Abstract
Exportin 1 (XPO1) is a crucial molecule of nucleocytoplasmic transport. Among others, it exports molecules important for oncogenesis from the nucleus to the cytoplasm. The expression of XPO1 is increased in numerous malignancies, which contributes to the abnormal localization of tumor suppressor proteins in the cytoplasm and subsequent cell cycle dysregulation. Selective inhibitors of nuclear export (SINEs) are novel anticancer agents that target XPO1, arrest tumor suppressor proteins in the nucleus, and induce apoptosis in cancer cells. Selinexor, a first-in-class SINE, has already been approved for the treatment of relapsed/refractory multiple myeloma and relapsed/refractory diffuse large B cell lymphoma not otherwise specified. It has also been proven effective in relapsed/refractory and previously untreated acute myeloid leukemia patients. In addition, numerous studies have yielded promising results in other malignancies of the hematopoietic system and solid tumors. However, future clinical use of selinexor and other SINEs may be hampered by their significant toxicity.
1. Introduction
The transport of molecules between the nucleus and the cytoplasm is a crucial process underlying the biology of eukaryotic cells. The nuclear envelope successfully separates DNA replication and RNA transcription in the nucleus from protein synthesis and their further modification in the cytoplasm. Small molecules can diffuse passively through the nuclear pore complex (NPC) [1,2]. However, most macromolecules require nucleocytoplasmic transport factors in order to reach the other side of the nuclear envelope [3]. These factors belong predominantly to the family of karyopherin-β. They are subdivided into exportins, which transport cargo to the cytosolic compartment, and importins, which are able to take cargo to the nuclear compartment [1,3,4].
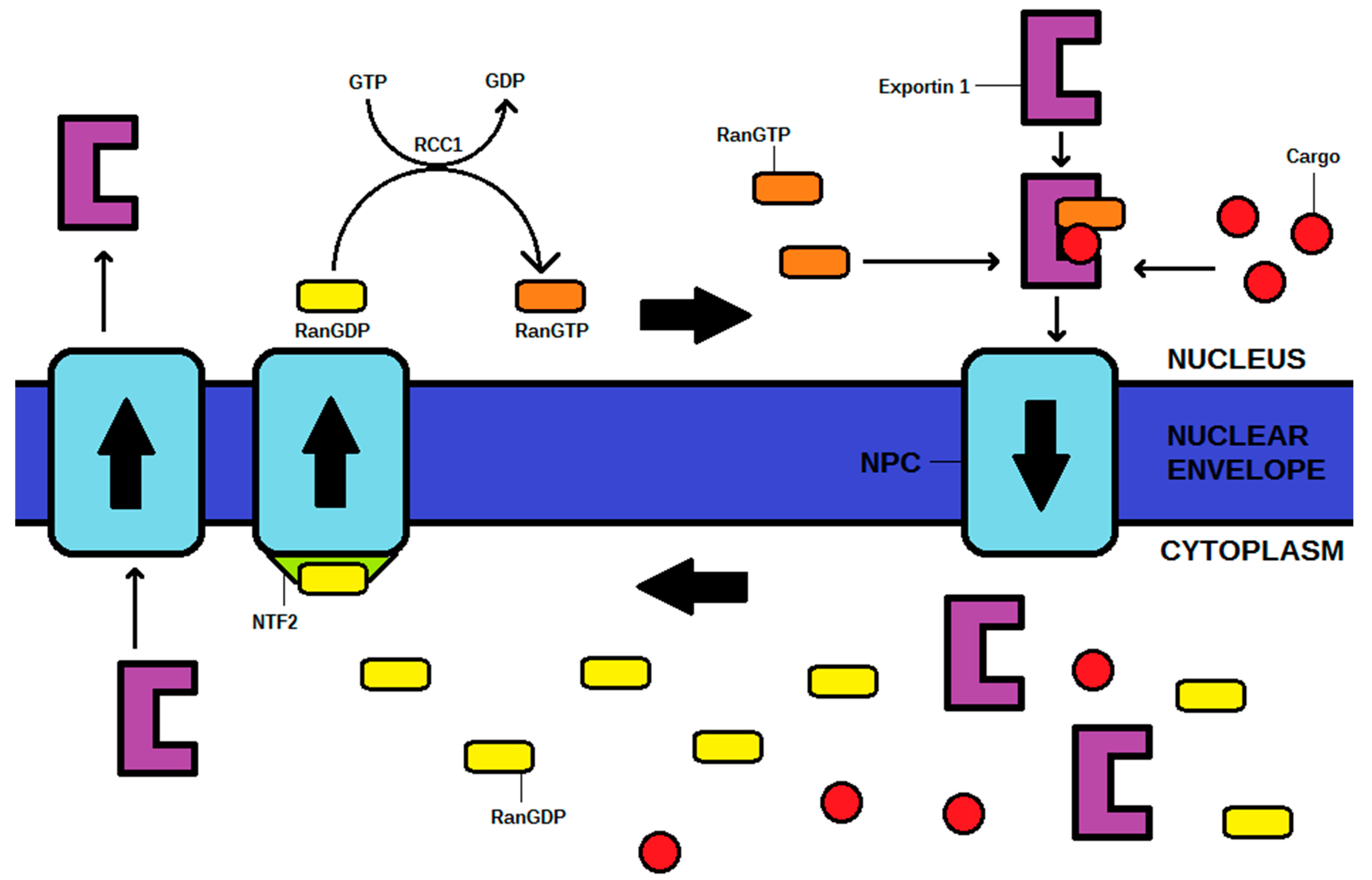
Nuclear export requires the conversion of RanGTP to RanGDP, and phosphate to provide energy for the process. Therefore, in order to reach the cytoplasm through NPC, an exportin, RanGTP, and a cargo must create a complex. After the process, RanGDP is transported back to the nucleus via NPC, in the presence of nuclear transport factor 2 (NTF2). The exportin itself shuttles back from the cytoplasm into the nucleus via NPC, where it is ready to begin another cycle. Afterward, RanGDP is phosphorylated in the nucleus to RanGTP by GTP, and therefore, further export is possible. A high concentration of RanGTP in the nucleus is granted by the regulator of chromosome condensation 1 (RCC1), an element essential to maintain the Ran cycle [1,2,3,5]. The Ran cycle is shown in Figure 1.
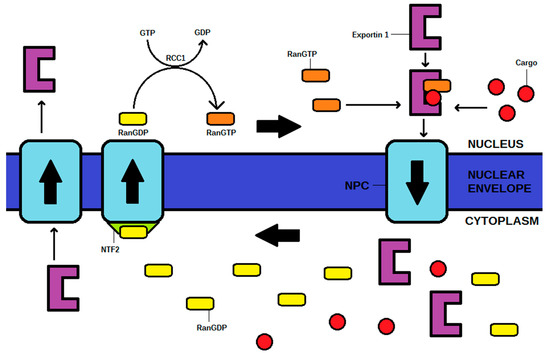
Figure 1.
The mechanism of the Ran cycle and exportin (exportin 1 as the example) transporting a cargo from the nucleus to cytoplasm in exportin 1-RanGTP-cargo complex. NPC—nuclear pore complex, NTF2—nuclear transport factor 2, RCC1—regulator of chromosome condensation 1.
Chromosome maintenance protein 1 (CRM1), also known as exportin 1 (XPO1), is the first protein confirmed to provide nuclear export [6] and the most crucial exportin known to date [3]. XPO1 transports over 200 different cargoes, such as transcriptional factors, translational factors, or kinases [7,8]. The comprehensive molecular mechanism of the exportin 1–RanGTP cargo complex creation has not been fully explained yet. Notably, transported cargoes must contain leucine-rich nuclear export signals (NES), while XPO1 in an unbound state has a ring-like hydrophobic structure which contains a NES-binding domain [4]. Moreover, XPO1 contains a loop in one of HEAT repeats, which regulates cargo binding through an allosteric mechanism [3]. The architecture of XPO1 was first modeled in 2004 based on X-ray crystallography, homology modeling, and finally, electron microscopy [9].
Some of the transported proteins are protooncogene products or tumor suppressor proteins, and therefore, XPO1 might impact oncogenesis [7]. Furthermore, the overexpression or increased activity of XPO1 is a common phenomenon in cancer cells, leading to nuclear deprivation of critical suppressor and regulatory proteins, such as p53, p21, IκB, RB, p27 [4,8,10]. As a result, these proteins are no more effective in counteracting genetic aberrations after being exported. Therefore, the cell cycle becomes dysregulated, leading to abnormal proliferation [8]. For instance, such a phenomenon was confirmed in breast cancer with BRCA1 mutation, where the altered localization of the BRCA1 protein product in the cytosolic compartment promotes metastasis [11].
Furthermore, XPO1 exports miRNAs that regulate cellular quiescence, which is the reversible state of proliferative arrest [12]. However, this is only an alternative pathway, as exportin 5 seems to play a more significant role in miRNA transport than exportin 1 [13]. The role of miRNA transport in oncogenesis is still poorly understood, although it is claimed that genetic abnormalities, such as deletions, might downregulate miRNA expression in some cancers. It leads to the dysregulation of quiescence/proliferation checkpoints and creates an opportunity for growth factors to increase proliferation [14]. Such a phenomenon occurs in chronic lymphocytic leukemia (CLL) with 13q14 deletions (more than half of CLL cases), where neoplastic cells are deprived of miRNA15 and miRNA16 [15].
Exportin 1 might also induce resistance to chemotherapy and to targeted cancer therapies by preventing the agents from achieving proper concentration in the nucleus [4]. For example, XPO1 is responsible for resistance to imatinib, ibrutinib, or cisplatin [5,10]. Overall, inducing pharmacotherapy resistance and escaping the cell cycle as a result of exporting tumor suppressor proteins both contribute to the poor prognosis of cancers with increased XPO1 expression [16].
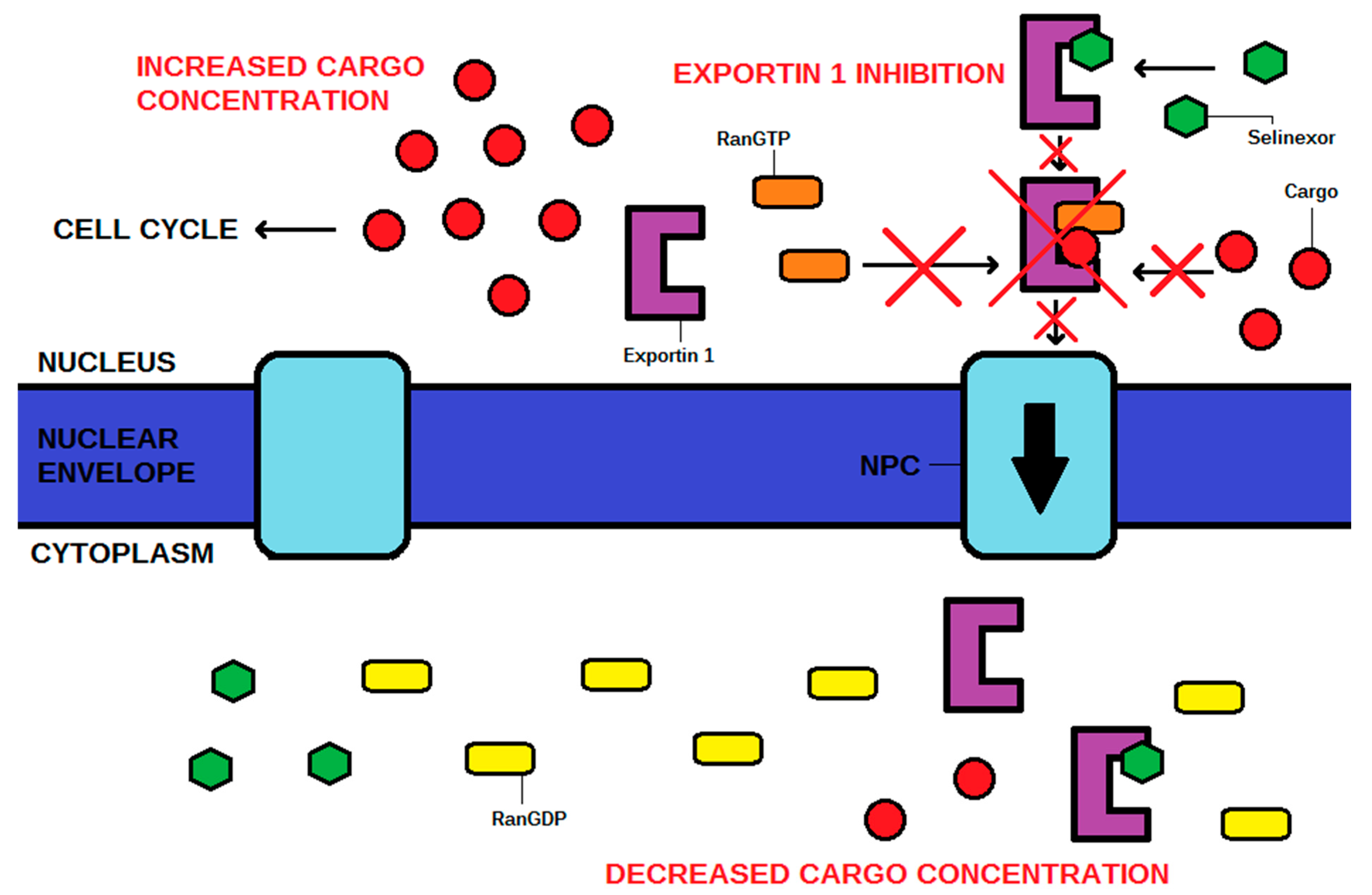
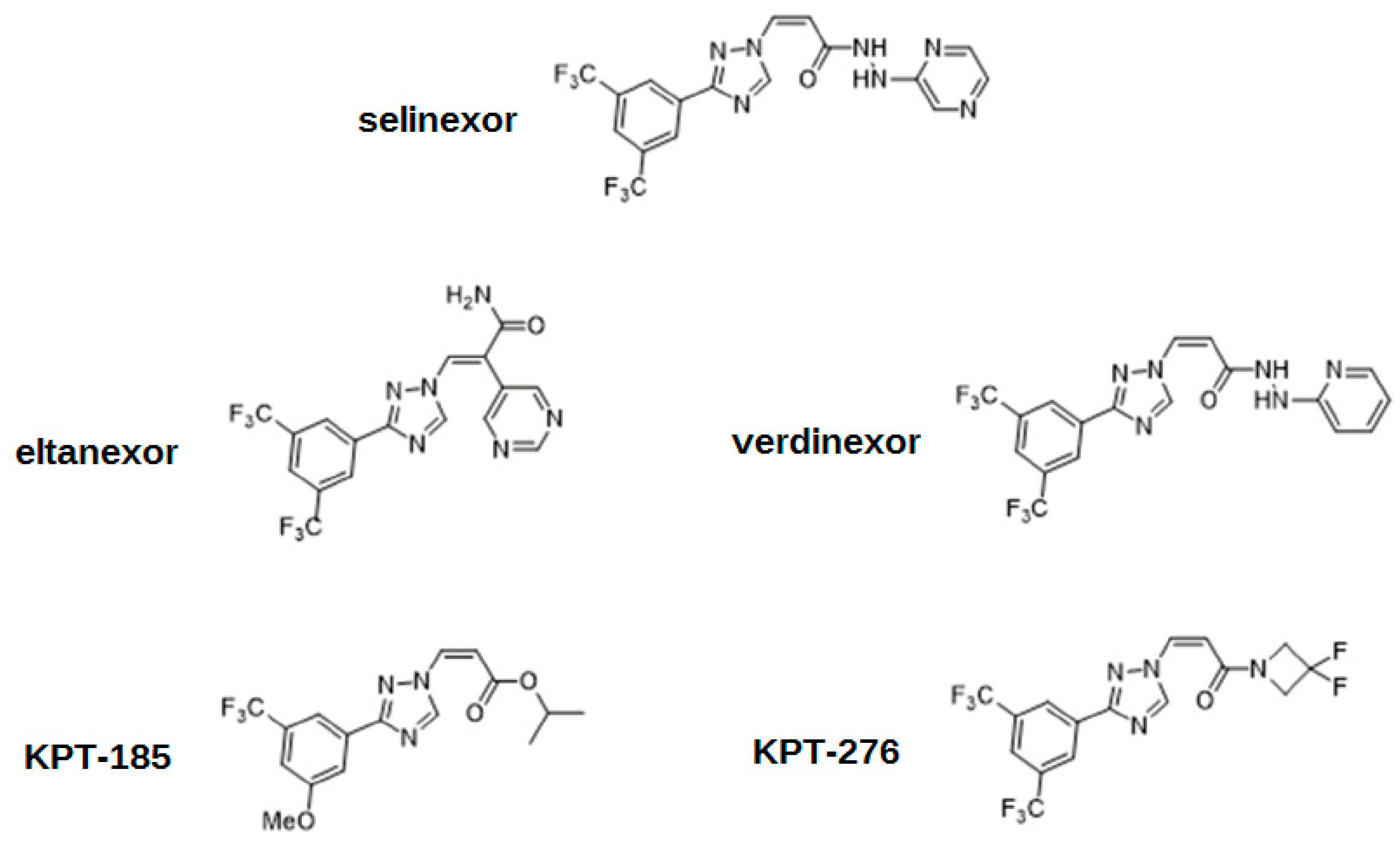
The action of XPO1 might be inactivated by an antibiotic leptomycin B, which attaches covalently to the cysteine-528 residue of the NES-binding domain [4,5,17]. Although successful in vitro and in vivo, this drug has never been used by clinicians due to its unacceptable toxicity, and subsequent recommendations to cease further clinical trials [5,8,18]. XPO1 inhibition has also been achieved using other molecules such as antibiotics (anguinomycins, ratjadones), natural substances (goniothalamin, valtrate, curcumin), and most importantly, using selinexor and other selective XPO1 inhibitors such as eltanexor [4]. These drugs, known as selective inhibitors of nuclear export (SINEs), have the same mechanism of action as leptomycin B, targeting the cysteine-528 of XPO1. SINE molecules contain hydrophobic trifluoromethyl groups buried deeply in the NES-binding domain of XPO1. However, the most significant part of their structures is the triazole scaffold, which forms covalent yet reversible bindings with the cysteine-528 of XPO1 [1]. The cellular mechanism of action is shown in Figure 2. The chemical structures of SINEs are shown in Figure 3.
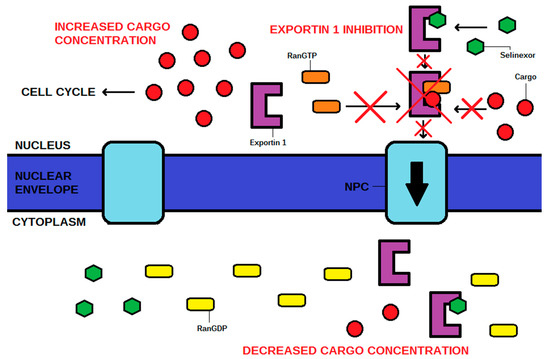
Figure 2.
The mechanism of action of selinexor, a selective inhibitor of nuclear export (SINE). The cargo is a tumor suppressor protein that rebuilds the cell cycle. NPC—nuclear pore complex.
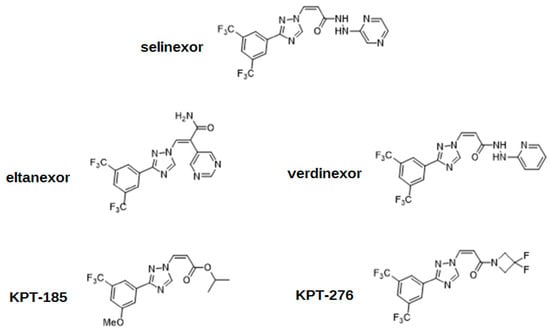
Figure 3.
The chemical structures of selective inhibitors of nuclear export (SINEs).
This review aims to summarize up-to-date clinical applications and preclinical findings, and to discuss the potential future role of this category of drugs. It is necessary to search for promising agents and new cancer treatment methods, as described malignancies of the hematopoietic system and solid tumors remain a challenge for clinicians, despite improvements in overall survival. In particular, the options for heavily pretreated patients with relapsed or refractory disease are still limited, as mentioned in the newest reviews and guidelines for multiple myeloma [19,20], diffuse large B-cell lymphoma [21,22], and acute myeloid leukemia [23,24].
2. Selinexor in R/R MM
Selinexor (KPT-330, brand name—Xpovio) is the first-in-class oral SINE targeted at exportin 1. The drug has been approved for the treatment of relapsed/refractory multiple myeloma (R/R MM) [25,26,27]. It was registered by the FDA in July 2019 in combination with dexamethasone for patients who have undergone at least four prior therapies and are refractory to at least two proteasome inhibitors (PIs), at least two immunomodulatory agents (IMIDs), and anti-CD38 monoclonal antibodies (MABs) [25,27]. Although these three classes of drugs are crucial in MM treatment, the majority of patients become refractory to them at some point of the therapy (“triple refractory MM”) [26]. Some patients undergo even more therapies (for instance, autologous stem cell transplantation) before selinexor is administered, with a median of seven lines of prior treatment revealed by some studies [28,29]. Management algorithms suggest considering selinexor in heavily pretreated MM patients as an alternative to BCL-2 inhibitor venetoclax, immunotherapy or CAR-T cell therapies [30]. In December 2020, selinexor was approved by the FDA in combination with bortezomib and dexamethasone for patients who have undergone one prior therapy only [31].
As SINE, selinexor affects cancer cells by trapping tumor suppressor proteins and oncoprotein mRNA in the nucleus and rebalancing the cell cycle [16]. Additionally, selinexor increases the expression of glucocorticoid receptors and inhibits the mTOR pathway synergistically with dexamethasone. Hence, an accelerated registration pathway was used for combination of selinexor with dexamethasone [29,32]. Moreover, selinexor inhibits DNA repair mechanisms in cancer cells and sensitizes these cells to DNA-damaging agents [33]. Therefore, clinical trials assessing the effectiveness of selinexor + dexamethasone and another additional agent (bortezomib [34,35], carfilizomib [36,37], pomalidomide [38] or doxorubicin [39]) have also been performed. Hence, it is still possible that the indications could be widened, and selinexor could be used in earlier stages of MM in other combinations, before the development of refractoriness to many other agents. Preclinical studies in this field are also ongoing [40]. The combination of venetoclax and selinexor was proven effective in R/R MM with translocation t(11;14) [41]. Moreover, selinexor was confirmed to overcome hypoxia-induced drug resistance to bortezomib, which supports the use of the currently approved combination: selinexor + bortezomib + dexamethasone [42].
Selinexor in combination with dexamethasone produced a 26% overall response rate (ORR) in R/R myeloma. The median duration of remission was 4.4 months, the median overall survival (OS) was 8.6 months, and the median progression-free survival (PFS) was 3.7 months [28,43]. Other studies yielded similar results (the ORR of 21%, the median duration of remission of 5 months) [29]. For the combination of selinexor + carfilizomib + dexamethasone, the overall response rate (ORR) was 38% and the median OS was 22.7 months [36,44]. The combination of selinexor + bortezomib + dexamethasone produced the ORR of 63% in patients with a median of three prior therapies [44,45]. However, these studies did not include a control group. The only phase III clinical trial assessed the efficacy of selinexor in combination with bortezomib and dexamethasone (SVd) versus bortezomib and dexamethasone (Vd) [34,46]. The median PFS was significantly longer (13.9 months vs. 9.5 months, p = 0.0075), and the ORR was significantly higher in SVd group (76% vs. 62%, p = 0.0012). The results were even more promising in terms of ORR, if only subgroups with high-risk cytogenetics were compared (ORR: 77% vs. 56%, p = 0.0008). However, no significant differences in terms of PFS have been observed in this subgroup. Moreover, no significant difference in OS between SVd and Vd groups has been found [34]. Considering all collected data, selinexor is still beneficial for patients with R/R MM, regardless of cytogenetic risk [47]. Interestingly, the latest cytogenetic analyses revealed that sensitivity to selinexor is strongly correlated with the expression of ABCC4 in MM cells, which implies the usefulness of ABCC4 as a predictive biomarker [48].
According to numerous studies, selinexor was not well tolerated by the patients. Due to adverse reactions, treatment was discontinued in 27% of cases. Over 50% of patients required dose reductions or dose delays, and fatal adverse effects occurred in 9% of cases [26]. Thrombocytopenia, anemia, leukopenia, neutropenia; hyponatremia; dyspnea, and upper respiratory infections occurred frequently. Less serious reactions were nausea, vomiting, diarrhea, decreased weight, and fatigue [26,38]. The occurrence of ocular adverse events was estimated at 20% [49], while neurological adverse events occurred in 25% of cases [50]. Due to the unsafe profile of selinexor, dedicated recommendations for dealing with adverse reactions have been prepared [51].
3. Selinexor in R/R DLBCL NOS
Selinexor was approved by FDA in June 2020 for the treatment of R/R diffuse large B cell lymphoma (DLBCL) not otherwise specified (NOS) after at least two lines of systemic therapy. As the third (or further) line of treatment, selinexor might be considered alternatively to CAR-T cell therapies and antibody-drug conjugates (ADCs) (polatuzumab vedotin, loncastuximab tesirine) [50].
Selinexor in monotherapy produced an ORR of 29%, where 38% of responses lasted at least 6 months. The median duration of remission of 9.3 months was observed, while the expected survival for R/R DLBCL patients is generally less than 6 months [50,52]. In another study, the ORR was estimated at 28%, the median PFS at 3.6 months, and the median OS at 9.1 months [53,54]. Notably, similar ORRs were achieved regardless of DLBCL subtype (germinal center B cell-like, non-germinal center B cell-like), but ORRs were lower for double-hit and triple-hit subtypes [55,56]. Patients with other non-Hodgkin lymphoma (NHL) types (follicular lymphoma, mantle cell lymphoma, and Richter transformation) also achieved an ORR of approximately 30% [50,55]. This would imply the possibility of creating new drug combinations in the near future. Moreover, in NHL, selinexor was proven to enhance the effectiveness of standard therapy R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) [57].
XPO1 overexpression was confirmed to worsen the prognosis of DLBCL patients with unfavorable cytogenetics (BCL-2 overexpression, double-hit, triple-hit) [56]. Moreover, targeting XPO1 with selinexor is only effective in the absence of the TP53 mutation. Otherwise, resistance is induced [58]. Therefore, selinexor does not seem to be beneficial for patients with unfavorable cytogenetic changes, but this phenomenon requires confirmation on larger groups of patients in further studies. Comprehensive genomic profiling in DLBCL revealed the recurrent mutation E571K of XPO1 [57]. Its role in pathogenesis and potential responsibility for inducing resistance is still unclear. However, it might be another target for future novel drugs.
Statistical analysis revealed that the adverse events of monotherapy with selinexor did not have a clinically meaningful negative impact on patients’ quality of life, despite the adverse events of grade 3 or 4 experienced in over 80% of cases [59]. Therefore, despite the unsafe profile of selinexor, adverse reactions during therapy should be considered manageable. It is worth noticing that the treatment response and stable disease groups were associated with significantly higher quality of life than the group of patients who experienced progressive disease. This would imply that the incidence and severity of adverse events during therapy had an impact on dose reductions or treatment discontinuation, and therefore affected the eventual outcome.
4. Selinexor in Other Hematologic Malignancies
Research on selinexor as an anticancer agent is not limited to its current indications. Its efficacy in acute myeloid leukemia (AML) has been assessed in numerous preclinical and clinical trials. Moreover, compassionate use in some cases has also been reported [60].
Selinexor with a CLAG (cladribine, cytarabine, filgrastim) regimen in R/R AML patients produced a complete remission (CR) rate of 45% [61]. In another study, the combination of selinexor and decitabine in R/R AML produced an ORR of 30%, a CR rate of 25%, a median OS of 5.9 months, and a median PFS of 5.9 months [62]. With selinexor as monotherapy, the ORR was 14%, the median OS among responders was 9.7 months, and the median PFS among responders was 5.1 months [63].
In previously untreated AML patients, selinexor added to standard therapy (daunorubicin + cytarabine: ‘3+7’) produced a significantly higher CR rate in comparison with standard therapy alone (80% vs. 59%, p = 0.018) [64]. Moreover, selinexor + ‘3+7’ was proven to be a safe regimen, and it produced a median OS of 10.3 months in previously untreated AML patients [65]. Maintenance therapy with selinexor after allogeneic stem cell transplantation in high-risk AML patients was also proven safe and effective [66]. A meta-analysis of the drug’s efficacy and safety in AML treatment is in progress [67]. Therefore, new approval for an XPO1 inhibitor in AML is probable in the near future. Moreover, an ORR of 26% was achieved in patients with myelodysplastic syndrome (MDS) or oligoblastic AML, which implies the possibility of using an XPO1 inhibitor at even earlier stages of the disease [64,68].
Moreover, in mouse AML models, selinexor was proven to synergize with topoisomerase inhibitors, increase sensitivity to idarubicin, and reduce DNA damage repair [69]. A synergistic anticancer effect with azacitidine was confirmed in AML cell lines [70].
Selinexor in combination with DICE (dexamethasone, ifosfamide, carboplatin, etoposide) produced an ORR of 82% and a 1-year survival of 67% in patients with R/R peripheral T cell lymphoma (PTCL) or natural killer/T cell lymphoma (NKTL) [71]. It is an alternative for other targeted agents in PTCL treatment (PI3K inhibitors, monoclonal antibodies, ADC—brentuximab vedotin) [72].
Selinexor was proven to synergize with ibrutinib, to increase OS in mouse CLL models, and to be effective in ibrutinib-resistant CLL in vitro [73]. The efficacy of selinexor in monotherapy was confirmed in CLL cells in vitro [74].
Selinexor combined with imatinib selectively targets chronic myeloid leukemia (CML) stem cells, implying the possibility of successfully eliminating residual disease in patients resistant to imatinib alone [75].
Examples of clinical studies involving selinexor in malignancies of the hematopoietic system are shown in Table 1.

Table 1.
Examples of clinical studies involving selinexor in malignancies of the hematopoietic system (alphabetical order). Abbreviations: AML—acute myeloid leukemia, CR—complete remission, DICE—dexamethasone, ifosfamide, carboplatin, etoposide, DLBCL—diffuse large B cell lymphoma, MDS—myelodysplastic syndrome, MM—multiple myeloma, NHL—non-Hodgkin lymphoma, ORR—overall response rate, PFS—progression-free survival, R-CHOP—rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, ‘3+7’—daunorubicin + cytarabine therapy.
5. Selinexor in Solid Tumors
Selinexor has been approved for malignancies of the hematopoietic system, and current research is mainly focused on its use in these diseases. However, it has also been considered for the therapy of solid tumors [76].
Selinexor has demonstrated anticancer activity in advanced gynecological malignancies [77,78]. In a phase I clinical study of selinexor with carboplatin and paclitaxel (CP) in patients with ovarian or endometrial cancer, the ORR was 57%, and the regimen was proven safe [77]. Furthermore, in the phase II clinical study of patients with recurrent ovarian, endometrial or cervical cancer, single-agent selinexor produced the CR rates of 30%, 35%, and 24%, respectively, and a median OS of 7.3 months, 7.0 months, and 5.0 months, respectively. Moreover, in this study, selinexor was safe for and tolerated by the patients. The majority of adverse events were mild (grade 1 or 2), reversible and fully manageable with supportive care [78].
Selinexor reduced angiogenesis, tumor growth, and the incidence of metastases and increased the OS in preclinical models of prostate cancer [79]. However, clinical activity could not be fully assessed, as the phase II clinical study of patients with refractory, castration-resistant metastatic prostate cancer was terminated before completion due to unacceptable toxicity of selinexor in combination with abiraterone and enzalutamide [80].
Selinexor was proven to decrease the concentration of hypoxia-inducible factor 1 (HIF-1) and, subsequently, to decrease radioresistance in human osteosarcoma cell lines [81]. Notably, selinexor successfully inhibited the growth of xenografts derived from other sarcoma types (liposarcoma, leiomyosarcoma, rhabdomyosarcoma, gastrointestinal stromal tumor, undifferentiated sarcomas) [82]. However, despite potential activity in numerous preclinical studies of sarcoma cell lines [83,84], the phase I clinical study did not yield promising results. Single-agent selinexor did not produce an objective response (by RECIST) in any of the evaluated patients [85].
Additionally, despite significant tumor reduction, no partial or complete responses were observed in the phase II clinical study of single-agent selinexor in patients with recurrent or metastatic salivary gland tumors [86].
There are numerous in vitro/in vivo preclinical studies that have assessed the efficacy of selinexor in these and other solid tumors. The examples are presented in Table 2. However, despite promising results and a further improvement in our understanding of the underlying molecular background, actual clinical prospects are still limited, and the safety profile is questionable.

Table 2.
Examples of preclinical and clinical studies involving selinexor in solid tumors (alphabetical order). Abbreviations: CR—complete remission, OS—overall survival, PFS—progression-free survival.
6. Other SINEs
Eltanexor (KPT-8602) is a second-generation SINE proven to inhibit XPO1 in malignancies of the hematopoietic system in preclinical models in vitro and in vivo. In AML and DLBCL patient-derived xenografts, eltanexor exerts a significant synergistic effect when co-administered with a BCL-2 inhibitor (venetoclax) [102]. Moreover, the drug demonstrates potent activity in both B cell and T cell acute lymphoblastic leukemia (ALL) models in vivo [103], and synergizes with dexamethasone in these malignancies in vitro [104].
In the phase I clinical trial, oral high-dose eltanexor produced an ORR of 40%, a median PFS of 4.5 months, and a median OS of 17.8 months in patients with R/R MM [105]. These results are promising, but they require a comparative study with selinexor in order to determine actual usefulness in the clinic. In patients with high-risk MDS that is refractory to hypomethylating agents (azacitidine), eltanexor produced an ORR of 53%, a CR of 47% and a median OS of 9.9 months [106]. Eltanexor has also been investigated in preclinical models of solid tumors, with promising results in glioblastoma [107] and castration-resistant prostate cancer [108]. The severity and incidence of adverse reactions associated with eltanexor were lower than for selinexor in R/R MM patients. The majority of events were cytopenias and gastrointestinal abnormalities, and rarely, mild neurological abnormalities. Patient withdrawal due to adverse events was only 8% [105].
KPT-185 is a SINE that has demonstrated anticancer activity in mantle cell lymphoma (MCL) in vitro [109] and in MM in vitro [110]. However, the efficacy was lower in MCL cells with high expression of TP53 [111]. In in vitro and in vivo preclinical models of AML, KPT-185 induced the apoptosis and downregulation of the FLT3 oncogene [112]. This activity has also been shown in non-small-cell lung cancer in vitro and in vivo [113].
KPT-276 is a SINE of similar properties to KPT-185. Both agents have demonstrated co-activity in MCL [114] and NHL [115] cell lines. Moreover, KPT-276 was proven effective in MM in vitro and in vivo, which contributed to the initiation of the phase 1 clinical trial in this indication [116].
Verdinexor (KPT-335) has demonstrated anticancer activity in preclinical models of esophageal cancer [117] and neuroblastoma [118] in vitro and in vivo. However, the research on this SINE is mainly focused on antiviral activity against the influenza A virus [119] and RSV [120]. Interestingly, according to the most recent studies, selinexor has demonstrated antiviral activity against Merkel cell carcinoma virus [121,122]. Apparently, the potential of this category of drugs is not limited to neoplastic diseases.
Other examples of using SINEs in preclinical models of malignancies of the hematopoietic system are felezonexor (SL-401) in CML, AML, MM, and Hodgkin lymphoma [123]; KPT-251 in AML [124]; and CBS9106 in MM [125]. Although all these drugs significantly decreased the proliferation of cancer cells in vitro, no clinical trials have been initiated for many years, and these drugs are far from clinical application. The most probable candidate for a second-generation SINE in cancer therapy is eltanexor. Its efficacy and safe profile have already been proven by the phase I clinical studies in the years 2021–2022. Hopefully, a safer alternative for selinexor will find its place in clinics in the near future. The chemical structures of selinexor and other SINEs are shown in Figure 3. Examples of studies involving other SINEs in neoplastic diseases are shown in Table 3.

Table 3.
Examples of studies involving selective inhibitors of nuclear export other than selinexor in malignancies of the hematopoietic system and solid tumors (alphabetical order). Abbreviations: ALL—acute lymphoblastic leukemia, AML—acute myeloid leukemia, DLBCL—diffuse large B cell lymphoma, MCL—mantle cell lymphoma, MM—multiple myeloma, NHL—non-Hodgkin lymphoma, NSCLC—non-small-cell lung carcinoma, ORR—overall response rate, OS—overall survival.
7. Summary and Future Directions
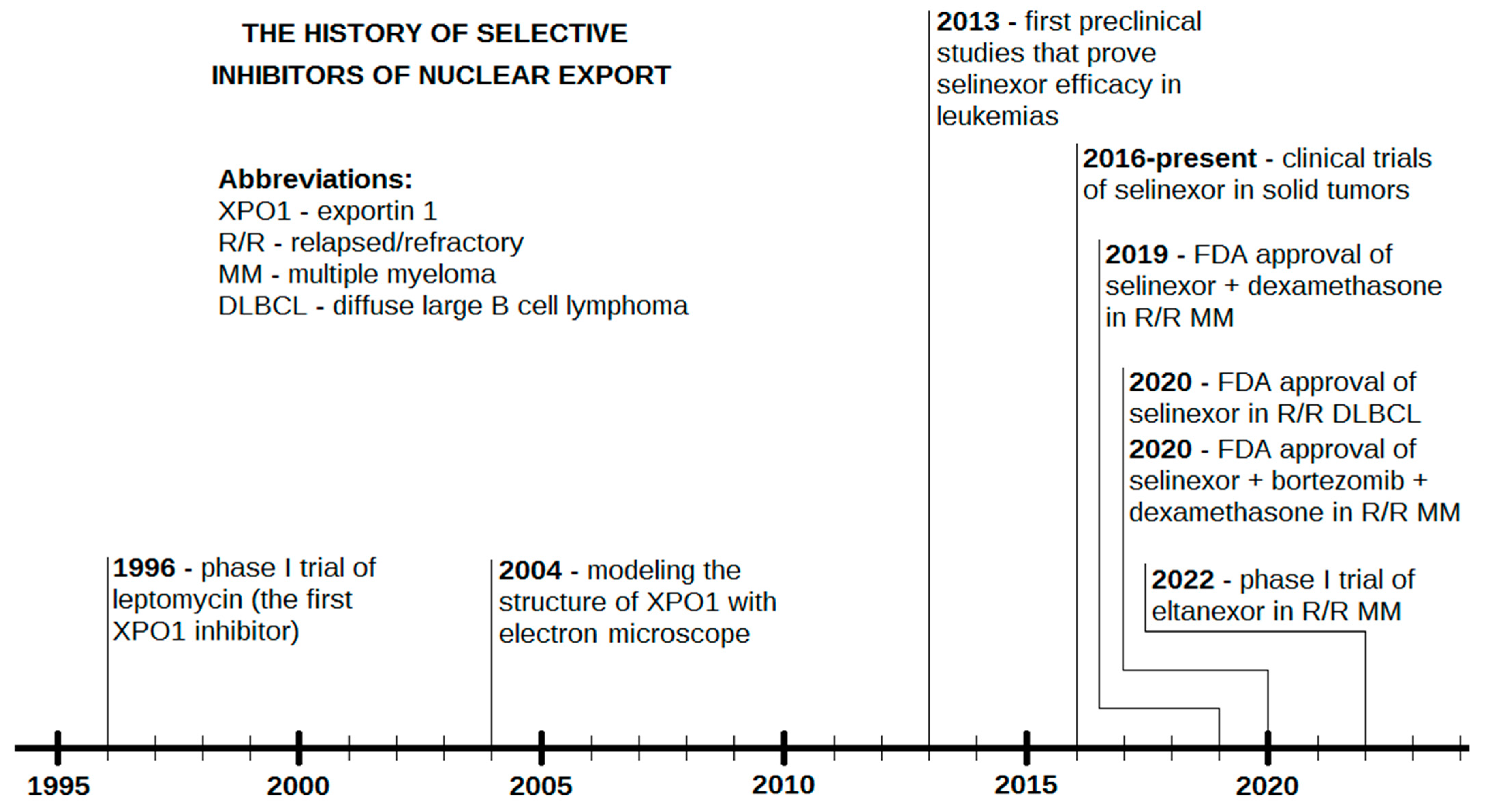
Selinexor is a promising agent already registered for the second or further line therapy of R/R MM [31] and in the third or further line therapy of DLBCL NOS [50]. The drug has already been and is still being assessed in numerous clinical and preclinical studies. Significant achievements have been demonstrated in R/R AML [64,65], which might contribute to the approval of selinexor for this indication in the near future. Although research has mainly focused on malignancies of the hematopoietic system [126], using XPO1 as a target may also be useful in solid tumors (Table 2). Moreover, due to numerous novel SINEs with promising anticancer activity, it is possible that selinexor will not be the only agent with this mechanism of action in the clinic. Eltanexor, whose efficacy has already been documented in a phase I clinical trial for R/R MM, is a possible candidate for a registered second-generation SINE in the longer term [105]. A timeline of the crucial facts and events in the history of SINEs is shown in Figure 4.
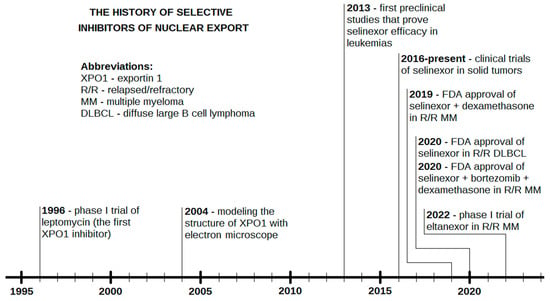
Figure 4.
The history of selective inhibitors of nuclear export (SINEs).
On the other hand, the unsafe toxicity profile of selinexor might be a potential barrier to its clinical application. The ratio and wide spectrum of adverse events in R/R MM patients led to dedicated guidelines based on independent clinical trials [51]. In DLBCL patients, the impact on quality of life was not that explicit [59]. Moreover, unacceptable toxicity was the reason for the termination of the phase II clinical trial in patients with castration-resistant metastatic prostate cancer [80]. On the other hand, a phase II clinical trial in patients with advanced gynecological malignancies yielded more promising results [78]. According to the only clinical trial, eltanexor had a safer toxicity profile than selinexor [105]. Other SINEs have not been investigated yet, but hopefully they will show similarities to eltanexor, leading to another SINE being applied in clinics. This would be a significant event, as the options for patients with R/R malignancies of the hematopoietic system and solid tumors are still limited.
However, even the approval and successful clinical application of next-generation SINEs would probably not constitute a breakthrough, due to the heterogeneity of neoplasms. The wide spectrum of genetic abnormalities and different protein expression profiles has a decisive impact on patients’ response to treatment and prognosis in DLBCL [127] or AML [128], as examples. Therefore, especially in relapsed/refractory diseases, personalized therapy is necessary to maximize clinical benefits. However, the more unique options are available, the more we can offer to patients, and this is the purpose of novel, promising categories of drugs such as SINEs. Considering the recent success of selinexor in different malignancies and current attempts to optimize new candidates, this category of drugs is likely to play a greater role in the future, if its toxicity profile and accessibility are improved [129].
XPO1 is a vulnerable target in different malignancies of the hematopoietic system, especially MM and solid tumors [116]. However, although XPO1 is crucial, it is not the only molecule that regulates nuclear export [130]. Multiple exportins have already been identified. Therefore, targeting one of them might not fully exploit the potential of anticancer activity. Examples of these exportins include Cse1, Pse1, Kap123, Sxm1 and Mtr10 [130]. There are also potential prospects of finding promising nuclear import inhibitors [131], the examples of which include karyostatin 1A, importazole, ivermectine, and mifepristone. Although these molecules are widely used as biological tools to identify cargo proteins, none of them have entered clinical trials for neoplastic disorders thus far [131].
Overall, nuclear transport has fundamental importance for a variety of pathophysiological processes. Therefore, understanding the interplay of exportins and importins will be crucial for the further perfection of compounds affecting nuclear transport. Hopefully, this will result in even more success of the aforementioned category of agents in the future.
Author Contributions
Both authors contributed to the work and meet the ICMJE criteria of authorship. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fung, H.Y.; Chook, Y.M. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin. Cancer Biol. 2014, 27, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Liu, H. The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig. 2019, 25, 6. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Bono, F.; Jinek, M.; Conti, E. Structural biology of nucleocytoplasmic transport. Annu. Rev. Biochem. 2007, 76, 647–671. [Google Scholar] [CrossRef]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The nuclear export protein XPO1—From biology to targeted therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef]
- El-Tanani, M.; Dakir, E.-H.; Raynor, B.; Morgan, R. Mechanisms of Nuclear Export in Cancer and Resistance to Chemotherapy. Cancers 2016, 8, 35. [Google Scholar] [CrossRef]
- Stade, K.; Ford, C.S.; Guthrie, C.; Weis, K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 1997, 90, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Kang, T.; Thakurta, T.G.; Ng, C.; Rogers, A.N.; Larsen, M.R.; Lapierre, L.R. Exportin 1 modulates life span by regulating nucleolar dynamics via the autophagy protein LGG-1/GABARAP. Sci. Adv. 2022, 8, 1604. [Google Scholar] [CrossRef]
- Crochiere, M.L.; Baloglu, E.; Klebanov, B.; Donovan, S.; Del Alamo, D.; Lee, M.; Kauffman, M.; Shacham, S.; Landesman, Y. A method for quantification of exportin-1 (XPO1) occupancy by Selective Inhibitor of Nuclear Export (SINE) compounds. Oncotarget 2016, 7, 1863–1877. [Google Scholar] [CrossRef]
- Petosa, C.; Schoehn, G.; Askjaer, P.; Bauer, U.; Moulin, M.; Steuerwald, U.; Soler-López, M.; Baudin, F.; Mattaj, I.W.; Müller, C.W. Architecture of CRM1/Exportin 1 Suggests How Cooperativity Is Achieved during Formation of a Nuclear Export Complex. Mol. Cell 2004, 16, 761–775. [Google Scholar] [CrossRef]
- Turner, J.G.; Sullivan, D.M. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr. Med. Chem. 2008, 15, 2648–2655. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Wang, H.; Wang, T.; Yang, Q.; Mo, X.; Brogi, E.; Haffty, B.G.; Chakravarthy, A.B.; Xia, F. Association between cytosolic expression of BRCA1 and metastatic risk in breast cancer. Br. J. Cancer 2015, 113, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Hayes, K.E.; Barr, J.A.; Harold, A.D.; Xie, M.; Bukhari, S.I.A.; Vasudevan, S.; Steitz, J.A.; DiMaio, D. An Exportin-1-dependent microRNA biogenesis pathway during human cell quiescence. Proc. Natl. Acad. Sci. USA 2017, 114, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. MicroRNA precursors in motion: Exportin-5 mediates their nuclear export. Trends Cell Biol. 2004, 14, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Zaidi, S.K.; Liu, C.-G.; Stein, J.L.; van Wijnen, A.J.; Croce, C.M.; Stein, G.S. MicroRNAs 221 and 222 bypass quiescence and compromise cell survival. Cancer Res. 2008, 68, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Senapedis, W.; McCauley, D.; Baloglu, E.; Shacham, S.; Festuccia, C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J. Hematol. Oncol. 2014, 7, 85. [Google Scholar] [CrossRef]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef]
- Newlands, E.S.; Rustin, G.J.; Brampton, M.H. Phase I trial of elactocin. Br. J. Cancer 1996, 74, 648–649. [Google Scholar] [CrossRef]
- Tanenbaum, B.; Miett, T.; Patel, S.A. The emerging therapeutic landscape of relapsed/refractory multiple myeloma. Ann. Hematol. 2023, 102, 1–11. [Google Scholar] [CrossRef]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. NCCN Guidelines Insights: Multiple Myeloma, Version 3.2022. J. Natl. Compr. Canc. Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef]
- Goldfinger, M.; Cooper, D.L. Refractory DLBCL: Challenges and Treatment. Clin. Lymphoma Myeloma Leuk. 2022, 22, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Poletto, S.; Novo, M.; Paruzzo, L.; Frascione, P.M.M.; Vitolo, U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat. Rev. 2022, 110, 102443. [Google Scholar] [CrossRef] [PubMed]
- Jiffry, M.Z.M.; Kloss, R.; Ahmed-Khan, M.; Carmona-Pires, F.; Okam, N.; Weeraddana, P.; Dharmaratna, D.; Dandwani, M.; Moin, K. A review of treatment options employed in relapsed/refractory A. ML. Hematology 2023, 28, 2196482. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.J.; Francis, A.; Kuchenbauer, F.; Sanford, D. Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 6245–6259. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Selinexor: First Global Approval. Drugs 2019, 79, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J.; Noonan, K.R.; Faiman, B.; Gleason, C.; Nooka, A.K.; Costa, L.J.; Jagannath, S.; Richardson, P.G.; Siegel, D.; Chari, A.; et al. Consensus Recommendations for the Clinical Management of Patients with Multiple Myeloma Treated with Selinexor. Clin. Lymphoma Myeloma Leuk. 2020, 20, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Madduri, D.; Richard, S.; Chari, A. Selinexor in relapsed/refractory multiple myeloma. Ther. Adv. Hematol. 2020, 11, 2040620720930629. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Vogl, D.T.; Dingli, D.; Cornell, R.F.; Huff, C.A.; Jagannath, S.; Bhutani, D.; Zonder, J.; Baz, R.; Nooka, A.; Richter, J.; et al. Selective Inhibition of Nuclear Export with Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 859–866. [Google Scholar] [CrossRef]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef]
- Delforge, M.; Raddoux, J.; Antonis, C.; Clement, C.; Kint, N.; Vanhellemont, A.; Bravetti, J.; Vandenberghe, P. Selinexor, Bortezomib and Dexamethasone: An Effective Salvage Regimen for Heavily Pretreated Myeloma Patients. Onco Targets Ther. 2022, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Argueta, C.; Kashyap, T.; Klebanov, B.; Unger, T.J.; Guo, C.; Harrington, S.; Baloglu, E.; Lee, M.; Senapedis, W.; Shacham, S.; et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget 2018, 9, 25529–25544. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Argueta, C.; Unger, T.; Klebanov, B.; Debler, S.; Senapedis, W.; Crochiere, M.L.; Lee, M.S.; Kauffman, M.; Shacham, S.; et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget 2018, 9, 30773–30786. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Mateos, M.V.; Gavriatopoulou, M.; Facon, T.; Auner, H.W.; Leleu, X.; Hájek, R.; Dimopoulos, M.A.; Delimpasi, S.; Simonova, M.; Špička, I.; et al. Effect of prior treatments on selinexor, bortezomib, and dexamethasone in previously treated multiple myeloma. J. Hematol. Oncol. 2021, 14, 59. [Google Scholar] [CrossRef]
- Jakubowiak, A.J.; Jasielec, J.K.; Rosenbaum, C.A.; Cole, C.E.; Chari, A.; Mikhael, J.; Nam, J.; McIver, A.; Severson, E.; Stephens, L.A.; et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br. J. Haematol. 2019, 186, 549–560. [Google Scholar] [CrossRef]
- Derman, B.A.; Chari, A.; Zonder, J.; Major, A.; Stefka, A.T.; Jiang, K.; Karrison, T.; Jasielec, J.; Jakubowiak, A. A phase I study of selinexor combined with weekly carfilizomib and dexamethasone in relapsed/refractory multiple myeloma. Eur. J. Hematol. 2023, 110, 564–570. [Google Scholar] [CrossRef]
- Chen, C.I.; Bahlis, N.; Gasparetto, C.; Tuchman, S.A.; Lipe, B.C.; Baljevic, M.; Kotb, R.; Sutherland, H.J.; Bensinger, W.I.; Sebag, M.; et al. Selinexor, Pomalidomide, and Dexamethasone (SPd) in Patients with Relapsed or Refractory Multiple Myeloma. Blood 2019, 134, 141. [Google Scholar] [CrossRef]
- Baz, R.; Zonder, J.A.; Shain, K.H.; Alsina, M.; Brayer, J.B. Phase I/II Study of Liposomal Doxorubicin (DOX) in Combination with Selinexor (SEL) and Dexamethasone (Dex) for Relapsed and Refractory Multiple Myeloma (RRMM). Blood 2017, 130, 3095. [Google Scholar]
- Gandhi, U.H.; Senapedis, W.; Baloglu, E.; Unger, T.J.; Chari, A.; Vogl, D.; Cornell, R.F. Clinical Implications of Targeting XPO1-mediated Nuclear Export in Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2018, 18, 335–345. [Google Scholar] [CrossRef]
- Nguyen, N.; Chaudhry, S.; Totiger, T.M.; Diaz, R.; Roberts, E.; Montoya, S.; Pardo, G.; Pardo, A.; Afaghani, J.; Affer, M.; et al. Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t(11;14). npj Precis. Oncol. 2022, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Azab, F.; de la Puente, P.; Landesman, Y.; Azab, A.K. Selinexor Overcomes Hypoxia-Induced Drug Resistance in Multiple Myeloma. Transl. Oncol. 2017, 10, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.; Hari, P.; Tang, S.; Biran, N.; Callander, N.; Chari, A.; Chhabra, S.; Fiala, M.A.; Gahvari, Z.; Gandhi, U.; et al. Overall survival of patients with triple-class refractory multiple myeloma treated with selinexor plus dexamethasone vs. standard of care in MAMMOTH. Am. J. Hematol. 2021, 96, 5–8. [Google Scholar]
- Peterson, T.J.; Orozco, J.; Buege, M. Selinexor: A First-in-Class Nuclear Export Inhibitor for Management of Multiply Relapsed Multiple Myeloma. Ann. Pharmacother. 2020, 54, 577–582. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Sutherland, H.; White, D.; Sebag, M.; Lentzsch, S.; Kotb, R.; Venner, C.P.; Gasparetto, C.; Del Col, A.; Neri, P.; et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood 2018, 132, 2546–2554. [Google Scholar] [CrossRef]
- Syed, Y.Y. Selinexor-Bortezomib-Dexamethasone: A Review in Previously Treated Multiple Myeloma. Target. Oncol. 2023, 18, 303–310. [Google Scholar] [CrossRef]
- Richard, S.; Chari, A.; Delimpasi, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Dimopoulos, M.A.; Pylypenko, H.; Auner, H.W.; et al. Selinexor, bortezomib, and dexamethasone versus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by cytogenetic risk. Am. J. Hematol. 2021, 96, 1120–1130. [Google Scholar] [CrossRef]
- Hu, F.; Chen, X.-Q.; Li, X.-P.; Lu, Y.-X.; Chen, S.-L.; Wang, D.-W.; Liang, Y.; Dai, Y.-J. Drug resistance biomarker ABCC4 of selinexor and immune feature in multiple myeloma. Int. Immunopharmacol. 2022, 108, 108722. [Google Scholar] [CrossRef]
- Al-Zubidi, N.; Gombos, D.S.; Hong, D.S.; Subbiah, V.; Fu, S.; Ahnert, J.R.; Piha-Paul, S.A.; Tsimberidou, A.M.; Karp, D.D.; Bernstam, F.M.; et al. Overview of Ocular Side Effects of Selinexor. Oncologist 2021, 26, 619–623. [Google Scholar] [CrossRef]
- Kasamon, Y.L.; Price, L.S.L.; Okusanya, O.O.; Richardson, N.C.; Li, R.J.; Ma, L.; Wu, Y.-T.; Theoret, M.; Pazdur, R.; Gormley, N.J. FDA Approval Summary: Selinexor for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Oncologist 2021, 26, 879–886. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Chari, A.; Chen, C.; Bahlis, N.; Vogl, D.T.; Jakubowiak, A.; Dingli, D.; Cornell, R.F.; Hofmeister, C.C.; Siegel, D.; et al. Integrated safety profile of selinexor in multiple myeloma: Experience from 437 patients enrolled in clinical trials. Leukemia 2020, 34, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Maerevoet, M.; Zijlstra, J.M.; Follows, G.; Casasnovas, R.O.; Vermaat, J.S.P.; Kalakonda, N.; Goy, A.; Choquet, S.; Van Den Neste, E.; Hill, B.; et al. Survival among patients with relapsed/refractory diffuse large B cell lymphoma treated with single-agent selinexor in the SADAL study. J. Hematol. Oncol. 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Ben-Barouch, S.; Kuruvilla, J. Selinexor (KTP-330)—A selective inhibitor of nuclear export (SINE): Anti-tumor activity in diffuse large B-cell lymphoma (DLBCL). Expert Opin. Investig. Drugs 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Kalakonda, N.; Maerevoet, M.; Cavallo, F.; Follows, G.; Goy, A.; Vermaat, J.S.P.; Casasnovas, O.; Hamad, N.; Zijlstra, J.M.; Bakhshi, S.; et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020, 7, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.; Savona, M.; Baz, R.; Mau-Sørensen, M.; Gabrail, N.; Garzon, R.; Stone, R.; Wang, M.; Savoie, L.; Martin, P.; et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood 2017, 129, 3175–3183. [Google Scholar] [CrossRef]
- Casasnovas, R.-O.; Follows, G.; Zijlstra, J.M.; Vermaat, J.S.; Kalakonda, N.; Choquet, S.; Neste, E.V.D.; Hill, B.; Thieblemont, C.; Cavallo, F.; et al. Comparison of the Effectiveness and Safety of the Oral Selective Inhibitor of Nuclear Export, Selinexor, in Diffuse Large B Cell Lymphoma Subtypes. Clin. Lymphoma Myeloma Leuk. 2022, 22, 24–33. [Google Scholar] [CrossRef]
- Seymour, E.K.; Khan, H.Y.; Li, Y.; Chaker, M.; Muqbil, I.; Aboukameel, A.; Ramchandren, R.; Houde, C.; Sterbis, G.; Yang, J.; et al. Selinexor in Combination with R-CHOP for Frontline Treatment of Non-Hodgkin Lymphoma: Results of a Phase I Study. Clin. Cancer Res. 2021, 27, 3307–3316. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, M.; Xu-Monette, Z.Y.; Pham, L.V.; Tzankov, A.; Visco, C.; Fang, X.; Bhagat, G.; Zhu, F.; Dybkaer, K.; et al. XPO1 expression worsens the prognosis of unfavorable DLBCL that can be effectively targeted by selinexor in the absence of mutant p53. J. Hematol. Oncol. 2020, 13, 148. [Google Scholar] [CrossRef]
- Shah, J.; Shacham, S.; Kauffman, M.; Daniele, P.; Tomaras, D.; Tremblay, G.; Casasnovas, R.-O.; Maerevoet, M.; Zijlstra, J.; Follows, G.; et al. Health-related quality of life and utility outcomes with selinexor in relapsed/refractory diffuse large B-cell lymphoma. Future Oncol. 2021, 17, 1295–1310. [Google Scholar] [CrossRef]
- Parikh, K.; Cang, S.; Sekhri, A.; Liu, D. Selective inhibitors of nuclear export (SINE)--a novel class of anti-cancer agents. J. Hematol. Oncol. 2014, 7, 78. [Google Scholar] [CrossRef]
- Abboud, R.; Chendamarai, E.; Rettig, M.P.; Trinkaus, K.M.; Riedell, P.A.; Abboud, C.N.; Ghobadi, A.; Pusic, I.; Stockerl-Goldstein, K.; Schroeder, M.A.; et al. Selinexor combined with cladribine, cytarabine, and filgrastim in relapsed or refractory acute myeloid leukemia. Haematologica 2020, 105, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, B.; Zhao, Q.; Mims, A.S.; Vasu, S.; Behbehani, G.K.; Larkin, K.; Blachly, J.S.; Blum, W.; Klisovic, R.B.; Ruppert, A.S.; et al. Selinexor in combination with decitabine in patients with acute myeloid leukemia: Results from a phase 1 study. Leuk. Lymphoma 2020, 61, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Savona, M.; Baz, R.; Andreeff, M.; Gabrail, N.; Gutierrez, M.; Savoie, L.; Mau-Sørensen, M.; Wagner-Johnston, N.; Yee, K.; et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood 2017, 129, 3165–3174. [Google Scholar] [CrossRef]
- Janssen, J.; Löwenberg, B.; Manz, M.; Biemond, B.; Westerweel, P. Addition of the nuclear export inhibitor selinexor to standard intensive treatment for elderly patients with AML and high risk MDS. Leukemia 2022, 36, 2189–2195. [Google Scholar] [CrossRef]
- Sweet, K.; Komrokji, R.; Padron, E.; Cubitt, C.L.; Turner, J.G.; Zhou, J.; List, A.F.; Sallman, D.A.; Dawson, J.L.; Sullivan, D.M.; et al. Phase I Clinical Trial of Selinexor in Combination with Daunorubicin and Cytarabine in Previously Untreated Poor-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2020, 26, 54–60. [Google Scholar] [CrossRef]
- Cooperrider, J.H.; Fulton, N.; Artz, A.S.; Larson, R.A.; Stock, W.; Kosuri, S.; Bishop, M.; Liu, H. Phase I trial of maintenance selinexor after allogeneic hematopoietic stem cell transplantation for patients with acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2020, 55, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yin, X.; Si, Y.; Wang, Y.; Wang, J.; Cui, S. Efficacy and safety of selinexor in the treatment of AML: A protocol for systematic review and meta-analysis. Medicine 2021, 100, 27884. [Google Scholar] [CrossRef]
- Taylor, J.; Mi, X.; Penson, A.V.; Paffenholz, S.V.; Alvarez, K.; Sigler, A.; Chung, S.S.; Rampal, R.K.; Park, J.H.; Stein, E.M.; et al. Safety and activity of selinexor in patients with myelodysplastic syndromes or oligoblastic acute myeloid leukaemia refractory to hypomethylating agents: A single-centre, single-arm, phase 2 trial. Lancet Haematol. 2020, 7, 566–574. [Google Scholar] [CrossRef]
- Ranganathan, P.; Kashyap, T.; Yu, X.; Meng, X.; Lai, T.-H.; McNeil, B.; Bhatnagar, B.; Shacham, S.; Kauffman, M.; Dorrance, A.M.; et al. XPO1 Inhibition using Selinexor Synergizes with Chemotherapy in Acute Myeloid Leukemia by Targeting DNA Repair and Restoring Topoisomerase IIα to the Nucleus. Clin. Cancer Res. 2016, 22, 6142–6152. [Google Scholar] [CrossRef]
- Long, H.; Hou, Y.; Li, J.; Song, C.; Ge, Z. Azacitidine Is Synergistically Lethal with XPO1 Inhibitor Selinexor in Acute Myeloid Leukemia by Targeting XPO1/eIF4E/c-Myc Signaling. Int. J. Mol. Sci. 2023, 24, 6816. [Google Scholar] [CrossRef]
- Tang, T.; Martin, P.; Somasundaram, N.; Lim, C.; Tao, M.; Poon, E.; Yunon, M.J.; Toh, S.Q.; Yan, S.X.; Farid, M.; et al. Phase I study of selinexor in combination with dexamethasone, ifosfamide, carboplatin, etoposide chemotherapy in patients with relapsed or refractory peripheral T-cell or natural-killer/T-cell lymphoma. Haematologica 2021, 106, 3170–3175. [Google Scholar] [CrossRef]
- Broccoli, A.; Argnani, L.; Zinzani, P.L. Peripheral T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease. Cancer Treat. Rev. 2017, 60, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hing, Z.A.; Mantel, R.; Beckwith, K.A.; Guinn, D.; Williams, E.; Smith, L.L.; Williams, K.; Johnson, A.J.; Lehman, A.M.; Byrd, J.C.; et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood 2015, 125, 3128–3132. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, B.; Miao, Y.; Li, Y.; Qin, S.; Liang, J.; Kong, Y.; Zhang, X.; Tang, J.; Xia, Y.; et al. Prognostic value and therapeutic targeting of XPO1 in chronic lymphocytic leukemia. Clin. Exp. Med. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Than, H.; Pomicter, A.D.; Yan, D.; Beaver, L.P.; Eiring, A.M.; Heaton, W.L.; Senina, A.; Clair, P.M.; Shacham, S.; Mason, C.C.; et al. Coordinated inhibition of nuclear export and Bcr-Abl1 selectively targets chronic myeloid leukemia stem cells. Leukemia 2020, 34, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.S.; Garzon, R.; Lapalombella, R. Selinexor for advanced hematologic malignancies. Leuk. Lymphoma 2020, 61, 2335–2350. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.M.; Grisham, R.N.; Cadoo, K.; Kyi, C.; Tew, W.P.; Friedman, C.F.; O’Cearbhaill, R.E.; Zamarin, D.; Zhou, Q.; Iasonos, A.; et al. A phase I open-label study of selinexor with paclitaxel and carboplatin in patients with advanced ovarian or endometrial cancers. Gynecol. Oncol. 2021, 160, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Lund, B.; Peen, U.; Umajuridze, Z.; Mau-Sorensen, M.; Kranich, A.; Van Nieuwenhuysen, E.; Haslund, C.; Nottrup, T.; Han, S.; et al. Phase 2 study of the Exportin 1 inhibitor selinexor in patients with recurrent gynecological malignancies. Gynecol. Oncol. 2020, 156, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Tortoreto, M.; Mancini, A.; Addis, A.; Di Cesare, E.; Lenzi, A.; Landesman, Y.; McCauley, D.; Kauffman, M.; Shacham, S.; et al. XPO1/CRM1-selective inhibitors of nuclear export (SINE) reduce tumor spreading and improve overall survival in preclinical models of prostate cancer (PCa). J. Hematol. Oncol. 2014, 7, 46. [Google Scholar] [CrossRef]
- Wei, X.X.; Siegel, A.P.; Aggarwal, R.; Lin, A.M.; Friedlander, T.W.; Fong, L.; Kim, W.; Louttit, M.; Chang, E.; Zhang, L.; et al. A Phase II Trial of Selinexor, an Oral Selective Inhibitor of Nuclear Export Compound, in Abiraterone- and/or Enzalutamide-Refractory Metastatic Castration-Resistant Prostate Cancer. Oncologist 2018, 23, 656–664. [Google Scholar] [CrossRef]
- Marretta, A.L.; Di Lorenzo, G.; Ribera, D.; Cannella, L.; von Arx, C.; Bracigliano, A.; Clemente, O.; Tafuto, R.; Pizzolorusso, A.; Tafuto, S. Selinexor and the Selective Inhibition of Nuclear Export: A New Perspective on the Treatment of Sarcomas and Other Solid and Non-Solid Tumors. Pharmaceutics 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Zhang, Y.-X.; Czaplinski, J.T.; Anatone, A.J.; Sicinska, E.T.; Fletcher, J.A.; Demetri, G.D.; Wagner, A.J. Preclinical activity of selinexor, an inhibitor of XPO1, in sarcoma. Oncotarget 2016, 7, 16581–16592. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Kanojia, D.; Mayakonda, A.; Said, J.W.; Doan, N.B.; Chien, W.; Ganesan, T.S.; Chuang, L.S.H.; Venkatachalam, N.; Baloglu, E.; et al. Molecular mechanism and therapeutic implications of selinexor (KPT-330) in liposarcoma. Oncotarget 2017, 8, 7521–7532. [Google Scholar] [CrossRef] [PubMed]
- Thirasastr, P.; Somaiah, N. Overview of systemic therapy options in liposarcoma, with a focus on the activity of selinexor, a selective inhibitor of nuclear export in dedifferentiated liposarcoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221081073. [Google Scholar] [CrossRef]
- Gounder, M.M.; Zer, A.; Tap, W.D.; Salah, S.; Dickson, M.A.; Gupta, A.A.; Keohan, M.L.; Loong, H.; D’angelo, S.P.; Baker, S.; et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients with Advanced Refractory Bone or Soft Tissue Sarcoma. J. Clin. Oncol. 2016, 34, 3166–3174. [Google Scholar] [CrossRef]
- Hernando-Calvo, A.; Malone, E.; Day, D.; Prawira, A.; Weinreb, I. Selinexor for the treatment of recurrent or metastatic salivary gland tumors: Results from the GEMS-001 clinical trial. J. Exp. Clin. Cancer Res. 2022. preprint NCT02069730. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Mayakonda, A.; Ganesan, T.S.; Sadhanandhan, B.; Suresh, S.; Sneha, S.; Nagare, R.P.; Said, J.W.; Doan, N.B.; et al. Selinexor (KPT-330) has antitumor activity against anaplastic thyroid carcinoma in vitro and in vivo and enhances sensitivity to doxorubicin. Sci. Rep. 2017, 7, 9749. [Google Scholar] [CrossRef]
- Baek, H.B.; Lombard, A.P.; Libertini, S.J.; Fernandez-Rubio, A.; Vinall, R.; Gandour-Edwards, R.; Nakagawa, R.; Vidallo, K.; Nishida, K.; Siddiqui, S.; et al. XPO1 inhibition by selinexor induces potent cytotoxicity against high grade bladder malignancies. Oncotarget 2018, 9, 34567–34581. [Google Scholar] [CrossRef]
- Arango, N.P.; Yuca, E.; Zhao, M.; Evans, K.W.; Scott, S.; Kim, C.; Gonzalez-Angulo, A.M.; Janku, F.; Ueno, N.T.; Tripathy, D.; et al. Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 2017, 19, 93. [Google Scholar] [CrossRef]
- Marijon, H.; Gery, S.; Chang, H.; Landesman, Y.; Shacham, S.; Lee, D.H.; de Gramont, A.; Koeffler, H.P. Selinexor, a selective inhibitor of nuclear export, enhances the anti-tumor activity of olaparib in triple negative breast cancer regardless of BRCA1 mutation status. Oncotarget 2021, 12, 1749–1762. [Google Scholar] [CrossRef]
- Walker, C.J.; Chang, H.; Henegar, L.; Kashyap, T.; Shacham, S.; Sommer, J.; Wick, M.J.; Levy, J.; Landesman, Y. Selinexor inhibits growth of patient derived chordomas in vivo as a single agent and in combination with abemaciclib through diverse mechanisms. Front. Oncol. 2022, 12, 808021. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, W.; Zhong, Y.; Hou, X.; Fang, S.; Liu, C.-Y.; Wang, G.; Yu, T.; Huang, Y.-Y.; Ouyang, X.; et al. Nuclear Export of Ubiquitinated Proteins Determines the Sensitivity of Colorectal Cancer to Proteasome Inhibitor. Mol. Cancer Ther. 2017, 16, 717–728. [Google Scholar] [CrossRef]
- Ferreiro-Neira, I.; Torres, N.E.; Liesenfeld, L.F.; Chan, C.H.; Penson, T.; Landesman, Y.; Senapedis, W.; Shacham, S.; Hong, T.S.; Cusack, J.C. XPO1 Inhibition Enhances Radiation Response in Preclinical Models of Rectal Cancer. Clin. Cancer Res. 2016, 22, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Subhash, V.V.; Yeo, M.S.; Wang, L.; Tan, S.H.; Wong, F.Y.; Thuya, W.L.; Tan, W.L.; Peethala, P.C.; Soe, M.Y.; Tan, D.S.P.; et al. Anti-tumor efficacy of Selinexor (KPT-330) in gastric cancer is dependent on nuclear accumulation of p53 tumor suppressor. Sci. Rep. 2018, 8, 12248. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Ramkissoon, S.H.; McCauley, D.; Jones, K.; Perry, J.A.; Hsu, J.H.-R.; Ramkissoon, L.A.; Maire, C.L.; Hubbell-Engler, B.; Knoff, D.S.; et al. Preclinical antitumor efficacy of selective exportin 1 inhibitors in glioblastoma. Neuro Oncol. 2015, 17, 697–707. [Google Scholar] [CrossRef]
- Wahba, A.; Rath, B.H.; O’Neill, J.W.; Camphausen, K.; Tofilon, P.J. The XPO1 Inhibitor Selinexor Inhibits Translation and Enhances the Radiosensitivity of Glioblastoma Cells Grown In Vitro and In Vivo. Mol. Cancer Ther. 2018, 17, 1717–1726. [Google Scholar] [CrossRef]
- Saenz-Ponce, N.; Pillay, R.; de Long, L.M.; Kashyap, T.; Argueta, C.; Landesman, Y.; Hazar-Rethinam, M.; Boros, S.; Panizza, B.; Jacquemyn, M.; et al. Targeting the XPO1-dependent nuclear export of E2F7 reverses anthracycline resistance in head and neck squamous cell carcinomas. Sci. Transl. Med. 2018, 10, 7223. [Google Scholar] [CrossRef]
- Von Fallois, M.; Kosyna, F.K.; Mandl, M.; Landesman, Y.; Dunst, J.; Depping, R. Selinexor decreases HIF-1α via inhibition of CRM1 in human osteosarcoma and hepatoma cells associated with an increased radiosensitivity. J. Cancer Res. Clin. Oncol. 2021, 147, 2025–2033. [Google Scholar] [CrossRef]
- Rosen, J.C.; Weiss, J.; Pham, N.-A.; Li, Q.; Martins-Filho, S.N.; Wang, Y.; Tsao, M.-S.; Moghal, N. Antitumor efficacy of XPO1 inhibitor Selinexor in KRAS-mutant lung adenocarcinoma patient-derived xenografts. Transl. Oncol. 2021, 14, 101179. [Google Scholar] [CrossRef]
- Galinski, B.; Luxemburg, M.; Landesman, Y.; Pawel, B.; Johnson, K.J.; Master, S.R.; Freeman, K.W.; Loeb, D.M.; Hébert, J.M.; Weiser, D.A. XPO1 inhibition with selinexor synergizes with proteasome inhibition in neuroblastoma by targeting nuclear export of IkB. Transl. Oncol. 2021, 14, 101114. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Landesman, Y.; Friedlander, S.; Shacham, S.; Kauffman, M.; Weiss, R.H. Specific inhibition of the nuclear exporter exportin-1 attenuates kidney cancer growth. PLoS ONE 2014, 9, 113867. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Friedlander, S.Y.; Arrate, M.P.; Chang, H.; Gorska, A.E.; Fuller, L.D.; Ramsey, H.E.; Kashyap, T.; Argueta, C.; Debler, S.; et al. Venetoclax response is enhanced by selective inhibitor of nuclear export compounds in hematologic malignancies. Blood Adv. 2020, 4, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, T.; De Bie, J.; Neggers, J.E.; Jacquemyn, M.; Vanstreels, E.; Schmid-Burgk, J.L.; Hornung, V.; Baloglu, E.; Landesman, Y.; Senapedis, W.; et al. The Second-Generation Exportin-1 Inhibitor KPT-8602 Demonstrates Potent Activity against Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2017, 23, 2528–2541. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, D.; Demeyer, S.; Prieto, C.; de Bock, C.E.; De Bie, J.; Gielen, O.; Jacobs, K.; Mentens, N.; Verhoeven, B.M.; Uyttebroeck, A.; et al. The XPO1 Inhibitor KPT-8602 Synergizes with Dexamethasone in Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2020, 26, 5747–5758. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.F.; Baz, R.; Richter, J.R.; Rossi, A.; Vogl, D.T.; Chen, C.; Shustik, C.; Alvarez, M.J.; Shen, Y.; Unger, T.J.; et al. A phase 1 clinical trial of oral eltanexor in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2022, 97, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Mohan, S.; Knupp, J.; Chamoun, K.; de Jonge, A.; Yang, F.; Baloglu, E.; Shah, J.; Kauffman, M.G.; Shacham, S.; et al. Oral eltanexor treatment of patients with higher-risk myelodysplastic syndrome refractory to hypomethylating agents. J. Hematol. Oncol. 2022, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Otte, K.; Zhao, K.; Braun, M.; Neubauer, A.; Raifer, H.; Helmprobst, F.; Barrera, F.O.; Nimsky, C.; Bartsch, J.W.; Rusch, T. Eltanexor Effectively Reduces Viability of Glioblastoma and Glioblastoma Stem-Like Cells at Nano-Molar Concentrations and Sensitizes to Radiotherapy and Temozolomide. Biomedicines 2022, 10, 2145. [Google Scholar] [CrossRef]
- Uddin, H.; Li, Y.; Khan, H.Y.; Muqbil, I.; Aboukameel, A.; Sexton, R.E.; Reddy, S.; Landesman, Y.; Kashyap, T.; Azmi, A.S.; et al. Nuclear Export Inhibitor KPT-8602 Synergizes with PARP Inhibitors in Escalating Apoptosis in Castration Resistant Cancer Cells. Int. J. Mol. Sci. 2021, 22, 6676. [Google Scholar] [CrossRef]
- Tabe, Y.; Kojima, K.; Jin, L.; Miida, T.; Shacham, S.; Kauffman, M.; Andreeff, M. Molecular Mechanisms of Antitumor Activity of the Selective Inhibitor of Nuclear Export (SINE) CRM1 Antagonist KPT-185 in Mantle Cell Lymphoma. Blood 2012, 120, 2438. [Google Scholar] [CrossRef]
- Kong, S.-Y.; Landesman, Y.; Jakubikova, J.; Sellitto, M.A.; Cagnetta, A.; Cea, M.; Chen, M.C.; Cottini, F.; McMillin, U.W.; Acharya, C.; et al. Blockade of Nuclear Export Protein CRM1 (chromosomal region maintenance 1, XPO1) by a Novel, Potent and Selective CRM1 Inhibitor KPT-185 Induces Significant Antitumor Activity Against Human Multiple Myeloma. Blood 2011, 118, 2913. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ishizawa, J.; Ruvolo, V.; Dilip, A.; Quintás-Cardama, A.; McDonnell, T.J.; Neelapu, S.S.; Kwak, L.W.; Shacham, S.; Kauffman, M.; et al. Induction of p53-mediated transcription and apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma. Cancer Sci. 2014, 105, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Yu, X.; Na, C.; Santhanam, R.; Shacham, S.; Kauffman, M.; Walker, A.; Klisovic, R.; Blum, W.; Caligiuri, M.; et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012, 120, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, X.; Wang, J.; Yao, J.; Shi, Y. Antitumor effects of a novel chromosome region maintenance 1 (CRM1) inhibitor on non-small cell lung cancer cells in vitro and in mouse tumor xenografts. PLoS ONE 2014, 9, 89848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, M.; Tamayo, A.T.; Shacham, S.; Kauffman, M.; Lee, J.; Zhang, L.; Ou, Z.; Li, C.; Sun, L.; et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp. Hematol. 2013, 41, 67–78. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Shen, Y.; Zhang, N.; Wang, S.; Yao, J.; Shi, Y. CRM1 as a new therapeutic target for non-Hodgkin lymphoma. Leuk. Res. 2015, 39, 38–46. [Google Scholar] [CrossRef]
- Schmidt, J.; Braggio, E.; Kortuem, K.M.; Egan, J.B.; Zhu, Y.X.; Xin, C.S.; Tiedemann, R.E.; Palmer, S.E.; Garbitt, V.M.; McCauley, D.; et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia 2013, 27, 2357–2365. [Google Scholar] [CrossRef]
- Ou, L.; Wang, X.; Cheng, S.; Zhang, M.; Cui, R.; Hu, C.; Liu, S.; Tang, Q.; Peng, Y.; Chai, R.; et al. Verdinexor, a Selective Inhibitor of Nuclear Exportin 1, Inhibits the Proliferation and Migration of Esophageal Cancer via XPO1/c-Myc/FOSL1 Axis. Int. J. Biol. Sci. 2022, 18, 276–291. [Google Scholar] [CrossRef]
- Pan, L.; Cheng, C.; Duan, P.; Chen, K.; Wu, Y.; Wu, Z. XPO1/CRM1 is a promising prognostic indicator for neuroblastoma and represented a therapeutic target by selective inhibitor verdinexor. J. Exp. Clin. Cancer Res. 2021, 40, 255. [Google Scholar] [CrossRef]
- Perwitasari, O.; Johnson, S.; Yan, X.; Howerth, E.; Shacham, S.; Landesman, Y.; Baloglu, E.; McCauley, D.; Tamir, S.; Tompkins, S.M.; et al. Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo. J. Virol. 2014, 88, 10228–10243. [Google Scholar] [CrossRef]
- Pickens, J.A.; Tripp, R.A. Verdinexor Targeting of CRM1 is a Promising Therapeutic Approach against RSV and Influenza Viruses. Viruses 2018, 10, 48. [Google Scholar] [CrossRef]
- Landes, J.R.; Bartley, B.R.; A Moore, S.; He, Q.; Simonette, R.; Rady, P.L.; Doan, H.Q.; Tyring, S.K. Effect of selinexor on lipogenesis in virus-positive Merkel cell carcinoma cell lines. Clin. Exp. Dermatol. 2023, llad081. [Google Scholar] [CrossRef] [PubMed]
- Bartley, B.R.; Simonette, R.A.; Rady, P.L.; Doan, H.Q.; Tyring, S.K. Molecular evidence for selinexor as a treatment for Merkel cell polyomavirus (MCPyV)-positive Merkel cell carcinoma. Int. J. Dermatol. 2023, 62, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Gionco, J.; Chen, J.; Lindsay, R.; Macri, V.; Brooks, C.L. SL-401, a Targeted Therapy Directed to the Interleukin-3 Receptor (CD123), and SL-801, a Reversible Inhibitor of Exportin-1 (XPO1), Display Synergistic Anti-Tumor Activity against Hematologic Malignancies In Vitro. Blood 2016, 128, 4724. [Google Scholar] [CrossRef]
- Etchin, J.; Sun, Q.; Kentsis, A.; Farmer, A.; Zhang, Z.C.; Sanda, T.; Mansour, M.; Barceló, C.; McCauley, D.; Kauffman, M.; et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 2013, 27, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Saito, N.; Sato, T.; Suzuki, A.; Hasegawa, Y.; Friedman, J.; Kufe, D.W.; Vonhoff, D.D.; Iwami, T.; Kawabe, T. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood 2011, 118, 3922–3931. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Leanza, R.; Musolino, C. Selective Inhibitors of Nuclear Export in the Treatment of Hematologic Malignancies. Clin. Lymphoma Myeloma Leuk. 2019, 19, 689–698. [Google Scholar] [CrossRef]
- Trkulja, K.L.; Manji, F.; Kuruvilla, J.; Laister, R.C. Nuclear Export in Non-Hodgkin Lymphoma and Implications for Targeted XPO1 Inhibitors. Biomolecules 2023, 13, 111. [Google Scholar] [CrossRef]
- Ranieri, R.; Pianigiani, G.; Sciabolacci, S.; Perriello, V.M.; Marra, A.; Cardinali, V.; Pierangeli, S.; Milano, F.; Gionfriddo, I.; Brunetti, L.; et al. Current status and future perspectives in targeted therapy of NPM1-mutated A.ML. Luekemia 2022, 36, 2351–2367. [Google Scholar] [CrossRef]
- Abramson, H.N. Recent Advances in the Application of Small Molecules in the Treatment of Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 2645. [Google Scholar] [CrossRef]
- Ullman, K.S.; Powers, M.A.; Forbes, D.J. Nuclear export receptors: From importin to exportin. Cell 1997, 90, 967–970. [Google Scholar] [CrossRef]
- Kosyna, F.K.; Depping, R. Controlling the Gatekeeper: Therapeutic Targeting of Nuclear Transport. Cells 2018, 7, 221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).