Zilucoplan: A Newly Approved Macrocyclic Peptide for Treatment of Anti-Acetylcholine Receptor Positive Myasthenia Gravis
Abstract
1. Introduction
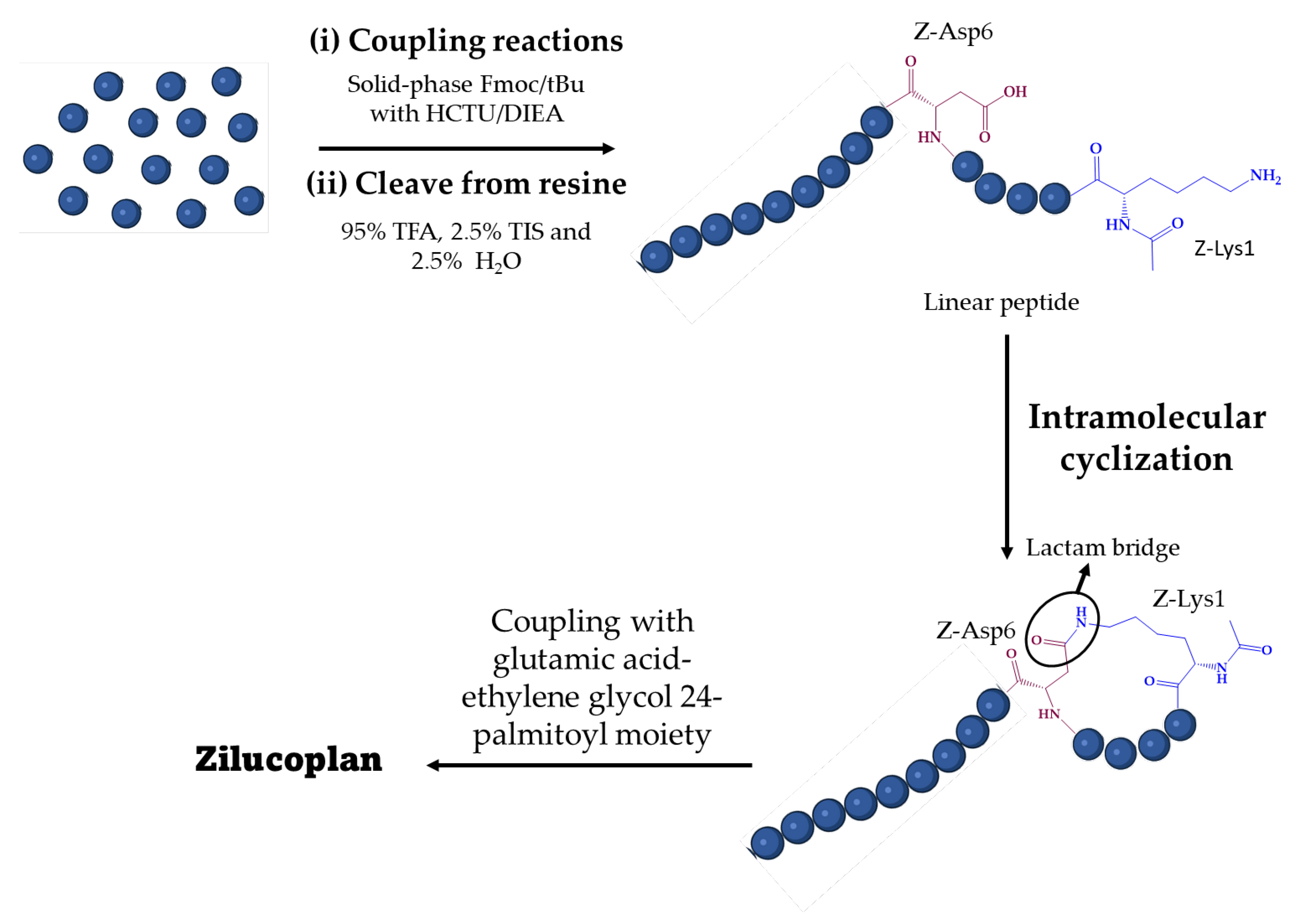
2. Synthesis of Zilucoplan
2.1. Generalities
2.2. Reported Procedures
3. Zilucoplan and Myasthenia Gravis
3.1. The Disease
3.2. Therapeutic Strategies
3.3. Zilucoplan
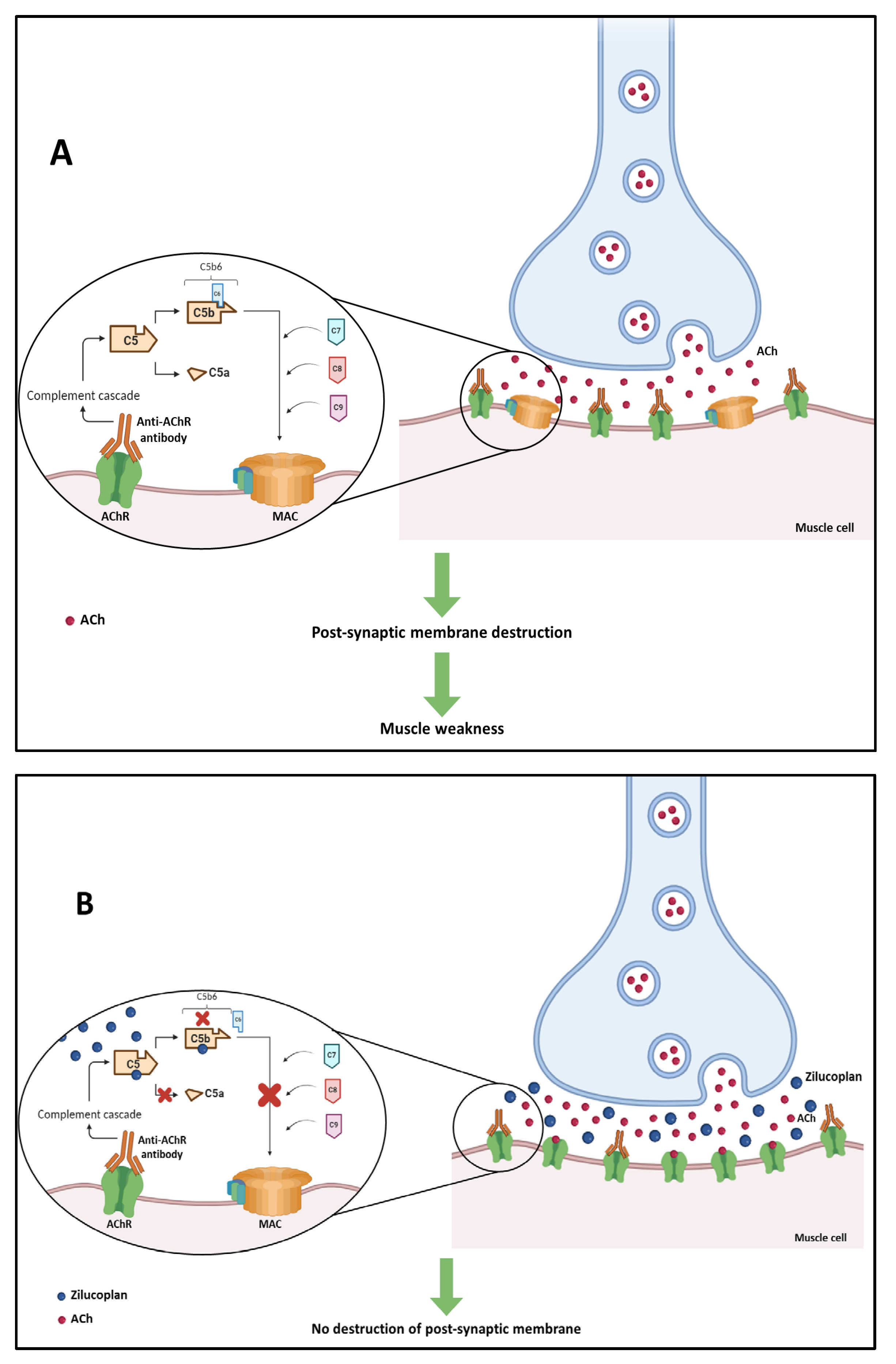
3.3.1. Mechanism of Action
3.3.2. Indication
3.3.3. Clinical Trials
3.3.4. Ongoing Clinical Trials
4. Zilucoplan and Other Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Santana, C.; Pinheiro, A.; Anjos, L.; Barth, T.; Júnior, O.; Fontes, W.; Castro, M. Marine Depsipeptides as Promising Pharmacotherapeutic Agents. Curr. Protein Pept. Sci. 2017, 18, 72–91. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Du, Z.; Fan, B.; Dai, Q.; Wang, L.; Guo, J.; Ye, Z.; Cui, N.; Chen, J.; Tan, K.; Li, R.; et al. Supramolecular peptide nanostructures: Self-assembly and biomedical applications. Giant 2022, 9, 100082. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H. Recent Progress on Cyclic Peptides’ Assembly and Biomedical Applications. ChemBioChem 2023, 24, e202300018. [Google Scholar] [CrossRef]
- Kotadiya, D.D.; Patel, P.; Patel, H.D. Cell-Penetrating Peptides: A Powerful Tool for Targeted Drug Delivery. Curr. Drug Deliv. 2024, 21, 368–388. [Google Scholar] [CrossRef]
- Choi, J.S.; Joo, S.H. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2020, 28, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules 2021, 26, 3380. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed]
- PepTherDia. Available online: http://peptherdia.herokuapp.com/list (accessed on 28 November 2023).
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the Treatment of Infected Wounds. Nature 1944, 154, 703–703. [Google Scholar] [CrossRef]
- Gall, Y.M.; Konashev, M.B. The discovery of Gramicidin S: The Intellectual Transformation of G.F. Gause from Biologist to Researcher of Antibiotics and on its Meaning for the Fate of Russian Genetics. Hist. Philos. Life Sci. 2001, 23, 137–150. [Google Scholar] [PubMed]
- Klinker, K.P.; Borgert, S.J. Beyond Vancomycin: The Tail of the Lipoglycopeptides. Clin. Ther. 2015, 37, 2619–2636. [Google Scholar] [CrossRef]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611. [Google Scholar] [CrossRef]
- Syed, Y.Y. Rezafungin: First Approval. Drugs 2023, 83, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Zain, J. Romidepsin in the treatment of cutaneous T-cell lymphoma. J. Blood Med. 2011, 2, 37–47. [Google Scholar]
- Layer, P.; Stanghellini, V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2014, 39, 371–384. [Google Scholar] [CrossRef]
- FDA. Novel Drug Approvals for 2023. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023 (accessed on 26 November 2023).
- Shirley, M. Zilucoplan: First Approval. Drugs 2024, 84, 99–104. [Google Scholar] [CrossRef]
- Howard, J.F.; Vissing, J.; Gilhus, N.E.; Leite, M.I.; Utsugisawa, K.; Duda, P.W.; Farzaneh-Far, R.; Murai, H.; Wiendl, H. Zilucoplan: An Investigational Complement C5 Inhibitor for the Treatment of Acetylcholine Receptor Autoantibody–Positive Generalized Myasthenia Gravis. Expert Opin. Investig. Drugs 2021, 30, 483–493. [Google Scholar] [CrossRef]
- Gorman, D.M.; Lee, J.; Payne, C.D.; Woodruff, T.M.; Clark, R.J. Chemical synthesis and characterisation of the complement C5 inhibitory peptide zilucoplan. Amino Acids 2021, 53, 143–147. [Google Scholar] [CrossRef]
- Tang, G.Q.; Tang, Y.; Dhamnaskar, K.; Ma, Z.; Zhu, N.; Cong, B.; Sayegh, C.; Ricardo, A. Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front. Immunol. 2023, 14, 1213920. [Google Scholar] [CrossRef] [PubMed]
- Guillen Schlippe, Y.V.; Hartman, M.C.T.; Josephson, K.; Szostak, J.W. In Vitro Selection of Highly Modified Cyclic Peptides That Act as Tight Binding Inhibitors. J. Am. Chem. Soc. 2012, 134, 10469–10477. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hartman, M.C.T. In Vitro Selection of Unnatural Cyclic Peptide Libraries via mRNA Display. In Ribosome Display and Related Technologies: Methods and Protocols; Douthwaite, J.A., Jackson, R.H., Eds.; Springer: New York, NY, USA, 2012; pp. 367–390. [Google Scholar]
- Ma, Z.; Hartman, M.C. In vitro selection of unnatural cyclic peptide libraries via mRNA display. Methods Mol. Biol. 2012, 805, 367–390. [Google Scholar]
- Ricardo, A.; Arata, M.; DeMarco, S.; Dhamnaskar, K.; Hammer, R.; Fridkis-Hareli, M.; Rajagopal, V.; Seyb, K.; Tang, G.-Q.; Tobe, S.; et al. Preclinical Evaluation of RA101495, a Potent Cyclic Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Blood 2015, 126, 939–939. [Google Scholar] [CrossRef]
- EMA. EU/3/22/2650: Orphan Designation for the Treatment of Myasthenia Gravis. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-22-2650 (accessed on 28 November 2023).
- Giannuzzi, V.; Conte, R.; Landi, A.; Ottomano, S.A.; Bonifazi, D.; Baiardi, P.; Bonifazi, F.; Ceci, A. Orphan medicinal products in Europe and United States to cover needs of patients with rare diseases: An increased common effort is to be foreseen. Orphanet J. Rare Dis. 2017, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Kanwal, J.; Musaddiq, S.; Khakwani, S. Bioactive Peptides and Their Natural Sources. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 75–97. [Google Scholar]
- Guo, R.; Guo, G.; Wang, A.; Xu, G.; Lai, R.; Jin, H. Spider-Venom Peptides: Structure, Bioactivity, Strategy, and Research Applications. Molecules 2024, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Taktaz, F.; Sousa, E.; Fernandes, C.; Kijjoa, A. Peptides from Marine-Derived Fungi: Chemistry and Biological Activities. Mar. Drugs 2023, 21, 510. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Ding, K.; Roller, P. Recent Progress of Synthetic Studies to Peptide and Peptidomimetic Cyclization. Curr. Org. Chem. 2008, 12, 1502–1542. [Google Scholar] [CrossRef]
- Hamada, Y.; Shioiri, T. Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides. Chem. Rev. 2005, 105, 4441–4482. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Morcinek-Orłowska, J.; Pierzynowska, K.; Gaffke, L.; Węgrzyn, G. Phage display and other peptide display technologies. FEMS Microbiol. Rev. 2021, 46, fuab052. [Google Scholar] [CrossRef]
- Deyle, K.; Kong, X.-D.; Heinis, C. Phage Selection of Cyclic Peptides for Application in Research and Drug Development. Acc. Chem. Res. 2017, 50, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wiedmann, M.M.; Suga, H. RNA Display Methods for the Discovery of Bioactive Macrocycles. Chem. Rev. 2019, 119, 10360–10391. [Google Scholar] [CrossRef]
- Duffy, F.J.; Devocelle, M.; Shields, D.C. Computational Approaches to Developing Short Cyclic Peptide Modulators of Protein–Protein Interactions. In Computational Peptidology; Zhou, P., Huang, J., Eds.; Humana Press: New York, NY, USA, 2015; pp. 241–271. [Google Scholar]
- Frecer, V.; Ho, B.; Ding, J.L. De Novo Design of Potent Antimicrobial Peptides. Antimicrob. Agents Chemother. 2004, 48, 3349–3357. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Watson, P.R.; Craven, T.W.; Li, X.; Rettie, S.; Pardo-Avila, F.; Bera, A.K.; Mulligan, V.K.; Lu, P.; Ford, A.S.; et al. Anchor extension: A structure-guided approach to design cyclic peptides targeting enzyme active sites. Nat. Commun. 2021, 12, 3384. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Delafield, D.G.; Li, L. Improved structural elucidation of peptide isomers and their receptors using advanced ion mobility-mass spectrometry. Trends Anal. Chem. 2020, 124, 115546. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Pilon, A.C.; Pinto, M.E.F.; Dias, N.B.; Freire, R.T.; Castro-Gamboa, I.; Bolzani, V.d.S. Computational methods for NMR and MS for structure elucidation I: Software for basic NMR. Phys. Sci. Rev. 2019, 4, 20180108. [Google Scholar] [CrossRef]
- Fernandes, C.; Ribeiro, R.; Pinto, M.; Kijjoa, A. Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022). Molecules 2023, 28, 615. [Google Scholar] [CrossRef] [PubMed]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Peptide-drug conjugates: A new paradigm for targeted cancer therapy. Eur. J. Med. Chem. 2024, 265, 116119. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Srinivasan, N.R.; Govindarajan, G. Antibiotic-Peptide Conjugation Against Multi-drug Resistant Pathogens: A Comprehensive Review for Therapeutics and Drug Delivery Strategies. Int. J. Pept. Res. Ther. 2023, 29, 91. [Google Scholar] [CrossRef]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- De Waard, M.; Sabatier, J.-M. CHAPTER 59-Structure-Function Strategies to Improve the Pharmacological Value of Animal Toxins. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Burlington, MA, USA, 2006; pp. 415–419. [Google Scholar]
- Moulahoum, H.; Ghorbanizamani, F.; Tok, K.; Zihnioglu, F. On the Cyclization of Non-cyclic Peptides for Biological Applications: Inspiration from Naturally Cyclic Peptides. ChemistrySelect 2023, 8, e202301335. [Google Scholar] [CrossRef]
- Costa, L.; Sousa, E.; Fernandes, C. Cyclic Peptides in Pipeline: What Future for These Great Molecules? Pharmaceuticals 2023, 16, 996. [Google Scholar] [CrossRef] [PubMed]
- Northfield, S.E.; Wang, C.K.; Schroeder, C.I.; Durek, T.; Kan, M.-W.; Swedberg, J.E.; Craik, D.J. Disulfide-rich macrocyclic peptides as templates in drug design. Eur. J. Med. Chem. 2014, 77, 248–257. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef]
- Chow, H.Y.; Zhang, Y.; Matheson, E.; Li, X. Ligation Technologies for the Synthesis of Cyclic Peptides. Chem. Rev. 2019, 119, 9971–10001. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Liu, S.-Z.; Cheng, X.-Z.; Ding, W.-Z.; Zhu, T.; Chen, B. Recent progress of on-resin cyclization for the synthesis of clycopeptidomimetics. Chin. Chem. Lett. 2016, 27, 1731–1739. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B.H. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lam, H.Y.; Zhang, Y.; Chan, C.K. Salicylaldehyde Ester-Induced Chemoselective Peptide Ligations: Enabling Generation of Natural Peptidic Linkages at the Serine/Threonine Sites. Org. Lett. 2010, 12, 1724–1727. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W.; Fox, R.M.; Baucom, K.D. Chemoselective Amide Ligations by Decarboxylative Condensations of N-Alkylhydroxylamines and α-Ketoacids. Angew. Chem. Int. Ed. 2006, 45, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.L.; Kiessling, L.L.; Raines, R.T. Staudinger Ligation: A Peptide from a Thioester and Azide. Org. Lett. 2000, 2, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “Traceless” Staudinger Ligation for the Chemoselective Synthesis of Amide Bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef]
- Wills, R.; Adebomi, V.; Raj, M. Site-Selective Peptide Macrocyclization. ChemBioChem 2021, 22, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Adebomi, V.; Cohen, R.D.; Wills, R.; Chavers, H.A.H.; Martin, G.E.; Raj, M. CyClick Chemistry for the Synthesis of Cyclic Peptides. Angew. Chem. Int. Ed. 2019, 58, 19073–19080. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.N.; Mitchell, J.P.; Roberts, K.D. The synthesis of cyclic peptides. J. Chem. Soc. Perkin Trans. 1 2001, 471–484. [Google Scholar] [CrossRef]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. Int. Ed. 2024, 63, e202308251. [Google Scholar] [CrossRef]
- Ribeiro, R.; Costa, L.; Pinto, E.; Sousa, E.; Fernandes, C. Therapeutic Potential of Marine-Derived Cyclic Peptides as Antiparasitic Agents. Mar. Drugs 2023, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine Cyclic Peptides: Antimicrobial Activity and Synthetic Strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Helmy, N.M.; Parang, K. Cyclic Peptides with Antifungal Properties Derived from Bacteria, Fungi, Plants, and Synthetic Sources. Pharmaceuticals 2023, 16, 892. [Google Scholar] [CrossRef]
- Ramadhani, D.; Maharani, R.; Gazzali, A.M.; Muchtaridi, M. Cyclic Peptides for the Treatment of Cancers: A Review. Molecules 2022, 27, 4428. [Google Scholar] [CrossRef] [PubMed]
- Boroojerdi, B.; Duda, P.; Brock, M.K. Treatment of Myasthenia Gravis with Zilucoplan. Patent WO2023215587A1, 9 November 2023. [Google Scholar]
- Kohli, R.M.; Walsh, C.T.; Burkart, M.D. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 2002, 418, 658–661. [Google Scholar] [CrossRef]
- Duda, P.; Farzaneh-far, R.; Ma, Z.; Zhu, N.; Thackaberry, E.; Ricardo, A. Neurological Disease Treatment with Zilucoplan. WO2020086506A1, 30 April 2020. [Google Scholar]
- Arata, M.D.; Dhamnaskar, K.A.; Elbaum, D.; Josephson, K.; Larson, K.C.; Ma, Z.; Nims, N.E.; Ricardo, A.; Seyb, K.; Tang, Q.; et al. Modulation of Complement Activity. WO2015191951A2, 17 December 2015. [Google Scholar]
- Ma, Z.; Zhu, N.; Thackaberry, E.; Farzaneh-far, R.; Ricardo, A. Modulators of Complement Activity. Patent WO2020185541A2, 14 February 2020. [Google Scholar]
- Li, W.; O’Brien-Simpson, N.; Hossain, M.; Wade, J. The 9-Fluorenylmethoxycarbonyl (Fmoc) Group in Chemical Peptide Synthesis–Its Past, Present, and Future. Aust. J. Chem. 2019, 73, 271–276. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nature Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Boccanegra, B.; Carollo, M.; Bottani, E.; Mantuano, P.; Trifirò, G.; De Luca, A. Myasthenia Gravis Treatment: From Old Drugs to Innovative Therapies with a Glimpse into the Future. CNS Drugs 2024, 38, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 526494. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, A.; Sneddon, S.; de Vries, C.S. The Incidence of Myasthenia Gravis: A Systematic Literature Review. Neuroepidemiology 2010, 34, 171–183. [Google Scholar] [CrossRef]
- Sanderson, N.S.R. Complement and myasthenia gravis. Mol. Immunol. 2022, 151, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Varma, A. Myasthenia Gravis: A Systematic Review. Cureus 2023, 15, e50017. [Google Scholar] [CrossRef]
- Grob, D.; Brunner, N.; Namba, T.; Pagala, M. Lifetime course of myasthenia gravis. Muscle Nerve 2008, 37, 141–149. [Google Scholar] [CrossRef]
- Oosterhuis, H.J. The natural course of myasthenia gravis: A long term follow up study. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Hehir, M.K.; Silvestri, N.J. Generalized Myasthenia Gravis: Classification, Clinical Presentation, Natural History, and Epidemiology. Neurol. Clin. 2018, 36, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis—autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002, 2, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hoch, W.; McConville, J.; Helms, S.; Newsom-Davis, J.; Melms, A.; Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 2001, 7, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, O.; Hamuro, J.; Motomura, M.; Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor–related protein 4 in myasthenia gravis. Ann. Neurol. 2011, 69, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zisimopoulou, P.; Evangelakou, P.; Tzartos, J.; Lazaridis, K.; Zouvelou, V.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 2014, 52, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.H.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpen, K.; Verrips, A.; Losen, M.; et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, J.; Gao, Z.; Liu, B.; Zhang, C.; Yu, W.; Yang, J.; Shen, Y.; Qi, L.; Yao, X.; et al. Myasthenia gravis: Molecular mechanisms and promising therapeutic strategies. Biochem. Pharmacol. 2023, 218, 115872. [Google Scholar] [CrossRef]
- Hara, H.; Hayashi, K.; Ohta, K.; Itoh, N.; Nishitani, H.; Ohta, M. Detection and characterization of blocking-type anti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin. Chem. 1993, 39, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.B.; Angus, C.W.; Adams, R.N.; Michelson, J.D.; Hoffman, G.J. Myasthenic Antibodies Cross-Link Acetylcholine Receptors to Accelerate Degradation. N. Engl. J. Med. 1978, 298, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Loutrari, H.; Kokla, A.; Tzartos, S.J. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur. J. Immunol. 1992, 22, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Rødgaard, A.; Nielsen, F.C.; Djurup, R.; Somnier, F.; Gammeltoft, S. Acetylcholine receptor antibody in myasthenia gravis: Predominance of IgG subclasses 1 and 3. Clin. Exp. Immunol. 1987, 67, 82–88. [Google Scholar] [PubMed]
- Deymeer, F. History of Myasthenia Gravis Revisited. Noro Psikiyatr. Arsivi 2021, 58, 154–162. [Google Scholar] [CrossRef]
- Alhaidar, M.K.; Abumurad, S.; Soliven, B.; Rezania, K. Current Treatment of Myasthenia Gravis. J. Clin. Med. 2022, 11, 1597. [Google Scholar] [CrossRef]
- Estephan, E.P.; Baima, J.P.S.; Zambon, A.A. Myasthenia gravis in clinical practice. Arq. Neuropsiquiatr. 2022, 80, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.K. Plasma Exchange for Treatment of Myasthenia Gravis: Pathophysiologic Basis and Clinical Experience. Ther. Apher. 2000, 4, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Guptill, J.T.; Juel, V.C.; Massey, J.M.; Anderson, A.C.; Chopra, M.; Yi, J.S.; Esfandiari, E.; Buchanan, T.; Smith, B.; Atherfold, P.; et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity 2016, 49, 472–479. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Wang, S.-H.; Chien, P.-J.; Shih, C.-M.; Chiu, H.-C. Changes in serum cytokine levels during plasmapheresis in patients with myasthenia gravis. Eur. J. Neurol. 2009, 16, 1318–1322. [Google Scholar] [CrossRef]
- Huda, R. New Approaches to Targeting B Cells for Myasthenia Gravis Therapy. Front. Immunol. 2020, 11, 240. [Google Scholar] [CrossRef]
- Huda, R.; Tüzün, E.; Christadoss, P. Targeting complement system to treat myasthenia gravis. Rev. Neurosci. 2014, 25, 575–583. [Google Scholar] [CrossRef]
- Pandey, S.; Maharana, J.; Li, X.X.; Woodruff, T.M.; Shukla, A.K. Emerging Insights into the Structure and Function of Complement C5a Receptors. Trends Biochem. Sci. 2020, 45, 693–705. [Google Scholar] [CrossRef] [PubMed]
- EMA. Zilbrysq. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/zilbrysq (accessed on 28 November 2023).
- UCB Announces Approval of RYSTIGGO[®] (Rozanolixizumab) and ZILBRYSQ[®] (Zilucoplan) for the Treatment of Adult Patients with Generalized Myasthenia Gravis in Japan. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-approval-of-RYSTIGGOR-rozanolixizumab-and-ZILBRYSQR-zilucoplan-for-the-treatment-of-adult-patients-with-generalized-myasthenia-gravis-in-Japan (accessed on 12 December 2023).
- UCB. UCB Announces U.S. FDA Approval of ZILBRYSQ[®] (zilucoplan) for the Treatment of Adults with Generalized Myasthenia Gravis. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-US-FDA-approval-of-ZILBRYSQR-zilucoplan-for-the-treatment-of-adults-with-generalized-myasthenia-gravis (accessed on 26 November 2023).
- US FDA. Zilbrysq NDA Approval. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216834Orig1s000ltr.pdf (accessed on 26 November 2023).
- UCB Announces European Commission Approval of ZILBRYSQ® (zilucoplan) for the Treatment of Adults with Generalized Myasthenia Gravis. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-European-Commission-approval-of-ZILBRYSQRV-zilucoplan-for-the-treatment-of-adults-with-generalized-Myasthenia-Gravis (accessed on 26 November 2023).
- Howard, J.F.; Nowak, R.J.; Wolfe, G.I.; Freimer, M.L.; Vu, T.H.; Hinton, J.L.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Farzaneh-Far, R.; et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients With Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020, 77, 582–592. [Google Scholar] [CrossRef]
- Johnston, J.; Ricardo, A.; Arata, M.; Lickliter, J.; DeMarco, S.; Fahrner, R.; Hammer, R.; Newstat, B.; Roychowdhury, D.; Tobe, S.; et al. A phase 1 single-ascending dose clinical study of RA101495, a subcutaneously administered synthetic macrocyclic peptide inhibitor of complement C5 for treatment of paroxysmal nocturnal hemoglobinuria. In Proceedings of the 21st Congress of the European Hematology Association, Copenhagen, Denmark, 9–12 June 2016; pp. 247–248. [Google Scholar]
- Johnston, J.; Ricardo, A.; Arata, M.; Lickliter, J.; DeMarco, S.J.; Fahrner, R.; Hammer, R.P.; Newstat, B.; Roychowdhury, D.; Tobe, S.; et al. A phase 1 multiple-dose clinical study of RA101495, a subcutaneously administered synthetic macrolytic peptide inhibitor of complement C5 for treatment of paroxysmal noctural hemogloginuria. In Proceedings of the 21st Congress of the European Hematology Association, Copenhagen, Denmark, 9–12 June 2016; pp. 415–416. [Google Scholar]
- Howard, J.F., Jr.; Nowak, R.J.; Wolfe, G.I.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Kaminski, H.J. Zilucoplan, a Subcutaneously Self-Administered Peptide Inhibitor of Complement Component 5 (C5), for the Treatment of Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial and Open-Label Long-Term Extension. Neurology 2019, 93, e530. [Google Scholar]
- Howard, J.F., Jr.; Bresch, S.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; Maniaol, A.; Mantegazza, R.; et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023, 22, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Utsugisawa, K.; Deguchi, K.; Konno, S.; Masuda, M.; Minami, N.; Murai, H.; Suzuki, S.; Suzuki, Y.; Tsujino, A.; Uzawa, A.; et al. Efficacy and safety of zilucoplan in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the phase III randomized RAISE study. Clin. Exp. Neuroimmunol. 2023, 15, 45–54. [Google Scholar] [CrossRef]
- Farmakidis, C.; Leite, M.; Bresch, S.; Freimer, M.; Genge, A.; Hewamadduma, C.; Hussain, Y.; Maniaol, A.; Mantegazza, R.; Śmiłowski, M.; et al. P273 Long-term safety, efficacy & self-injection satisfaction with zilucoplan in myasthenia gravis: RAISE-XT interim analysis. Neuromuscul. Disord. 2023, 33, S178. [Google Scholar]
- Open-Label Extension of Zilucoplan in Subjects With Generalized Myasthenia Gravis (RAISE-XT). Available online: https://clinicaltrials.gov/study/NCT04225871?cond=NCT04225871&limit=50&rank=1 (accessed on 2 December 2023).
- An Open-Label Study to Evaluate the Safety, Tolerability, and Efficacy of Subcutaneous Zilucoplan in Participants With Generalized Myasthenia Gravis Who Were Previously Receiving Intravenous Complement Component 5 Inhibitors. Available online: https://clinicaltrials.gov/study/NCT05514873?cond=Zilucoplan&limit=50&page=1&rank=2 (accessed on 2 December 2023).
- A Study to Evaluate Subcutaneous Zilucoplan in Pediatric Participants With Generalized Myasthenia Gravis (ziMyG). Available online: https://clinicaltrials.gov/study/NCT06055959?cond=Zilucoplan&limit=50&page=1&rank=4 (accessed on 2 December 2023).
- Mammen, A.L.; Amato, A.A.; Dimachkie, M.M.; Chinoy, H.; Hussain, Y.; Lilleker, J.B.; Pinal-Fernandez, I.; Allenbach, Y.; Boroojerdi, B.; Vanderkelen, M.; et al. Zilucoplan in immune-mediated necrotising myopathy: A phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2023, 5, e67–e76. [Google Scholar] [CrossRef]
- Declercq, J.; Bosteels, C.; Van Damme, K.; De Leeuw, E.; Maes, B.; Vandecauter, A.; Vermeersch, S.; Delporte, A.; Demeyere, B.; Vuylsteke, M.; et al. Zilucoplan in patients with acute hypoxic respiratory failure due to COVID-19 (ZILU-COV): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 934. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, E.; Van Damme, K.F.; Declercq, J.; Bosteels, C.; Maes, B.; Tavernier, S.J.; Detalle, L.; Smart, T.; Glatt, S.; Debeuf, N.; et al. Efficacy and safety of the investigational complement C5 inhibitor zilucoplan in patients hospitalized with COVID-19: An open-label randomized controlled trial. Respir. Res. 2022, 23, 202. [Google Scholar] [CrossRef] [PubMed]



| Alternative Names | Zilucoplan; RA101495; Zilbrysq® |
|---|---|
| IUPAC name | (2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,5S,8S,11S,14S,22S)-22-acetamido-11-benzyl-8-(3-carbamimidamidopropyl)-5-(2-carboxyethyl)-3,6,9,12,16,23-hexaoxo-2-propan-2-yl-1,4,7,10,13,17-hexazacyclotricosane-14-carbonyl]-methylamino]-3-carboxypropanoyl]amino]-3,3-dimethylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(1H-pyrrolo[2,3-b]pyridin-3-yl)propanoyl]amino]-4-carboxybutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-2-cyclohexylacetyl]amino]-6-[3-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[[(4S)-4-carboxy-4-(hexadecanoylamino)butanoyl]amino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]hexanoic acid |
| Amino acid composition | L-Lys, L-Val, L-Glu, L-Arg, L-Phe, L-Asp, L- L-NMe-Asp, L-tButyl-Gly, L-Tyr, L-7-aza-Trp, L-Glu, L-Tyr, L-Pro, L-Cyclohexyl-Gly, and L-Lys |
| Mechanism of action | Inhibitor of terminal complement cascade activation |
| Route of administration | Subcutaneous injection |
| Indication | Phase | Status | Location | Identifier |
|---|---|---|---|---|
| Healthy subjects | I | Completed | ------ | ------ |
| Healthy subjects | I | Completed | ------ | ------ |
| Generalized myasthenia gravis | II | Completed | USA | NCT03315130 |
| Generalized myasthenia gravis | III | Completed | Multinational | NCT04115293 |
| Generalized myasthenia gravis | III | Active, not recruiting | Multinational | NCT04225871 |
| Generalized myasthenia gravis | III | Active, not recruiting | USA | NCT05514873 |
| Generalized myasthenia gravis | II and III | Not yet recruiting | ------ | NCT06055959 |
| Indication | Phase | Status | Location | Identifier |
|---|---|---|---|---|
| Immune-mediated necrotising myopathy | II | Terminated | Multinational | NCT04025632 |
| COVID-19 | II | Completed | Belgium | NCT04382755 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.; Fernandes, C. Zilucoplan: A Newly Approved Macrocyclic Peptide for Treatment of Anti-Acetylcholine Receptor Positive Myasthenia Gravis. Drugs Drug Candidates 2024, 3, 311-327. https://doi.org/10.3390/ddc3020018
Costa L, Fernandes C. Zilucoplan: A Newly Approved Macrocyclic Peptide for Treatment of Anti-Acetylcholine Receptor Positive Myasthenia Gravis. Drugs and Drug Candidates. 2024; 3(2):311-327. https://doi.org/10.3390/ddc3020018
Chicago/Turabian StyleCosta, Lia, and Carla Fernandes. 2024. "Zilucoplan: A Newly Approved Macrocyclic Peptide for Treatment of Anti-Acetylcholine Receptor Positive Myasthenia Gravis" Drugs and Drug Candidates 3, no. 2: 311-327. https://doi.org/10.3390/ddc3020018
APA StyleCosta, L., & Fernandes, C. (2024). Zilucoplan: A Newly Approved Macrocyclic Peptide for Treatment of Anti-Acetylcholine Receptor Positive Myasthenia Gravis. Drugs and Drug Candidates, 3(2), 311-327. https://doi.org/10.3390/ddc3020018







