Synergistic Interaction of Glycyrrhizin with Norfloxacin Displays ROS-Induced Bactericidal Activity against Multidrug-Resistant Staphylococcus aureus
Abstract
1. Introduction
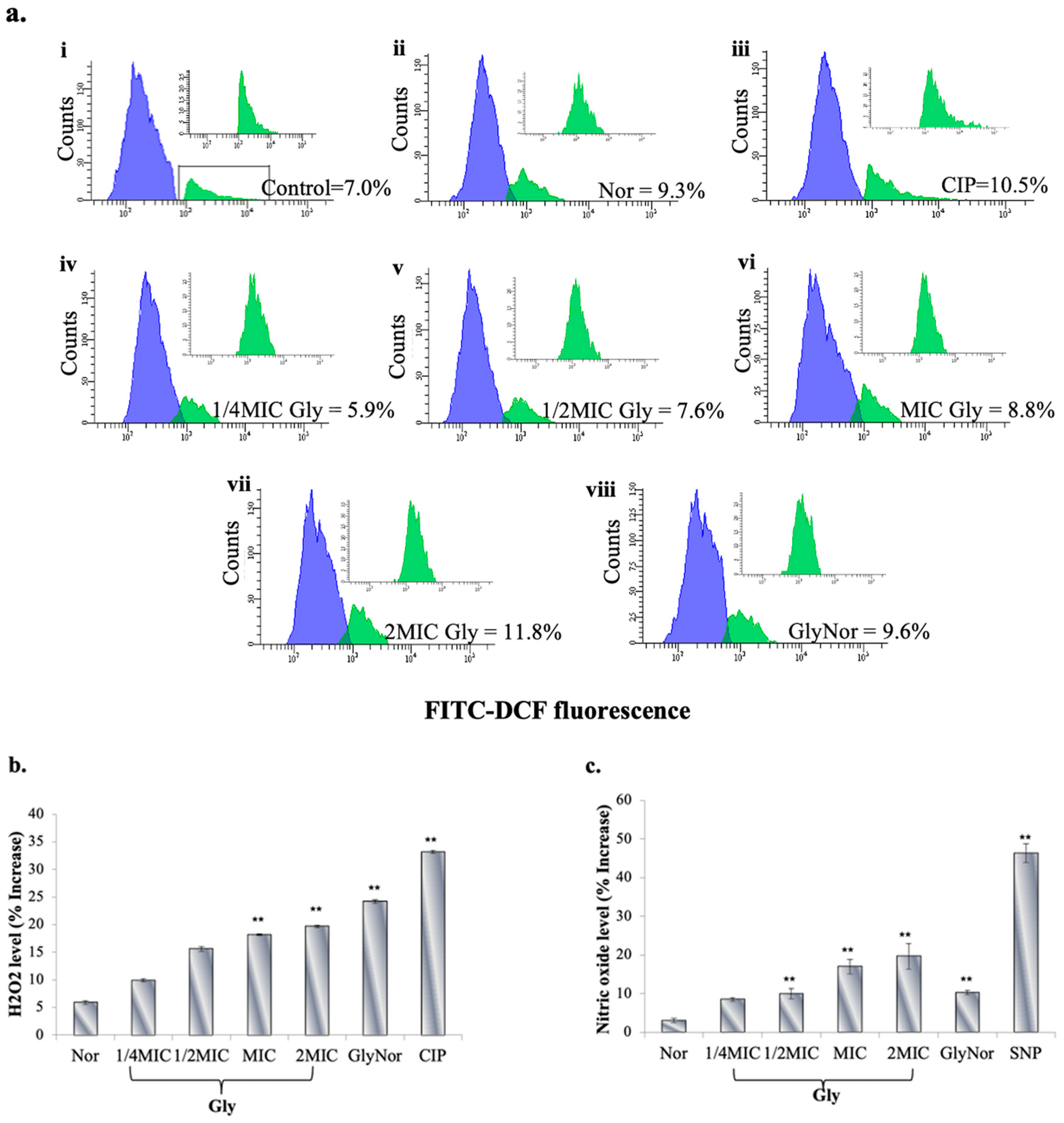
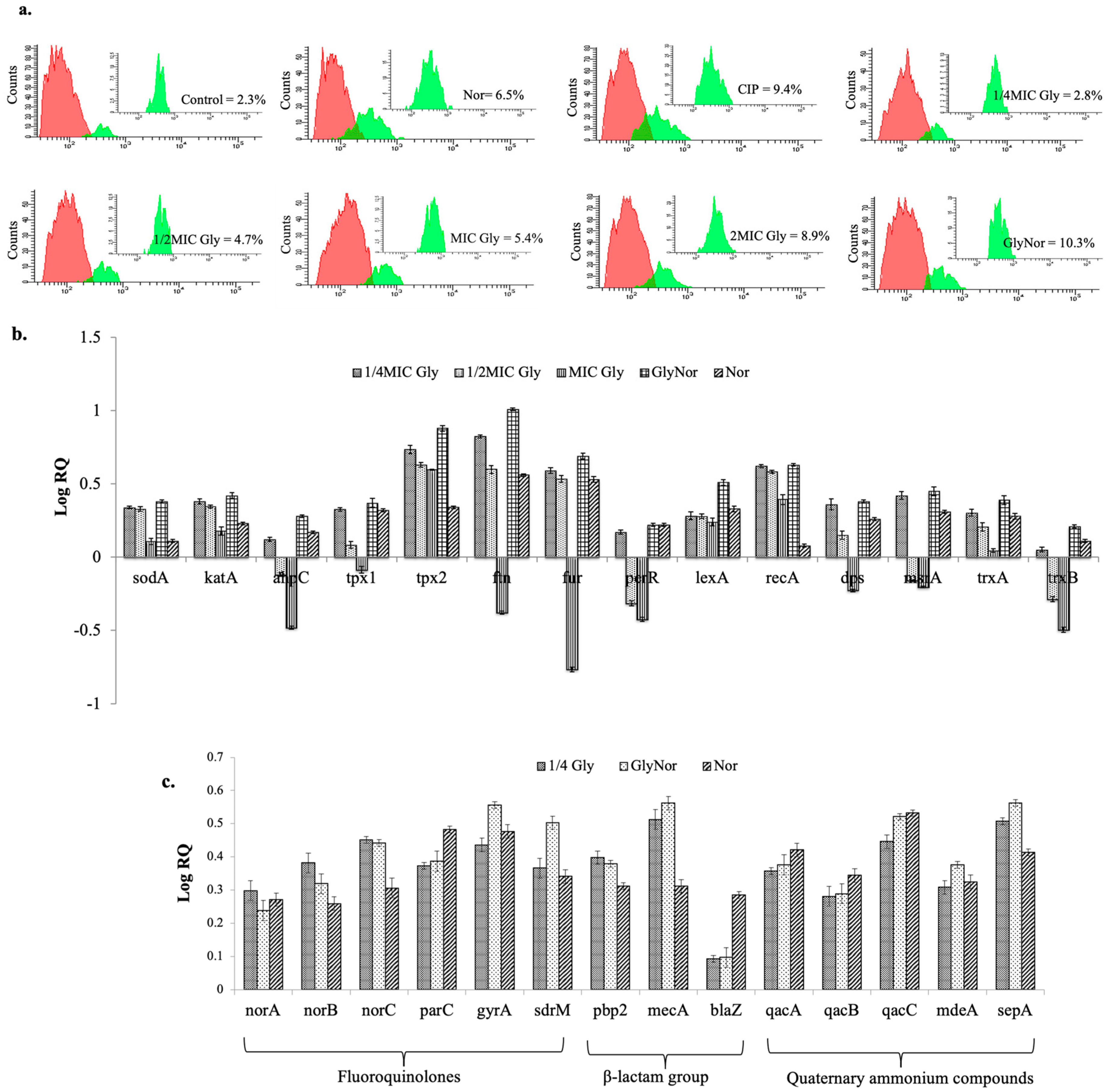
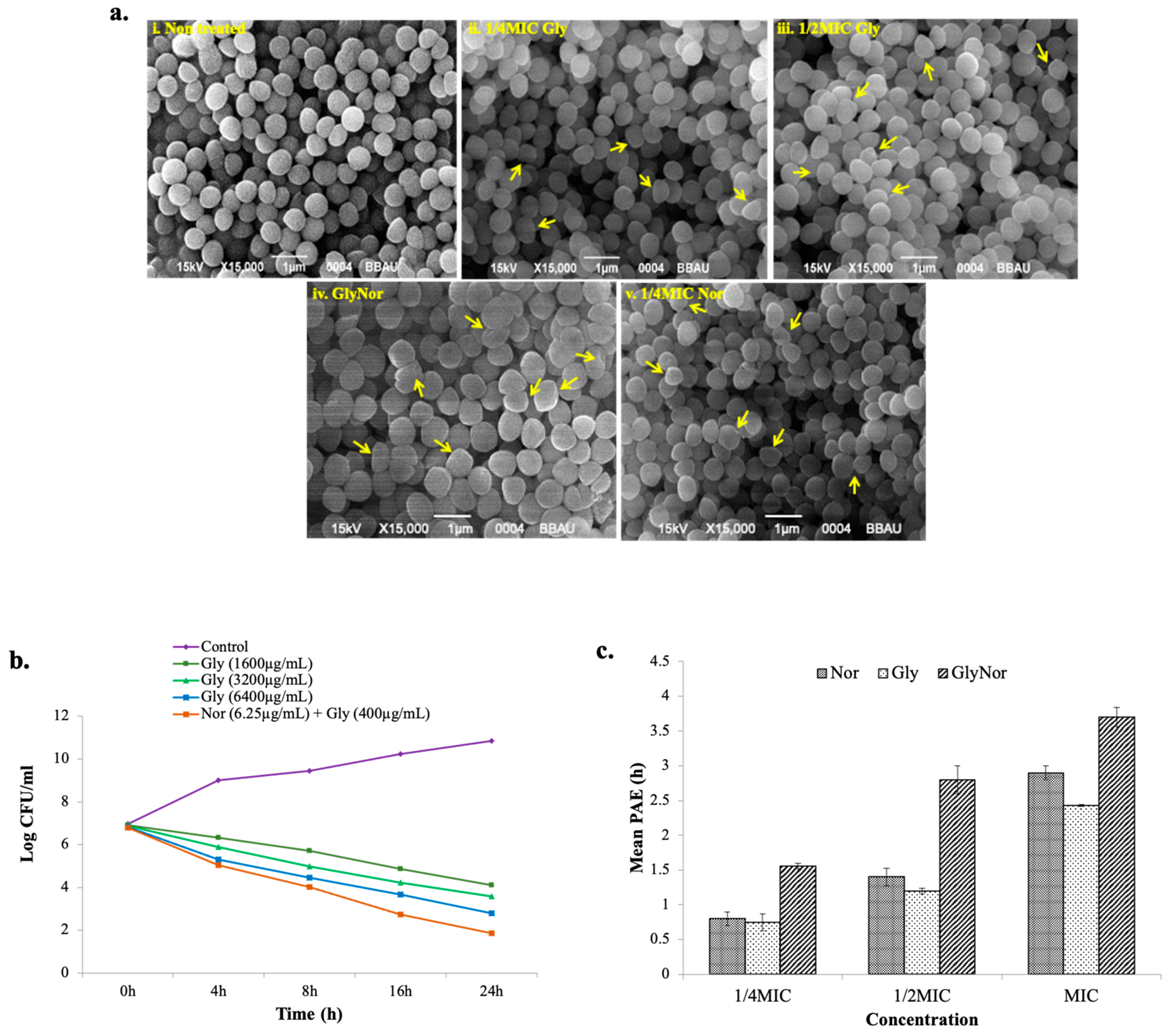
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Strains and Growth Conditions
3.2. In Vitro Combination Assay
3.3. Measurement of Reactive Oxygen Species
3.3.1. Spectro Fluorimeter Assay
3.3.2. Flow Cytometry
3.4. Nitrite Determination Assay
3.5. Lipid Peroxidation
3.6. Ethidium Bromide Efflux Studies
3.7. Cytoplasmic Leakage Assay
3.8. Membrane Depolarization Assay
3.9. Membrane Integrity Assay
3.10. Membrane Fluidity Assay
3.11. DNA Fragmentation (TUNEL) Assay
3.12. Scanning Electron Microscope Assay
3.13. qRT-PCR Analysis
3.14. Time-Kill Studies
3.15. Post-Antibiotic Effect (PAE)
3.16. Selection of Resistant Mutants In Vitro
3.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Das, S.; Dasgupta, A.; Chopra, S. Drug repurposing: A new front in the war against Staphylococcus aureus. Future Microbiol. 2016, 11, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Huang, T.-Y.; Chen, J.-L.; Hsiao, C.-T.; Kuo, L.-T.; Huang, K.-C. Bacteriology and mortality of necrotizing fasciitis in a tertiary coastal hospital with comparing risk indicators of methicillin-resistant Staphylococcus aureus and Vibrio vulnificus infections: A prospective study. BMC Infect. Dis. 2021, 21, 771. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef]
- Tatiraju, D.V.; Bagade, V.B.; Karambelkar, P.J.; Jadhav, V.M.; Kadam, V. Natural bioenhancers: An overview. J. Pharmacogn. Phytochem. 2013, 2, 55–60. [Google Scholar]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Kim, A.V.; Shelepova, E.A.; Selyutina, O.Y.; Meteleva, E.S.; Dushkin, A.V.; Medvedev, N.N.; Polyakov, N.E.; Lyakhov, N.Z. Glycyrrhizin-Assisted Transport of Praziquantel Anthelmintic Drug through the Lipid Membrane: An Experiment and MD Simulation. Mol. Pharm. 2019, 16, 3188–3198. [Google Scholar] [CrossRef]
- Kim, A.V.; Shelepova, E.A.; Evseenko, V.I.; Dushkin, A.V.; Medvedev, N.N.; Polyakov, N.E. Mechanism of the enhancing effect of glycyrrhizin on nifedipine penetration through a lipid membrane. J. Mol. Liq. 2021, 344, 117759. [Google Scholar] [CrossRef]
- Khanuja, S.P.S.; Kumar, S.; Arya, J.S.; Shasany, A.K.; Singh, M.; Awasthi, S.; Gupta, S.C.; Darokar, M.P.; Rahman, L.U. Composition Comprising Pharmaceutical/Nutraceutical Agent and a Bioenhancer Obtained from Glycyrrhiza glabra. U.S. Patent 6,979,471, 27 December 2005. [Google Scholar]
- Farooqui, A.; Khan, F.; Khan, I.; Ansari, I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G0/G1 in HPV18+ human cervical cancer HeLa cell line. Biomed. Pharmacother. 2018, 97, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Son, D.; Chung, T.-H.; Lee, Y.-J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Dudhatra, G.B.; Mody, S.K.; Awale, M.M.; Patel, H.B.; Modi, C.M.; Kumar, A.; Kamani, D.R.; Chauhan, B.N. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Sci. World J. 2012, 2012, 637953. [Google Scholar] [CrossRef]
- Rasool, M.; Iqbal, J.; Malik, A.; Ramzan, H.S.; Qureshi, M.S.; Asif, M.; Qazi, M.H.; Kamal, M.A.; Chaudhary, A.G.A.; Al-Qahtani, M.H.; et al. Hepatoprotective Effects of Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) in Combination: A Possible Synergy. Evid. Based Complement. Altern. Med. 2014, 2014, 641597. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin Use for Multi-Drug Resistant Pseudomonas aeruginosa: In Vitro and In Vivo Studies. Investig. Opthalmol. Vis. Sci. 2019, 60, 2978–2989. [Google Scholar] [CrossRef]
- Huan, C.; Xu, Y.; Zhang, W.; Guo, T.; Pan, H.; Gao, S. Research Progress on the Antiviral Activity of Glycyrrhizin and its Derivatives in Liquorice. Front. Pharmacol. 2021, 12, 680674. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037. [Google Scholar] [CrossRef]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; De Buschiazzo, P.M.; Ríos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Niki, E. Assessment of Antioxidant Capacity in vitro and in vivo. Free. Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V. Emerging Prospective of Phytomolecules as Antioxidants against Chronic Diseases. ECS Trans. 2022, 107, 9571–9580. [Google Scholar] [CrossRef]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Leutz, A.; Doerr, H.W.; Cinatl, J., Jr. Glycyrrhizin exerts antioxidative effects in H5N1 influenza a virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE 2011, 6, e19705. [Google Scholar] [CrossRef]
- Farrukh, M.R.; Nissar, U.-A.; Kaiser, P.J.; Afnan, Q.; Sharma, P.R.; Bhushan, S.; Tasduq, S.A. Glycyrrhizic acid (GA) inhibits reactive oxygen species mediated photodamage by blocking ER stress and MAPK pathway in UV-B irradiated human skin fibroblasts. J. Photochem. Photobiol. B 2015, 148, 351–357. [Google Scholar] [CrossRef]
- Selyutina, O.; Polyakov, N.E.; Korneev, D.; Zaitsev, B. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 848–855. [Google Scholar] [CrossRef]
- D’avolio, A.; Pensi, D.; Baietto, L.; Pacini, G.; Di Perri, G.; De Rosa, F.G. Daptomycin Pharmacokinetics and Pharmacodynamics in Septic and Critically Ill Patients. Drugs 2016, 76, 1161–1174. [Google Scholar] [CrossRef]
- Liapikou, A.; Dimakou, K.; Toumbis, M. Telavancin in the treatment of Staphylococcus aureus hospital-acquired and ventilator-associated pneumonia: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2016, 10, 368–378. [Google Scholar] [CrossRef]
- Vanamala, K.; Tatiparti, K.; Bhise, K.; Sau, S.; Scheetz, M.H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Novel approaches for the treatment of methicillin-resistant Staphylococcus aureus: Using nanoparticles to overcome multidrug resistance. Drug Discov. Today 2020, 26, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, R.; Singh, A.; Kumar, A. Antibacterial potential of selected phytomolecules: An experimental study. Microbiol. Immunol. 2021, 65, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pal, A.; Darokar, M.P. A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic. Biol. Med. 2015, 87, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antimicrobial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef]
- Kristiansen, M.M.; Leandro, C.; Ordway, D.; Martins, M.; Viveiros, M.; Pacheco, T.; Molnar, J.; E Kristiansen, J.; Amaral, L. Thioridazine reduces resistance of methicillin-resistant staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. Vivo 2006, 20, 361–366. [Google Scholar]
- Novy, P.; Urban, J.; Leuner, O.; Vadlejch, J.; Kokoska, L. In vitro synergistic effects of baicalin with oxytetracycline and tetracycline against Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 1298–1300. [Google Scholar] [CrossRef]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Chueca, B.; Pagán, R.; García-Gonzalo, D. Oxygenated monoterpenes citral and carvacrol cause oxidative damage in Escherichia coli without the involvement of tricarboxylic acid cycle and Fenton reaction. Int. J. Food Microbiol. 2014, 189, 126–131. [Google Scholar] [CrossRef]
- Woodmansee, A.N.; Imlay, J.A. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol. Microbiol. 2003, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Martinez, L.R.; Mihu, M.R.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS ONE 2009, 4, e7804. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.; Naibi, A.; Abdulazeez, I.; Alege, I.; Mohammed, A.; Bello, S.; Yusuf, I.; Ibitoye, O.; Muritala, H. Involvement of oxidative stress in bactericidal activity of 2-(2-nitrovinyl) furan against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Microb. Pathog. 2016, 91, 107–114. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Niki, E.; Kamiya, Y.; Miki, M.; Tamai, H.; Mino, M. Free radical chain oxidation and hemolysis of erythrocytes by molecular oxygen and their inhibition by vitamin E. J. Nutr. Sci. Vitaminol. 1986, 32, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Markham, P.N.; A Neyfakh, A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1996, 40, 2673–2674. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-b-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Sims, P.J.; Waggoner, A.S.; Wang, C.H.; Hoffman, J.F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 1974, 13, 3315–3330. [Google Scholar] [CrossRef]
- Berti, A.D.; Wergin, J.E.; Girdaukas, G.G.; Hetzel, S.J.; Sakoulas, G.; Rose, W.E. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5046–5053. [Google Scholar] [CrossRef]
- Erental, A.; Sharon, I.; Engelberg, K.H. Two programmed cell death systems in Escherichia coli: An apoptotic-like death is inhibited by the mazEF-mediated death pathway. PLoS Biol. 2012, 10, e1001281. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Cell. Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Velamakanni, S.; Yao, Y.; Gutmann, D.A.P.; van Veen, H.W. Multidrug Transport by the ABC Transporter Sav1866 from Staphylococcus aureus. Biochemistry 2008, 47, 9300–9308. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Ann. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Gudmundsson, S. The post antibiotic effect. Clin. Microbiol. Newsl. 1991, 3, 403–431. [Google Scholar]
- Heisig, P.; Tschorny, R. Characterization of fluoroquinolone resistant mutants of Escherichia coli selected in vitro. Antimicrob. Agents Chemother. 1994, 38, 1284–1291. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically-Eight Edition: Approved Standard M07-A8; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Baek, S.H.; Cho, Y.; Lee, J.; Choi, B.Y.; Choi, Y.; Park, J.S.; Kim, H.; Sul, J.; Kim, E.; Park, J.H.; et al. Intracellular and Mitochondrial Reactive Oxygen Species Measurement in Primary Cultured Neurons. Bio-Protocol 2018, 8, e2871. [Google Scholar] [CrossRef]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.V.; Antunes, F.; Pires, J.; Silva-Herdade, A.; Pinto, M.L. A Comparison of Different Approaches to Quantify Nitric Oxide Release from NO-Releasing Materials in Relevant Biological Media. Molecules 2020, 25, 2580. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, M.; Sravanthi, M.; Hemalatha, K.P.J. Evaluation of antioxidant activity of two Indian medicinal plants. J. Phytol. 2011, 3, 86–91. [Google Scholar]
- Brenwald, N.P.; Gill, M.J. Wise R. Prevalence of putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1998, 42, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Oonmetta-Aree, J.; Suzuki, T.; Gasaluck, P.; Eumkeb, G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT 2006, 39, 1214–1220. [Google Scholar] [CrossRef]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of Daptomycin Bactericidal Activity and Membrane Depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Gant, V.A.; Warnes, G.; Phillips, I.; Savidge, G.F. The application of flow cytometry to the study of bacterial responses to antibiotics. J. Med. Microbiol. 1993, 39, 147–154. [Google Scholar] [CrossRef]
- Chamberlain, N.R.; Mehrtens, B.G.; Xiong, Z.; A Kapral, F.; Boardman, J.L.; I Rearick, J. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [CrossRef]
- Golding, C.G.; Lamboo, L.L.; Beniac, D.R.; Booth, T.F. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci. Rep. 2016, 6, 26516. [Google Scholar] [CrossRef]
- Chini, V.; Foka, A.; Dimitracopoulos, G.; Spiliopoulou, I. Absolute and relative real time PCR in the quantification of tst gene expression among methicillin-resistant Staphylococcus aureus: Evaluation by two mathematical models. Lett. Appl. Microbiol. 2007, 45, 479–484. [Google Scholar] [CrossRef]
- McKay, G.A.; Beaulieu, S.; Arhin, F.F.; Belley, A.; Sarmiento, I.; Parr, J.T.; Moeck, G. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 2009, 63, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Food Sci. Technol. 1997, 30, 609–615. [Google Scholar]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactyli-fera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Siddhurrajir, P.; Mohan, P.S.; Becker, K. Studies on the antioxidant activity of Indian laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002, 79, 61–67. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Hamtasin, C.; Mahabusarakam, W.; Voravuthikunchai, S.P. Inhibitory effect of Eleutherine americana Merr. extract on Staphylococcus aureus isolated from food. J. Food Sci. 2009, 1, 31–36. [Google Scholar] [CrossRef]
- Casero, C.; Estévez-Braun, A.; Ravelo, A.G.; Demo, M.; Méndez-Álvarez, S.; Machín, F. Achyrofuran is an antibacterial agent capable of killing methicillin-resistant vancomycin-intermediate Staphylococcus aureus in the nanomolar range. Phytomed 2013, 20, 133–138. [Google Scholar] [CrossRef]

| Agent | SA 4627 | SA 3721 | SA 4753 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alone | Combination (Ab/Gly) | Fold Reduction | Alone | Combination (Ab/Gly) | Fold Reduction | Alone | Combination (Ab/Gly) | Fold Reduction | |
| Glycyrrhizin | 1600 | - | - | 800 | - | - | 800 | - | - |
| Norfloxacin | 200 | 6.25/400 | 32/4 | 100 | 3.12/400 | 32/2 | 100 | 1.56/400 | 64/2 |
| Oxacillin | 800 | 50/400 | 16/4 | 400 | 50/400 | 8/2 | 400 | 50/400 | 8/2 |
| Vancomycin | 3.12 | 0.78/800 | 4/2 | 0.78 | 0.04/400 | 16/2 | 0.78 | 0.19/400 | 4/2 |
| Teicoplanin | 3.12 | 0.78/200 | 4/8 | 3.12 | 0.78/800 | 4/0 | 3.12 | 1.56/400 | 2/2 |
| Mutation Frequency of S. aureus (SA 96) with Nor and Gly | |||||
|---|---|---|---|---|---|
| Agent | MIC | 2MIC | 4MIC | 8MIC | 16MIC |
| Gly | 5.3 × 10−10 | 3.9 × 10−10 | 2.1 × 10−10 | 0.4 × 10−10 | <10−10 |
| Nor | 3.2 × 10−10 | 1.8 × 10−10 | 0.6 × 10−10 | <10−10 | <10−10 Ref. [34] |
| Nor + Gly (100 µg/mL) | 2.9 × 10−10 | 0.5 × 10−10 | <10−10 | <10−10 | <10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Pal, A.; Darokar, M.P. Synergistic Interaction of Glycyrrhizin with Norfloxacin Displays ROS-Induced Bactericidal Activity against Multidrug-Resistant Staphylococcus aureus. Drugs Drug Candidates 2023, 2, 295-310. https://doi.org/10.3390/ddc2020016
Singh V, Pal A, Darokar MP. Synergistic Interaction of Glycyrrhizin with Norfloxacin Displays ROS-Induced Bactericidal Activity against Multidrug-Resistant Staphylococcus aureus. Drugs and Drug Candidates. 2023; 2(2):295-310. https://doi.org/10.3390/ddc2020016
Chicago/Turabian StyleSingh, Vigyasa, Anirban Pal, and Mahendra P. Darokar. 2023. "Synergistic Interaction of Glycyrrhizin with Norfloxacin Displays ROS-Induced Bactericidal Activity against Multidrug-Resistant Staphylococcus aureus" Drugs and Drug Candidates 2, no. 2: 295-310. https://doi.org/10.3390/ddc2020016
APA StyleSingh, V., Pal, A., & Darokar, M. P. (2023). Synergistic Interaction of Glycyrrhizin with Norfloxacin Displays ROS-Induced Bactericidal Activity against Multidrug-Resistant Staphylococcus aureus. Drugs and Drug Candidates, 2(2), 295-310. https://doi.org/10.3390/ddc2020016







