Modulatory Effect of Croton heliotropiifolius Kunth Ethanolic Extract on Norfloxacin Resistance in Staphylococcus aureus
Abstract
1. Introduction
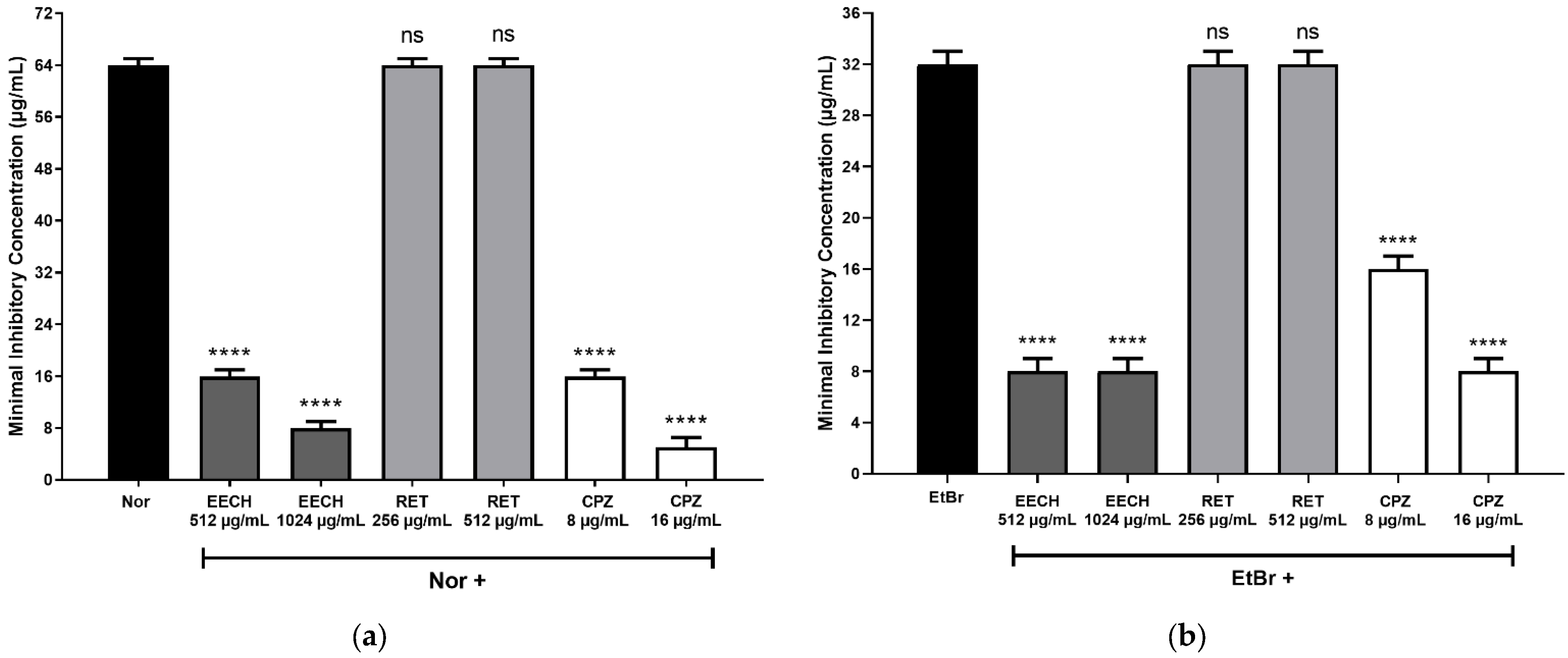
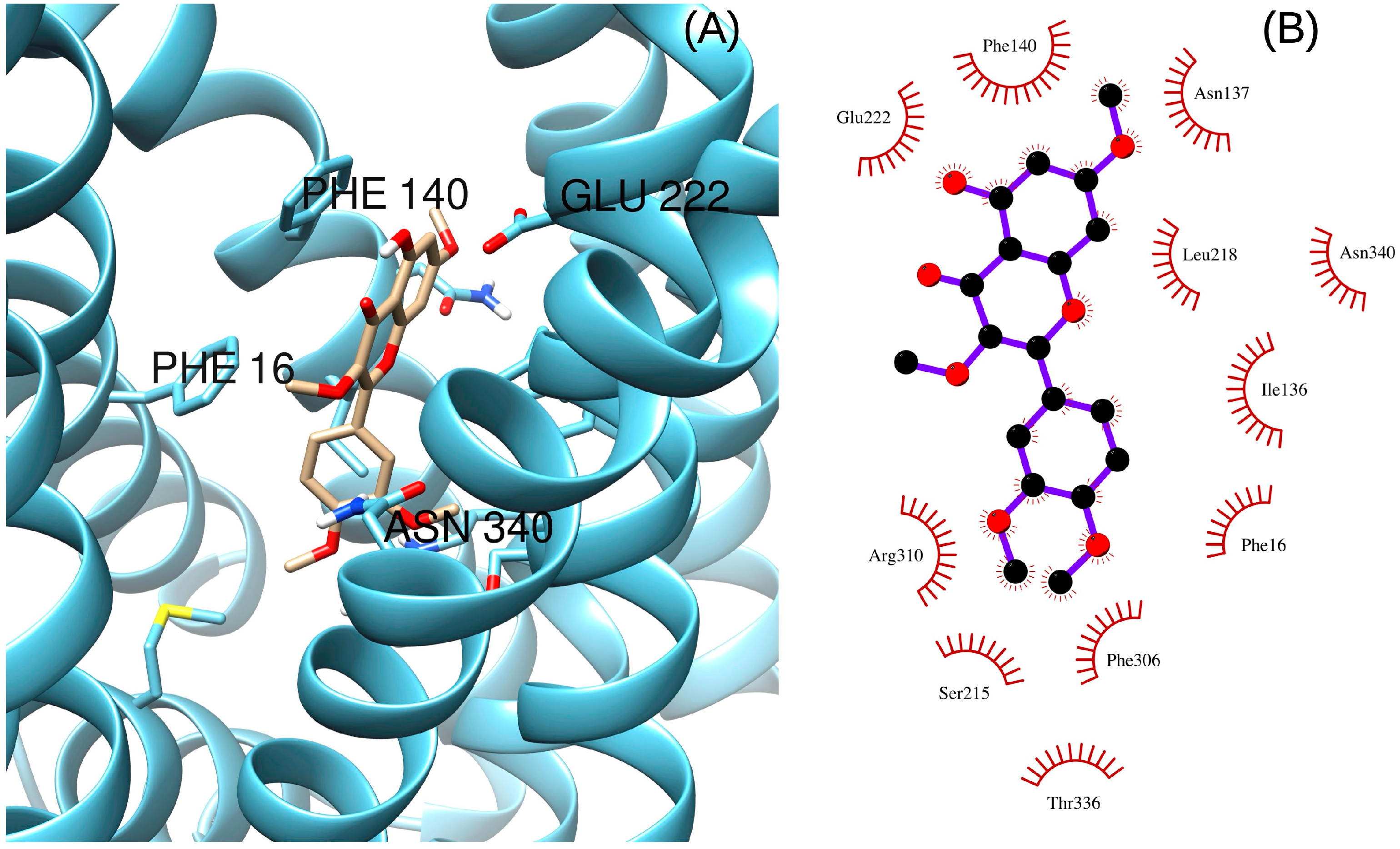
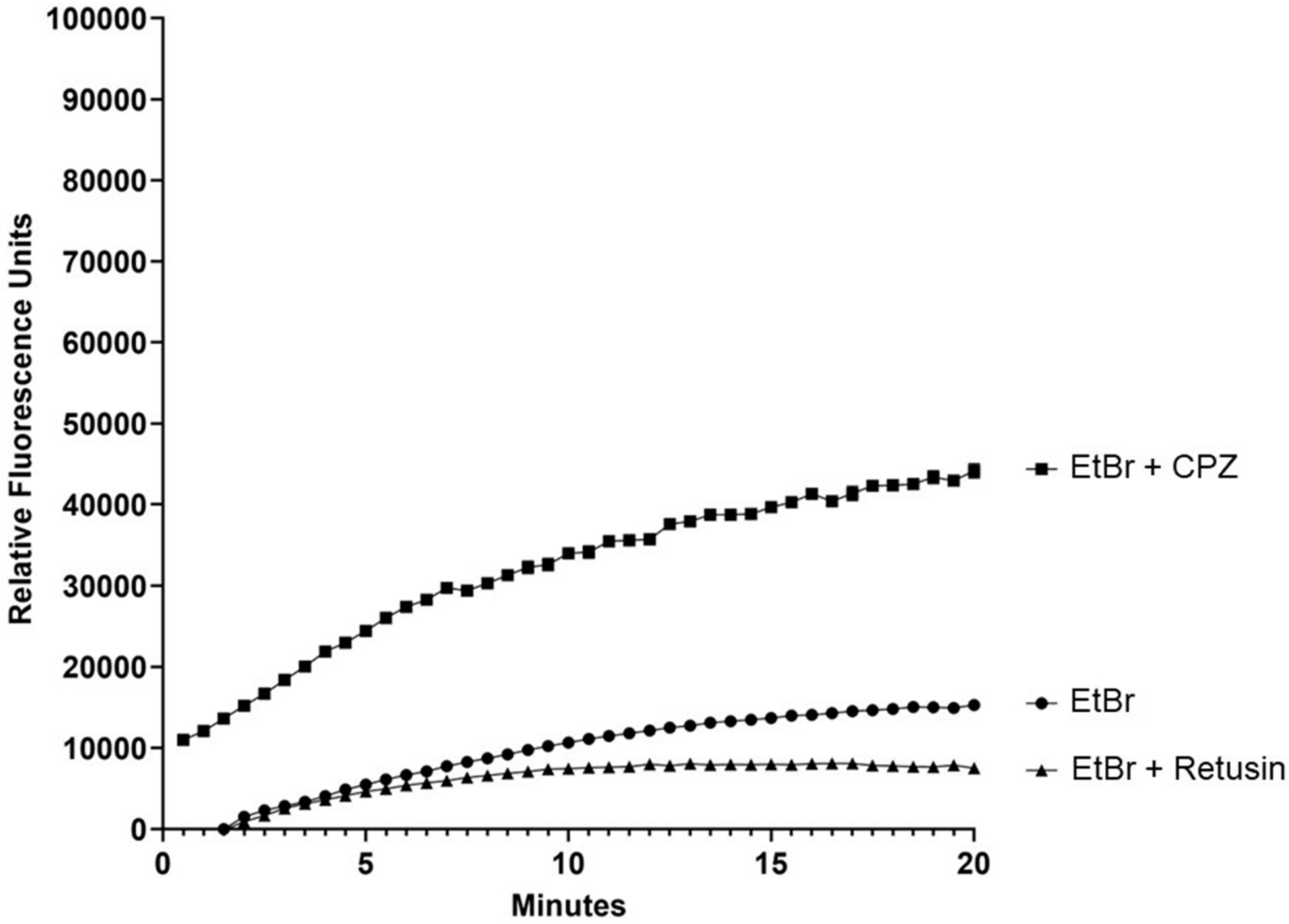
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Nuclear Magnetic Resonance (NMR) Analysis
3.3. Strains and Chemicals
3.4. Assays for Evaluation of the Intrinsic Antimicrobial Activity
3.5. Assays for Evaluation of the Modulating Effect of Antibiotic-Resistance
3.6. Statistical Analysis
3.7. Docking Procedure
3.8. Fluorometry Assays for Evaluation of the Inhibition of Ethidium Bromide Efflux
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chua, K.Y.L.; Howden, B.P.; Jiang, J.-H.; Stinear, T.; Peleg, A.Y. Population genetics and the evolution of virulence in Staphylococcus aureus. Infect. Genet. Evol. 2014, 21, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist. Updat. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Rao, M.; Padyana, S.; Dipin, K.M.; Kumar, S.; Nayak, B.B.; Varela, M.F. Antimicrobial compounds of plant origin as efflux pump inhibitors: New avenues for controlling multidrug resistant pathogens. J. Antimicrob. Agents 2018, 4, 1–6. [Google Scholar]
- Beytur, A.; Yakupogullari, Y.; Oguz, F.; Otlu, B.; Kaysadu, H. Oral amoxicillin-clavulanic acid treatment in urinary tract infections caused by extended-spectrum beta-lactamase–producing organisms. Jundishapur J. Microbiol. 2014, 8, e13792. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Silva, R.L.P.; Costa, M.S.; Menezes, I.R.A.; Calixto-Júnior, J.T.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Leal-Balbino, T.C.; et al. Evaluation of the tannic acid inhibitory effect against the NorA efflux pump of Staphylococcus aureus. Microb. Pathog. 2016, 97, 9–13. [Google Scholar] [CrossRef]
- Bharate, J.B.; Singh, S.; Wani, A.; Sharma, S.; Joshi, P.; Khan, I.A. Discovery of 4-acetyl-3-(4-fluorophenyl)-1-(p-tolyl)-5-methylpyrrole as a dual inhibitor of human P-glycoprotein and Staphylococcus aureus NorA efflux pump. Org. Biomol. Chem. 2015, 13, 5424–5431. [Google Scholar] [CrossRef]
- Costa, L.M.; de Macedo, E.V.; Oliveira, F.A.A.; Ferreira, J.H.L.; Gutierrez, S.J.C.; Peláez, W.J.; Lima, F.C.A.; de Siqueira Júnior, J.P.; Coutinho, H.D.M.; Kaatz, G.W.; et al. Inhibition of the NorA efflux pump of Staphylococcus aureus by synthetic riparins. J. Appl. Microbiol. 2016, 121, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.R.S.; Scherf, J.R.; Freitas, T.S.; Menezes, I.R.A.; Pereira, R.L.S.; Santos, J.F.S.; Jesus, S.S.P.; Lopes, T.P.; Silveira, Z.S.; Oliveira-Tintino, C.D.M.; et al. Effect of Carvacrol and Thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus aureus strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Silva, H.T.; Magnani, M.; de Siqueira, S.; de Souza, E.L.; de Siqueira-Júnior, J.P. Fruit flavonoids as modulators of norfloxacin resistance in Staphylococcus aureus that overexpresses norA. LWT-Food Sci. Technol. 2017, 85, 324–326. [Google Scholar] [CrossRef]
- Sun, Z.-L.; Sun, S.-C.; He, J.-M.; Lan, J.-E.; Gibbons, S.; Mu, Q. Synergism of sophoraflavanone G with norfloxacin against effluxing antibiotic-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2020, 56, 106098. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Donato, I.A.; Bezerra, S.R.; Santos, H.S.; Bandeira, P.N.; do Nascimento, M.T.R.; Guedes, J.M.; Freitas, P.R.; de Araújo, A.C.J.; de Freitas, T.S.; et al. Synthesis, spectroscopic characterization, and antibacterial activity of chalcone (2E)-1-(3′-aminophenyl)-3-(4-dimethylaminophenyl)-prop-2-en-1-one against multiresistant Staphylococcus aureus carrier of efflux pump mechanisms and β-lactamase. Fundam. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.S.; Xavier, J.C.; Pereira, R.L.S.; Rocha, J.E.; Campina, F.F.; de Araújo Neto, J.B.; Silva, M.M.C.; Barbosa, C.R.S.; Marinho, E.S.; Nogueira, C.E.S.; et al. In vitro and in silico studies of chalcones derived from natural acetophenone inhibitors of NorA and MepA multidrug efflux pumps in Staphylococcus aureus. Microb. Pathog. 2021, 161, 105286. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.G.; Slotved, H.-C.; Mølgaard, P.; Olsen, C.E.; Christensen, S.B. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorganic Med. Chem. 2012, 20, 4514–4521. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Latacz, G.; Kaczor, A.; Czekajewska, J.; Żesławska, E.; Chudzik, A.; Karczewska, E.; Nitek, W.; Kieć-Kononowicz, K.; Handzlik, J. Phenylpiperazine 5,5-Dimethylhydantoin derivatives as first synthetic inhibitors of Msr(A) efflux pump in Staphylococcus epidermidis. Molecules 2020, 25, 3788. [Google Scholar] [CrossRef] [PubMed]
- Nasato, M.C.; Krautler, M.I.L.; Pereira, P.S.; Costa, J.G.M.D.; Rodrigues, F.F.G.; Teixeira, A.M.R.; Ribeiro-Filho, J.; Tintino, S.R.; de Menezes, I.R.A.; Coutinho, H.D.M.; et al. The 1,8-naphthyridines sulfonamides are NorA efflux pump inhibitors. J. Glob. Antimicrob. Resist. 2021, 24, 233–240. [Google Scholar]
- Felicetti, T.; Cannalire, R.; Nizi, M.G.; Tabarrini, O.; Massari, S.; Barreca, M.L.; Manfroni, G.; Schindler, B.D.; Cecchetti, V.; Kaatz, G.W.; et al. Studies on 2-phenylquinoline Staphylococcus aureus NorA efflux pump inhibitors: New insights on the C-6 position. Eur. J. Med. Chem. 2018, 155, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef]
- Buonerba, F.; Lepri, S.; Goracci, L.; Schindler, B.D.; Seo, S.M.; Kaatz, G.W.; Cruciani, G. Improved potency of indole-based NorA Efflux Pump Inhibitors: From serendipity toward rational design and development. J. Med. Chem. 2017, 60, 517–523. [Google Scholar] [CrossRef]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef]
- Cavalcanti, D.R.; Albuquerque, U.P. The “Hidden Diversity” of Medicinal Plants in Northeastern Brazil: Diagnosis and Prospects for Conservation and Biological Prospecting. Evid-Based Compl. Altern. Med. 2013, 2013, 102714. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.; Sales, M.F.; Gomes, A.P.S.; Carneiro-Torres, D.S. Sinopse das espécies de Croton L. (Euphorbiaceae) no estado de Pernambuco, Brasil. Acta Bot. Bras. 2010, 24, 441–453. [Google Scholar] [CrossRef]
- Crepaldi, C.G.; Campos, J.L.A.; Albuquerque, U.P.; Sales, M.P. Richness and ethnobotany of the family Euphorbiaceae in a tropical semiarid landscape of Northeastern Brazil. S. Afr. J. Bot. 2016, 102, 157–165. [Google Scholar] [CrossRef]
- Queiroz, M.M.F.; Queiroz, E.F.; Zeraik, M.L.; Marti, G.; Favre-Godal, Q.; Simoes-Pires, C.; Marcour, L.; Carrupt, P.A.; Cuendet, M.; Paulo, M.Q.; et al. Antifungals and acetylcholinesterase inhibitors from the stem bark of Croton heliotropiifolius. Phytochem. Lett. 2014, 10, 88–93. [Google Scholar] [CrossRef]
- Malafaia, C.B.; Jardelino, A.C.S.; Silva, A.G.; Souza, E.B.; Macedo, A.J.; Correia, M.T.S.; Silva, M.V. Effects of Caatinga Plant Extracts in Planktonic Growthand Biofilm Formation in Ralstonia solanacearum. Microb. Ecol. 2018, 75, 555–561. [Google Scholar] [CrossRef]
- Silva, T.; Alves, A.C.L.; Azevedo, F.R.; Marco, C.A.; Santos, H.R.; Alves, W.S. Efeito larvicida de óleos essenciais de plantas medicinais sobre larvas de Aedes aegypti L. (Diptera: Culicidae). Rev. Verde Agroecol. Desenvolv. Sustent. 2017, 12, 256–260. [Google Scholar] [CrossRef]
- Dória, G.A.A.; Silva, W.J.; Carvalho, G.A.; Alves, P.B.; Cavalcanti, S.C.H. A study of the larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm. Biol. 2010, 48, 615–620. [Google Scholar] [CrossRef]
- Magalhães, C.R.I.; Oliveira, C.R.F.; Matos, C.H.C.; Brito, S.S.S.; Magalhães, T.A.; Ferraz, M.S.S. Potencial inseticida de óleos essenciais sobre Tribolium castaneum em milho armazenado. Rev. Bras. Plantas Med. 2015, 17, 1150–1158. [Google Scholar] [CrossRef][Green Version]
- Alencar-Filho, J.M.T.; Araujo, L.C.; Oliveira, A.P.; Guimarães, A.L.; Pacheco, A.G.M.; Silva, F.S.; Calvacanti, L.S.; Lucchese, A.M.; Almeida, J.R.G.S.; Araujo, E.C.C. Chemical composition and antibacterial activity of essential oil from leaves of Croton heliotropiifolius in different seasons of the year. Rev. Bras. Farmacogn. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Araújo, F.M.; Dantas, M.C.S.M.; Silva, L.S.; Aona, L.Y.S.; Tavares, I.F.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crop. Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef] [PubMed]
- Ndunda, B.; Langat, M.K.; Midiwo, J.O.; Omosa, L.K. Diterpenoid derivatives of Kenyan Croton sylvaticus. Nat. Prod. Commun. 2015, 10, 557–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Li, J.G.; Li, G.Q.; Xu, J.J.; Wu, X.; Ye, W.C.; Li, Y.L. Clerodane diterpenoids from Croton crassifolius. J. Nat. Prod. 2012, 75, 2188–2192. [Google Scholar] [CrossRef]
- Barreto, M.B.; Gomes, C.L.; Freitas, J.V.B.D.; Pinto, F.D.C.L.; Silveira, E.R.; Gramosa, N.V.; Torres, D.S.C. Flavonoides e terpenoides de Croton muscicarpa (Euphorbiaceae). Quim. Nova 2013, 36, 675–679. [Google Scholar] [CrossRef]
- Zou, G.A.; Su, Z.H.; Zhang, H.W.; Wang, Y.; Yang, J.S.; Zou, Z.M. Flavonoids from the stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules 2010, 15, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, R.; Díaz, B.K.; Aguilar, M.I.; Diego, N.; Lotina-Hennsen, B. Pachypodol from Croton ciliatoglanduliferus Ort. as Water-Splitting Enzyme Inhibitor on Thylakoids. J. Agric. Food Chem. 2006, 54, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Howes, M.J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- Vanvuuren, S.F.; Viljoen, A.M. In vitro evidence of phyto-synergy for plant part combinations of Croton gratissimus (Euphorbiaceae) used in African traditional healing. J. Ethnopharmacol. 2008, 119, 700–704. [Google Scholar] [CrossRef]
- Selowa, S.C.; Shai, L.J.; Masoko, M.P.; Makgotho, S.R.; Magano, S.R. Antibacterial activity of extracts of three Croton species collected in Mpumalanga region in South Africa. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 98–103. [Google Scholar] [CrossRef]
- Rodrigues, F.F.G.; Costa, J.G.M.; Coutinho, H.D.M. Synergy effects of the antibiotics gentamicin and the essential oil of Croton zehntneri. Phytomedicine 2009, 16, 1052–1055. [Google Scholar] [CrossRef]
- Diniz, A.C.B.; Astarita, L.V.; Santarém, E.R. Alteração dos metabólitos secundários em plantas de Hypericum perforatum L. (Hypericaceae) submetidas à secagem e ao congelamento. Acta Bot. Bras. 2007, 21, 443–450. [Google Scholar] [CrossRef]
- Neyfakh, A.A.; Borsch, C.M.; Kaatz, G.W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 1993, 37, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Markham, P.N.; Westhaus, E.; Klyachko, K.; Johnson, M.E.; Neyfakh, A.A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Palazzotti, D.; Bissaro, M.; Bolcato, G.; Astolfi, A.; Felicetti, T.; Sabatini, S.; Sturlese, M.; Cecchetti, V.; Barreca, M.L.; Moro, S. Deciphering the Molecular Recognition Mechanism of Multidrug Resistance Staphylococcus aureus NorA Efflux Pump Using a Supervised Molecular Dynamics Approach. Int. J. Mol. Sci. 2019, 20, 4041. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.B.; de Sousa, J.N.; Costa, L.M.; Oliveira, F.A.A.; dos Santos, R.C.; Nunes, A.S.S.; da Silva, W.O.; Cordeiro, P.J.M.; Neto, J.S.L.; de Siqueira-Júnior, J.P.; et al. Antimicrobial activity of Phyllanthus amarus Schumach. & Thonn and inhibition of the NorA efflux pump of Staphylococcus aureus by Phyllanthin. Microb. Pathog. 2019, 130, 242–246. [Google Scholar]
- Rezende-Júnior, L.M.; Andrade, L.M.S.; Leal, A.L.A.B.; Mesquita, A.B.S.; Santos, A.L.P.A.; Lima-Neto, J.S.; Siqueira-Júnior, J.P.; Nogueira, C.E.S.; Kaatz, G.W.; Coutinho, H.D.M.; et al. Chalcones isolated from Arrabidaea brachypoda flowers as inhibitors of NorA and mepa multidrug efflux pumps of Staphylococcus aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Maia, G.L.A.; Falcão-Silva, V.S.; Aquino, P.G.V.; Araújo-Júnior, J.X.; Tavares, J.F.; Silva, M.S.; Rodrigues, L.C.; Siqueira-Júnior, J.P.; Barbosa-Filho, J.M. Flavonoids from Praxelis clematidea R.M. King and Robinson modulate bacterial drug resistance. Molecules 2011, 16, 4828–4835. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.F.S.; Tintino, S.R.; Silva, A.R.P.; Barbosa, C.R.S.; Scherf, J.R.; Silveira, Z.S.; Freitas, T.S.; Lacerda Neto, L.J.; Barros, L.M.; Menezes, I.R.A.; et al. Enhancement of the antibiotic activity by quercetin against Staphylococcus aureus efflux pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Leal, A.L.A.B.; Machado, A.J.T.; Bezerra, C.F.; Inácio, C.E.S.; Rocha, J.E.; Sales, D.L.; de Freitas, T.S.; Almeida, W.O.; Amaral, W.; da Silva, L.E.; et al. Chemical identification and antimicrobial potential of essential oil of Piper rivinoides Kunth (BETIS-WHITE). Food Chem. Toxicol. 2019, 131, 110559. [Google Scholar] [CrossRef]
- Silva, S.W.C.; Monção, N.B.N.; Araújo, B.Q.; Arcanjo, D.D.R.; Ferreira, J.H.L.; Lima Neto, J.S.; Citó, A.M.G.L.; Siqueira-Júnior, J.P.; Kaatz, G.W.; Barreto, H.M. Antimicrobial activity of Mimosa caesalpiniifolia Benth and its interaction with antibiotics against Staphylococcus aureus strains overexpressing efflux pump genes. Lett. Appl. Microbiol. 2019, 69, 57–63. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Matias, E.F.F.; Santos, K.K.A.; Tintino, S.R.; Sousa, C.E.S.; Guedes, G.M.M.; Santos, F.A.D.; Costa, J.G.M.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the norfloxacin antibiotic activity by gaseous contact with the essential oil of Croton zehntneri. J. Young Pharm. 2010, 2, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.D.; Matias, E.F.; Santos, K.K.; Santos, F.A.; Morais-Braga, M.F.; Sousa, T.M.; Andrade, J.C.; Souza, C.E.; Titino, S.R.; Guedes, G.M.; et al. Modulation of the norfloxacin resistance in Staphylococcus aureus by Croton campestris A. and Ocimum gratissimum L. Biomedica 2011, 31, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.D.M.; Lavor, A.K.L.S.; Matias, E.F.F.; Alves, E.F.; Santos, B.S.; Figueredo, F.G.; Lima, L.F.; Leite, N.F.; Sobral-Sousa, C.E.; Andrade, J.C.; et al. In vitro potentiation of the aminoglycoside antibiotic activity by Croton campestris A. against multiresistant bacteria. J. Biol. Act. Prod. Nat. 2015, 5, 322–330. [Google Scholar]
- Kaatz, G.W.; Seo, S.M. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993, 37, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and unexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
- Hospital, A.; Andrio, P.; Fenollosa, C.; Cicin-Sain, D.; Orozco, M.; Gelpí, J.L. MDWeb and MDMoby: An integrated web-based platform for molecular dynamics simulations. Bioinformatics 2012, 28, 1278–1279. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; O’Brien, L.; Wahiduzzaman, M.; Foster, T.J. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob. Agents Chemother. 2000, 44, 1404–1406. [Google Scholar] [CrossRef]

| Metabolites | Assignment | δ 1H (mult., J in Hz) | δ 13C | HMBC Correlations 13C (ppm) |
|---|---|---|---|---|
| Retusin | C-6 | 6.36 (d, 2.4) | 98.1 | 92.3 (C-8), 106.2 (C-10), 156.8 (C-10), 165.6 (C-7) |
| C-8 | 6.45 (d, 2.4) | 92.3 | 98.1 (C-6), 106.2 (C-10), 165.6 (C-7) | |
| C-2′ | 7.69 (d, 2.3) | 111.7 | 122.2 (C-5′), 151.1 (C-2), 151.4 (C-4′) | |
| C-5′ | 6.99 (d, 8.8) | 110.8 | 122.9 (C-1′), 149.2 (C-3′) | |
| C-6′ | 7.74 (dd, 8.8 and 2.3) | 122.2 | 111.7 (C-2′), 151.4 (C-4′) | |
| OCH3-3 | 3.86 (s) | 60.3 | 139.1 (C-3) | |
| OCH3-7 | 3.87 (s) | 56.0 | 165.6 (C-7) | |
| OCH3-3′ | 3.97 (s) | 55.9 | 149.2 (C-3′) | |
| OCH3-4′ | 3.98 (s) | 55.8 | 151.4 (C-4′) | |
| OH-5 | 12.64 (s) | - | 161.9 (C-5) | |
| Clerodane diterpenes | CH-3 | 5.11 (m) | 124.4 | 16.0 (C-18), 39.8 (C-5) |
| CH3-17 | 0.86 (d, 6.5) | 22.7 | 22.8 (C-7), 28.1 (C-8), 39.1 (C-9) | |
| CH3-17 | 1.04 (d, 6.5) | 28.6 | 20.3 (C-7), 27.8 (C-8), 29.6 (C-9) | |
| CH3-18 | 1.59 (s) | 16.0 | 39.8 (C-5), 124.4 (C-3), 135.0 (C-4) |
| MIC (μg/mL) | ||||
|---|---|---|---|---|
| CEPAS | EECH | CPZ | NOR | EtBr |
| Staphylococcus aureus SA1199B | 16,384 | 128 | 64 | 32 |
| Staphylococcus aureus ATCC 25923 | 8192 | - | - | - |
| Streptococcus pyogenes ATCC 19615 | 4096 | - | - | - |
| Escherichia coli ATCC 25922 | 4096 | - | - | - |
| Pseudomonas aeruginosa ATCC 27853 | 16,384 | - | - | - |
| Salmonella Typhimurium ATCC 14028 | 16,384 | - | - | - |
| Candida albicans ATCC 10231 | 16,384 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, S.B.d.; Alcântara, F.A.d.O.; Leal, A.L.A.B.; Veloso, K.H.d.S.; Sousa, L.d.R.; Oliveira, A.P.d.; Santos, A.D.d.C.; Dutra, L.M.; Almeida, J.R.G.d.S.; Nogueira, C.E.S.; et al. Modulatory Effect of Croton heliotropiifolius Kunth Ethanolic Extract on Norfloxacin Resistance in Staphylococcus aureus. Drugs Drug Candidates 2024, 3, 1-12. https://doi.org/10.3390/ddc3010001
Brito SBd, Alcântara FAdO, Leal ALAB, Veloso KHdS, Sousa LdR, Oliveira APd, Santos ADdC, Dutra LM, Almeida JRGdS, Nogueira CES, et al. Modulatory Effect of Croton heliotropiifolius Kunth Ethanolic Extract on Norfloxacin Resistance in Staphylococcus aureus. Drugs and Drug Candidates. 2024; 3(1):1-12. https://doi.org/10.3390/ddc3010001
Chicago/Turabian StyleBrito, Samara Barbosa de, Felipe Araújo de Oliveira Alcântara, Antonio Linkoln Alves Borges Leal, Kaliny Henri da Silva Veloso, Leonardo da Rocha Sousa, Ana Paula de Oliveira, Alan Diego da Conceição Santos, Lívia Macedo Dutra, Jackson Roberto Guedes da Silva Almeida, Carlos Emídio Sampaio Nogueira, and et al. 2024. "Modulatory Effect of Croton heliotropiifolius Kunth Ethanolic Extract on Norfloxacin Resistance in Staphylococcus aureus" Drugs and Drug Candidates 3, no. 1: 1-12. https://doi.org/10.3390/ddc3010001
APA StyleBrito, S. B. d., Alcântara, F. A. d. O., Leal, A. L. A. B., Veloso, K. H. d. S., Sousa, L. d. R., Oliveira, A. P. d., Santos, A. D. d. C., Dutra, L. M., Almeida, J. R. G. d. S., Nogueira, C. E. S., Souza, J. S. N. d., Cruz-Martins, N., Arcanjo, D. D. R., & Barreto, H. M. (2024). Modulatory Effect of Croton heliotropiifolius Kunth Ethanolic Extract on Norfloxacin Resistance in Staphylococcus aureus. Drugs and Drug Candidates, 3(1), 1-12. https://doi.org/10.3390/ddc3010001









