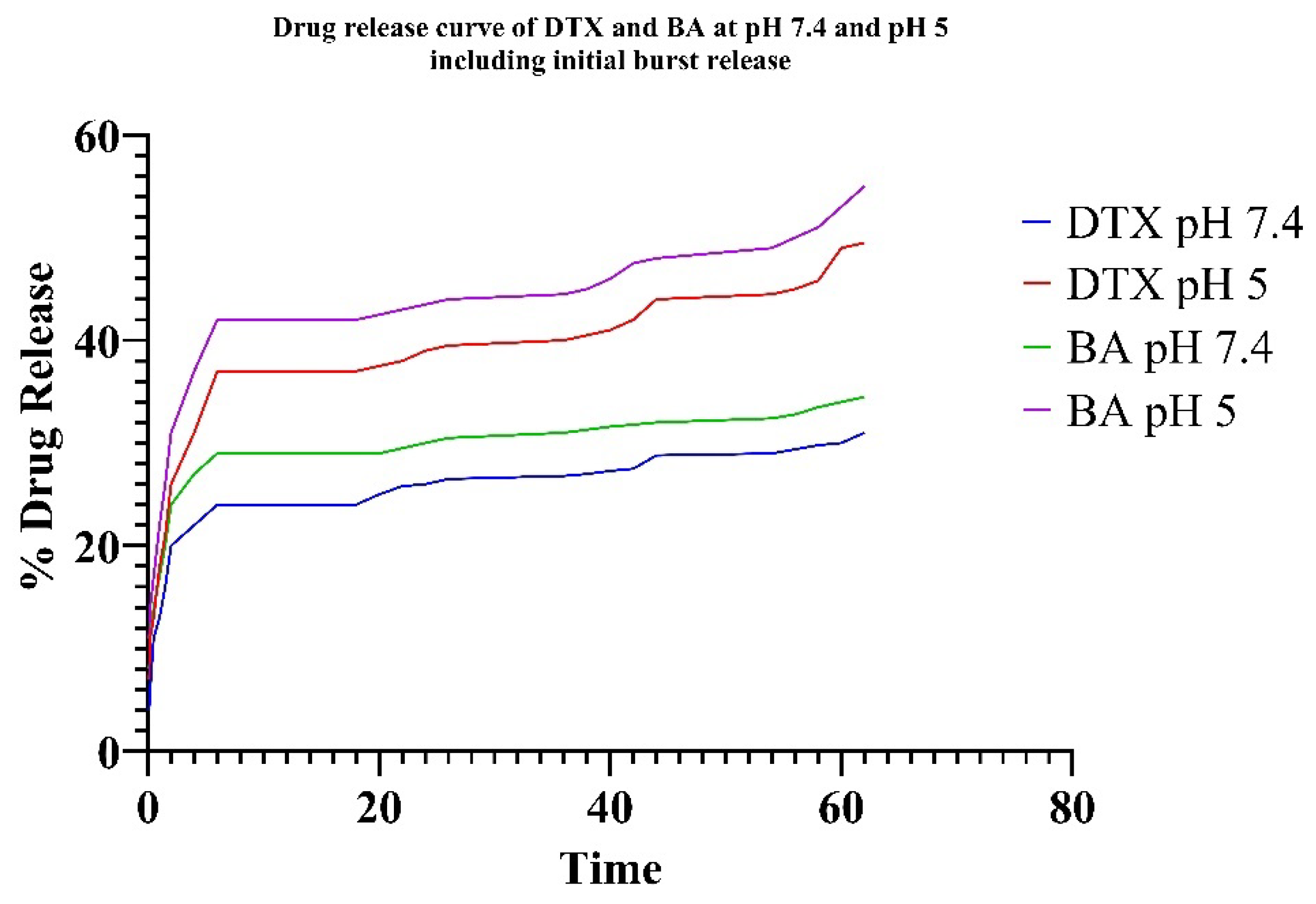
In vitro drug release was observed under two different conditions (pH 7.4 = physiological pH and pH 5 = tumor microenvironment pH). The pH of the tumor microenvironment is normally acidic (pH 5) due to the metabolic activities of cancer cells [
27]. So, both pH values were considered to study the drug release. Drug release data show the sustained release nature of the formulation. Drug release data show the initial burst release of the drug, which leads to a fast initial concentration in the blood followed by reaching a plateau value after 1.5 h to confirm the sustained release. Also, both the drugs showed better release % in pH 5 compared to pH 7.4, confirming the suitability of the formulation in the tumor microenvironment. For PLGA NPs across all examined release media, the highest R
2 values were obtained using the Higuchi model, indicating that the release from the NPs is primarily diffusion-controlled. Although empirical modeling confirms diffusion as the main release mechanism, semi-empirical models help to identify the type of diffusion. The Peppas–Sahlin model allows for the assessment of k1 and k2, which corresponds to the Fickian kinetic constant and the matrix swelling kinetic constant, respectively. If the ratio of |k1|/|k2| exceeds 1, drug release is primarily diffusion-driven; if it is less than 1, the release is predominantly influenced by matrix swelling. The experimental data fit well with both the Korsmeyer–Peppas and Peppas–Sahlin models, with R
2 values exceeding 0.95. Additionally, the “
n” values related to NP release are below 0.45, regardless of the pH of the release medium, indicating that Fickian diffusion predominantly controls the release mechanism. For the same NPs, the |k1|/|k2| ratio was significantly greater than 1, further confirming that the release is governed by Fickian diffusion [
28].
By stabilizing microtubules and preventing their disintegration, DTX inhibits the division of lung cancer cells, resulting in cell cycle arrest and apoptosis. Through mitochondrial pathways, BA triggers apoptosis and strengthens the immune system’s defense against cancerous cells. When combined, they have a synergistic effect, as BA contains immunomodulatory and apoptotic induction qualities, along with DTX’s ability to stabilize microtubules, providing a powerful therapy option for lung cancer [
29]. In vitro cytotoxicity analysis through an MTT assay showed a significant reduction in IC
50 values of DTX-BA-NPs against the A549 cell line compared to the free DTX and BA and single-drug NPs, i.e., DTX-NPs and BA-NPs, as shown in the results. Combinatorial NPs of DTX and BA showed lower IC
50 values compared to individual DTX-NPs and BA-NPs for 24, 48, and 72 h. For synergy calculation, a modified formula based on the Chou–Talalay method was used. The Combination Index (CI) values for DTX-BA-NPs show synergy at all time points (CI < 1). Notably, synergy intensifies over time, with CI values decreasing from 0.80 at 24 h to 0.30 at 72 h. This suggests that the combined treatment’s efficacy improves with prolonged exposure. For free DTX and BA free-drug solutions, passive diffusion may be the uptake route; however, NPs may be absorbed by cellular endocytic pathways and exhibit superior absorption [
30].
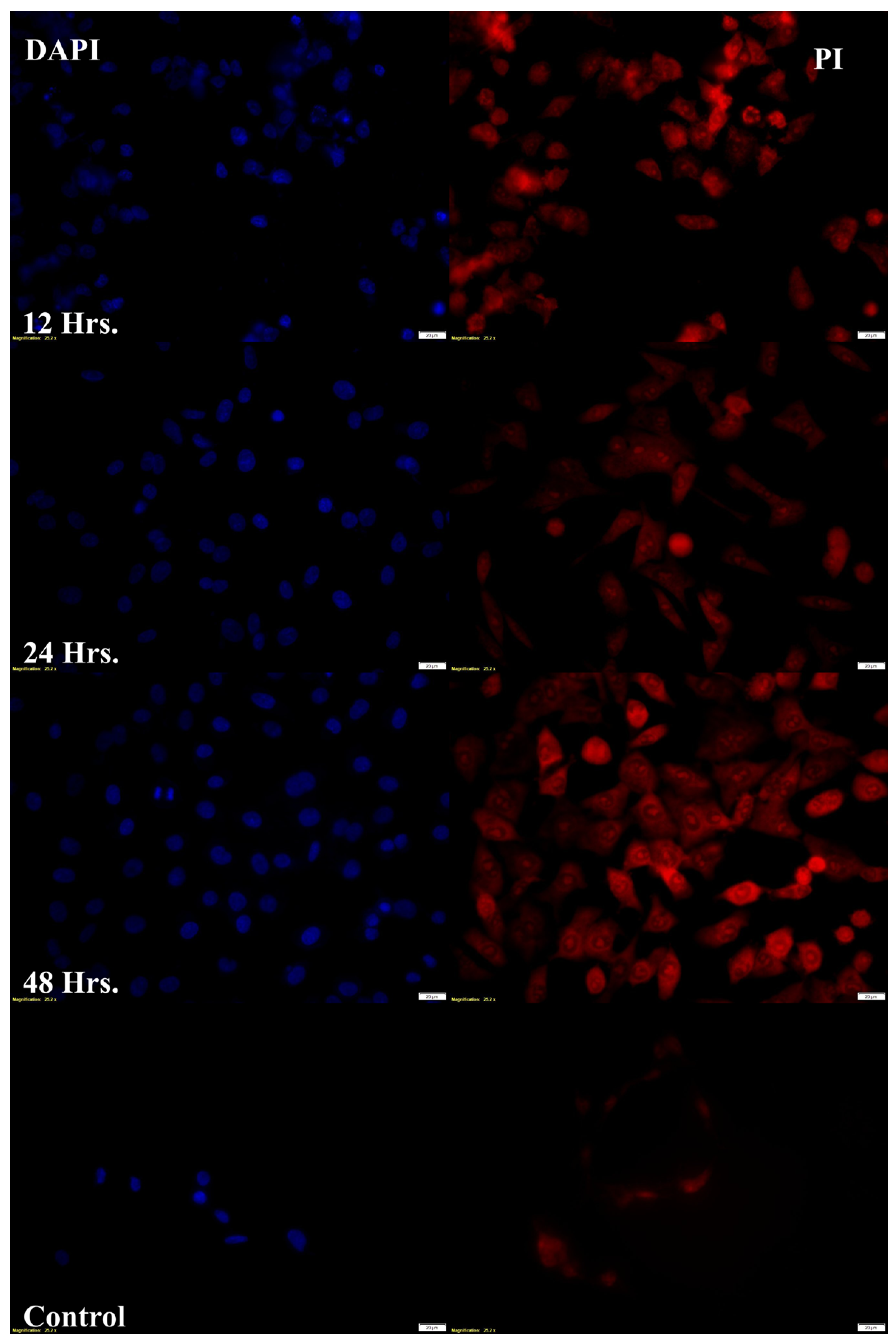
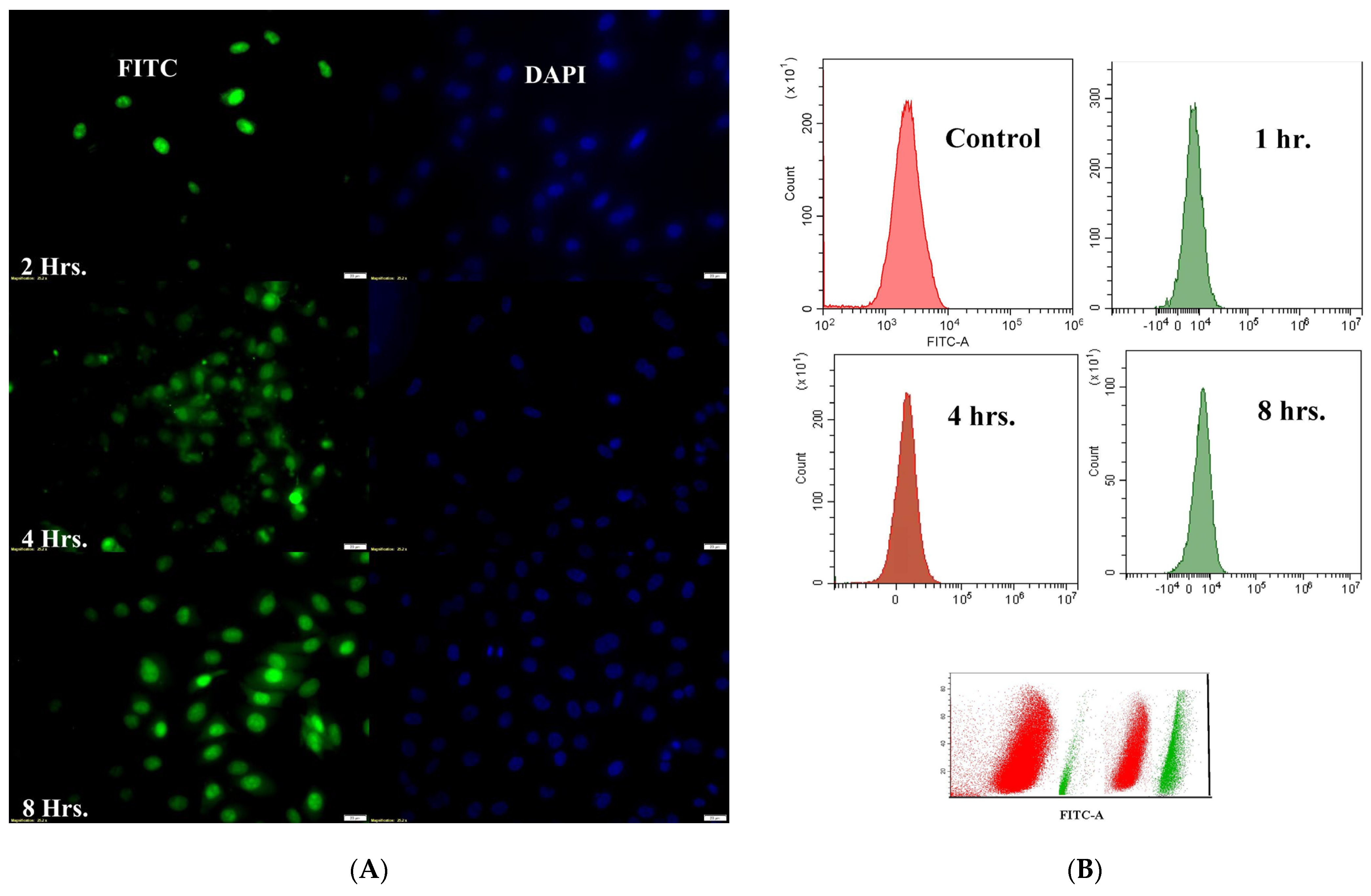
Target-specific cellular internalization is a critical challenge when using nanoformulation to deliver therapeutic molecules. The body’s natural defense mechanism, phagocytosis, removes micron-sized particulate compositions by classifying them as “foreign particles”. However, nanoparticles can stay in the bloodstream for much longer periods since macrophages generally do not identify them. Additionally, they can be pinocytosed inside macrophages, a behavior that depends on the extracellular concentration [
31]. The capacity of DTX-BA-NPs to reach the nucleus was confirmed by fluorescence microscopic analysis, which showed a predominant accumulation of fluorescently labeled nanoparticles (FITC-DTX-BA-NP) in the perinuclear region (
Figure 11A). Fluorescence image analysis confirms that PLGA nanoparticles successfully enter tumor cells. However, since PLGA lacks inherent specificity for tumor cells, further research is needed to enhance targeted delivery. Modifying PLGA nanoparticles with targeting ligands or antibodies, such as folic acid, LHRH, or hyaluronic acid, could improve specificity and enhance delivery to cancer cells. These modifications may help increase the therapeutic efficacy by ensuring that the nanoparticles preferentially target tumor cells, reducing off-target effects and improving overall treatment outcomes. An MTT assay was conducted on normal human bronchial epithelial cells (BEAS-2B) across a concentration range of 50 to 5 µM. The results showed that cell viability remained above 80% at all concentrations, indicating a favorable safety profile in normal cells (
Supplementary Table S7). This suggests that the nanoparticles exhibit greater specificity toward tumor cell lines compared to normal cells, further supporting their potential as a targeted therapeutic option in cancer treatment while minimizing harm to healthy tissues. Fluorescence microscopy also confirmed cell death with red or orange spots (PI stain). Treatment with DTX-BA-NPs for 12, 24, and 48 h confirmed cell death with an orange color (
Figure 10A). The quantity of orange cells increased as treatment duration increased, and some cells turned scarlet, signifying cell death. Certain cells’ cytoplasm showed noticeable orange patches upon high magnification and laser modification, indicating the creation of acidic vacuoles [
32].
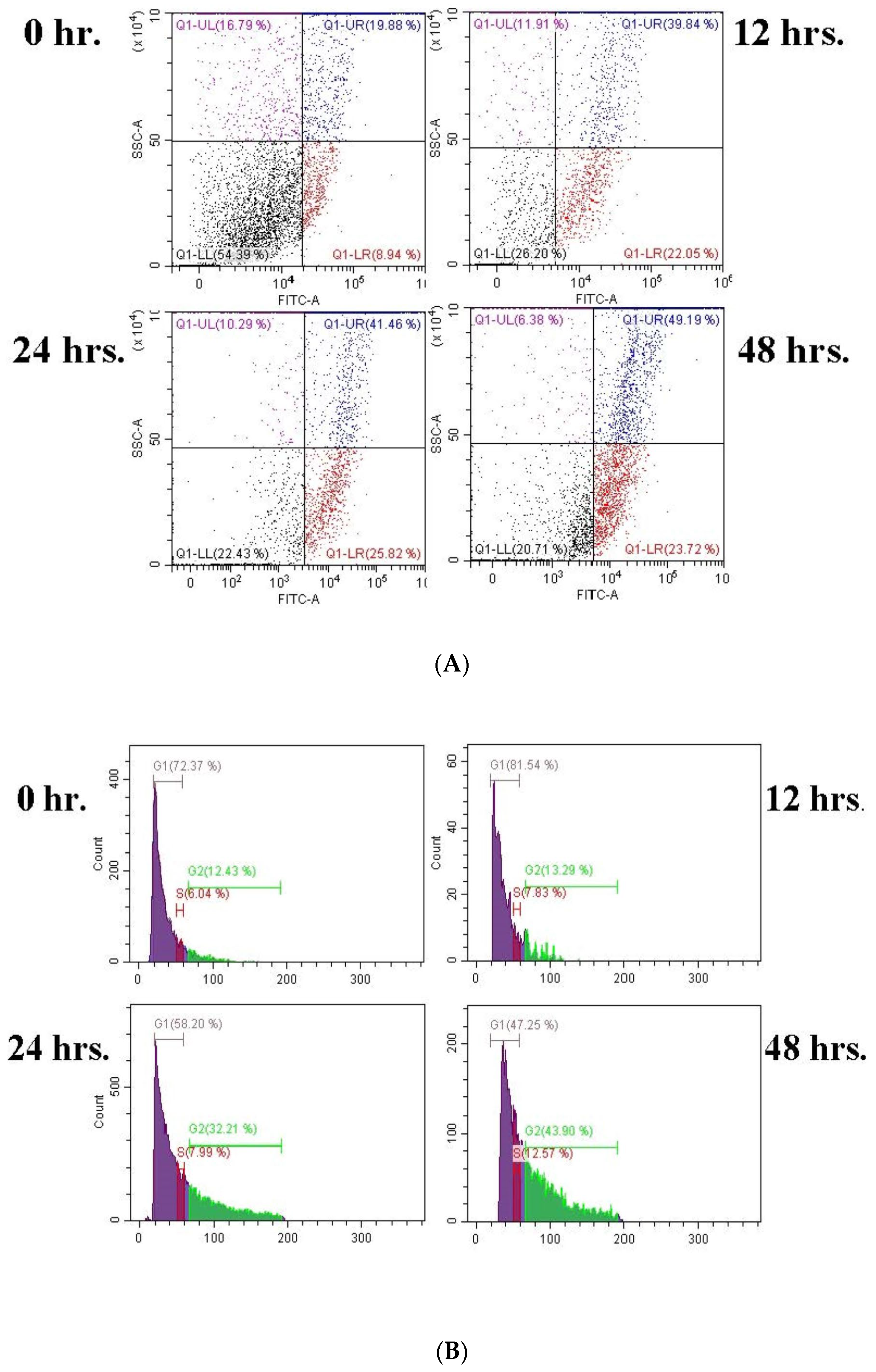
DTX and BA both cause cell cycle arrest in the G2/M phase. With the increase in treatment time, as shown in the results, the number of cells increases in the G2 phase. An extension of the treatment duration increased the G2/M ratio. This result suggested that, following treatment with DTX-BA-NPs, cells are blocked in the interphase (G2), facilitating the arrest of mitotic cell division. The primary means of obstruction in the cell cycle seems to be chromosomal vandalism, which is incapable of being redesigned and carried out to induce programmed cell death [
33].
The potential of DTX and BA in treating lung cancer can be successfully assessed by molecular docking of the ligands with Bcl-2 protein and PI3K gamma and analyzing their binding affinities and interactions. DTX stabilizes microtubules and prevents cancer cell division, which can be ascertained by looking at the tubulin-associated protein Bcl-2. The ability of BA to trigger apoptosis in cancer cells can be demonstrated using the protein PI3K, which is associated with apoptotic pathways. By predicting the drug efficacy, specificity, and synergistic effects, the in silico approach offers important insights into improving targeted lung cancer therapy and co-encapsulating the drugs more effectively [
23]. Both DTX and BA showed high negative binding scores with the two most probable lung cancer proteins (Bcl-2 and PI3K) confirming high binding with both proteins and thus proved effective in lung cancer treatment (
Supplementary Table S6).
Future clinical studies should focus on optimizing nanoparticle formulations, assessing pharmacokinetics, and evaluating long-term safety and efficacy. Additionally, exploring combination therapies with immunotherapies could further enhance treatment responses in lung cancer patients. If successfully translated, DTX-BA-NPs could significantly advance the field of lung cancer therapy, offering a promising alternative to standard chemotherapy. The regulatory challenges surrounding the co-encapsulation of DTX and BA in NP-based formulations for lung cancer therapy are intricate, encompassing both safety and efficacy concerns. Rigorous toxicological and preclinical studies must address nanoparticle biodistribution, toxicity, and therapeutic superiority over conventional treatments. Ensuring batch consistency, sterility, and stability in compliance with Good Manufacturing Practices (GMPs) is crucial. Regulatory approval hinges on comprehensive data submissions covering chemistry, manufacturing, and control (CMC) aspects, with alignment with guidelines for combination therapies and nanomedicines. Early regulatory engagement through pre-IND meetings can streamline the process [
3].
4. Materials and Methods
Poly-D, L-lactic-co-glycolic acid (PLGA) with a copolymer ratio of D, L-lactide to glycolide of 50:50 (molecular weight 50,000–75,000) was purchased from Sigma-Aldrich, (Mumbai, Maharastra, India) and betulinic acid was gifted by the natural product department, CSIR-NEIST, Jorhat. Polyvinyl alcohol (PVA) was purchased from Loba Chemie Pvt., Ltd. (Mumbai, India). HPLC-grade acetone and methanol were purchased from Sisco Research Laboratories Pvt., Ltd. (SRL), (Andheri (East), Mumbai, Maharastra, India). Fluorescein isothiocyanate (FITC) and 4′,6-diamidino-2-phenylindole (DAPI) were procured from Sigma Aldrich (Mumbai, Maharastra, India) and Invitrogen (Bengaluru, Karnataka, India), respectively. All other chemicals and solvents were of analytical grade and purchased from Merck India (Mumbai, Maharastra, India), and the A549 lung cancer cell line was from NCCS, Pune, Maharastra, India.
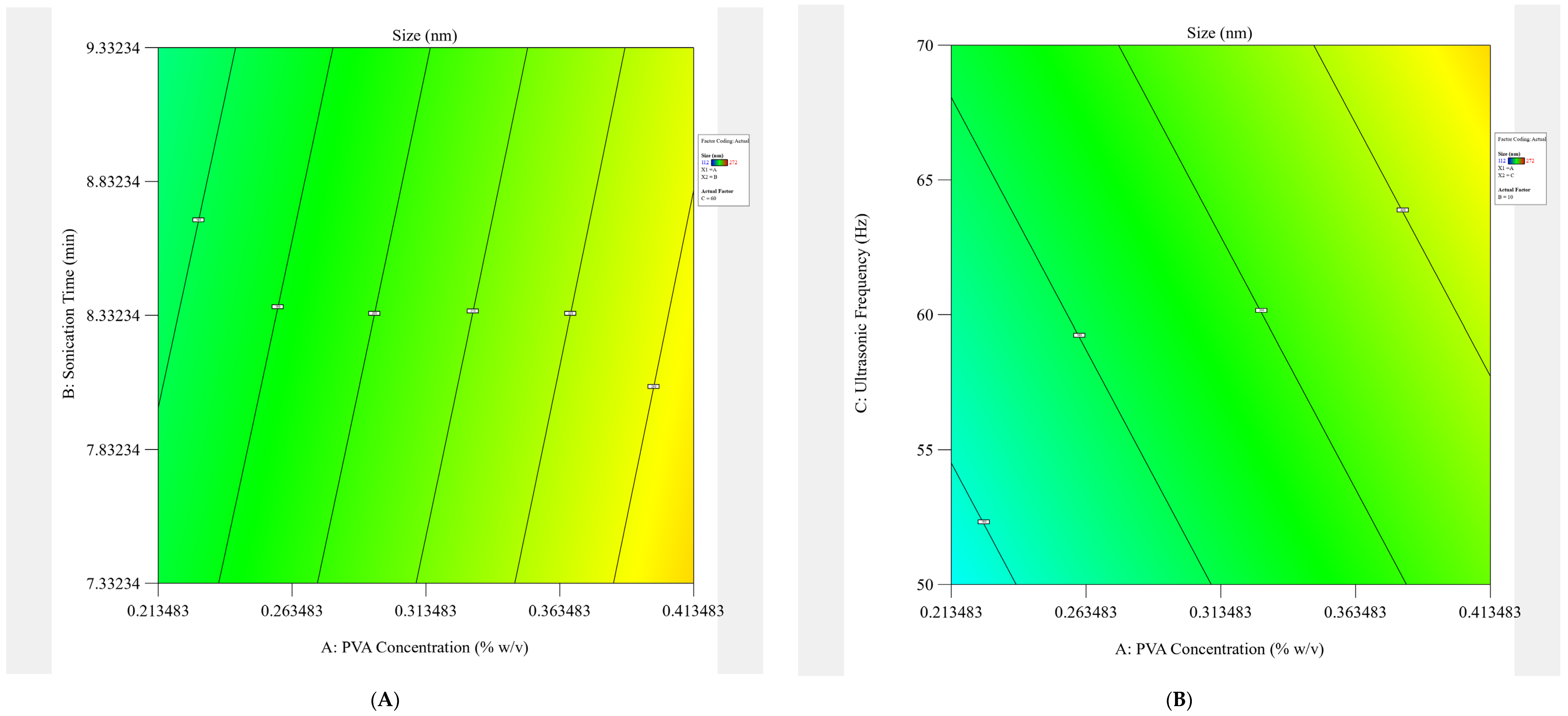
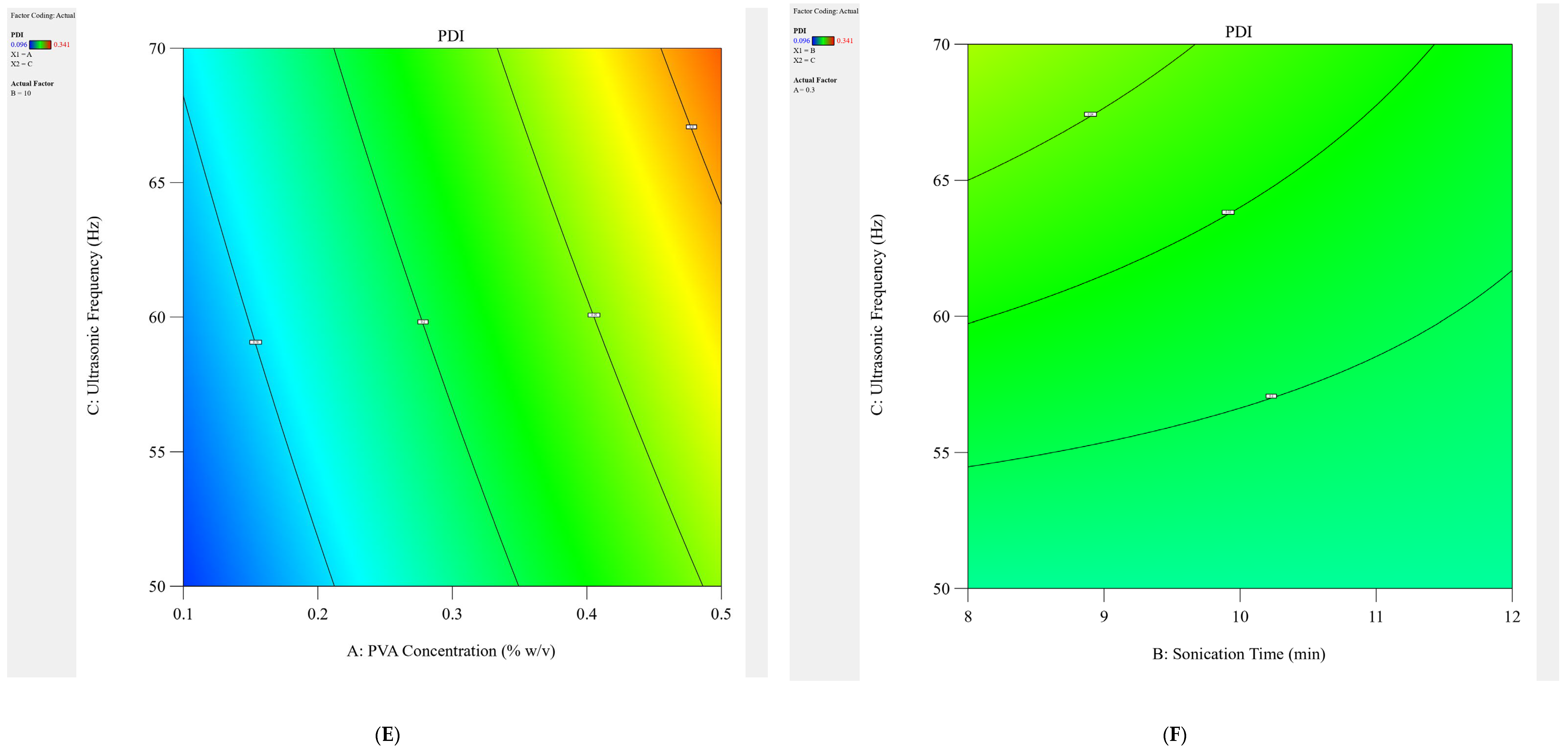
4.1. Experimental Designing
The design of the experiment (DoE) was constructed in this study using Design Expert
® Software (Version 13.0.5.0, Stat-Ease Inc, Minneapolis, MN, USA) by adapting the 2
3 factorial design approaches to optimize the blank NP
S. Before loading drugs into the NPs, the preparation process of NPs was optimized according to selected parameters. The independent parameters for optimization were PVA concentration (A = X
1), sonication time (B = X
2), and ultrasonic frequency (C = X
3). The levels for every formulation variable were also established via preliminary investigations. Furthermore, the architecture is suitable for building second-order polynomial models and studying quadratic response surfaces. The selected responses were the Size (Y1) and PDI (Y2) of the study. Eight experimental runs of the DTX-BA-NPs formulations, as indicated in
Table 5, were created after each independent variable was assigned a high and low-level value.
4.2. Optimization of 23 Factorial Designs Using Design Expert® Software Version 13
The model was run in triplicate for each of the eight possible formulations after the findings for the dependent variables, size (Y1) and PDI (Y2), were entered into the response columns of the experimental design program. When “
p” values were less than 0.05, the statistical analysis of the data was deemed significant for any factor. To investigate the impact of independent variables (PVA concentration, sonication time, and ultrasonic frequency) on dependent variables (Size and PDI), a complete factorial design with 2
3 variables was chosen. Plots of contour (2-D) provide direct visualization of the link between the response and independent variables, where A, B, and C are the coded levels of independent variables, Y1 is the measured response (Size), and Y2 is the measured response (PDI) associated with each factor level combination. The interaction terms are denoted by the terms AB, BC, and AC. The average outcome of gradually increasing each factor’s value from low to high is represented by the primary effects (A, B, and C). The testing settings and factorial design parameters are displayed in
Table 5.
4.3. Preparation of DTX-BA-NPs
DTX-BA-NPs were prepared by a double-emulsion solvent evaporation technique as described previously with minor modifications [
34]. All the dependent variable values were obtained from the process optimization after the design of experiment analysis by 2
3 design on Design Expert software version 13 and used for the formulation. In brief, BA (2 mg) was dissolved in acetone and methanol in a 3:1 ratio (2 mL) with sonication and gentle heating, then cooled, and again the same solution of DTX was added followed by sonication to dissolve. The DTX-BA solution was treated with PLGA (30 mg). The solution was added to an aqueous solution (30 mL) of PVA (0.1%
w/
v) and immediately sonicated (for 11.45 min) under cold conditions using a microtip probe sonicator set at 55 W of energy output (50 Hz) (Fisherbrand™ Model 705 Sonic, Mumbai, Maharastra, India). The oil-in-water emulsion was allowed to stir at room temperature for 6–8 h for the removal of acetone and methanol. The prepared DTX-BA-NPs were retrieved by ultracentrifugation (35,000 rpm, 5 °C) for 20 min (Thermo Scientific Sorvall MTX 150 Micro-Ultracentrifuge, Mumbai, Maharastra, India) and washed thrice with milli-Q water (Millipore Corp., Billerica, MA, USA) to remove excess PVA and unencapsulated free DTX and BA. The pellet obtained was lyophilized for 6 h to obtain a free-flowing powder. Image depiction of the formulation procedure is shown in
Figure 14.
In a similar way, blank nanoparticles were developed without the addition of drugs. FITC was employed as a fluorescent marker, and to produce FITC-labelled blank NPs, 0.1 mL of its stock solution (0.5% w/v in ethanol) was added to the PVA solution during emulsification. The prepared nanoparticles were lyophilized for further use.
4.4. Characterization of NPs
4.4.1. Particle Size Distribution and Zeta Potential Analysis
First, 2–3 mg of lyophilized NPs was taken in a microcentrifuge tube and 2 mL of milli-Q water was used for even redispersion using a bath sonicator for 70–80 min at a controlled temperature between 15 and 0 °C. The average particle size, size distribution, and polydispersity index (PDI) of the prepared nanoparticles were determined by dynamic light scattering technology with proper dilution (if required) with milli-Q water in zetasizer instruments (Zetasizer Nano ZSP—Malvern Panalytical, New Delhi, India). Zeta Potential was measured at 25 °C using the same instrument. All the measurements were performed in triplicate, and the average values were calculated and reported [
20].
4.4.2. High-Resolution Transmission Electron Microscopy (HRTEM)
Lyophilized NPs were redispersed in milli-Q water using a bath sonicator for 80 min at a controlled temperature. After that, 2 µL of the dispersed NP solution was added to a TEM grid of mesh sized 600 mesh × 42 μM with a pitch of copper and air dried for 1 h and then kept in a desiccator for 24 h. After that, the grid was subjected to HRTEM (JEOL, Tokyo, Japan, Model: JEM-2100 Plus Electron Microscope) and operated at about 210–240 V with a frequency range of 50–60 Hz [
35].
4.4.3. Filed Emission Scanning Electron Microscopy (FESEM)
Prepared lyophilized NPs (2–3 mg) were redispersed in milli-Q water with bath sonication at a controlled temperature for 80 min. After that, 50 µL of the dispersed NP solution was added dropwise to a coverslip and left to air dry for 2 h, and after that, they were kept in a desiccator for 24 h. The dried samples were then subjected to FESEM imaging by gold surface coating technology in nitrogen environments (Carl ZEISS Microscopy, Jena Germany, Model: ZEISS, SIGMA) with 20,000× and 40,000× magnifications, operated at 20 kV accelerating voltage [
36].
4.4.4. HPLC Method Development of DTX and BA
The stock solution of a 1 mg/mL concentration of DTX solution was prepared in acetone. BA was dissolved in acetone and methanol in a 1:1 ratio by taking 1 mg/mL as the stock, and different concentration ranges were prepared as 400, 200, 100, 80, 60, 40, 20, and 10 µg/mL with methanol.
For DTX and BA, water–ACN (
v/
v) was used in various ratios to obtain the proper ratio in both isocratic and gradient systems along with retention time. For the stationary phase, the Waters Symmetry
® C18 5 μM Column (4.62 × 250 mm) was used. For DTX 5/45, 10/55, 13/67, 17/82, and 22/95, gradient systems (time/ACN) were used according to the time the ACN concentration was changed. For BA 5/90, 7/85, 9/75, and 12/60, time/ACN was used. After optimization of the gradient system, an isocratic system was developed and run for both drugs. The flow rate was also chosen from 0.5 mL/min to 1 mL/min. For DTX and BA determination, 220, 235, 245, and 275 nm wavelengths and 205, 210, 220, and 235 nm were used, respectively [
37,
38].
4.4.5. Drug Loading and Encapsulation Efficiency of DTX-BA-NPs
The concentrations of DTX and BA in NPs were determined using the Waters Corporation ACQUITY Arc System equipped with a Waters 2487 dual wavelength absorbance detector and a Waters Symmetry
® C18 5 μM Column (4.62 × 250 mm). For DTX and BA, water–ACN (
v/
v) was used in various ratios to obtain the proper ratio in both isocratic and gradient systems along with retention time with a flow rate of 0.5 mL/min. An aliquot of the DTX-BA-NPs suspension was added to a mixture of water–ACN (1:1, 1 mL), vortexed, sonicated, and centrifuged; the clear supernatant was filtered and analyzed by HPLC. Measurement was performed using standard solutions of DTX and BA in methanol (10–400 μg/mL). The % yield, drug loading, and encapsulation efficiency were calculated using Equations (3), (4), and (5), respectively [
20].
4.4.6. Fourier Transform Infrared Spectroscopy (FTIR)
To determine the possible chemical interaction occurring between the drug and the PLGA polymer, the FTIR spectra of DTX, BA, the physical mixture of DTX, BA, and PLGA, DTX-BA-NPs, and PLGA in the range of 400–4000 cm
−1 were studied, employing an FTIR spectrophotometer (Perkin Elmer, Model-Spectrum 100Thane, Maharastra, India). For the IR study, the KBr plate method was used. Drugs and excipients were assorted with IR-grade KBr at a ratio of 1:1000 and then a KBR thin plate was obtained using a hydraulic press at 100 psi pressure for 2 min. KBR plates were then placed in the IR sample holder and spectra were taken [
39].
4.4.7. In Vitro Drug Release
The prepared NPs (10 mg) were redispersed again after lyophilization in 20 mL of phosphate buffer saline of pH 7.4 and 5 containing Tween 80 (50 μL) and subsidized in a magnetic stirrer at 4000 RPM for 7 days at 37 °C. The withdrawn samples were then centrifuged and the supernatant was collected and filtered through a 0.45 µ membrane filter and checked in HPLC for release data using the same method as described in the drug-loading method. A previously generated calibration curve was used to create a cumulative % release of drugs vs. time graph. The release profile of NPs was interpreted using a variety of kinetic models, including first-order, zero-order, Korsmeyer–Peppas, Highuchi, and Hixson–Crowell models, and curve fitting was completed using Origin Pro software version 10 [
39].
4.4.8. Long-Term Stability Study
Lyophilized NPs were stored in a sample tube at
t RT, 4 ± 2 °C, and −20 °C for 3 months after preparation. After the storage period, NPs were re-dispersed in milli-Q water, and bath sonication was performed for 70 min at 15 °C. After that, the dispersed NP solutions were subjected to size and zeta potential tests to determine the change in charge voltage compared to the charge of fresh NPs for the stability study [
40].
4.5. Cell Line and Culture Maintenance
The adherent adenocarcinoma human alveolar basal epithelial A459 lung cancer cell line was obtained from the National Centre for Cell Science (NCCS) in Pune, India. These cells were cultured in a high-glucose dulbecco’s Modified Eagle Medium (DMEM) medium, supplemented with 10% fetal Bovine Serum (FBS), 50 IU/mL penicillin G, and 50 µg/mL streptomycin, and maintained in a humidified incubator with a 5% CO2 atmosphere. Sub-culturing was performed when the cells reached 90% confluence. Cell viability was assessed using the trypan blue exclusion method as needed [
41].
4.6. In Vitro Cytotoxicity Assay Using MTT
The cytotoxic effects of the free drugs (DTX, BA), DTX-NPs, BA-NPs, and DTX-BA-NPs on the A549 lung cancer cell line were evaluated using the MTT assay. A549 cells (2 × 10
4) were seeded into the wells of a 96-well plate and incubated overnight at 37 °C in a 5% CO
2 environment with supplemented DMEM media. Following incubation, the media was removed, and the cells were treated with 200 μL of media containing either the free-drug suspension (concentration range of 1–50 μmol) or the NPs suspension (equivalent free-drug concentration range of 1–50 μmol) across different groups for 24, 48, and 72 h. The cells in the control group did not receive any medication treatment. Twenty microliters of the MTT solution (5 mg/mL in Phosphate Buffer Saline (PBS)) were added to each well after the treatment period was over, and it was incubated for 3 to 4 h at 37 °C. After removing the MTT solution, 100 μL of dimethyl sulphoxide (DMSO) was added to each well and shaken for 15 min to dissolve the formazan crystals that had formed in the cells as a result of the reaction between the MTT and the enzyme mitochondrial reductase. After that, the plate was examined at 570 nm in a microplate reader (Bio-Rad, Hercules, CA, USA). Cell viability % was plotted against DTX-NP, BA-NP, and DTX-BA-NP concentrations and the IC
50 value was determined. The IC
50 value, representing the significance of the results, was determined [
39].
Combination index (CI) calculation for determining the synergism, antagonism, or additivity of drug combinations was performed by comparing the IC50 values of the combination treatment (DTX-BA-NPs) against the IC50 values of individual treatments (free DTX, Free BA, DTX-NPs, and BA-NPs) using Equation (6). Equation (6) is an adaption of the CI formula based on the Chou–Talalay method [
42].
4.7. Cell Death by Dual Cell Staining
Cell death induced by NPs was observed using fluorescence microscopy after treating A549 cells with an IC
50 concentration of NPs. In brief, 5 × 10
4 cells were seeded on each coverslip and placed in 35 mm cell culture dishes. The cells were then treated with NPs at their IC
50 concentration for 12, 24, and 48 h, while the control group received only media. First, after incubation, the coverslip was cleansed with PBS and stained with PI (4 μg/mL) for incubation for 10 min. Then, the coverslip was removed and wiped with PBS, and scrubbed cells were fixed using 4% paraformaldehyde for 15 min at 4 °C. The fixed cells were washed again and stained with DAPI (300 ng/mL) in the dark for 15 min. Finally, stained cells were mounted on a slide with antifade and observed under fluorescence microscopy (Horiba, Model: Fluorlolog-3) [
43].
4.8. Cellular Uptake Study
The intracellular uptake of NPs by A549 cells was studied in vitro using confocal laser microscopy. For confocal laser microscopy, approximately 1 × 10
4 cells were seeded on a coverslip, placed in a 35 mm tissue culture dish with media, and incubated overnight at 37 °C. The cells were then treated with the IC
50 concentration of FITC-labeled NPs in different groups for 1, 4, and 8 h. After treatment, the cells were washed with PBS, fixed with 70% ice-cold ethanol, co-stained with DAPI (for the nucleus), and mounted on a slide with glycerol for observation under a confocal laser microscope (Leica Microsystems, Leica TCS SP8, Wetzlar, Germany). Filters for FITC (Ex/Em 495/519 nm) and DAPI (Ex/Em 359/461 nm) were used to capture binary color images [
44].
4.9. Apoptosis and Cell Cycle Analysis
Apoptosis and cell cycle analyses of NPs were conducted using flow cytometry after treating A549 cells with the IC
50 concentration of NPs and staining with the annexin V-FITC apoptosis detection kit. Briefly, 5 × 10
4 cells were seeded on each coverslip and placed in 35 mm cell culture dishes. The cells were treated with NPs at their IC
50 concentration for 12, 24, and 48 h, while the control group received only media. After the time gap cells were trypsinized and centrifuged to collect the palate at 2000 RPM for 3 min. Cells were resuspended in 500 μL of 1X Binding Buffer. Then, 5 μL of Annexin V-FITC detection kit solutions was appended, escorted by incubation at room temperature for 5 min in the shade. The binding of Annexin V-FITC was analyzed by flow cytometry (Ex/Em = 488/530 nm) using a FITC signal detector (usually FL1) to see cell death or cell cycle arrest phases (Beckman Coulter, USA, Model: MoFlo XDP&CytoFLEX S) [
45].
4.10. In-Silico Docking Studies
The SMILES ID of the two drugs DTX and BA were obtained from the PubChem database (PubChem CID 148124 and 64971, respectively). The X-ray crystal structure of the Bcl-2 protein (PDB ID: 4MAN) with a resolution of 2.07Å and PI3K gamma (PDB ID: 3L08) with a resolution of 2.7 Å were selected as the target proteins and downloaded from the RCSB-PDB website in .pdb format. The target proteins were prepared and docking was performed with the BIOVIA Discovery Studio Client 2022 software. The active binding site was defined for both the protein in chain A, the active site coordinates of Bcl-2 (x = −11.76, y = 10.20, z = 8.70) and PI3K (x = 24.92, y = 15.61, z = 21.36) were identified, and molecular docking of the ligands with the proteins was performed to determine the binding efficacy to the target proteins [
22].
4.11. Statistical Analysis
The results are presented as mean ± SD. The statistical analysis was performed using Student’s t-test in Origin Pro version 10 software. The differences were considered significant at a p value of <0.05 unless stated differently.