Abstract
Zilucoplan is a synthetic macrocyclic peptide approved by the Food and Drug Administration (FDA), in October 2023, for the treatment of generalized myasthenia gravis. It is considered as an orphan drug that causes the inhibition of terminal complement cascade activation with a dual mechanism of action preventing the formation of the membrane attack complex (MAC) and the destruction of the neuromuscular junction. This drug has been demonstrated to be able to treat the generalized myasthenia gravis without significant adverse effects, with good efficacy, safety, and tolerability profile. Zilucoplan is not only innovative and promising in the therapeutics of generalized myasthenia gravis, but it could also be beneficial for the treatment of other diseases as well as a model for synthesis of analogues to improve pharmacological profile.
1. Introduction
Peptides are at the top of current research for new selective, potent, and safe therapeutic agents [1,2]. Peptides are a unique class of molecules composed of two or more residues of amino acids with structurally diverse chemical compositions, sizes, and shapes. They can be considered between small molecules and large biological molecules such as proteins [3].
Over the last decades, this class of compounds have been captivated great attention in different fields, including pharmaceutical [4,5,6], nutraceutical [7,8], and cosmeceutical [9,10] industries. Another relevant application of peptides is in drug delivery considering the capability of these molecules to organize themselves to form nanostructures. Peptide nanostructures proved to have ability to form stable drug-complexes, displaying high drug load capacity and protection [11,12]. In addition, cell penetrating peptides demonstrated to be a powerful tool for targeted drug delivery [13]. Peptides are also used as imaging and disease diagnosis tools [14] as well as chiral stationary phases for the chromatographic enantioseparation of chiral analytes, due to their high capacity for chiral recognition [15,16].
Until December 2023, 119 peptides had been approved by the competent regulatory authorities as new drugs and, among them, around 46% are cyclic peptides [17]. Gramicidin S (antibiotic), discovered during the Second World War, was the first cyclic peptide to be used in therapeutics [18,19]. Other remarkable examples of cyclic peptides as drugs are vancomycin, telavancin, dalbavancin, and oritavancin (antibiotics) [20], anidulafungin and rezafungin (antifungals) [21,22], lanreotide, pasireotide, and romidepsin (anticancer drugs) [23], and linaclotide (for gastrointestinal (GI) disorders) [24].
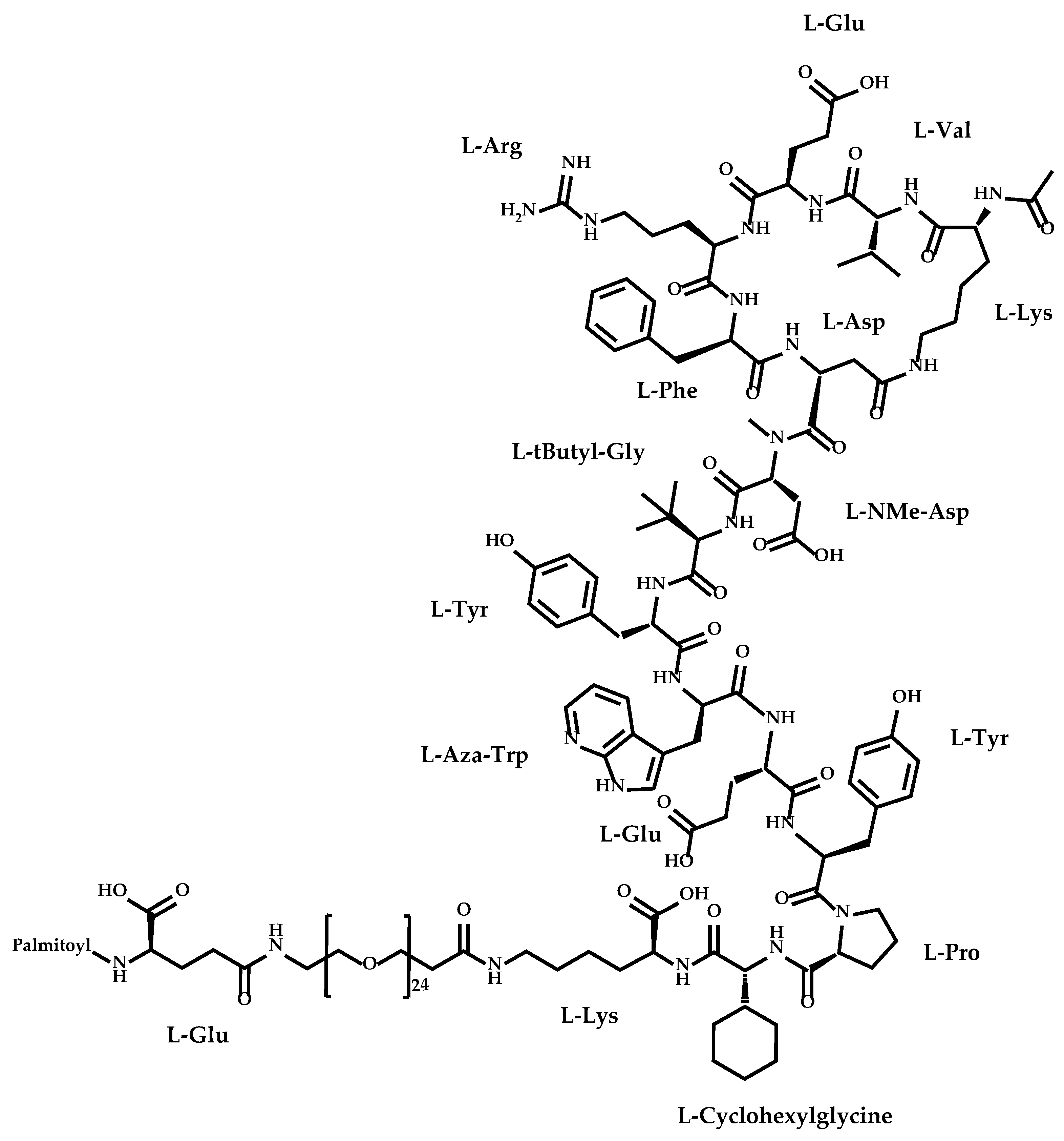
The newly approved cyclic peptide was zilucoplan (Figure 1), a macrocyclic peptide used for treatment of generalized myasthenia gravis [25,26].

Figure 1.
Structure of zilucoplan.
Zilucoplan (Table 1), also designated as RA101495, is the active principle of Zilbrysq®, commercialized by UCB Pharma S.A. It is a 3.5 kDa synthetic macrocyclic peptide composed of 15 amino acid residues, including four unnatural amino acids [27]. The amino acid residues composition is: L-Lys, L-Val, L-Glu, L-Arg, L-Phe, L-Asp, L-L-NMe-Asp, L-tButyl-Gly, L-Tyr, L-7-aza-Trp, L-Glu, L-Tyr, L-Pro, L-Cyclohexyl-Gly, and L-Lys.

Table 1.
Summary of some properties of zilucoplan.
Polyethylene glycol 24, γ-glutamic acid and an N-terminal palmitic acid moiety were attached to the side chain of a L-Lys residue (Figure 1) [28]. The addition of a C16 lipid via a short monodisperse polyethylene glycol linker provides a pharmacokinetic profile consistent with daily dosing in humans [29].
Zilucoplan was developed through a targeted screen using the innovative extreme diversity mRNA display platform [30,31,32,33]. This drug received the designation of orphan drug for the treatment of myasthenia gravis in the European Union in July 2018 from the European Medicines Agency (EMA) [34]. The EMA defines an orphan drug as a drug that is used for the diagnosis, prevention, or treatment of a life-threatening or chronically/seriously debilitating condition that affects no more than 5 in 10,000 people in the European Union [35].
The aim and novelty of this review is to compile all the specific information about zilucoplan.
2. Synthesis of Zilucoplan
2.1. Generalities
Many peptides are obtained from natural sources [36], both terrestrial [37] and marine [38,39]. Chemical synthesis is also a remarkable source of peptides [40,41] and, in addition to classical solid-phase and solution-phase techniques [42], the introduction of sustainable and innovative processes was carried out for synthesis and purification methodologies [43,44]. The development of display techniques also stimulated the scientific world toward the development and/or optimization of peptides [45,46,47]. One great benefit of this approach is the possibility of creation of non-natural peptide libraries with improved pharmacokinetic properties and lower toxicity [30]. Nowadays, there is a great diversity of display techniques, both in vivo and in vitro, capable of generating libraries of peptides, to find interactions between peptides and targets, and to identify and isolate the peptides of interest [46]. Computer-aided methods were put at the service for rational planning and discovering of new peptides, being possible to perform virtual screening of many molecules [48,49,50]. The advancements of analytical structural characterization methods also contributed for the growing interest of this class of compounds [51,52,53,54]. Peptides are also interesting models for molecular modifications [55,56] and conjugation with other molecules [57,58] to improved properties and/or for targeted therapy.
Considering drug design and development, what makes peptides so suitable to be used as drugs is a combination of multiple favourable properties as their high selectivity, potency and target specificity, in addition to their low immunogenicity, accumulation in tissues, and side effects [59,60]. Nevertheless, these molecules have some limitations that need to be overcome such as poor oral bioavailability, low membrane permeability, and short half-life, among others [3]. Research has been in a continuous effort to offer solutions to improve peptide stabilization. First, structural modifications through chemical synthesis are an excellent solution. Examples of such modifications include replacing L-amino acids with D-amino acids, using chemically modified amino acids and the selective chemical modification of the peptide (e.g., N-acetylation or C-amidation) or replacing the peptide bond by a pseudo-peptide bond [61,62]. Another strategy adopted to increase the stability of these molecules is cyclization [4]. Cyclic peptides show both enhanced pharmacokinetic and pharmacodynamic properties compared to linear peptides [63]. Cyclization decreases conformational changes of the molecule, by reduction of spatial vibrations, and increases the surface area for interaction with the biological target, improving the binding affinity and selectivity. Moreover, absorption and membrane permeability were also improved due to the rigidification of the peptide structure that decreases the energy barrier required for the molecule to adapt to the membrane environment and bind to transport proteins to enter the cell by passive diffusion or active transport. Cyclization also affords better metabolic stability, because cyclic peptides are resistant to the action of both exopeptidases and endopeptidases [14,60,64]. In addition to cyclization, there are also disulfide bonds which play a very important role in peptides as they allow constrained and defined structures to be created [65]. Thus, it is possible to further enhance the structural and metabolic stability, selectivity, potency and permeability of the peptides, since the structure becomes even more rigid than it would be with cyclization exclusively [66]. The full potential and advantages of cyclic peptides containing disulfide bonds have already been explored in some scientific articles [65,66].
Cyclization is the most challenging step to obtain cyclic peptides; therefore, some strategies have been developed to facilitate this process [38]. In addition to the classical cyclization process in solution, using coupling reagents and protecting groups [67,68], new methods of cyclization have emerged, including native chemical ligation [69], serine/threonine ligation [70], KAHA ligation [71], Staudinger’s ligation [72,73], enzyme-mediated cyclization [74], Cyclick strategy [75], on-resin cyclization [41,76], among others. These strategies ensure that the difficulties inherent to cyclization in solution can be overcome, namely the possibility of C-terminal epimerization, oligomerization, and low coupling efficiency according to the ring size and linear peptide sequence. In addition, the full protection of the side chains is not required and a chemoselective bond between two amino acid residues is obtained, allowing an increase of the efficiency of cyclization [67,68]. However, it is important to highlight that some of these strategies are still not widely used, since the presence of certain functional groups on the amino acid residues is mandatory for cyclization to take place, which can be unfeasible for obtaining a desired cyclic peptide structure [64].
The rewards of cyclization, combined with the intrinsic and unique characteristics of peptides, make cyclic peptides very attractive molecules for the development of new drugs with diverse biological activities [64,77]. Most of the cyclic peptides were explored as antimicrobial and anticancer agents [78,79,80,81].
2.2. Reported Procedures
Zilucoplan has been obtained by total synthesis [28,82]. As mentioned before, chemical synthesis is an important source of peptides for drug discovery and development. Numerous advantages characterize the synthetic strategy, including the possibility of obtaining high quantities of peptides required for large-scale biological assays and/or drug development. Another noteworthy benefit of synthesis is to allow the preparation of peptides comprising the desired structure as well as to achieve structurally diverse analogues and derivatives for structure–activity relationship studies [40,41,42,83].
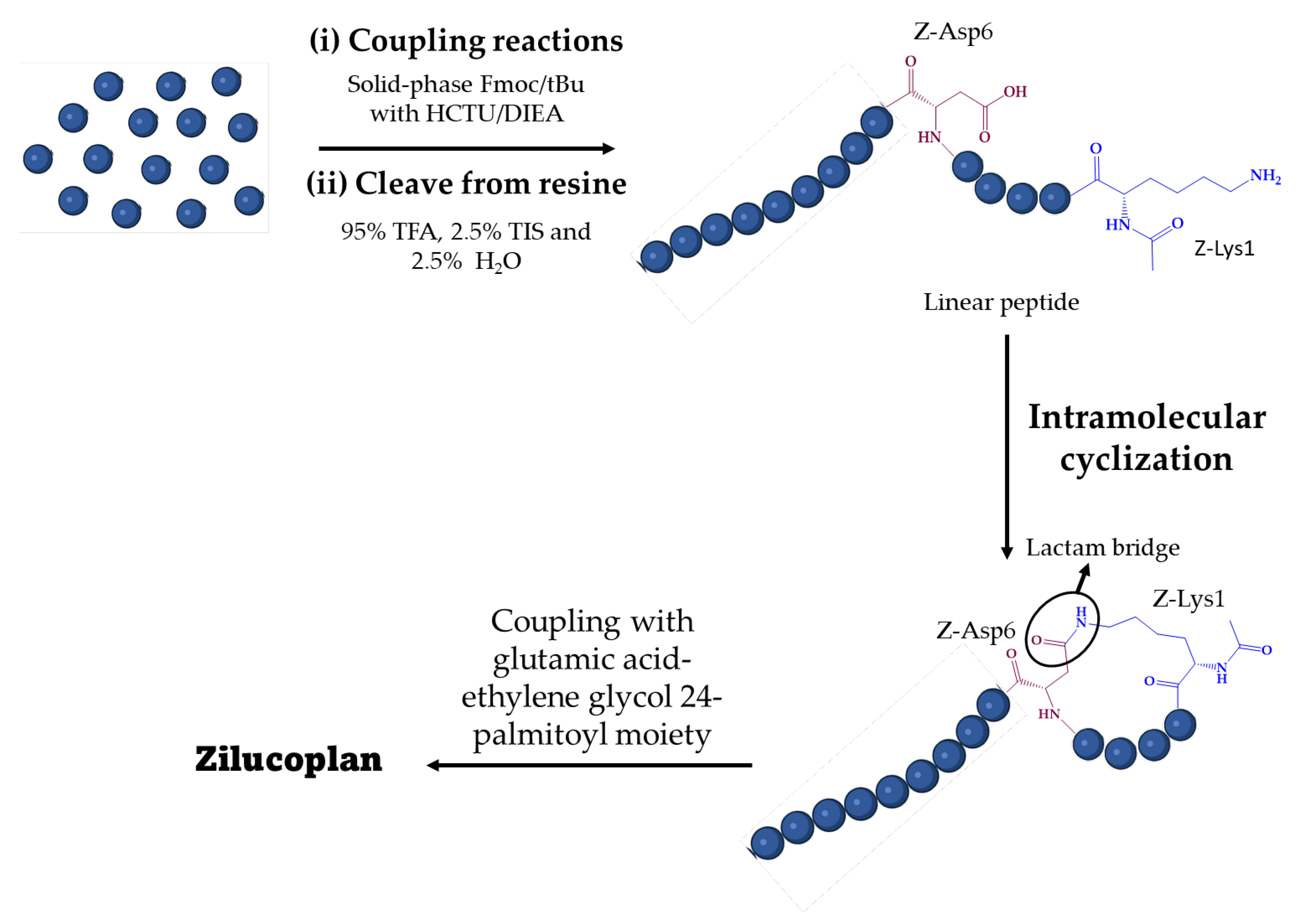
A brief description of the synthesis of zilucoplan is reported in patent literature [82,84,85,86] and in an original article by Gorman et al. [28]. Zilucoplan was synthesized in three steps (Figure 2). First, a linear peptide sequence was synthesized by coupling amino acid residues [82]. In general, peptide synthesis can be carried out in solution, in solid phase, or by using these two strategies simultaneously [79]. Both include two key steps for the formation of a peptide bond between two amino acid residues: the activation of the carboxyl group by coupling reagents and the temporary use of protecting groups to direct the reaction to the desired direction [87]. The two main strategies are fluorenylmethyloxycarbonyl (Fmoc)/tert-butyl (tBu) and tert-butyloxycarbonyl (Boc)/benzyl (Bn) [88].

Figure 2.
General synthetic pathway for zilucoplan. HCTU: 2-(6-chloro-l-H-benzotriazole-lyl)-1,1,3,3-tetramethylaminium hexafluorophosphate; DIEA: diisopropyl ethylamine; Fmoc: fluorenylmethyloxycarbonyl; tBu: tert-butyl; TFA: trifluoroacetic acid; TIS: triisopropylsilane.
For zilucoplan, this step was carried out using a standard solid-phase Fmoc/tBu method. The synthesis was performed in a Liberty automated microwave peptide synthesizer following standard protocols with Rink amide resin. The coupling agent used was 2-(6-chloro-l-H-benzotriazole-lyl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HCTU) with diisopropyl ethylamine (DIEA) as catalytic base [82].
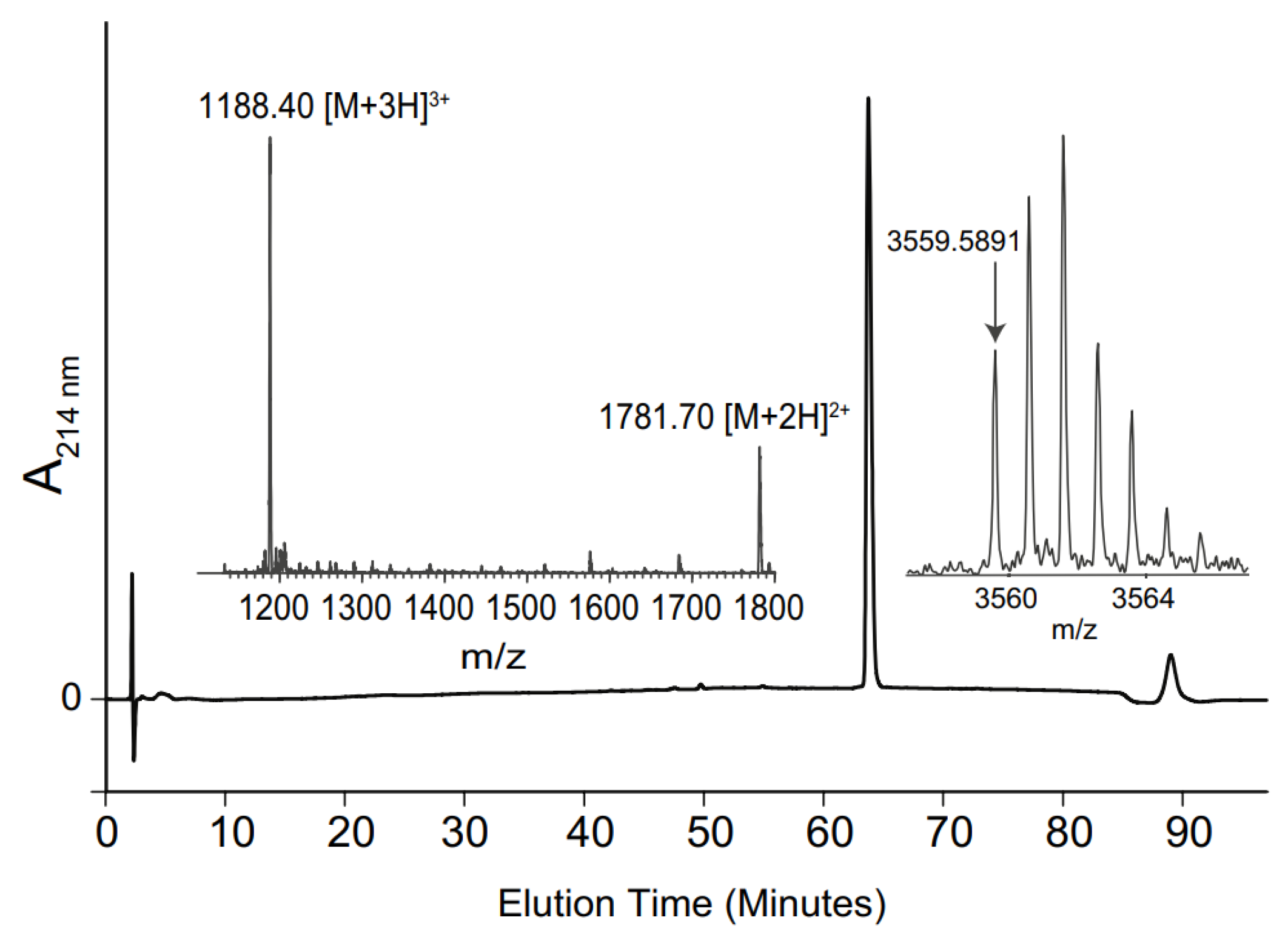
After this process, the polypeptide chain was cleaved from the resin and side chainprotecting groups were removed by treatment with 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIS) and 2.5% water for 3 h at room temperature and was isolated by precipitation with ether [82]. The crude linear polypeptide was purified by preparative reverse phase high-performance liquid chromatography (RP-HPLC) using a C18 column [82]. The pure linear peptide was analyzed by liquid chromatography-mass spectrometry (LC-MS) (Figure 3). RP-HPLC was performed with an increasing gradient of 1% buffer B (90% acetonitrile, 0.05% TFA) in buffer A (0.05% TFA) per minute [28].

Figure 3.
RP-HPLC chromatogram, electrospray ionization mass spectrometry (ESI–MS) spectrum and MALDI-MS spectrum of zilucoplan [28].
The next synthetic step was the intramolecular cyclization of the linear peptide [82]. Cyclization of a peptide can occur in four different ways, depending on the amino acid residues present: head-to-tail cyclization, head-to-side chain cyclization, side-chain-to-tail cyclization, and side-chain-to-side-chain cyclization [89]. For zilucoplan, a head-to-side chain cyclization was carried out via a lactam bridge between the side chains of the Z-Lysl and Z-Asp6 residues. Finally, a glutamic acid-ethylene glycol24-palmitoyl moiety was coupled to a second Lys sidechain affording the zilucoplan [82].
The planar structure of zilucoplan was established by comprehensive analyses of two-Dimensional 1H-1H TOCSY and NOESY experiments, and the secondary structure was identified via the determination of secondary Hα shifts from the closest naturally occurring residues random coil values [28].
3. Zilucoplan and Myasthenia Gravis
3.1. The Disease
Myasthenia gravis is a chronic, unpredictable, and antibody-mediated organ specific autoimmune disease commonly associated with thymic changes [90,91]. Systematic epidemiological studies revealed that its annual incidence is estimated to range from 7–23 cases per million, while the prevalence varies from 70–320 cases per million [92]. Nevertheless, although the incidence of this disease may vary according to the geographical region in question, its prevalence has been increasing over the last seven decades [93]. This trend may be explained by the evolution of knowledge about the disease and the development of diagnostic methods [94]. Nevertheless, the mortality rate has decreased significantly since the introduction of various treatment options [93].
This debilitating rare disease is associated with loss of muscle strength and is worsened by physical exercise or repeated use of the muscles [95,96]. This disease first manifests itself with eye symptoms such as ptosis, diplopia (double vision), or blurred vision [97]. Normally, around 80% of patients with these symptoms progress to generalized myasthenia gravis with generalized symptoms in two or three years [98,99,100]. These generalized symptoms are related to bulbar manifestations like dysphagia, weakness and fatigue [97]. In more than 40% of patients, there is muscular respiratory weakness that causes dyspnoea on exertion or orthopnoea [100]. Around 15% to 20% of patients will suffer a myasthenic crisis, which results in respiratory failure, requiring ventilation [97,100]. Prolonged muscle weakness can lead to obesity and respiratory infections. Other comorbidities of this disease can be the appearance of other autoimmune diseases and thymic malignancies [101].
The damage caused by this disease is due to autoantibodies targeting certain components of the neuromuscular junction (NMJ) [102]. Depending on the components targeted by the autoantibodies, this disease is subdivided into different subtypes: the most common subtype is characterized by autoantibodies targeting nicotinic acetylcholine receptors (AChR), this is the subtype that affects around 85% of patients; there is also the case where autoantibodies target muscle-specific kinase (MuSK) or lipoprotein receptor-related protein 4 (LRP4) these situations affect around 15% of patients [103,104,105,106]. There is also a more unusual situation in which circulating autoantibodies to known targets are not detected; this only happens in a small portion of patients [91].
AChR MG autoantibodies belong to subtypes that mediate tissue damage at the NMJ, while MuSK MG autoantibodies belong to subtypes that, by interfering with protein–protein interactions at the NMJ, cause direct disruption of AChR signaling [107,108]. When diagnosing the disease, it is extremely important to identify the subtype of the disease, since each subtype has different immunopathologies and the treatments required will therefore be different [91]. This review will only explore MG caused by autoantibodies targeting AChR.
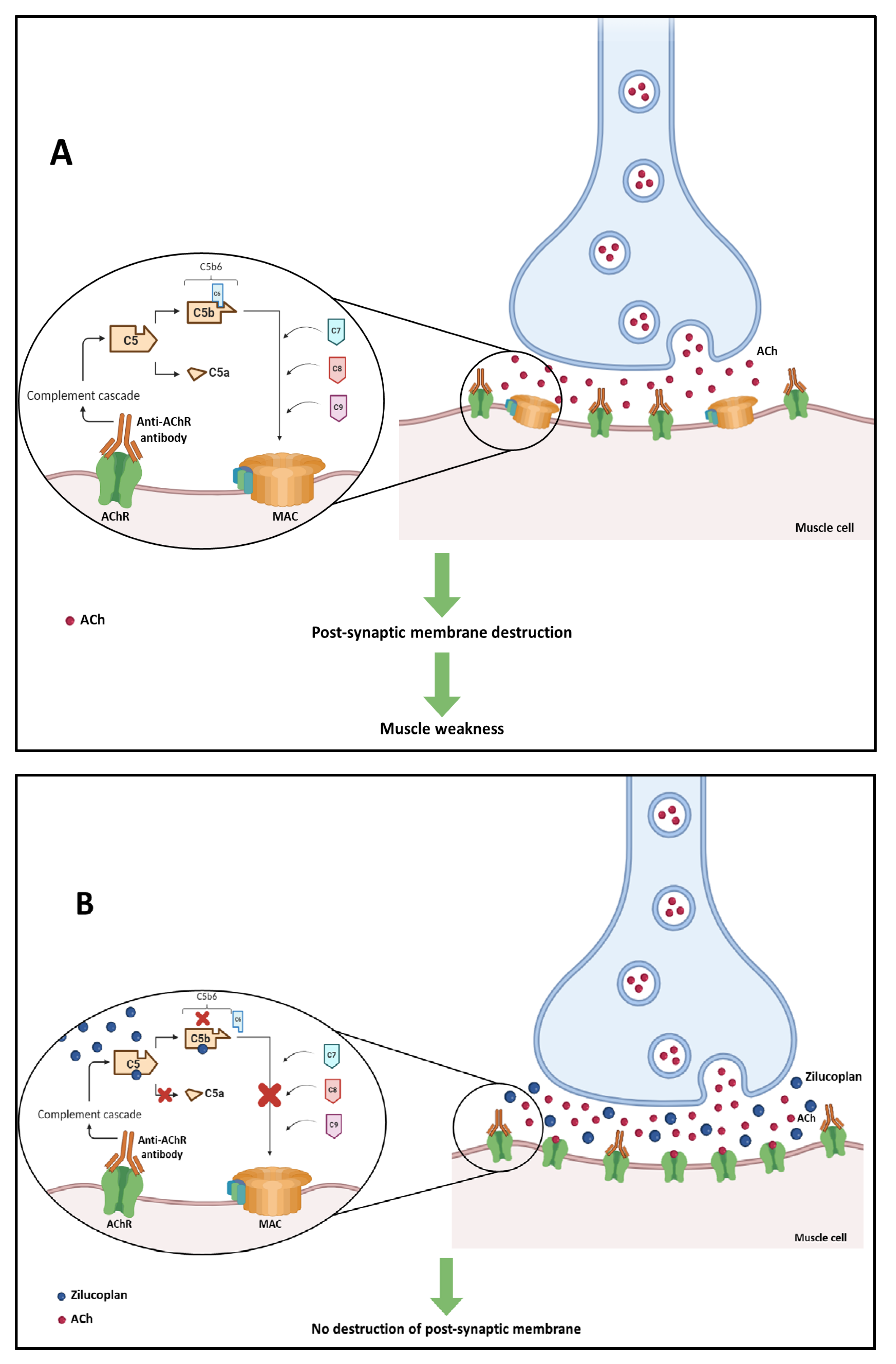
Three pathogenic mechanisms of AChR auto-antibodies have already been identified: Firstly, there is the mechanism in which AChR auto-antibodies inhibit the binding of acetylcholine (ACh) to its receptors by binding to the active site of the receptor or by binding close to the active site. This will result in a decrease in ACh-dependent signaling at the NMJ [109]. The second mechanism consists of auto-antibody-mediated crosslinking, which results in the internalization of the AChR, decreasing the number of these receptors at the NMJ. This mechanism is known as antigen modulation [110,111]. The third mechanism is related to the immunoglobulin effector functions of AChR auto-antibodies. One of these main functions is the activation of the complement cascade, which leads to the formation of the membrane attack complex (MAC) and causes damage to the postsynaptic membrane and the destruction of synaptic components [112]. The latter is the predominant mechanism and will be explored below [93].
In this mechanism, AChR autoantibodies ACh receptors located in the post-synaptic membrane of neuromuscular junctions, which significantly reduces the number of these receptors. This attack triggers the activation of the antibody-dependent complement system which leads to the formation of MAC. The accumulation of MAC in the post-synaptic membrane of neuromuscular junctions is responsible for their destruction, which leads to muscle weakness (Figure 4A) [108].
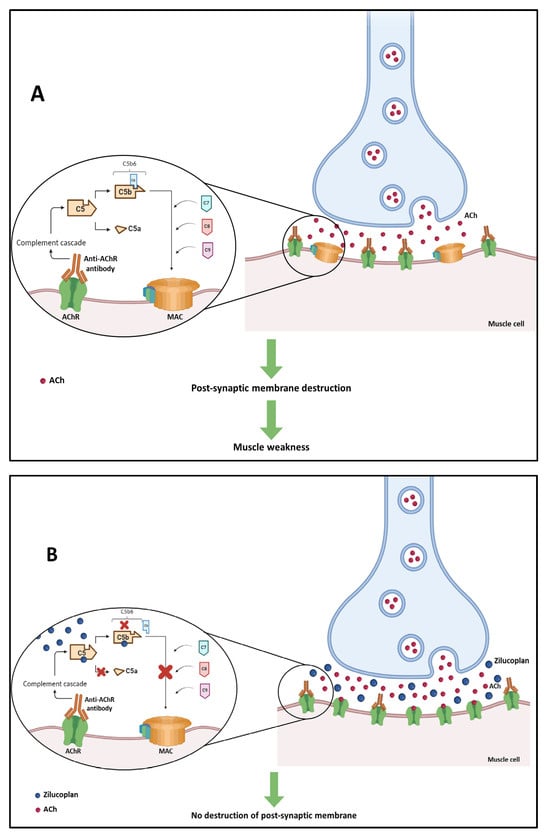
Figure 4.
(A) Activation of the antibody-dependent complement system in generalized myasthenia gravis by attack of anti-acetylcholine receptor (anti-AChR) antibodies, leading to the destruction of the post-synaptic membrane of neuromuscular junctions. (B) Mechanism of action of zilucoplan blocking the complement cascade and preventing the formation of membrane attack complex (MAC) and the destruction of the neuromuscular junction.
Initially, scarce information was available about this disease or its physiological causes and, as a result, the prognosis for patients was very serious and the mortality rate was high. However, as scientific knowledge progressed, the causes and mechanism of this disease were discovered and, as a consequence, treatments began to be developed [113]. In recent decades, there has been great progress in the treatment of myasthenia gravis [90], being considered one of the most treatable autoimmune diseases [114].
3.2. Therapeutic Strategies
Currently, therapeutic strategies for myasthenia gravis disease are divided into two main groups [108]. One group includes traditional therapies such as the use of non-immunosuppressants such as pyridostigmine, which is an acetylcholinesterase (AChE) inhibitor that increases the amount of ACh in the synapses [115]. Among the traditional therapies there are also immunosuppressants such as corticosteroids, calcineurin inhibitors (cyclosporine A, tacrolimus), antimetabolites (cyclophosphamide, mycophenolate mofetil and methotrexate), and intravenous immunoglobulin (IVIG) therapy, with a diverse range of mechanisms of action [108,116]. Plasma exchange (PLEX) is another therapeutic approach, consisting of the direct removal of autoantibodies and complement components involved in the pathogenesis of the disease, in addition with the decrease of pro-inflammatory cytokines levels [117,118,119]. The traditional therapies are used to control the symptoms of muscle weakness [27].
On the other hand, there are therapies that use biological molecules that exert therapeutic effects in less time and have fewer side effects, when compared with traditional therapies, because its mechanism of action is more targeted [114]. Therapies using biologicals include B-cell-targeted drugs (rituximab, obinutuzumab, belimumab and inebilizumab), T-cell-targeted drugs (iscalimab, tocilizumab), complement inhibitors (eculizumab and ravulizumab), proteosome inhibitors (bortezomib and mezagitamab), and neonatal Fc receptor antibody therapy (efgartigimod, rozanolixizumab, nipocalimab and batoclimab) [108,120]. The development of biologicals can be considered as a turning point in the treatment of myasthenia gravis [114]. However, all these options have numerous side effects that need to be constantly monitored. In the case of the use of complement system inhibitor antibodies (like eculizumab and ravulizumab) it is even more complicated since these are associated with opportunistic infections like meningococcal infection, and can trigger immunogenicity problems that can reduce the effectiveness of the treatment [108,121]. These hurdles can be overcome with a non-antibody-based complement inhibition strategy like zilucoplan [121]. Zilucoplan, a newly approved drug, emerges as an innovative and promising solution for the treatment of this disease.
3.3. Zilucoplan
3.3.1. Mechanism of Action
Zilucoplan is an inhibitor of terminal complement cascade activation, which is initiated by anti-AChR antibodies. This drug has a dual mechanism of action: it binds to complement 5 (C5) with high affinity and specificity, preventing the conversion of this complement into C5a and C5b by C5 convertase, and it binds to the previously formed C5b moiety, sterically preventing its interaction with C6 (Figure 4B) [29]. Both C5a and C5b metabolites are associated with host defense, with C5a propagating leukocyte chemotaxis and cytokine release through its receptors C5aR1 and C5aR2 [122], and C5b initiating the formation of MAC.
The dual mechanism of zilucoplan prevents the assembly and activity of MAC. Since the MAC complex is not formed, there is no destruction of the postsynaptic membrane [29].
3.3.2. Indication
In September 2023, the Committee for Medicinal Products for Human Use (CHMP) of the EMA issued a positive opinion recommending the granting of a marketing authorization for Zilbrysq®, intended for the treatment of myasthenia gravis [123]. On 25 September 2023, zilucoplan was first approved by the Japanese Ministry of Health, Labour and Welfare (MHLW) for the treatment of adults with generalized myasthenia gravis in adults who are AChR antibody-positive and inappropriately respond to steroids or other immunosuppressants [124]. Then, it was approved in the USA, on 17 October 2023, [25,125,126] and in the EU, on 4 December 2023, for the treatment of generalized myasthenia gravis in adult patients who are anti-AChR antibody positive [127].
It is administered via subcutaneous self-injection once a day [128]. This is an advantage over the other C5 inhibitors that are currently available since they are administered intravenously. For patients, self-administered subcutaneous treatment is more convenient because it prevents the need for regular clinic visits, and consequently, improves patients’ autonomy and reduces daily life interference. In addition, if rescue therapy is required, the treatment with a peptide C5 inhibitor has the additional benefit of allowing the simultaneous use of plasma exchange and intravenous immunoglobulin, without need for supplemental dosing [27].
3.3.3. Clinical Trials
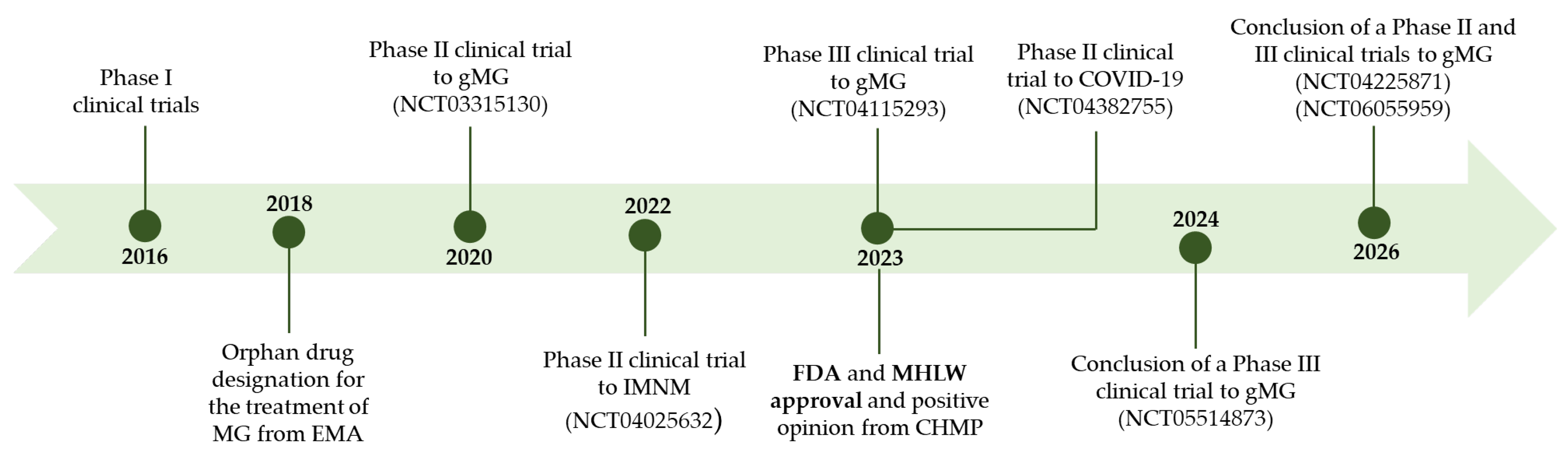
This drug completed several clinical trials before it was approved, which are summarized in Figure 5.

Figure 5.
Pipeline of zilucoplan. gMG: generalized myasthenia gravis.
Phase I clinical trials were carried out to assess the pharmacokinetic and pharmacodynamic properties of zilucoplan and its safety and tolerability after subcutaneous injection [129,130]. The first study was a Phase I, randomized, double-blind, placebo-controlled, single-escalating-dose study in which single doses of zilucoplan (0.05 mg/kg to 0.4 mg/kg) were administered [129]. This trial involved 22 healthy volunteers. From a pharmacokinetic point of view, this study has shown that zilucoplan plasma levels are consistent with the values predicted by in silico pharmacokinetic models. Furthermore, the relationship between the maximum concentration in plasma and the dose administered was linear, demonstrating dose-dependent exposure. At all doses administered, the t1/2 was approximately 7 days [129].
With regard to pharmacodynamics, this study showed that zilucoplan has a rapid dose-dependent inhibition of haemolysis and suppression of complement activity [129].
Furthermore, this study concluded that zilucoplan is apparently safe and well tolerated. This preliminary trial suggested that with low-dose administration, steady-state levels of haemolysis suppression of over 90% can be achieved [129].
The second Phase I trial was a randomized, double-blind, placebo-controlled, multiple-dose study, in which multiple doses of zilucoplan (0.2 mg/kg) were administered over 7 days [130]. This trial involved six healthy volunteers. Regarding pharmacokinetics, it was possible to conclude that the drug levels achieved were consistent with the prediction made in an in silico pharmacokinetic model and were in agreement with the values observed in the previous Phase I trial [129,130]. Plasma levels of zilucoplan showed low variability in exposure in all subjects and peak drug levels were observed after 3 h in all participants. In addition, the results obtained allowed the prediction that with the administration of a dose of 0.2 mg/kg, zilucoplan plasma concentrations reach steady-state on day 11 [130]. From a pharmacodynamic point of view, it was concluded that the inhibition of haemolysis was rapid and sustained in 95% with the administration of daily doses of 0.2 mg/kg for 7 days. As for the suppression of complement activity, at the dose and for the period tested this drug demonstrated the capacity for rapid, complete, and sustained suppression. Furthermore, this trial showed that zilucoplan is safe and well tolerated for 7 days at the dose tested [130].
A Phase II clinical trial (NCT03315130) was carried out to evaluate the clinical effects of zilucoplan when administered via subcutaneous injection to adult patients with moderate to severe generalized myasthenia gravis [128]. This was a randomized, double-blind, placebo-controlled, multicenter clinical trial in which 44 patients with generalized myasthenia gravis took part. During the study, the patients maintained the therapy that they were using to treat generalized myasthenia gravis [128].
In this study, doses of 0.1 mg/kg and 0.3 mg/kg were tested and injected daily for 12 weeks. The 0.3 mg/kg dose was the one that showed the most rapid improvement in the disease. With this study it was possible to conclude that zilucoplan administered at a dose of 0.3 mg/kg is an effective complement inhibitor for the majority of patients with moderate to severe generalized myasthenia gravis. Moreover, safety and tolerability profiles were favourable [128].
After these 12 weeks, the patients who took part in this Phase II trial were eligible to be enrolled in an open-label extension period with the aim of confirming the dose to be used in a Phase III trial. Once again, zilucoplan showed a good safety and tolerability profile for both doses and the dose that allowed for the most significant results in the shortest period of time was 0.3 mg/kg; thus, this was the chosen dose [128,131].
A Phase III trial (RAISE) (NCT04115293) evaluated the safety and efficacy of zilucoplan when administered to patients with AChR autoantibody positive generalized myasthenia gravis [132]. RAISE was a randomized, double-blind, and placebo-controlled trial. This trial was carried out in Europe, Japan, and North America and involved 174 patients with AChR autoantibody positive generalized myasthenia gravis. In this study, zilucoplan was administered once a day at a dose of 0.3 mg/kg by subcutaneous self-injection. Zilucoplan demonstrated rapid and significant improvements in the treatment of generalized myasthenia gravis and proved to be effective. In this trial, similar percentages of patients in the zilucoplan and placebo groups experienced any-grade treatment-emergent adverse events (TEAEs) (77 vs. 70%), serious TEAEs (13 vs. 15%) and severe TEAEs (12 vs. 13%). The most frequently described TEAEs in the zilucoplan and placebo groups were injection-site pain (9 vs. 3%), injection-site bruising (16 vs. 9%), headache (15 vs. 16%), diarrhoea (10 vs. 2%), and myasthenia gravis worsening (10 vs. 9%). In the zilucoplan and placebo groups, 5% and 2% of patients, respectively, discontinued treatment due to TEAEs [132]. The favourable safety profile that was identified in the Phase II trial was confirmed in this Phase III trial as it was well tolerated [132]. The same conclusions were drawn when analyzing the results in the subgroup of Japanese patients, which was described separately [133]. In this subgroup, zilucoplan also showed a significant improvement in the disease with a favourable safety and tolerability profile [133].
3.3.4. Ongoing Clinical Trials
After approval, clinical trials are still ongoing with zilucoplan to assess the possibility of this drug being used in other situations. A Phase III clinical trial (RAISE-XT) is currently underway, which is an open-label extension study to assess the long-term efficacy of zilucoplan and to continue monitoring its safety and efficacy (NCT04225871) [134]. This trial is expected to be completed in 2026 [135].
There is also an ongoing Phase III trial to assess the safety and tolerability of switching from intravenous C5 inhibitors to the subcutaneous inhibitor (zilucoplan) (NCT05514873) [136].
A Phase II and III trial is underway to study the pharmacokinetics, pharmacodynamics, safety, tolerability, immunogenicity, and activity of zilucoplan in pediatric patients with generalized myasthenia gravis (NCT06055959) [137].
Table 2 summarizes some relevant information regarding all the clinical trials of zilucoplan for generalized myasthenia gravis.

Table 2.
Summary of clinical trials of zilucoplan for generalized myasthenia gravis.
4. Zilucoplan and Other Diseases
In addition to the indication for which it was approved, zilucoplan has been the object of clinical trials to assess its effectiveness in other diseases.
A Phase II clinical trial (NCT04025632) was also carried out to assess the efficacy, tolerability, and safety of zilucoplan in patients with immune-mediated necrotising myopathy [138]. It was a multicentre, randomized, double-blind, placebo-controlled study in which 27 patients were enrolled. In this trial, zilucoplan was administered subcutaneously daily for 8 weeks at a dose of 0.3 mg/kg, as this dose had been shown to be effective in inhibiting the terminal complement cascade in patients with generalized myasthenia gravis [138]. As shown in previous trials, zilucoplan was well tolerated by the patients and it was confirmed that zilucoplan can inhibit the terminal complement cascade. Despite this, no improvements were seen in the outcomes assessed for immune-mediated necrotising myopathy, which suggests that activation of the complement cascade is not the main mechanism in this disease [138].
Another Phase II trial (NCT04382755) was carried out with zilucoplan to assess its efficacy and safety in hospitalized COVID-19 patients [139]. It was a multi-center randomized controlled open-label trial in which 32.4 mg of zilucoplan was given subcutaneously every day for 14 days to 81 patients. During this period and for the following 14 days, the patients also received daily 2 g of ceftriaxone intravenously [140].
This study demonstrated that zilucoplan was safe, although it did not show significant improvements in oxygenation parameters after 6 and 15 days of treatment. Further studies will be carried out in larger patient populations to elucidate the clinical efficacy of zilucoplan [140].
Table 3 summarizes some relevant information regarding all the clinical trials of zilucoplan for other diseases.

Table 3.
Summary of clinical trials of zilucoplan for other diseases.
5. Conclusions
The treatment of myasthenia gravis is associated with several constraints considering the limitations of the available drugs, as they only treat the symptoms of the disease, are associated to many adverse effects, and can even cause immunogenicity. Therefore, the strategy of using non-antibody inhibitors of the complement system is a good therapeutic solution, as they can directly treat the disease with minor adverse effects. Zilucoplan, a macrocyclic peptide recently approved for treatment of myasthenia gravis, is considered as an orphan drug with an innovative mechanism of action. It is an inhibitor of the terminal complement cascade activation by inhibiting C5. Zilucoplan can be self-administered subcutaneously just once a day and demonstrated efficacy, good tolerability, and safety in several clinical trials. This peptide represents a new hope for the effective treatment of generalized myasthenia gravis, as it can treat the disease with safety and without the risk of immunogenicity. The drug is a welcome addition to targeted biological treatments in MG, being better tolerated and safer than other options, although it was not effective in some 30% of patients in the Phase III RAISE trial; this underlines the fact that other immunological mechanisms beside complement activation are active in myasthenia gravis along with some specific patient-related factors. Furthermore, zilucoplan could also be useful for the treatment of other diseases as well as an inspiration for the synthesis of analogues improving pharmacological profile. Thus, this discovery may open the way for development of other drugs with the same mechanism of action. In addition, this cyclic peptide also evidenced that the chemical class of peptide molecules has a great therapeutic potential to be exploited with one more contribution that reached the therapeutic market with great success.
Author Contributions
Conceptualization: C.F.; Data collection and analysis: L.C.; Writing—original draft preparation: L.C.; Writing—reviewing and editing: C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by national funds through FCT (Foundation for Science and Technology) within the scope of UIDB/04423/2020, UIDP/04423/2020 (Group of Marine Natural Products and Medicinal Chemistry–CIIMAR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Santana, C.; Pinheiro, A.; Anjos, L.; Barth, T.; Júnior, O.; Fontes, W.; Castro, M. Marine Depsipeptides as Promising Pharmacotherapeutic Agents. Curr. Protein Pept. Sci. 2017, 18, 72–91. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Du, Z.; Fan, B.; Dai, Q.; Wang, L.; Guo, J.; Ye, Z.; Cui, N.; Chen, J.; Tan, K.; Li, R.; et al. Supramolecular peptide nanostructures: Self-assembly and biomedical applications. Giant 2022, 9, 100082. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H. Recent Progress on Cyclic Peptides’ Assembly and Biomedical Applications. ChemBioChem 2023, 24, e202300018. [Google Scholar] [CrossRef]
- Kotadiya, D.D.; Patel, P.; Patel, H.D. Cell-Penetrating Peptides: A Powerful Tool for Targeted Drug Delivery. Curr. Drug Deliv. 2024, 21, 368–388. [Google Scholar] [CrossRef]
- Choi, J.S.; Joo, S.H. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2020, 28, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules 2021, 26, 3380. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed]
- PepTherDia. Available online: http://peptherdia.herokuapp.com/list (accessed on 28 November 2023).
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the Treatment of Infected Wounds. Nature 1944, 154, 703–703. [Google Scholar] [CrossRef]
- Gall, Y.M.; Konashev, M.B. The discovery of Gramicidin S: The Intellectual Transformation of G.F. Gause from Biologist to Researcher of Antibiotics and on its Meaning for the Fate of Russian Genetics. Hist. Philos. Life Sci. 2001, 23, 137–150. [Google Scholar] [PubMed]
- Klinker, K.P.; Borgert, S.J. Beyond Vancomycin: The Tail of the Lipoglycopeptides. Clin. Ther. 2015, 37, 2619–2636. [Google Scholar] [CrossRef]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611. [Google Scholar] [CrossRef]
- Syed, Y.Y. Rezafungin: First Approval. Drugs 2023, 83, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Zain, J. Romidepsin in the treatment of cutaneous T-cell lymphoma. J. Blood Med. 2011, 2, 37–47. [Google Scholar]
- Layer, P.; Stanghellini, V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2014, 39, 371–384. [Google Scholar] [CrossRef]
- FDA. Novel Drug Approvals for 2023. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023 (accessed on 26 November 2023).
- Shirley, M. Zilucoplan: First Approval. Drugs 2024, 84, 99–104. [Google Scholar] [CrossRef]
- Howard, J.F.; Vissing, J.; Gilhus, N.E.; Leite, M.I.; Utsugisawa, K.; Duda, P.W.; Farzaneh-Far, R.; Murai, H.; Wiendl, H. Zilucoplan: An Investigational Complement C5 Inhibitor for the Treatment of Acetylcholine Receptor Autoantibody–Positive Generalized Myasthenia Gravis. Expert Opin. Investig. Drugs 2021, 30, 483–493. [Google Scholar] [CrossRef]
- Gorman, D.M.; Lee, J.; Payne, C.D.; Woodruff, T.M.; Clark, R.J. Chemical synthesis and characterisation of the complement C5 inhibitory peptide zilucoplan. Amino Acids 2021, 53, 143–147. [Google Scholar] [CrossRef]
- Tang, G.Q.; Tang, Y.; Dhamnaskar, K.; Ma, Z.; Zhu, N.; Cong, B.; Sayegh, C.; Ricardo, A. Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front. Immunol. 2023, 14, 1213920. [Google Scholar] [CrossRef] [PubMed]
- Guillen Schlippe, Y.V.; Hartman, M.C.T.; Josephson, K.; Szostak, J.W. In Vitro Selection of Highly Modified Cyclic Peptides That Act as Tight Binding Inhibitors. J. Am. Chem. Soc. 2012, 134, 10469–10477. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hartman, M.C.T. In Vitro Selection of Unnatural Cyclic Peptide Libraries via mRNA Display. In Ribosome Display and Related Technologies: Methods and Protocols; Douthwaite, J.A., Jackson, R.H., Eds.; Springer: New York, NY, USA, 2012; pp. 367–390. [Google Scholar]
- Ma, Z.; Hartman, M.C. In vitro selection of unnatural cyclic peptide libraries via mRNA display. Methods Mol. Biol. 2012, 805, 367–390. [Google Scholar]
- Ricardo, A.; Arata, M.; DeMarco, S.; Dhamnaskar, K.; Hammer, R.; Fridkis-Hareli, M.; Rajagopal, V.; Seyb, K.; Tang, G.-Q.; Tobe, S.; et al. Preclinical Evaluation of RA101495, a Potent Cyclic Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Blood 2015, 126, 939–939. [Google Scholar] [CrossRef]
- EMA. EU/3/22/2650: Orphan Designation for the Treatment of Myasthenia Gravis. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-22-2650 (accessed on 28 November 2023).
- Giannuzzi, V.; Conte, R.; Landi, A.; Ottomano, S.A.; Bonifazi, D.; Baiardi, P.; Bonifazi, F.; Ceci, A. Orphan medicinal products in Europe and United States to cover needs of patients with rare diseases: An increased common effort is to be foreseen. Orphanet J. Rare Dis. 2017, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Kanwal, J.; Musaddiq, S.; Khakwani, S. Bioactive Peptides and Their Natural Sources. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 75–97. [Google Scholar]
- Guo, R.; Guo, G.; Wang, A.; Xu, G.; Lai, R.; Jin, H. Spider-Venom Peptides: Structure, Bioactivity, Strategy, and Research Applications. Molecules 2024, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Taktaz, F.; Sousa, E.; Fernandes, C.; Kijjoa, A. Peptides from Marine-Derived Fungi: Chemistry and Biological Activities. Mar. Drugs 2023, 21, 510. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Ding, K.; Roller, P. Recent Progress of Synthetic Studies to Peptide and Peptidomimetic Cyclization. Curr. Org. Chem. 2008, 12, 1502–1542. [Google Scholar] [CrossRef]
- Hamada, Y.; Shioiri, T. Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides. Chem. Rev. 2005, 105, 4441–4482. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Morcinek-Orłowska, J.; Pierzynowska, K.; Gaffke, L.; Węgrzyn, G. Phage display and other peptide display technologies. FEMS Microbiol. Rev. 2021, 46, fuab052. [Google Scholar] [CrossRef]
- Deyle, K.; Kong, X.-D.; Heinis, C. Phage Selection of Cyclic Peptides for Application in Research and Drug Development. Acc. Chem. Res. 2017, 50, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wiedmann, M.M.; Suga, H. RNA Display Methods for the Discovery of Bioactive Macrocycles. Chem. Rev. 2019, 119, 10360–10391. [Google Scholar] [CrossRef]
- Duffy, F.J.; Devocelle, M.; Shields, D.C. Computational Approaches to Developing Short Cyclic Peptide Modulators of Protein–Protein Interactions. In Computational Peptidology; Zhou, P., Huang, J., Eds.; Humana Press: New York, NY, USA, 2015; pp. 241–271. [Google Scholar]
- Frecer, V.; Ho, B.; Ding, J.L. De Novo Design of Potent Antimicrobial Peptides. Antimicrob. Agents Chemother. 2004, 48, 3349–3357. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Watson, P.R.; Craven, T.W.; Li, X.; Rettie, S.; Pardo-Avila, F.; Bera, A.K.; Mulligan, V.K.; Lu, P.; Ford, A.S.; et al. Anchor extension: A structure-guided approach to design cyclic peptides targeting enzyme active sites. Nat. Commun. 2021, 12, 3384. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Delafield, D.G.; Li, L. Improved structural elucidation of peptide isomers and their receptors using advanced ion mobility-mass spectrometry. Trends Anal. Chem. 2020, 124, 115546. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Pilon, A.C.; Pinto, M.E.F.; Dias, N.B.; Freire, R.T.; Castro-Gamboa, I.; Bolzani, V.d.S. Computational methods for NMR and MS for structure elucidation I: Software for basic NMR. Phys. Sci. Rev. 2019, 4, 20180108. [Google Scholar] [CrossRef]
- Fernandes, C.; Ribeiro, R.; Pinto, M.; Kijjoa, A. Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022). Molecules 2023, 28, 615. [Google Scholar] [CrossRef] [PubMed]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Peptide-drug conjugates: A new paradigm for targeted cancer therapy. Eur. J. Med. Chem. 2024, 265, 116119. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Srinivasan, N.R.; Govindarajan, G. Antibiotic-Peptide Conjugation Against Multi-drug Resistant Pathogens: A Comprehensive Review for Therapeutics and Drug Delivery Strategies. Int. J. Pept. Res. Ther. 2023, 29, 91. [Google Scholar] [CrossRef]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- De Waard, M.; Sabatier, J.-M. CHAPTER 59-Structure-Function Strategies to Improve the Pharmacological Value of Animal Toxins. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Burlington, MA, USA, 2006; pp. 415–419. [Google Scholar]
- Moulahoum, H.; Ghorbanizamani, F.; Tok, K.; Zihnioglu, F. On the Cyclization of Non-cyclic Peptides for Biological Applications: Inspiration from Naturally Cyclic Peptides. ChemistrySelect 2023, 8, e202301335. [Google Scholar] [CrossRef]
- Costa, L.; Sousa, E.; Fernandes, C. Cyclic Peptides in Pipeline: What Future for These Great Molecules? Pharmaceuticals 2023, 16, 996. [Google Scholar] [CrossRef] [PubMed]
- Northfield, S.E.; Wang, C.K.; Schroeder, C.I.; Durek, T.; Kan, M.-W.; Swedberg, J.E.; Craik, D.J. Disulfide-rich macrocyclic peptides as templates in drug design. Eur. J. Med. Chem. 2014, 77, 248–257. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef]
- Chow, H.Y.; Zhang, Y.; Matheson, E.; Li, X. Ligation Technologies for the Synthesis of Cyclic Peptides. Chem. Rev. 2019, 119, 9971–10001. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Liu, S.-Z.; Cheng, X.-Z.; Ding, W.-Z.; Zhu, T.; Chen, B. Recent progress of on-resin cyclization for the synthesis of clycopeptidomimetics. Chin. Chem. Lett. 2016, 27, 1731–1739. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B.H. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lam, H.Y.; Zhang, Y.; Chan, C.K. Salicylaldehyde Ester-Induced Chemoselective Peptide Ligations: Enabling Generation of Natural Peptidic Linkages at the Serine/Threonine Sites. Org. Lett. 2010, 12, 1724–1727. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W.; Fox, R.M.; Baucom, K.D. Chemoselective Amide Ligations by Decarboxylative Condensations of N-Alkylhydroxylamines and α-Ketoacids. Angew. Chem. Int. Ed. 2006, 45, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.L.; Kiessling, L.L.; Raines, R.T. Staudinger Ligation: A Peptide from a Thioester and Azide. Org. Lett. 2000, 2, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “Traceless” Staudinger Ligation for the Chemoselective Synthesis of Amide Bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef]
- Wills, R.; Adebomi, V.; Raj, M. Site-Selective Peptide Macrocyclization. ChemBioChem 2021, 22, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Adebomi, V.; Cohen, R.D.; Wills, R.; Chavers, H.A.H.; Martin, G.E.; Raj, M. CyClick Chemistry for the Synthesis of Cyclic Peptides. Angew. Chem. Int. Ed. 2019, 58, 19073–19080. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.N.; Mitchell, J.P.; Roberts, K.D. The synthesis of cyclic peptides. J. Chem. Soc. Perkin Trans. 1 2001, 471–484. [Google Scholar] [CrossRef]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. Int. Ed. 2024, 63, e202308251. [Google Scholar] [CrossRef]
- Ribeiro, R.; Costa, L.; Pinto, E.; Sousa, E.; Fernandes, C. Therapeutic Potential of Marine-Derived Cyclic Peptides as Antiparasitic Agents. Mar. Drugs 2023, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine Cyclic Peptides: Antimicrobial Activity and Synthetic Strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Helmy, N.M.; Parang, K. Cyclic Peptides with Antifungal Properties Derived from Bacteria, Fungi, Plants, and Synthetic Sources. Pharmaceuticals 2023, 16, 892. [Google Scholar] [CrossRef]
- Ramadhani, D.; Maharani, R.; Gazzali, A.M.; Muchtaridi, M. Cyclic Peptides for the Treatment of Cancers: A Review. Molecules 2022, 27, 4428. [Google Scholar] [CrossRef] [PubMed]
- Boroojerdi, B.; Duda, P.; Brock, M.K. Treatment of Myasthenia Gravis with Zilucoplan. Patent WO2023215587A1, 9 November 2023. [Google Scholar]
- Kohli, R.M.; Walsh, C.T.; Burkart, M.D. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 2002, 418, 658–661. [Google Scholar] [CrossRef]
- Duda, P.; Farzaneh-far, R.; Ma, Z.; Zhu, N.; Thackaberry, E.; Ricardo, A. Neurological Disease Treatment with Zilucoplan. WO2020086506A1, 30 April 2020. [Google Scholar]
- Arata, M.D.; Dhamnaskar, K.A.; Elbaum, D.; Josephson, K.; Larson, K.C.; Ma, Z.; Nims, N.E.; Ricardo, A.; Seyb, K.; Tang, Q.; et al. Modulation of Complement Activity. WO2015191951A2, 17 December 2015. [Google Scholar]
- Ma, Z.; Zhu, N.; Thackaberry, E.; Farzaneh-far, R.; Ricardo, A. Modulators of Complement Activity. Patent WO2020185541A2, 14 February 2020. [Google Scholar]
- Li, W.; O’Brien-Simpson, N.; Hossain, M.; Wade, J. The 9-Fluorenylmethoxycarbonyl (Fmoc) Group in Chemical Peptide Synthesis–Its Past, Present, and Future. Aust. J. Chem. 2019, 73, 271–276. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nature Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Boccanegra, B.; Carollo, M.; Bottani, E.; Mantuano, P.; Trifirò, G.; De Luca, A. Myasthenia Gravis Treatment: From Old Drugs to Innovative Therapies with a Glimpse into the Future. CNS Drugs 2024, 38, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 526494. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, A.; Sneddon, S.; de Vries, C.S. The Incidence of Myasthenia Gravis: A Systematic Literature Review. Neuroepidemiology 2010, 34, 171–183. [Google Scholar] [CrossRef]
- Sanderson, N.S.R. Complement and myasthenia gravis. Mol. Immunol. 2022, 151, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Varma, A. Myasthenia Gravis: A Systematic Review. Cureus 2023, 15, e50017. [Google Scholar] [CrossRef]
- Grob, D.; Brunner, N.; Namba, T.; Pagala, M. Lifetime course of myasthenia gravis. Muscle Nerve 2008, 37, 141–149. [Google Scholar] [CrossRef]
- Oosterhuis, H.J. The natural course of myasthenia gravis: A long term follow up study. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Hehir, M.K.; Silvestri, N.J. Generalized Myasthenia Gravis: Classification, Clinical Presentation, Natural History, and Epidemiology. Neurol. Clin. 2018, 36, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis—autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002, 2, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hoch, W.; McConville, J.; Helms, S.; Newsom-Davis, J.; Melms, A.; Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 2001, 7, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, O.; Hamuro, J.; Motomura, M.; Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor–related protein 4 in myasthenia gravis. Ann. Neurol. 2011, 69, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zisimopoulou, P.; Evangelakou, P.; Tzartos, J.; Lazaridis, K.; Zouvelou, V.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 2014, 52, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.H.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpen, K.; Verrips, A.; Losen, M.; et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, J.; Gao, Z.; Liu, B.; Zhang, C.; Yu, W.; Yang, J.; Shen, Y.; Qi, L.; Yao, X.; et al. Myasthenia gravis: Molecular mechanisms and promising therapeutic strategies. Biochem. Pharmacol. 2023, 218, 115872. [Google Scholar] [CrossRef]
- Hara, H.; Hayashi, K.; Ohta, K.; Itoh, N.; Nishitani, H.; Ohta, M. Detection and characterization of blocking-type anti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin. Chem. 1993, 39, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.B.; Angus, C.W.; Adams, R.N.; Michelson, J.D.; Hoffman, G.J. Myasthenic Antibodies Cross-Link Acetylcholine Receptors to Accelerate Degradation. N. Engl. J. Med. 1978, 298, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Loutrari, H.; Kokla, A.; Tzartos, S.J. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur. J. Immunol. 1992, 22, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Rødgaard, A.; Nielsen, F.C.; Djurup, R.; Somnier, F.; Gammeltoft, S. Acetylcholine receptor antibody in myasthenia gravis: Predominance of IgG subclasses 1 and 3. Clin. Exp. Immunol. 1987, 67, 82–88. [Google Scholar] [PubMed]
- Deymeer, F. History of Myasthenia Gravis Revisited. Noro Psikiyatr. Arsivi 2021, 58, 154–162. [Google Scholar] [CrossRef]
- Alhaidar, M.K.; Abumurad, S.; Soliven, B.; Rezania, K. Current Treatment of Myasthenia Gravis. J. Clin. Med. 2022, 11, 1597. [Google Scholar] [CrossRef]
- Estephan, E.P.; Baima, J.P.S.; Zambon, A.A. Myasthenia gravis in clinical practice. Arq. Neuropsiquiatr. 2022, 80, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.K. Plasma Exchange for Treatment of Myasthenia Gravis: Pathophysiologic Basis and Clinical Experience. Ther. Apher. 2000, 4, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Guptill, J.T.; Juel, V.C.; Massey, J.M.; Anderson, A.C.; Chopra, M.; Yi, J.S.; Esfandiari, E.; Buchanan, T.; Smith, B.; Atherfold, P.; et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity 2016, 49, 472–479. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Wang, S.-H.; Chien, P.-J.; Shih, C.-M.; Chiu, H.-C. Changes in serum cytokine levels during plasmapheresis in patients with myasthenia gravis. Eur. J. Neurol. 2009, 16, 1318–1322. [Google Scholar] [CrossRef]
- Huda, R. New Approaches to Targeting B Cells for Myasthenia Gravis Therapy. Front. Immunol. 2020, 11, 240. [Google Scholar] [CrossRef]
- Huda, R.; Tüzün, E.; Christadoss, P. Targeting complement system to treat myasthenia gravis. Rev. Neurosci. 2014, 25, 575–583. [Google Scholar] [CrossRef]
- Pandey, S.; Maharana, J.; Li, X.X.; Woodruff, T.M.; Shukla, A.K. Emerging Insights into the Structure and Function of Complement C5a Receptors. Trends Biochem. Sci. 2020, 45, 693–705. [Google Scholar] [CrossRef] [PubMed]
- EMA. Zilbrysq. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/zilbrysq (accessed on 28 November 2023).
- UCB Announces Approval of RYSTIGGO[®] (Rozanolixizumab) and ZILBRYSQ[®] (Zilucoplan) for the Treatment of Adult Patients with Generalized Myasthenia Gravis in Japan. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-approval-of-RYSTIGGOR-rozanolixizumab-and-ZILBRYSQR-zilucoplan-for-the-treatment-of-adult-patients-with-generalized-myasthenia-gravis-in-Japan (accessed on 12 December 2023).
- UCB. UCB Announces U.S. FDA Approval of ZILBRYSQ[®] (zilucoplan) for the Treatment of Adults with Generalized Myasthenia Gravis. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-US-FDA-approval-of-ZILBRYSQR-zilucoplan-for-the-treatment-of-adults-with-generalized-myasthenia-gravis (accessed on 26 November 2023).
- US FDA. Zilbrysq NDA Approval. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216834Orig1s000ltr.pdf (accessed on 26 November 2023).
- UCB Announces European Commission Approval of ZILBRYSQ® (zilucoplan) for the Treatment of Adults with Generalized Myasthenia Gravis. Available online: https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-European-Commission-approval-of-ZILBRYSQRV-zilucoplan-for-the-treatment-of-adults-with-generalized-Myasthenia-Gravis (accessed on 26 November 2023).
- Howard, J.F.; Nowak, R.J.; Wolfe, G.I.; Freimer, M.L.; Vu, T.H.; Hinton, J.L.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Farzaneh-Far, R.; et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients With Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020, 77, 582–592. [Google Scholar] [CrossRef]
- Johnston, J.; Ricardo, A.; Arata, M.; Lickliter, J.; DeMarco, S.; Fahrner, R.; Hammer, R.; Newstat, B.; Roychowdhury, D.; Tobe, S.; et al. A phase 1 single-ascending dose clinical study of RA101495, a subcutaneously administered synthetic macrocyclic peptide inhibitor of complement C5 for treatment of paroxysmal nocturnal hemoglobinuria. In Proceedings of the 21st Congress of the European Hematology Association, Copenhagen, Denmark, 9–12 June 2016; pp. 247–248. [Google Scholar]
- Johnston, J.; Ricardo, A.; Arata, M.; Lickliter, J.; DeMarco, S.J.; Fahrner, R.; Hammer, R.P.; Newstat, B.; Roychowdhury, D.; Tobe, S.; et al. A phase 1 multiple-dose clinical study of RA101495, a subcutaneously administered synthetic macrolytic peptide inhibitor of complement C5 for treatment of paroxysmal noctural hemogloginuria. In Proceedings of the 21st Congress of the European Hematology Association, Copenhagen, Denmark, 9–12 June 2016; pp. 415–416. [Google Scholar]
- Howard, J.F., Jr.; Nowak, R.J.; Wolfe, G.I.; Benatar, M.; Duda, P.W.; MacDougall, J.E.; Kaminski, H.J. Zilucoplan, a Subcutaneously Self-Administered Peptide Inhibitor of Complement Component 5 (C5), for the Treatment of Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial and Open-Label Long-Term Extension. Neurology 2019, 93, e530. [Google Scholar]
- Howard, J.F., Jr.; Bresch, S.; Genge, A.; Hewamadduma, C.; Hinton, J.; Hussain, Y.; Juntas-Morales, R.; Kaminski, H.J.; Maniaol, A.; Mantegazza, R.; et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023, 22, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Utsugisawa, K.; Deguchi, K.; Konno, S.; Masuda, M.; Minami, N.; Murai, H.; Suzuki, S.; Suzuki, Y.; Tsujino, A.; Uzawa, A.; et al. Efficacy and safety of zilucoplan in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the phase III randomized RAISE study. Clin. Exp. Neuroimmunol. 2023, 15, 45–54. [Google Scholar] [CrossRef]
- Farmakidis, C.; Leite, M.; Bresch, S.; Freimer, M.; Genge, A.; Hewamadduma, C.; Hussain, Y.; Maniaol, A.; Mantegazza, R.; Śmiłowski, M.; et al. P273 Long-term safety, efficacy & self-injection satisfaction with zilucoplan in myasthenia gravis: RAISE-XT interim analysis. Neuromuscul. Disord. 2023, 33, S178. [Google Scholar]
- Open-Label Extension of Zilucoplan in Subjects With Generalized Myasthenia Gravis (RAISE-XT). Available online: https://clinicaltrials.gov/study/NCT04225871?cond=NCT04225871&limit=50&rank=1 (accessed on 2 December 2023).
- An Open-Label Study to Evaluate the Safety, Tolerability, and Efficacy of Subcutaneous Zilucoplan in Participants With Generalized Myasthenia Gravis Who Were Previously Receiving Intravenous Complement Component 5 Inhibitors. Available online: https://clinicaltrials.gov/study/NCT05514873?cond=Zilucoplan&limit=50&page=1&rank=2 (accessed on 2 December 2023).
- A Study to Evaluate Subcutaneous Zilucoplan in Pediatric Participants With Generalized Myasthenia Gravis (ziMyG). Available online: https://clinicaltrials.gov/study/NCT06055959?cond=Zilucoplan&limit=50&page=1&rank=4 (accessed on 2 December 2023).
- Mammen, A.L.; Amato, A.A.; Dimachkie, M.M.; Chinoy, H.; Hussain, Y.; Lilleker, J.B.; Pinal-Fernandez, I.; Allenbach, Y.; Boroojerdi, B.; Vanderkelen, M.; et al. Zilucoplan in immune-mediated necrotising myopathy: A phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2023, 5, e67–e76. [Google Scholar] [CrossRef]
- Declercq, J.; Bosteels, C.; Van Damme, K.; De Leeuw, E.; Maes, B.; Vandecauter, A.; Vermeersch, S.; Delporte, A.; Demeyere, B.; Vuylsteke, M.; et al. Zilucoplan in patients with acute hypoxic respiratory failure due to COVID-19 (ZILU-COV): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 934. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, E.; Van Damme, K.F.; Declercq, J.; Bosteels, C.; Maes, B.; Tavernier, S.J.; Detalle, L.; Smart, T.; Glatt, S.; Debeuf, N.; et al. Efficacy and safety of the investigational complement C5 inhibitor zilucoplan in patients hospitalized with COVID-19: An open-label randomized controlled trial. Respir. Res. 2022, 23, 202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).