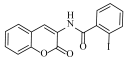
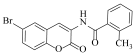
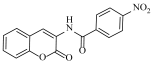
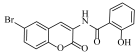
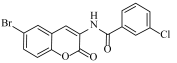
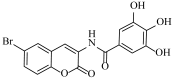
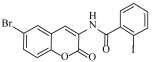
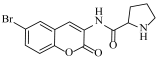
Abstract
Pseudomonas aeruginosa, a life-threatening bacteria listed as a priority pathogen by World Health Organization WHO, is known to cause severe nosocomial infections and fatality in immunocompromised individuals through its quorum sensing (QS) mediated biofilm formation. P. aeruginosa’s antibiotic-resistant biofilms are highly challenging to the existing antibiotic treatment options. There is an urgent clinical need to develop novel alternative therapeutic molecules such as antibiofilm and antiquorum sensing agents to counter the emergence of an unprecedented pace of antibiotic resistance of pathogens. In this context, a library of seventy 3-amidocoumarin derivatives was designed, and docking studies were performed against the P. aeruginosa LasR receptor using AutoDock 4.0. Based on docking results, a final series of sixteen 3-amidocoumarin derivatives (4a–p) were synthesized and evaluated for antibiofilm activity in vitro. Eight compounds significantly inhibited the formation of P. aeruginosa PAO1 biofilm. Compounds 4f, 4l and 4o showed maximum % inhibition in antibiotic-resistant P. aeruginosa PAO1 biofilm formation in the range of 80% to 86%. Further, the structure–activity relationship (SAR) studies revealed that the presence of electron-donating and bromo substituents at benzamido and coumarin moieties, respectively, effectively enhances the antibiofilm activity. In addition, the binding interactions between the synthesized compounds and active sites of the LasR QS receptor (Protein Data Bank Code: 2uv0) in P. aeruginosa were also investigated by molecular docking. The high binding affinities indicate that these compounds might be suitable for development into potent inhibitors of QS and biofilm disruptors.
1. Introduction
Bacterial biofilms are highly structured complex communities of bacteria held together by a self-produced extracellular polymeric matrix mainly composed of polysaccharides, secreted proteins and extracellular DNAs. Biofilms, which can be formed on biotic and abiotic surfaces, provide chemical and biological protection to the bacterium, making them physiologically distinct from planktonic cells []. The protective shield of biofilm gives additional resistance to the bacteria to tolerate harsh conditions and also makes them resistant to antibiotics, host–defense systems and external stresses. Current estimates show that more than 80% of bacterial infections are accompanied by biofilm formation, which contributes to pathogenesis, especially in chronic infections []. Biofilm can enhance the antibiotic resistance of microbial cells up to 10–1000 times more than planktonic cells []. Antibiotic-resistant bacterial infections not only cause huge morbidity and mortality across the world but also lead to social and economic instability for affected individuals and their families []. Decreased penetration of antibiotics, a decreased growth rate of the biofilm cells and/or a decreased metabolism of bacterial cells in biofilms may contribute to this tolerance []. Biofilm formation is a well-regulated multi-step process, and its eradication from the site of infection by the use of present antimicrobial therapy is not often possible [,].
Quorum sensing (QS) plays an important role in the production of biofilms and related virulence factors, which are closely related to the regulation of bacterial resistance [,]. It enables bacteria to communicate with their surroundings and regulate a variety of physiological functions in a cell-density-dependent manner via the production and release of chemical signaling molecules called autoinducers. When an autoinducer attains a critical threshold, the bacteria detect and respond to this signal by altering their gene expression []. QS signaling systems in bacteria can be broadly classified into three main groups. Gram-positive bacteria specifically produce autoinducing peptides (AIPs) as signaling molecules, whereas Gram-negative bacteria communicate using small molecules such as the acylated homoserine lactones (AHLs). The autoinducer-2 (AI-2) signal molecule has been detected in both Gram-negative and Gram-positive bacteria [,].
Over recent years, several natural and synthetic molecules have been reported in the literature that can cause disruption of the QS signaling cascade by mimicking signaling molecules, thereby inactivating the QS system by blocking the receptor and preventing gene expression and biofilm formation [,]. Brominated furanone firstly isolated from Delisea pulchra (marine algae) in 1993 showed potent QS inhibitory activity []. Since then, several halogenated lactones and non-lactone mimics have been reported to disrupt QS signaling and biofilm formation [,]. In 2019, Almohaywi et al. reported dihydropyrrolones as bacterial quorum sensing inhibitors (QSIs) []. The most active compound of this series exhibited 63.1% QS inhibition at 31.25 µM and 60% biofilm inhibition at 250 µM. Liu et al. recently reported the biofilm inhibitory activity of some aryl-substituted pyrrolidone derivatives with IC50 values in the range of 0.219 to 0.281 mM against Pseudomonas aeruginosa PAO1 []. Some other non-AHL-based QSIs, such as N-aryl glyoxamide-based small molecules, reduced biofilm formation in P. aeruginosa MH602 up to 71.2% at 250 µM [], some hydrazine-carboxamide hybrids have shown good antibiofilm activity against P. aeruginosa PAO1 at 100 µg/mL [], and some marine alkaloids have also been reported as inhibitors of biofilm formation [,]. El-Messery et al. described the antibiofilm activity of some amide chalcones, with IC50 values ranging from 2.4 to 8.6 µg/mL []. Certain cadiolide analogs also inhibited biofilm formation in Staphylococcus aureus and Enterococcus faecalis at low concentrations []. In a recent contribution, trifluoromethyl substituted salicylanilide derivative has been reported to reduce preformed biofilm in S. aureus better than vancomycin [].
A number of molecules belonging to the coumarin family have also been documented as antibiofilm and anti-QS agents (Figure 1) [,]. Some simple coumarin derivatives such as 6,7-dihydroxy, 7-hydroxy, 6-hydroxy and 3-hydroxycoumarin have been reported to cause 63, 61, 46 and 38% inhibition, respectively, of P. aeruginosa ATCC 27,853 biofilm formation at 100 µg/mL []. An important coumarin derivative warfarin, which is widely used as an anticoagulant, inhibited the biofilm formation in Escherichia coli by 50% at a concentration of 5 mM in the presence of lactoferrin and ampicillin []. These two compounds have been reported to promote biofilm formation in E. coli when administered at subinhibitory concentrations. A recent study described the inhibitory action of the coumarin scaffold on P. aeruginosa PAO1 biofilm formation in a dose-dependent manner; biofilm formation was inhibited by 9.98, 21.51, 33.16 and 46.10% at 31.25, 62.5, 125 and 250 μg/mL, respectively, with respect to the control []. Though a number of natural and synthetic compounds have been reported as inhibitors of QS and biofilm formation, so far, none of these have reached the clinics. Therefore, there is an urgent requirement for new antibiofilm agents. Here, we report the design and synthesis of 3-amidocoumarin as P. aeruginosa PAO1 biofilm inhibitors.
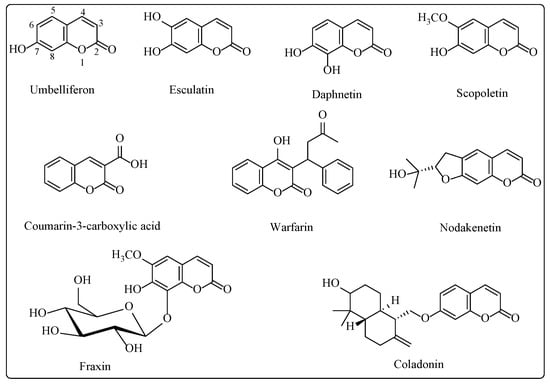
Figure 1.
Chemical structures of coumarin compounds possessing anti-QS and antibiofilm activity.
Inhibitor Design
Recent reports on the role of coumarins in the disruption of biofilm formation and QS signaling without disturbing planktonic cell growth have drawn the attention of many researchers to develop these molecules as novel antibiofilm agents. Studies also have revealed that substitutions at specific sites resulted in enhancement of activity while at other positions led to diminishing activity [,,]. Thus, suitably substituted coumarins provide a good choice for the development of antibiofilm agents. In our earlier investigations, we reported the antibacterial activity of a series of twenty-three amidocoumarins []. These compounds comprised coumarin heterocycle containing lactone ring and a substituted amido group at position 3, and both these moieties are also present in a number of antibiofilm agents and QSIs [Figure 2: color coding depicts different components: coumarin moiety (yellow), amide group (red ellipse), bromine (green) and lactone (blue rectangle)]. Therefore, we envisaged to evaluate the QS inhibitory activities of these amidocoumarins as a mechanistic approach for biofilm inhibition using Chromobacterium violaceum CV026 as a reporter strain by a method reported by Shukla et al. []. C. violaceum is a Gram-negative bacterium, which utilizes AHLs as signaling molecules for QS. Hence, this strain is widely used in research work related to QSI screening []. Out of twenty-three amidocoumarins tested, six compounds S1–S6 (Figure S1) showed anti-QS activity with zones of inhibition 14, 15, 14, 12, 16 and 14 mm, respectively, against C. violaceum. The remaining compounds of the series showed low/minimal activity, which suggested that compounds S1-S6 might have a specific biofilm formation inhibitory potential than the rest of the compounds. Therefore, we considered further substitutions/modifications of compounds S1–S6 to develop them as potent antibiofilm agents. Initially, a library of forty-eight compounds with appreciable diversity was designed by introducing 6,8-dicloro (a), 6-bromo (b), 6-chloro (c), 6-nitro (d), 8-methoxy (e), 6-methyl (f), 7-hydroxy (g) and 8-methoxy substituent (h) on the coumarin ring of S1–S6 precursor molecules, to obtain six sets, each containing eight molecules S1a–h to S6a–h (Supporting Information Tables S1–S6). All these compounds showed a good binding affinity with the active site of P. aeruginosa LasR (PDB Code: 2uvo) receptor with binding energies in the range of −8.25 to −11.95 kcal/mol. Among them, the binding energies of compounds with the 6-bromo substituent (S1b–S3b, S5b, S6b) were the best in each set with the exception of S4b. In previously reported work, bromine substituent has also delivered promising results for QS and biofilm inhibition [,]. We further designed twelve more compounds by introducing the bromo group on the C-5 and C-7 positions of the coumarin ring (Supporting Information Table S7, compounds S1i,j to S6i,j) of precursor molecules S1–S6, but most of these compounds exhibited low binding affinity (i.e., higher binding energy values) with the receptor as compared to their C-6 bromo analogs. Further, to obtain better SAR, we constructed five new coumarin derivatives S7–S11 by introducing different amido substituents at the C-3 position of the coumarin ring and also designed their 6-bromo derivatives S7a to S11a (Supporting Information Table S8). These compounds also exhibited good binding affinity with the LasR receptor. Finally, based on the docking results and synthetic accessibility, a series of sixteen 3-amidocoumarins containing five newly designed compounds with unsubstituted coumarin ring (S7–S11) and eleven bearing the 6-bromo substituent (S7a–11a and S1b–S6b) were synthesized for testing antibiofilm activity. Hereafter, the synthesized 3-amidocoumarins have been re-designated as 4a–4p and their detailed synthesis procedure, molecular docking and bioactivity results are described here. The docking poses of 4a-4p in complex with P. aeruginosa Las R receptor are shown in Figure S2.
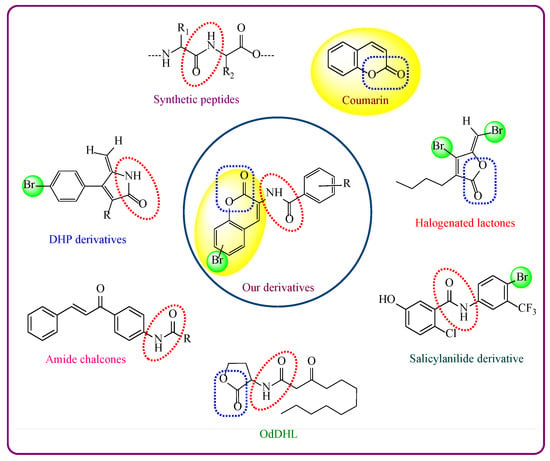
Figure 2.
Rational design of our synthesized compounds.
2. Results and Discussion
2.1. Chemistry
The synthetic route for the preparation of coumarin compounds is depicted in Scheme 1. 3-Nitrocoumarin 1a and 6-bromo-3-nitrocoumarin 1b were prepared via L-proline catalyzed condensation of ethyl nitroacetate and salicylaldehyde as reported earlier []. Nitrocoumarins 1a and 1b were reduced to 3-aminocoumarin 2a and 3-amino-6-bromocoumarin 2b, respectively, using acidified SnCl2. 3-Amidocoumarins 4a–p were prepared by reaction of 2a and 2b with suitable carboxylic acids 3a–p using PCl3 in acetonitrile under reflux for 5 to 6 h by slight modification in earlier reported method []. The structures of all the synthesized compounds were established on the basis of IR, 1H and 13C NMR and mass spectral data. Spectral data of compound 4a are shown in Figures S3–S6. The log P values were calculated using the ChemDraw 16.0 program (Table 1).
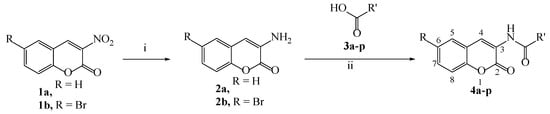
Scheme 1.
Synthesis of 3-amidocoumarins. (i) SnCl2, HCl, rt (ii) PCl3, CH3CN, reflux.

Table 1.
Synthesized amidocoumarin derivatives 4a–4p and their log P and binding energy (kcal/mol) values.
2.2. Biology
Initial screening of the coumarin compounds was performed with antimicrobial testing by the minimum inhibitory concentration (MIC) method. Results were not promising, and the compounds showed very poor antibacterial activity against the tested bacterial strains C. violaceum and P. aeruginosa. This inactivity further suggested that the compounds have no ability to interfere with the primary bacterial metabolism and have little effect on bacterial growth []. The compounds were next examined for biofilm inhibition activity against P. aeruginosa PAO1. Hentzer et al. reported that the genes responsible for the quorum sensing are present in the strain P. aeruginosa PAO1, which was considered in the present study []. Hence, the biofilm inhibitory activity of the synthesized compounds against P. aeruginosa may be due to the quorum quenching activity. An ideal QS inhibitor should possess high QS inhibition to minimize the production of bacterial virulence factors and biofilms while having minimal bacterial growth inhibition to reduce the likelihood of bacteria developing drug resistance.
The biofilm inhibition test was performed against the P. aeruginosa PAO1 bacterial strain using a crystal violet stain []. The assay results revealed significant biofilm inhibition by some of the compounds at 100 µg/mL. 4l, followed by 4o, 4f, 4a and 4h with 86%, 82%, 80%, 76% and 72% inhibition, respectively, were the most potent antagonists of biofilm formation (Figure 3). Importantly, 4f, 4g, 4h, and 4i with the 6-bromo-substituted coumarin ring displayed superior biofilm inhibition activity compared to their precursors 4a, 4b, 4c and 4d with the exception of 4j, which showed 50% inhibition in comparison to 4e (68%). These results suggest that bromo substitution is tolerated and enhances biofilm inhibitory activity. 4c, 4d, 4i, 4k, 4m and 4p showed biofilm inhibitory properties in the range of 50–72%, while 4n (log P = 5.26) with 30% inhibition was not effective in biofilm dispersal, which may be due to reduced cell permeability or steric hindrance caused by bulkier halogen atoms.
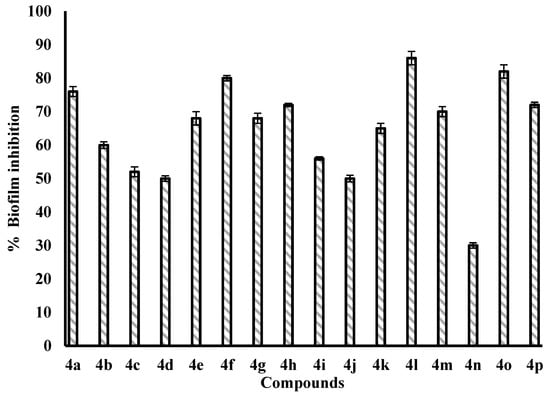
Figure 3.
Biofilm inhibition in P. aeruginosa PAO1 by the synthesized compounds under experimental conditions of 100 µg/mL concentration.
The nature of substituents present on the amido phenyl ring significantly influenced the biofilm activity. Compounds containing hydroxyl and amino groups (4a, 4f, 4l, 4m and 4o) showed greater biofilm inhibition, whereas substitution of chloro and nitro groups (4b, 4d, 4g, 4i and 4j) led to a decrease in biofilm inhibition. Compounds 4k and 4p with Ar-CH3 and proline groups displayed moderate activity with 65% and 72% inhibition. 4l bearing o-OH at the phenyl ring and bromo group at the C-6 position of the coumarin nucleus displayed the highest antibiofilm activity (86%) amongst all the synthesized coumarins. Overall, the synthesized compounds exhibited moderate to excellent levels of biofilm inhibition against P. aeruginosa. Some compounds were found to be superior in inhibiting the formation of biofilm than recently reported antibiofilm agents. At 100 µg/mL, seven compounds of the series exhibited more than 70% inhibition. These compounds also exhibited good affinity with the binding pocket of LasR protein with binding energy values less than −11 kcal/mole, which suggests that the biofilm inhibitory activity of these amidocoumarins might be due to the disruption in QS signaling.
2.3. Docking Studies
To identify binding orientations, all the compounds were docked into the crystal structure of P. aeruginosa LasR (PDB Code: 2uv0) with the autoinducer N-3-(oxododecanoyl)-L-homoserine lactone (OdDHL) [] using the AutoDock 4.0 (The Scripps Research Institute, La Jolla, CA, USA program. Compounds interacted with the amino acid residues of the active pocket, consisting of 17 amino acid residues including Leu36, Gly38, Leu39, Tyr64, Leu125, Gly126, Ala127, Trp60, Asp73, Tyr56, Ser129, Thr75, Ala105, Trp88, Leu110, Tyr93 and Arg61, with binding energy values ranging from −9.44 to −11.69 kcal/mol. The docking results of the top seven compounds are presented in Table 2. The docking results were mainly interpreted on the basis of (i) orientation of docked compounds relative to natural ligand OdDHL, (ii) intermolecular interactions present between the compound and target and (iii) binding energies of the docked compounds with the reference OdDHL.

Table 2.
Docking of compounds into the LasR receptor protein of P. aeruginosa.
The crystal structure of the P. aeruginosa LasR ligand-binding domain (LasR-LBD) bound to its autoinducer OdDHL reveals that the polar region, including the lactone ring and carbonyl groups, of OdDHL form six intermolecular H-bonds with LasR-LBD. Five direct H-bonds are formed between (i) the lactone carbonyl and NH of Trp60 (3.01 Å), (ii) the amide NH and the carboxyl group of Asp73 (2.76 Å) and Thr75 (3.39 Å), and (iii) amide 1-oxo with the hydroxyl groups of Ser129 (2.70 Å) and Tyr56 (2.65 Å). An indirect H-bond is formed between the 3-oxo group and a water molecule and then with Arg61. The long 12-carbon acyl chain buries into the cavity lined with several hydrophobic residues [].
We also manually positioned the autoinducer OdDHL into the receptor binding site for a better understanding of the binding interactions (Table 2). The docking analysis suggests that the lactone ring of OdDHL interacts with the lactone pocket and forms four H-bonds between (i) carbonyl lactone and Trp-60 (2.80Å), (ii) NH of amide and Asp-73 side chain (2.19Å), (iii) amide carbonyl and Ser129 side chain (2.73 Å), and (iv) acyl carbonyl and Try64 (3.01 Å). The 12-carbon acyl chain inserts into the hydrophobic pocket and adopts a similar conformation to that of the LasR-OdDHL crystal complex.
Amongst all the docked compounds, 4o having a binding energy of −11.69 kcal/mol showed the maximum binding affinity with the receptor (Figure 4). 4o contains three hydroxyl groups and forms seven H-bonds with the LasR receptor. The lactone carbonyl formed an H-bond with Arg61 (3.33 Å), and amide carbonyl formed two H-bonds, one with Ser129 (2.80 Å) and the second with Tyr56. The hydroxyl groups formed four H-bonds with Tyr93 (2.68, 2.10, and 2.89 Å) and Leu110 (2.22 Å) side chains. The trihydroxyphenyl ring was stabilized by π–π interaction with Trp88 and Tyr56 and electrostatic interactions with Trp88, Tyr93, Ala105, Leu110 and Thr75. Bromine interacted with Gly38, Gly126 and Ala127 through hydrophobic interactions. The molecule was stabilized by hydrophobic and electrostatic interaction by Val76, Leu36, Tyr64 and Asp73 amino acid residues.
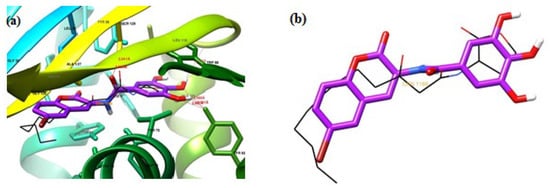
Figure 4.
(a) Binding mode of compound 4o (violet) within the LasR binding site in comparison with OdDHL (black lines). (b) 4o (violet) superimposed on the X-ray structure of OdDHL (black lines) from the LasR complex.
4l showed a binding energy value of −11.66 kcal/mol. The coumarin ring exhibited hydrophobic interaction with Ile52, Leu36, Gly38 and π–π stacking with Tyr64. The ortho-hydroxyphenyl ring also exhibited π–π stacking with Trp88 and hydrophobic interaction with Leu110, Tyr93 and Thr75. This compound displayed four H-bonds between (i) the carbonyl of the amido group and the hydroxyl of Ser129, (ii) the lactone carbonyl of coumarin and the terminal NH2 of Arg61, (iii) the lactone carbonyl of coumarin and the NH of Arg61, and (iv) the hydroxyl group and NH of Trp60. 4h with a binding energy of −11.65 kcal/mol was docked in a similar orientation as OdDHL, with the lactone ring being inserted in the lactone pocket, while the aromatic amido group occupies the hydrophobic pocket. However, the interactions were somewhat different than OdDHL. The lactone carbonyl formed two H-bonds with Arg61. The additional H-bond was formed between the lactone ring oxygen and the Trp60 side chain. The coumarin ring showed π-stacking with Tyr64 and Tyr56, whereas bromine exhibited interaction with Trp88. Aromatic rings showed hydrophobic interactions with Asp73, Thr75, Gly38, Leu39, Ala50 and Ala127. Amide carbonyl and NH and lactone carbonyl showed electrostatic interactions with Leu36. Iodine groups showed van der Waals contacts with Tyr47, Arg61 and Tyr64.
Compound 4f with a binding energy of −11.47 kcal/mol formed five H-bonds: two between amido carbonyl and Ser129 (2.85 Å) and Tyr56 (3.30 Å); likewise, in 4o, p-hydroxyl formed H-bonds with Tyr93 (2.95 Å) and Val111 (2.05 Å), and another H-bond was formed between lactone carbonyl and Arg61 (3.25 Å). The coumarin ring in 4f showed hydrophobic interactions with Ile52 and Gly38, whereas bromine exhibited van der Waals interactions with Gly38, Leu39, Leu125, Gly126 and Ala127. The coumarin and amide group was stabilized by electrostatic interactions with Tyr64, Leu36 and Asp73. The para-hydroxyphenyl ring of 4f formed a hydrophobic interaction with Thr75, Tyr56, Leu110 and π-stacking with Trp88. Compound 4a displayed similar H-bonding, electrostatic, hydrophobic and π–π stacking interactions like 4f. The binding energy of 4a was calculated as −11.36 kcal/mol. 4m showed similar interactions as that of 4f. The amino group of 4f formed a hydrogen bond interaction with Tyr93. 4k with a binding energy of −11.25 kcal/mol showed hydrophobic interactions with Tyr56, Trp60, Leu110, Thr75 and Tyr93 and π-stacking with Trp88.
The docking results of the LasR-synthesized compounds complex revealed that all the compounds docked in the ‘flipped orientation’ relative to OdDHL except 4h. Possibly, the presence of large iodine groups makes compound 4h more lipophilic and more suited to the hydrophobic ligand-binding pocket of the receptor resulting in a similar orientation as that of OdDHL. All the compounds formed H-bond between (i) lactone carbonyl and Arg61 and (ii) amide carbonyl and Ser129 except 4h.
3. Materials and Methods
3.1. Experimental
General: Reagents and starting materials were purchased from commercial suppliers (SRL (Gurgaon, India), Alfa Aesar (Haverhill, MA, USA), Sigma-Aldrich (St. Louis, MO, USA) and Spectrochem Pvt. Ltd. (Mumbai, India)) and used without any purification unless otherwise stated. The melting points were determined in open capillary tubes on Buchi B-510 apparatus. IR spectra were recorded on a JASCO FTIR 4700 in KBr from 400 to 4000 cm−1. Analytical thin-layer chromatography (TLC) was performed on 60 F254 silica gel precoated aluminum plates with a 0.25 mm thickness. The spots were located under a UV lamp (λmax = 254 nm) or stained with iodine or Dragendorff’s reagent solution. 1H and 13C NMR were recorded on a JEOL NMR spectrometer at 500 and 125 MHz, respectively. Chemical shifts for 1H NMR are reported as δ values, and coupling constants are in hertz (Hz). The following abbreviations were used for spin multiplicity: singlet (s), doublet (d), triplet (t), double doublet (dd), multiplet (m), broad singlet (bs), and if the pattern of splitting could not be interpreted easily, they are assigned as multiplet (m). Synthesized compounds were isolated by column chromatography on silica gel (60–120 mesh). Mass spectra were recorded on Sciex-X500R QTOF.
3.2. Chemistry
3.2.1. Preparation of 3-nitrocoumarin 1a and 6-bromo3-nitrocoumarin 1b
Compounds 1a and 1b were prepared based on a literature method []. 3-Nitrocoumarin 1a was prepared by reaction between salicylaldehyde (0.10 mL, 1 mmol) and ethyl nitroacetate (0.11 mL, 1 mmol) using L-proline (30 mol%) as catalyst in ethanol (3 mL), whereas, 6-bromo-3-nitrocoumarin 1b was prepared by reaction between 5-bromo-3-hydroxy benzaldehyde (0.20, 1 mmol) and ethyl nitroacetate (0.11 mL, 1 mmol).
Compound 1a: Yellow solid, yield: 85%, mp 142–143 °C (literature []: 141–142 °C); 1H NMR (CDCl3): 8.78 (s, 1H), 7.81 (d, J = 7.5 Hz, 1H), 7.73 (m, 1H), 7.46 (m, 2H).
Compound 1b: Pale yellow solid, yield: 74%, mp 198–199 °C (literature []: 200 °C); 1H NMR (CDCl3): 8.86 (s, 1H), 7.92 (d, J = 2.7 Hz, 1H), 7.63 (dd, J = 9.0 and 2.7 Hz, 1H), 7.38 (d, J = 9.0 Hz, 1H).
3.2.2. Preparation of 3-aminocoumarin 2a and 6-bromo-3-aminocoumarin 2b
Compounds 2a and 2b were prepared by reduction of compounds 1a and 1b, respectively, as described in previous work [].
Compound 2a: Off white solid; yield: 72%, mp 137–138 °C (literature []: 136–138 °C); 1H NMR (CDCl3): 7.33 (m, 2H), 7.18 (m, 2H), 6.67 (s, 1H), 4.29 (bs, 2H).
Compound 2b: Off white solid; yield: 68%, mp 207–208 °C (literature []: 208–209 °C); 1H NMR (CDCl3): 7.41(d, J = 2.5 Hz, 1 H, H-5), 7.29 (dd, J = 8.5 and 2.5 Hz, 1H, H-7), 7.16 (d, J = 8.7 Hz, 1H, H-8), 6.58 (s, 1H, H-4), 4.32 (bs, 2H, NH2).
3.2.3. General Procedure for the Synthesis of Compounds (4a–p)
3-aminocoumarin 2a (1 mmol) and appropriate aromatic acid 3a–e (1.1 mmol) were stirred in 5 mL of CH3CN at room temperature for half an hour. PCl3 (3 mmol) was added dropwise to the reaction mixture and refluxed for 5–6 h. After completion of reaction (monitored by TLC), the reaction mixture was quenched by adding ice-cold water (5 mL), and CH3CN was evaporated under reduced pressure. A 30 mL volume of water was added to the remaining aqueous layer and the product was extracted with CHCl3 (2 × 40 mL). The combined organic layers were washed twice with water (2 × 20 mL) and then with saturated NaHCO3 solution (20 mL) and finally with brine (20 mL). The organic layer was collected, dried over anhydrous Na2SO4 and evaporated under reduced pressure to afford the crude products. The crude products were recrystallized from ethanol to yield pure samples of 4a–4d. Compound 4e was purified by column chromatography using hexane: ethylacetate (7:3) as eluent.
3-amidocoumarins 4f–p were prepared by reacting 6-bromo-3-aminocoumarin 2b (1 mmol) with appropriate carboxylic acid 3f–p using the same procedure as described above for the synthesis of compounds 4a–4e. The crude products in the case of 4f–4i, 4k, 4l, 4n and 4o were recrystallized from ethanol. Compounds 4m, 4j and 4p were purified by coluumn chromatography using hexane:ethylacetate (7:3) as eluent.
4-Hydroxy-N-(2-oxo-2H-chromen-3-yl)benzamide (4a)
The title compound was prepared from 2a (0.16 g, 1.0 mmol) and 4-hydroxy benzoic acid 3a (0.15 g, 1.1 mmol) according to general procedure, to give off white solid. Yield: 48%; m.p.: 135–137 °C; Rf = 0.55 (hexane-ethyl acetate, 3:1); IR (KBr): 3445, 3078, 1710, 1606, 1515, 1426, 1273 cm−1; 1H NMR (DMSO-d6): δ 10.84 (s, 1H, OH), 8.58 (s, 1H, H-4), 8.28 (s, 1H, ArH), 8.11 (m, 1H, ArH), 7.66 (d, J = 7.5 Hz, 1H, H-5), 7.47 (m, 1H, H-7), 7.32 (m, 4H, H-6, H-8 and 2ArH); 13C NMR (DMSO-d6/CDCl3; 1:1): δ 164.7, 157.5, 157.3, 150.1, 149.8, 137.7, 130.0, 127.9, 127.3, 124.8, 123.4, 119.8, 119.0, 115.7. HRMS (ESI) m/z: calcd. for C16H11NO4 [M + H]+: 282.0766, found: 282.0463.
3-Chloro-N-(2-oxo-2H-chromen-3-yl)benzamide (4b)
The title compound was prepared from 2a (0.16 g, 1.0 mmol) and 2-chloro benzoic acid 3b (0.17 g, 1.1 mmol) according to general procedure, to yield a white solid. Yield: 65%; m.p.: 150–151 °C; Rf = 0.46 (hexane-ethyl acetate, 3:1); IR (KBr): 3378, 3079, 3039, 1714, 1675, 1594, 1368, 1239 cm−1; 1H NMR (CDCl3): δ 8.91 (s, 1H, NH), 8.85 (s, 1H, H-4), 7.75 (d, J = 7.0 Hz, 1H, H-5), 7.54 (d, J = 7.0 Hz, 1H, ArH), 7.45 (m, 2H, ArH and H-7), 7.38 (m, 1H, ArH), 7.33 (m, 3H, H-6, H-8 and ArH); 13C NMR (CDCl3): δ 165.3, 158.7, 150.1, 133.9, 132.4, 131.1, 130.8, 130.3, 130.0, 128.0, 127.4, 125.3, 124.1, 124.0, 119.8, 116.5. HRMS (ESI) m/z: calcd. for C16H10ClNO3 [M + H]+: 299.0349, found: 299.0343.
2-Iodo-N-(2-oxo-2H-chromen-3-yl)benzamide (4c)
The title compound was prepared from 2a (0.16 g, 1.0 mmol) and 2-iodo benzoic acid 3c (0.27 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 88%; m.p.: 124–126 °C; Rf = 0.42 (hexane-ethyl acetate, 3:1); IR (KBr): 3342, 3062, 1693, 1682, 1582, 1466, 1403, 1267 cm−1; 1H NMR (DMSO-d6): δ 9.11 (s, 1H, NH), 8.69 (s, 1H, H-4), 7.96 (m, 2H, ArH), 7.68 (m, 2H, H-5 and ArH), 7.46 (m, 2H, H-7 and ArH), 7.21 (m, 2H, H-6 and H-8); 13C NMR (DMSO-d6): δ 168.2, 158.7, 150.2, 148.0, 140.5, 137.0, 132.5, 131.3, 130.0, 128.2, 126.8, 125.1, 124.0, 119.6, 116.4, 94.0. HRMS (ESI) m/z: calcd. for C16H10INO3 [M + H]+: 391.9783, found: 391.4968.
4-Nitro-N-(2-oxo-2H-chromen-3-yl)benzamide (4d)
The title compound was prepared from 2a (0.16 g, 1.0 mmol) and 4-nitro benzoic acid 3d (0.18 g, 1.1 mmol) according to general procedure, to yield a pale yellow solid. Yield: 86%; m.p.: 166–168 °C; Rf = 0.39 (hexane-ethyl acetate, 3:1); IR (KBr): 3373, 3115, 1694, 1607, 1540, 1430, 1350, 1294 cm−1; 1H NMR (DMSO-d6): δ 8.93 (s, 1H, NH), 8.61 (s, 1H, H-4), 8.26 (m, 2H, ArH), 8.13 (m, 2H, ArH), 7.64 (d, J = 8.0 Hz, 1H, H-5), 7.49 (m, 1H, H-7), 7.31 (m, 2H, H-6 and H-8); 13C NMR (DMSO-d6): δ 163.9, 159.6, 151.6, 148.1, 141.2, 134.6, 129.9, 128.8, 128.2, 126.8, 125.4, 125.3, 123.2, 122.6, 121.6, 116.5. HRMS (ESI) m/z: calcd. for C16H10N2O5 [M + H]+: 311.0668, found: 311.0662.
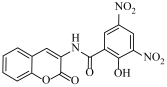
2-Hydroxy-3,5-dinitro-N-(2-oxo-2H-chromen-3-yl)benzamide (4e)
The title compound was prepared from 2a (0.16 g, 1.0 mmol) and 3,5-dinitrosalicylic acid 3e (0.25 g, 1.1 mmol) according to general procedure, to yield a light brown solid. Yield: 72%, Rf = 0.42 (hexane-ethyl acetate, 3:2); IR (KBr): 3016, 1716, 1672, 1529, 1337, 1265 cm−1; 1H NMR (DMSO-d6): δ 13.45 (s, 1H, OH), 8.79 (m, 2H, ArH), 8.53 (s, 1H, H-4), 7.66 (m, 1H, H-5), 7.43 (m, 1H, H-7), 7.29 (m, 2H, H-6 and H-8); 13C NMR (DMSO-d6): δ 169.1, 164.4, 158.1, 150.0, 141.3, 130.5, 129.8, 128.4, 128.3, 125.7, 125.5, 125,4, 123.1, 122.7, 120.4, 116.3. HRMS (ESI) m/z: calcd. for C16H9N3O8 [M + H]+: 372.0468, found: 372.0462.
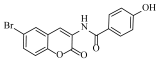
N-(6-bromo-2-oxo-2H-chromen-3-yl)-4-hydroxybenzamide (4f)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 4-hydroxy benzoic acid 3f (0.15 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 88%; m.p.: 193–195 °C; Rf = 0.48 (hexane-ethyl acetate, 3:1); IR (KBr): 3568, 3230, 3066, 1712, 1604, 1515, 1428, 1362, 1275 cm−1; 1H NMR (DMSO-d6): δ 10.1 (s, 1H, OH), 8.32 (s, 1H, H-4), 7.69 (s, 1H, H-5), 7.59 (m, 2H, Ar-H), 7.41 (m, 1H, H-7), 6.88 (d, J = 9.0 Hz, 1H, H-8), 6.75 (m, 2H, ArH); 13C NMR (DMSO-d6): δ 168.7, 167.5, 155.9, 151.0, 134.6, 133.1, 132.4, 130.6, 129.2, 125.3, 123.2, 122.8, 118.8, 118.7, 116.2, 110.7. HRMS (ESI) m/z: calcd. for C16H10BrNO4 [M + H]+: 359.9871, found: 359.4998.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-3-chlorobenzamide (4g)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 2-chloro benzoic acid 3g (0.17 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 58%; m.p.: >200 °C; Rf = 0.49 (hexane-ethyl acetate, 3:1); IR (KBr): 3400, 3003, 1750, 1604, 1476, 1425, 1230 cm−1; 1H NMR (DMSO-d6): δ 9.23 (s, 1H, NH), 8.22 (s, 1H, H-4), 7.98 (m, 2H, ArH), 7.67 (m, 2H, H-5 and ArH), 7.42 (m, 2H, H-7 and ArH), 6.82 (d, J = 8.5 Hz, 1H, H-8); 13C NMR (DMSO-d6): δ 168.6, 156.2, 154.9, 135.6, 134.2, 132.7, 132.4, 132.2, 131.8, 131.3, 121.8, 119.4, 119.1, 115.8, 112.3, 110.5. HRMS (ESI) m/z: calcd. for C16H9BrClNO3 [M + H]+: 377.9532, found: 377.9524.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-iodobenzamide (4h)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 2-iodo benzoic acid 3h (0.27 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 90%; m.p.: 135–138 °C; Rf = 0.42 (hexane-ethyl acetate, 3:1); IR (KBr): 3409, 3062, 1683, 1582, 1466, 1404, 1268 cm−1; 1H NMR (DMSO-d6): δ 8.27 (s, 1H, H-4), 7.97 (d, J = 7.5 Hz, 1H, ArH), 7.69 (m, 2H, H-5 and ArH), 7.46 (m, 2H, H-7 and ArH), 7.21 (m, 1H, ArH), 6.83 (d, J = 8.5 Hz, 1H, H-8); 13C NMR (DMSO-d6): δ 168.9, 161.4, 151.5, 142.2, 141.0, 137.4, 132.9, 131.6, 130.5, 128.7, 126.3, 124.8, 121.2, 119.6, 116.8, 94.5. HRMS (ESI) m/z: calcd. for C16H9BrINO3 [M + H]+: 469.8888, found: 469.8321.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-4-nitrobenzamide (4i)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 4-nitro benzoic acid 3i (0.18 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 60%; m.p.: >200 °C; Rf = 0.48 (hexane-ethyl acetate, 3:1); IR (KBr): 3117, 3063, 1694, 1607, 1542, 1430, 1351, 1294 cm−1; 1H NMR (DMSO-d6): δ 9.25 (s, 1H, NH), 8.26 (m, 3H, H-4 and ArH), 8.10 (m, 2H, ArH), 7.68 (d, J = 2.0 Hz, 1H, H-5), 7.37 (m, 1H, H-7), 6.91 (m, 1H, H-8); 13C NMR (DMSO-d6): δ 168.2, 161.6, 151.8, 150.7, 140.3, 137.6, 134.2, 130.2, 129.3, 126.8, 124.8, 122.1, 119.3, 118.2, 117.2. HRMS (ESI) m/z: calcd. for C16H9BrN2O5 [M + H]+: 388.9773, found: 388.9716.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxy-3,5-dinitrobenzamide (4j)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 3,5-dinitrosalicylic acid 3j (0.25 g, 1.1 mmol) according to general procedure, to yield a yellow solid. Yield: 68%; m.p.: >200 °C; Rf = 0.43 (hexane-ethyl acetate, 3:1); IR (KBr): 3259, 3071, 1695, 1600, 1569, 1494, 1415, 1322, 1271 cm−1; 1H NMR (DMSO-d6/CDCl3; 1:1): δ 13.32 (s, 1H, OH), 8.64 (m, 2H, ArH), 8.24 (s, 1H, H-4), 7.63 (s, 1H, H-5), 7.47 (m, 1H, H-7), 6.84 (m, 1H, H-8); 13C NMR (DMSO-d6/CDCl3; 1:1): δ 164.2, 161.2, 159.6, 149.9, 141.1, 137.1, 134.9, 131.2, 130.1, 126.3, 124.1, 123.1, 121.6, 119.2, 118.5, 117.1. HRMS (ESI) m/z: calcd. for C16H8BrN3O8 [M]+: 448.9495, found: 448.8767.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-methylbenzamide (4k)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and toluic acid 3k (0.14 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 96%; m.p.: 110–112 °C; Rf = 0.60 (hexane-ethyl acetate, 3:1); IR (KBr): 3300, 2949, 2809, 1739, 1693, 1610, 1430, 1226 cm−1; 1H NMR (DMSO-d6): δ 8.31 (s, 1H, H-4), 7.77 (m, 2H, H-5, ArH), 7.45 (m, 2H, H-7 and ArH), 7.24 (m, 1H, ArH), 6.71 (m, 2H, H-8 and ArH), 2.47 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 169.2, 156.2, 149.5, 139.4, 132.9, 132.4, 132.2, 131.7, 131.0, 130.6, 126.3, 124.4, 122.8, 120.2, 118.9, 110.5, 21.8. HRMS (ESI) m/z: calcd. for C17H12BrNO3 [M + H]+: 358.0079, found: 358.5027.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxybenzamide (4l)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 2-hydroxy benzoic acid 3l (0.15 g, 1.1 mmol) according to general procedure, to yield a white solid. Yield: 92%; m.p.: 205–207 °C; Rf = 0.42 (hexane-ethyl acetate, 3:1); IR (KBr): 3400, 3000, 1750, 1606, 1443, 1220 cm−1; 1H NMR (DMSO-d6): δ 9.54 (s,1H, OH), 8.31 (s, IH, H-4), 7.70 (m, 2H, H-5 and ArH), 7.45 (d, J = 8.5 Hz, 1H, H-7), 7.22 (m, 1H, ArH), 6.83 (d, J = 8.5 Hz, 1H, H-8), 6.71 (m, 2H, ArH); 13C NMR (DMSO-d6): δ 164.6, 160.2, 158.4, 151.2, 135.1, 134.2, 133.5, 132.5, 130.5, 128.6, 124.8, 123.2, 122.5, 121.9, 119.1, 112.2. HRMS (ESI) m/z: calcd. for C16H10BrNO4 [M + H]+: 359.9871, found: 359.9865.
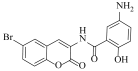
5-Amino-N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxybenzamide (4m)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 5-amino salicylic acid 3m (0.16 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 82%; m.p.: 220 °C; Rf = 0.42 (hexane-ethyl acetate, 3:2); IR (KBr): 3410, 3003, 749, 1605, 1477, 1425, 1266, 1230 cm−1; 1H NMR (DMSO-d6/CDCl3; 1:2): δ 10.2 (s, 1H, OH), 8.21 (s, 1H, H-4), 7.72 (s, 1H, H-5), 7.45 (d, J = 8.0 Hz, 1H, H-7), 7.21 (m, 1H, ArH), 6.87 (d, J = 8.0 Hz, 1H, H-8), 6.76 (m, 2H, ArH), 4.54 (bs, 2H, NH2); 13C NMR (DMSO-d6/CDCl3; 1:2): δ 166.6, 158.8, 153.7, 150.1, 139.3, 132.2, 130.7, 130.6, 130.0, 129.7, 124.8, 121.4, 120.8, 116.7, 116.5, 110.3. HRMS (ESI) m/z: calcd. for C16H11BrN2O4 [M + H]+: 374.9980, found: 374.4202.
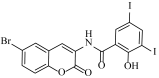
N-(6-bromo-2-oxo-2H-chromen-3-yl)-2-hydroxy-3,5-diiodobenzamide (4n)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and 3,5-diiodo salicylic acid 3n (0.42 g, 1.1 mmol) according to general procedure, to yield a white solid. Yield: 70%; m.p.: 195 °C; Rf = 0.38 (hexane-ethyl acetate, 3:1); IR (KBr): 3407, 3003, 1749, 1667, 1476, 1425, 1229 cm−1; 1H NMR (DMSO-d6/CDCl3; 1:1): δ 10.15 (s, 1H, OH), 8.22 (s, 1H, H-4), 8.13 (s, 1H, ArH), 7.99 (s, 1H, ArH), 7.72 (s, 1H, H-5), 7.45 (d, J = 8.5 Hz, 1H, H-7), 6.84 (d, J = 8.5 Hz, 1H, H-8); 13C NMR (DMSO-d6/CDCl3; 1:1): δ 170.4, 168.5, 162.0, 156.1, 149.4, 138.7, 132.8, 132.5, 132.3, 122.0, 118.9, 118.5, 110.6, 88.8, 79.5. HRMS (ESI) m/z: calcd. for C16H8BrI2NO4 [M + H]+: 611.7804, found: 611.7716.
N-(6-bromo-2-oxo-2H-chromen-3-yl)-3,4,5-trihydroxybenzamide (4o)
The title compound was prepared from 2p (0.24 g, 1.0 mmol) and gallic acid 3o (0.18 g, 1.1 mmol) according to general procedure, to yield an off white solid. Yield: 92%; m.p.: 155 °C; Rf = 0.41 (hexane-ethyl acetate, 3:2); IR (KBr): 3322, 3000, 1738, 1609, 1496, 1429, 1365, 1267 cm−1; 1H NMR (DMSO-d6): δ 9.74 (s, 1H, OH), 9.01 (s 1H, OH), 8.14 (s, 1H, H-4), 7.75 (s, 1H, H-5), 7.45 (m, 1H, H-7), 7.38 (m, 2H, ArH), 6.87 (m, 1H, H-8); 13C NMR (DMSO-d6): δ 165.8, 157.0, 152.8, 142.6, 141.4, 140.4, 134.3, 132.7, 130.2, 129.0, 125.0, 121.5, 118.5, 116.2, 117.0, 109.9. HRMS (ESI) m/z: calcd. for C16H10BrNO6 [M + H]+: 391.9769, found: 391.9221.
N-(6-bromo-2-oxo-2H-chromen-3-yl)pyrrolidine-2-carboxamide (4p)
The title compound was prepared from 2b (0.24 g, 1.0 mmol) and L-proline 3p (0.12 g, 1.1 mmol) according to general procedure, to yield a light brown solid. Yield: 66%; m.p.: 224 °C; Rf = 0.52 (hexane-ethyl acetate, 3:1); IR (KBr): 3400, 3006, 2951, 2725, 2610, 1750, 1604, 1476, 1316, 1266 cm−1; 1H NMR (CDCl3): δ 8.50 (s, 1H, NH), 8.13 (s, 1H, H-4), 7.68 (s, 1H, H-5), 7.43 (d, J = 8.5 Hz, 1H, H-7), 6.86 (d, J = 8.5 Hz, 1H, H-7), 4.07 (m, 1H, NCH), 3.59 (m, 2H, NCH2), 2.41 (m, 2H, CH2), 1.82 (m, 2H, CH2); 13C NMR (DMSO-d6/CDCl3; 1:2): δ 168.2, 160.1, 149.4, 137.1, 132.6, 130.2, 125.6, 121.2, 119.4, 116.8, 61.4, 45.4, 32.1, 24.2. HRMS (ESI) m/z: calcd. for C14H13BrN2O3 [M + H]+: 337.0188, found: 337.0194.
3.3. Biology
3.3.1. Evaluation of Antiquorum Sensing Activity of Compounds for Biofilm Inhibition
The antiquorum sensing activity of the compound series was evaluated by biosensor bioassay with the Chromobacterium violaceum CV026 strain using the agar well diffusion method []. A bacterial lawn was developed on agar plates by using freshly prepared C. violaceum culture in Luria Bertani (LB) medium. The wells were inoculated with 100 µL of stock solution of 2 mg/mL of compounds. These plates were incubated at 27 °C for 48 h. DMSO was used as negative control. The antiquorum sensing activity was determined in terms of the zone of inhibition (measured in mm) developed around the wells. Compounds showing positive results on agar plates were subjected to biofilm inhibition analysis.
3.3.2. Biofilm Formation
Biofilm of Gram-negative Pseudomonas aeruginosa PAO1 was formed on glass wool in Petri plates (25 mm) filled with freshly prepared 10 mL culture in nutrient broth. These plates were incubated under static conditions at 37 °C for 48 h. After incubation, biofilm grown on glass wool was observed and analyzed.
3.3.3. Biofilm Inhibition Analysis
Biofilm inhibition and degradation tests were carried out in 96-well microtiter plates using the crystal violet (CV) staining method as described by Shukla et al. []. A 100 µL volume of freshly prepared P. aeruginosa suspension was taken in a 96-well plate, and 100 µL of 0.2 mg/mL stock of the test compound was added to each well and incubated for 48 h at 37 °C. After incubation, planktonic cells were removed by removing excess media and washing the wells with distilled water. A 200 µL volume of 0.4% CV dye was further added to each well and incubated for 20 min to stain cells of the attached biofilm. This was followed by washing of wells with distilled water to remove excess dye, and plates were dried. For analysis, the biofilm was resolubilized in DMSO, and a reading was taken at 620 nm with an ELISA reader. The percentage biofilm inhibited by the test compounds was calculated by the following formula
3.4. Molecular Docking
All the synthesized compounds were docked using Auto-Dock Tools (ADT version 1.5.6, The Scripps Research Institute, La Jolla, CA, USA) and AutoDock version 4.0. The high-resolution crystal structure of P. aeruginosa the LasR ligand-binding domain bound to its autoinducer with PDB ID: 2UV0 was retrieved from the Protein Data Bank (www.rcsb.org (8 December 2021)). A self-predicted active site having grid X-Y-Z coordinates: 23.900, 15.715, 80.896 Å and spacing 1.000 Å was taken for the calculation. This active pocket consists of 17 amino acids. Initially, the 3D structures of ligands were drawn using ACD/ChemSketch and saved in MDL Molfiles [V2000] format. The MDL Molfiles were further converted into .pdb format using the Open Babel GUI. Then, the environment was created for protein preparation in ADT consecutively polar hydrogens, and Kollman charges were added. The formats of both the protein and ligand files were changed to pdbqt format as only this particular format is operational in ADT GUI. The docking tool operated on the Lamarckian genetic algorithm and yielded various docked poses, among which the top ten runs were considered on the basis of the scoring function. Root mean square deviation (RMSD) values differing by less than 0.5 Å were taken into account to generate docked ligands of our interest. The ligand–protein docked conformations were studied and evaluated on the basis of various parameters such as binding energy, hydrogen bonding and hydrophobic interactions existing between the binding ligand and receptor protein. For visualization of various docking poses, coordinates and interactions, we used the software Chimera 1.8.1 (University of California, San Francisco, CA, USA).
4. Conclusions
In conclusion, we synthesized a series of sixteen amidocoumarins as potential biofilm inhibitors. Compounds 4f, 4l and 4o inhibited the biofilm formation in antibiotic-resistant P. aeruginosa PAO1 in the range of 80 to 86% at 100 μg/mL compound. This study also provides preliminary SARs around the 3-amidocoumarin motif. The presence of the bromo group at the C-6 position of the coumarin ring was favorable for bioactivity. The electron-donating groups at amido moiety enhance the antibiofilm activity. while electron-withdrawing groups result in reduced antibiofilm properties of the compounds. The SAR data could further help in the development of newer 3-amidocoumarin-based potential biofilm inhibitors. The in silico study revealed that coumarins compounds occupied the active site of the LasR receptor. The binding energy from molecular docking ranged from −9.44 to −11.69 kcal/mol. The best results of the docking studies were obtained with compounds 4h, 4l and 4o. The results showed that this study might hold promise in the design and development of safe and effective inhibitors of biofilm and QS as an alternative therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ddc2020015/s1, Figure S1: Chemical structures of active compounds; Figure S2: Molecular docked complex of compounds 4a-4o with LasR (2uv0); Figure S3: IR spectrum of 4a; Figure S4: HRMS spectra of 4a; Figure S5: 1H NMR of 4a; Figure S6: 13C NMR of 4a. Table S1: Binding energies (kcal/mol) of designed ligands S1a-h; Table S2: Binding energies (kcal/mol) of designed ligands S2a-h; Table S3: Binding energies (kcal/mol) of designed ligands S3a-h; Table S4: Binding energies (kcal/mol) of designed ligands S4a-h; Table S5: Binding energies (kcal/mol) of designed ligands S5a-h; Table S6: Binding energies (kcal/mol) of designed ligands S6a-h; Table S7: Binding energies (kcal/mol) of designed ligands S1i,h to S6i,j; Table S8: Binding energies (kcal/mol) of designed ligands S7-S11 and S7a-S11a.
Author Contributions
Conceptualization, V.S. and D.K.; Methodology, R.K.S., V.S. and V.R.; Formal analysis, R.K.S., V.S. and D.K.; Investigation, R.K.S., V.S. and D.K.; Writing—Original Draft Preparation, R.K.S. and D.K.; Writing—Review and Editing, V.S., V.R. and D.K.; Funding Acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
R.K.S. is grateful to CSIR, New Delhi, India, for providing financial support in the form of Senior Research Fellowship (Letter No. 09/013 (0850)/2018-EMR-I). D.K. gratefully acknowledge Institution of Eminence (IoE), Banaras Hindu University (Dev. Scheme No. 6031) and Science and Engineering Research Board (SERB), India (Grant Number: EMR/2016/001396) for the funding. The authors are also thankful to the CISC-Banaras Hindu University for providing spectroscopic data of the developed compounds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the article and/or Supplementary Materials.
Conflicts of Interest
The authors declare no competing interests.
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Stoodley, L.H. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.; Greenberg, E. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Ramanathan, S.; Sivasubramanian, S.; Pandurangan, P.; Mani, G.; Madhu, D.; Lin, X. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S.A. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.-J.; Li, L.; Choi, B.-K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- Yang, S.; Abdel-Razek, O.A.; Cheng, F.; Bandyopadhyay, D.; Shetye, G.S.; Wang, G.; Luk, Y.-Y. Bicyclic brominated furanones: A new class of quorum sensing modulators that inhibit bacterial biofilm formation. Bioorg. Med. Chem. 2014, 22, 1313–1317. [Google Scholar] [CrossRef]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Chan, D.S.H.; Ho, K.K.K.; Rice, S.; Black, D.S.; Griffith, R.; Kumar, N. Dihydropyrrolones as bacterial quorum sensing inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1054–1059. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Qin, Y.; Zhang, N.; Teng, Y.; Venter, H.; Ma, S. Design and synthesis of aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors. Bioorg. Chem. 2020, 105, 104376. [Google Scholar] [CrossRef]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Bhadbhade, M.M.; Manefield, M.; Willcox, M.; Black, D.S.C.; Kumar, N. Synthesis and biological evaluation of novel acyclic and cyclic glyoxamide based derivatives as bacterial quorum sensing and biofilm inhibitors. Org. Biomol. Chem. 2017, 15, 5743–5755. [Google Scholar] [CrossRef]
- Singh, L.R.; Tripathi, V.C.; Raj, S.; Kumar, A.; Gupta, S.; Horam, S.; Upadhyay, A.; Kushwaha, P.; Arockiaraj, J.; Sashidhara, K.V.; et al. In-house chemical library repurposing: A case example for Pseudomonas aeruginosa antibiofilm activity and quorum sensing inhibition. Drug Dev. Res. 2018, 9, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs. 2011, 9, 2010–2035. [Google Scholar] [CrossRef] [PubMed]
- Herrera, K.M.S.; da Silva, F.K.; de Lima, W.G.; de Barbosa, S.C.; Gonçalves, A.M.M.N.; Viana, G.H.R.; Soares, A.C.; Ferreira, J.M.S. Antibacterial and antibiofilm activities of synthetic analogs of 3-alkylpyridine marine alkaloids. Med. Chem. Res. 2020, 29, 1084–1089. [Google Scholar] [CrossRef]
- El-Messery, S.M.; Habib, E.E.; Al-Rashood, S.T.A.; Hassan, G.S. Synthesis, antimicrobial, anti-biofilm evaluation, and molecular modelling study of new chalcone linked amines derivatives. J. Enzyme Inhib. Med. Chem. 2018, 33, 818–832. [Google Scholar] [CrossRef]
- Mairink, S.Z.; Barbosa, L.C.A.; Boukouvalas, J.; Pedroso, S.H.S.P.; Santos, S.G.; Magalhães, P.P.; Farias, L.M. Synthesis and evaluation of cadiolide analogues as inhibitors of bacterial biofilm formation. Med. Chem. Res. 2018, 27, 2426–2436. [Google Scholar] [CrossRef]
- Lal, J.; Kaul, G.; Akhir, A.; Ansari, S.B.; Chopra, S.; Reddy, D.N. Bio-evaluation of fluoro and trifluoromethyl-substituted salicylanilides against multidrug-resistant S. aureus. Med. Chem. Res. 2021, 30, 2301–2315. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.; Parages, M.L.; O’Gara, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef]
- D’Almeida, R.E.; Molina, R.D.I.; Viola, C.M.; Luciardi, M.C.; Peñalver, C.N.; Bardón, A.; Arena, M.E. Comparison of seven structurally related coumarins on the inhibition of quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 2017, 73, 37–42. [Google Scholar] [CrossRef]
- Ojima, Y.; Nunogami, S.; Taya, M. Antibiofilm effect of warfarin on biofilm formation of Escherichia coli promoted by antimicrobial treatment. J. Glob. Antimicrob. Resist. 2016, 7, 102–105. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; AlHarbi, W. Coumarin exhibits broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria: In vitro and in silico investigation. ACS Omega 2021, 6, 18823–18835. [Google Scholar] [CrossRef]
- Sharma, R.K.; Singh, V.; Tiwari, N.; Butcher, R.J.; Katiyar, D. Synthesis, antimicrobial and chitinase inhibitory activities of 3-amidocoumarins. Bioorg. Chem. 2020, 98, 103700. [Google Scholar] [CrossRef]
- Shukla, M.; Singh, V.; Habeeballah, H.; Alkhanani, M.F.; Lata, M.; Hussain, Y.; Mukherjee, M.; Pasupuleti, M.; Meena, A.; Mishra, B.N.; et al. Quorum quenching guided inhibition of mixed bacterial biofilms and virulence properties by protein derived from leaves of Carissa carandas. Front. Cell. Infect. Microbiol. 2022, 12, 836819. [Google Scholar] [CrossRef]
- Sharma, R.K.; Priyanka; Katiyar, D. L-proline catalyzed condensation of salicylaldehydes with ethyl nitroacetate: An efficient access to 3-nitrocoumarins. Monatsh. Chem. 2016, 147, 2157–2161. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef]
- Gavrilova, N.A.; Semichenko, E.S.; Korotchenko, O.S.; Suboch, G.A. Cyclocondensation of ethyl nitroacetate with 2-hydroxybenzaldehydes. Russ. J. Org. Chem. 2008, 44, 624–625. [Google Scholar] [CrossRef]
- Das, D.K.; Sarkar, S.; Khan, M.; Belal, M.; Khan, A.T. A mild and efficient method for large scale synthesis of 3-aminocoumarins and its further application for the preparation of 4-bromo-3-aminocoumarins. Tetrahedron Lett. 2014, 55, 4869–4874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).