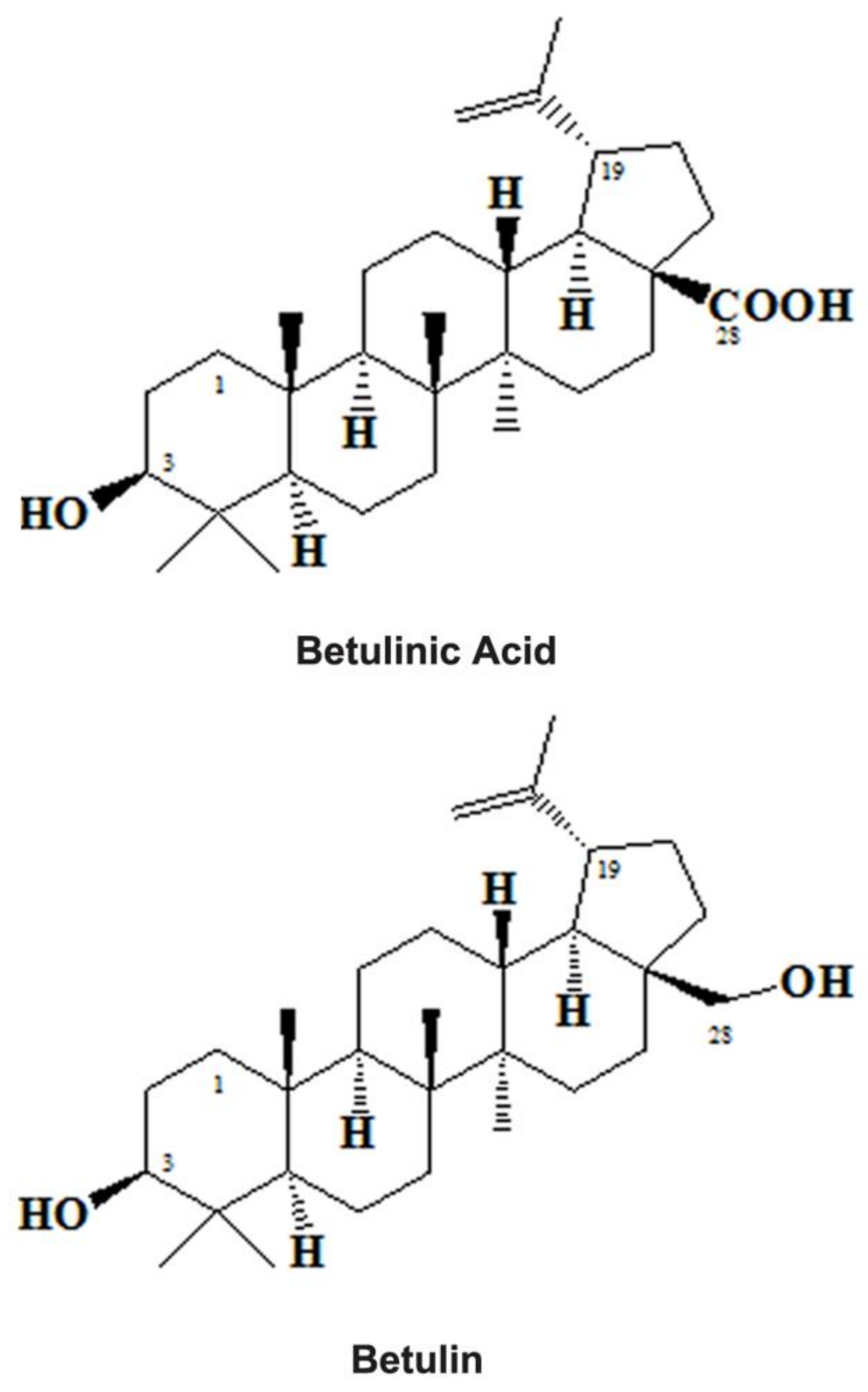
Betulinic Acid: Triterpenoid Derivative Induced NADPH-d Expression in the Urinary System with a Possible Renal Protective Role of Nitric Oxide
Abstract
1. Introduction
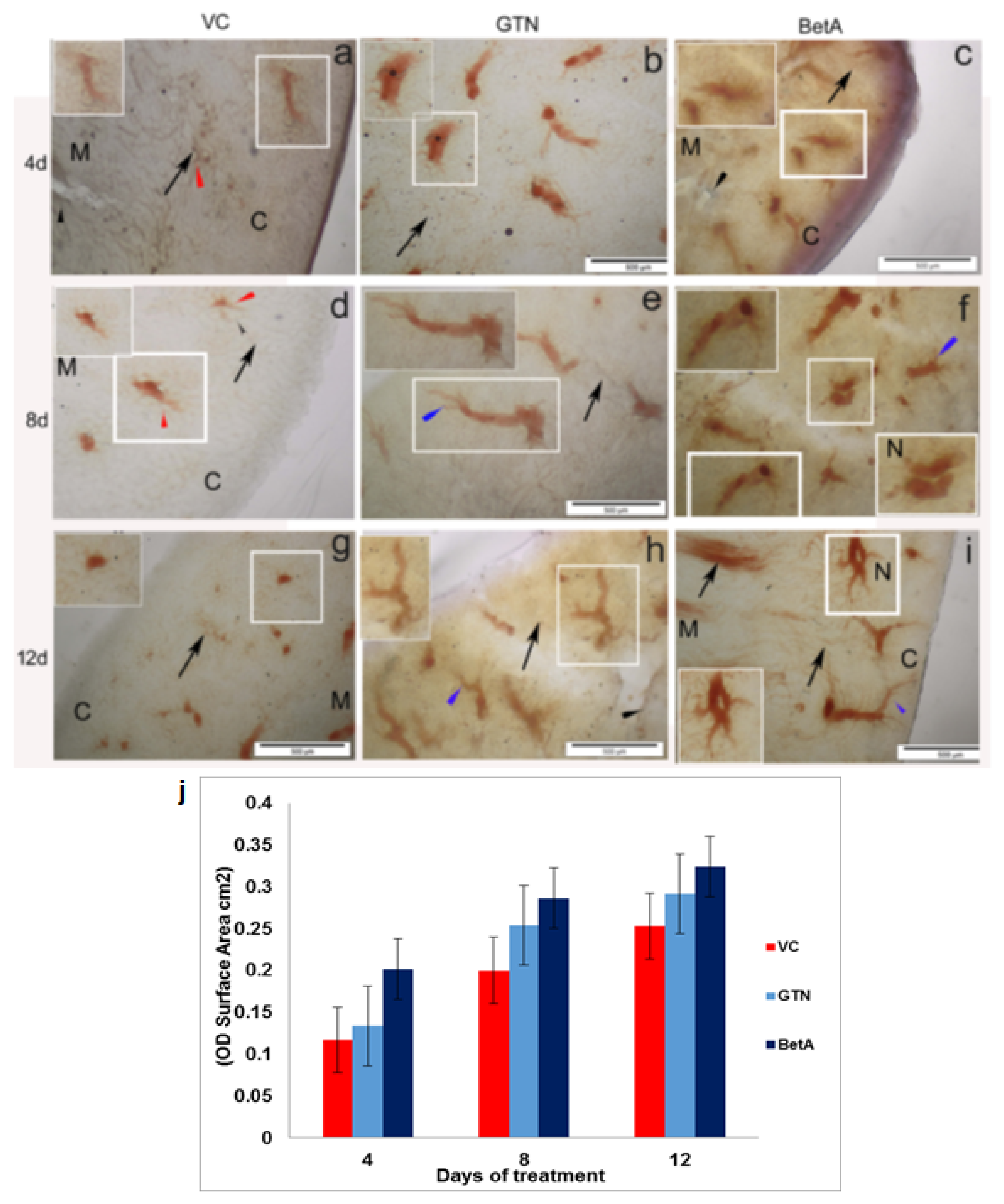
2. Results
3. Urinary Bladder
4. Discussion
5. Materials and Methods
5.1. Experimental Animals and Chemical Requirements
5.2. Animal Treatment and Sample Collection
5.3. NADPH-Diaphorase Histochemistry and Tissue Morphology Analysis
5.4. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BetA | Betulinic Acid |
| BE | Betulin |
| NADPH-d | NADPH-diaphorase |
| µM | micro-Molar |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| eNOS | endothelial nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| OH | Hydroxyl radical |
| O2− | Super Oxide |
| H2O2 | Hydrogen Peroxide |
| O3 | Ozone |
| HOCI | Hypochlorous acid |
| RO2 | Alkoxyl radical 2 |
| RO | Alkoxyl radical |
| CR | Chronic Renal |
| CRF | Chronic Renal Failure |
| SOD | Superoxide Dismutase |
| NOS1 | Neuronal nitric oxide synthase |
| NOS3 | Endothelial nitric oxide synthase |
| NOS2 | Inducible nitric oxide synthase |
| DN | Diabetic nephropathy |
| NPs | Natural products |
| HIV | Human Immunodeficiency Virus |
| DGAT | Diglyceride acyltransferase |
| NF-kB | Nuclear factor kappa |
| MEL-1 | Human metastatic Skin Cancer cell line 1 |
| UKM | Universiti Kebangsaan Malaysia |
| UKMAEC | Universiti Kebangsaan Malaysia (UKM) Animal Ethics Committee |
| GTN | Goniothalamin |
| DMSO | Dimethyl sulfoxide |
| ANOVA | Analysis of variance |
| cGMP | Cyclic guanosine monophosphate |
| cGTP | Cyclic guanosine diphosphate |
| CNS | Central Nervous System |
| TGF | Tubule-glomerular feedback |
| CKD | Chronic kidney disease |
| NO2 | Nitrite |
| NO3 | Nitrate |
| b. wt. | body weight |
| AMPK | 5’ AMP-activated protein kinas |
| CPB | Cardiopulmonary bypass |
| PCR | Polymerase chain reaction |
| IMCD | Inner medullary cortical collecting ducts |
| ROS | Reactive Oxygen Species |
| BK | Bradykinin |
| Ach | Acetylcholine |
| PKC | Protein kinase C |
| ECM | Extracellular matrix |
| TGF | Transforming growth factor |
| CTGF | Connective tissue growth factor |
References
- Ali, S.M.; Olivo, M. Nitric oxide mediated photo-induced cell death in human malignant cells. Int. J. Oncol. 2003, 22, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Rajkumar, J.; Seyed, M.A. Phytochemical screening, free radical scavenging, and antimicrobial potential of Chromolaena odorata leaf extracts against pathogenic bacterium in wound infections—A multispectrum perspective. Biocatal. Agric. Biotech. 2008, 15, 103–112. [Google Scholar] [CrossRef]
- Phaniendra, A.; Babu, D.J.; Latha, P. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.G.; Higgs, A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharm. Rev. 1991, 43, 109–142. [Google Scholar]
- Knowles, R.G.; Moncada, S. Nitric oxide synthase in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef]
- Pang, K.L.; Vijayaraghavan, K.; Badr, A.S.; Ali, S.M. Betulinic acid-induced expression of nicotinamide adenine dinucleotide phosphate-diaphorase in the immune organs of mice: A role of nitric oxide in immunomodulation. Mol. Med. Rep. 2018, 17, 3035–3041. [Google Scholar] [CrossRef]
- Khorashadi, M.; Bokoch, M.P.; Legrand, M. Is nitric oxide the forgotten nephroprotective treatment during cardiac surgery? Ann. Intensive Care 2020, 10, 22. [Google Scholar] [CrossRef]
- Ali, S.M.; Chan, A.S.; Leong, S.K. Localization of nitrergic neuronal and non-neuronal cells in the ultimobranchial glands of the chicken. Anat. Embryol. 1996, 193, 161–168. [Google Scholar] [CrossRef]
- Akbari, Z.; Rohani, M.H.; Behzadi, G. NADPH-d/NOS reactivity in the lumbar dorsal horn of congenitally hypothyroid pups before and after formalin pain induction. Int. J. Dev. Neurosci. 2009, 27, 779–787. [Google Scholar] [CrossRef]
- Syed, M.A.; Leong, S.K.; Chan, A.S. Localization of NADPH-diaphorase reactivity in the chick and mouse thyroid gland. Thyroid 1994, 4, 475–478. [Google Scholar] [CrossRef]
- Gulati, P.; Leong, S.K.; Chan, A.S. Ontogeny of NADPH-d expression in the thymic microenvironment of the chick embryo. Cell Tissue Res. 1998, 294, 335–343. [Google Scholar] [CrossRef]
- Kerschbaum, H.H.; Huang, S.; Xie, M.; Hermann, A. NADPH-diaphorase activity and nitric oxide synthase activity in the kidney of the clawed frog, Xenopus laevis. Cell Tissue Res. 2000, 301, 405–411. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Liang, K. Enhanced nitric oxide inacti vation and protein nitration by reactive oxygen species in chronic renal insufficiency. Hypertension 2002, 39, 135–141. [Google Scholar] [CrossRef]
- Deng, G.; Vaziri, N.D.; Jabbari, B.; Yan, X.X. Increased tyrosine nitration of the brain in chronic renal insufficiency: Reversal by antioxidant therapy. J. Am. Soc. Nephrol. 2001, 12, 1892–1898. [Google Scholar] [CrossRef]
- Dorko, F.; Špakovská, T.; Lovasová, K.; Patlevič, P.; Kluchová, D. NADPH-d activity in rat thymus after the application of retinoid acid. Eur. J. Histochem. 2012, 56, e7. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart. J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Andronowska, A.; Chruściel, M. Expression, and cellular distribution of NADPH-diaphorase and nitric oxide synthases in the porcine uterus during early pregnancy. Folia Histochem. Cytobiol. 2007, 45, 375–380. [Google Scholar]
- Lee, J. Nitric oxide in the kidney: Its physiological role and pathophysiological implications. Electrolyte Blood Press. 2008, 6, 27–34. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, K.J. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Caron, N.; Jadot, I.; Colombaro, V.; Federici, G.; Depommier, C.; Declèves, A.É. Evaluation of inducible nitric oxide synthase inhibition on kidney function and structure in high-fat diet-induced kidney disease. Exp. Physiol. 2018, 103, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, W.I.C.; de Wit, N.C.J.; Sertorio, J.T.C.; van Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Mik, E.G.; Johannes, T.; Payen, D.; Ince, C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol. Med. 2008, 14, 502–516. [Google Scholar] [CrossRef]
- Choi, J.Y.; Nam, S.A.; Jin, D.C.; Kim, J.; Cha, J.H. Expression, and cellular localization of inducible nitric oxide synthase in lipopolysaccharide-treated rat kidneys. J. Histochem. Cytochem. 2012, 60, 301–315. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Brodsky, S.V.; Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002, 61, 855–861. [Google Scholar] [CrossRef]
- Bank, N.; Aynedjian, H.S. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney. Int. 1993, 43, 1306–1312. [Google Scholar] [CrossRef]
- Komers, R.; Allen, T.J.; Cooper, M.E. Role of endothelium-derived nitric oxide in the pathogenesis of the renal hemodynamic changes of experimental diabetes. Diabetes 1993, 43, 1190–1197. [Google Scholar] [CrossRef]
- Musabayane, C.T. The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc. J. Afr. 2012, 23, 462–468. [Google Scholar] [CrossRef]
- Seyed, M.A.; Ayesha, S. Modern phytomedicine in treating Diabetic foot ulcer: Progress and opportunities. In Diabetic Foot Ulcer; Zubair, M., Ahmad, J., Malik, A., Talluri, M.R., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Disis, M.L. Immune regulation of cancer. J. Clin. Oncol. 2010, 28, 4531–4538. [Google Scholar] [CrossRef]
- Geetha, S.; Singh, V.; Ram, M.S.; Ilavazhagan, G.; Banerjee, P.K.; Sawhney, R.C. Immunomodulatory effects of seabuckthorn (Hippophae rhamnoides L.) against chromium (VI) induced immunosuppression. Mol. Cell. Biochem. 2005, 278, 101–109. [Google Scholar] [CrossRef]
- K Aravindaram, N.S. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef]
- Winkler, C.; Wirleitner, B.; Schroecksnadel, K.; Schennach, H.; Mur, E.; Fuchs, D. In vitro effects of two extracts and two pure alkaloid preparations of Uncaria tomentosa on peripheral blood mononuclear cells. Planta Med. 2004, 70, 205–210. [Google Scholar]
- B Patwardhan, M.G. Botanical immunodrugs: Scope and opportunities. Drug Discov. Today 2005, 10, 495–502. [Google Scholar] [CrossRef]
- Oveissi, V.; Ram, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Naseri, R.; Belwal, T.; Devkota, H.P.; Abbasabadi, Z.; Farzaei, M.H. Medicinal plants and their isolated phytochemicals for the management of chemotherapy-induced neuropathy: Therapeutic targets and clinical perspective. Daru 2019, 27, 389–406. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Seyed, M.A.; Siddiqua, A. Calotropis—A multi-potential plant to humankind: Special focus on its wound healing efficacy. Biocatal. Agric. Biotechnol. 2020, 28, 101725. [Google Scholar] [CrossRef]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 7–31. [Google Scholar] [CrossRef]
- Bruce, J. The role of natural products in evolution. In Recent Advances in Phytochemisty; Elsevier: Oxford, UK; Volume 34, pp. 1–24. 2022. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- McChesney, J.D.; Venkataraman, S.K.; Henri, J.T. Plant natural products: Back to the future or into extinction? Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef]
- James, J.T.; Dubery, I.A. Pentacyclic Triterpenoids from the Medicinal Herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S. Introductory chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2631. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar] [PubMed]
- Dehelean, C.A.; Soica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.G.; Luşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef]
- Kovalenko, L.P.; Balakshin, V.V.; Presnova, G.A.; Chistyakov, A.N.; Shipaeva, E.V.; Alekseeva, S.V.; Durnev, A.D. Immunotoxicity and allergenic properties of betulin-containing birch bark dry extract. Pharm. Chem. J. 2007, 41, 17–19. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene Glycosides from the Sea Cucumber Eupentacta fraudatrix. Structure and Biological Action of Cucumariosides A1, A3, A4, A5, A6, A12 and A15, Seven New Minor Non-sulfated Tetraosides and Unprecedented 25-Keto, 27-Norholostane Aglycone. Nat. Prod. Commun. 2012, 7, 517–525. [Google Scholar]
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and their cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533. [Google Scholar] [CrossRef]
- Moghaddam, M.G.; Ahmad, F.B.H.; Kermani, A.S. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef]
- Drag-Zalesinska, M.; Kulbacka, J.; Saczko, J.; Wysocka, T.; Zabel, M.; Surowiak, P.; Drag, M. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorg. Med. Chem. Lett. 2009, 19, 4814–4817. [Google Scholar] [CrossRef]
- Drag, M.; Surowiak, P.; Drag-Zalesinska, M.; Dietel, M.; Lage, H.; Oleksyszy, J. Comparision of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug-resistant cell lines. Molecules 2009, 14, 1639–1651. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Rzeski, W.; Paduch, R.; Szuster-Ciesielska, A.; Kaczor, J.; Wejksza, K.; Kandefer-Szerszeń, M. Differentialeffect of betulin and betulinic acid on cytokine productionin human whole blood cell cultures. Pol. J. Pharmacol. 2003, 55, 235–238. [Google Scholar]
- Tsai, J.C.; Peng, W.H.; Chiu, T.H.; Lai, S.C.; Lee, C.Y. Anti-inflammatory Effects of Scoparia dulcis L. and Betulinic Acid. Am. J. Chin. Med. 2011, 39, 943–956. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Kokpol, U.; Miles, D.H. The isolation from Sarracenia flava and partial synthesis of betulinaldehyde. Phytochemistry 1976, 15, 432–433. [Google Scholar] [CrossRef]
- Kingston, D.G.; Munjal, R.C. Plant anticancer agents. VIII. Constituents of Inga punctata. Lloydia 1978, 41, 499–500. [Google Scholar]
- Trumbull, E.R.; Bianchi, E.; Wiedhopf, D.J.E.R.M.; Cole, J.R. Tumor Inhibitory Agents from Vauquelinia corymbosa Rosaceae. J. Pharm. Sci. 1976, 65, 1407–1408. [Google Scholar] [CrossRef]
- Eiznhamer, D.A.; Xu, Z.Q. Betulinic acid: A promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar]
- Patlolla, J.M.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef]
- Costa, J.F.; Barbosa-Filho, J.M.; Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.; Soares, M.B. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.B.; Chen, I.S.; Lee, K.H. Anti-AIDS Agents, 11. Betulinic Acid and Platanic Acid as Anti-HIV Principles from Syzigium claviflorum, and the Anti-HIV Activity of Structurally Related Triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Chandramu, C.; Manohar, R.D.; Krupadanam, D.G.; Dashavantha, R.V. Isolation, characterization, and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother. Res. 2003, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.; Warhurst, D.C.; Kirby, G.C.; Simmonds, M.S. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother. Res. 1999, 13, 115–119. [Google Scholar] [CrossRef]
- Enwerem, N.M.; Okogun, J.I.; Wambebe, C.O.; Okorie, D.A.; Akah, P.A. Anthelmintic activity of the stem bark extracts of Berlina grandiflora and one of its active principles, Betulinic acid. Phytomedicine 2001, 8, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Tzakos, A.G.; Kontogianni, V.G.; Tsoumani, M.; Kyriakou, E.; Hwa, J.; Rodrigues, F.A.; Tselepis, A.D. Exploration of the Antiplatelet Activity Profile of Betulinic Acid on Human Platelets. J. Agric. Food Chem. 2012, 60, 6977–6983. [Google Scholar] [CrossRef]
- Xia, A.; Xue, Z.; Li, Y.; Wang, W.; Xia, J.; Wei, T.; Cao, J.; Zhou, W. Cardioprotective effect of betulinic Acid on myocardial ischemia reperfusion injury in rats. Evid. Based Complem. Altern. Med. 2014, 2014, 573745. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic Acid, a Natural Compound with Potent Anticancer Effects. Anticancer Drug 2010, 21, 215–227. [Google Scholar] [CrossRef]
- Melzig, M.F.; Bormann, H. Betulinic acid inhibits aminopeptidase N activity. Planta Med. 1998, 64, 655–657. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Mandal, S.; Mittra, B.; Sharma, S.; Mukhopadhyay, S.; Majumder, H.K. Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: Identification of the inhibitory step, the major functional group responsible and development of more potent derivatives. Med. Sci. Monit. 2002, 8, BR254–BR265. [Google Scholar]
- Lee, S.H.; Stephens, J.L.; Paul, K.S.; Englund, P.T. Fatty acid synthesis by elongases in trypanosomes. Cell 2006, 126, 691–699. [Google Scholar] [CrossRef]
- De Melo, C.L.; Queiroz, M.G.R.; Filho, A.C.V.A.; Rodrigues, A.M.; de Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic Acid, a Natural Pentacyclic Triterpenoid, Prevents Abdominal Fat Accumulation in Mice Fed a High-Fat Die. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, F.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008, 133, 1106–1117. [Google Scholar] [CrossRef]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisińska, B.; Kandefer-Szerszeń, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch. Pharm. 2006, 374, 11–20. [Google Scholar] [CrossRef]
- Huang, L.; Ho, P.; Chen, C.H. Activation, and inhibition of the proteasome by betulinic acid and its derivatives. FEBS Lett. 2007, 581, 4955–4959. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Heiss, E.H.; Kramer, M.P.; Atanasov, A.G.; Beres, H.; Schachner, D.; Dirsch, V.M. Glycolytic switch in response to betulinic acid in non-cancer cells. PLoS ONE 2014, 9, e115683. [Google Scholar] [CrossRef]
- Hope, B.T.; Michael, G.J.; Knigge, K.M.; Vincent, S.R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 2811–2814. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharm. 2006, 147 (Suppl. S1), S193–S201. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Devala, R.G.; Lakshmayya; Ramachandra, S.S. Nephroprotective and Nitric Oxide Scavenging Activity of Tubers of Momordica tuberosa in Rats. Avicenna J. Med. Biotechnol. 2011, 3, 87–93. [Google Scholar] [PubMed]
- Wang, P.; Li, Q.; Li, K.; Zhang, X.; Han, Z.; Wang, J.; Gao, D.; Li, J. Betulinic acid exerts immunoregulation and anti-tumor effect on cervical carcinoma (U14) tumor-bearing mice. Pharmazie 2012, 67, 733–739. [Google Scholar] [PubMed]
- Herrera, M.; Garvin, J.L. Recent Advances in the Regulation of Nitric Oxide in the Kidney. Hypertension 2005, 45, 1062–1067. [Google Scholar] [CrossRef]
- Ahmed, A.; Campbell, R.C. Epidemiology of chronic kidney disease in heart failure. Heart Fail. Clin. 2008, 4, 387–399. [Google Scholar] [CrossRef]
- Carlström, M.; Persson, A.E.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011, 89, 574–585. [Google Scholar] [CrossRef]
- Derbyshire, E.R.; Marletta, M.A. Biochemistry of soluble guanylate cyclase. Handb. Exp. Pharm. 2009, 191, 17–31. [Google Scholar]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef]
- Sausbier, M.; Schubert, R.; Voigt, V.; Hirneiss, C.; Pfeifer, A.; Korth, M.; Kleppisch, T.; Ruth, P.; Hofmann, F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ. Res. 2009, 87, 825–830. [Google Scholar] [CrossRef]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, renal blood flow and filtration. In StatPearls; Updated 26 July 2021; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482248/ (accessed on 25 July 2022).
- Kurtz, A.; Eckardt, K.U.; Neumann, R.; Kaissling, B.; le Hir, M.; Bauer, C. Site of erythropoietin formation. Contrib. Nephrol. 1989, 76, 14–23. [Google Scholar]
- Mundel, P.; Bachmann, S.; Bader, M.; Fischer, A.; Kummer, W.; Mayer, B.; Kriz, W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992, 42, 1017–1019. [Google Scholar] [CrossRef]
- Münter, K.; Hackenthal, E. The effects of endothelin on renovascular resistance and renin release. J. Hypertens. 1989, 7, S276–S277. [Google Scholar] [CrossRef]
- Szabo, C. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 1995, 3, 2–32. [Google Scholar]
- Mulligan, M.S.; Hovel, J.M.; Marletta, M.A.; Ward, P.A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc. Natl. Acad. Sci. USA 1991, 88, 6338–6342. [Google Scholar] [CrossRef]
- Kolb, H.; Kolb-Bachofen, V. Nitric oxide: A pathogenetic factor in autoimmunity. Immunol. Today 1992, 13, 157–160. [Google Scholar] [CrossRef]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharm. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Ali, S.M.; Chan, A.S.; Leong, S.K. Histochemical and immunohistochemical localisation of nitrergic neuronal and non-neuronal cells in the bursa of Fabricius of the chicken. Cell Tissue Res. 1996, 285, 273–279. [Google Scholar] [CrossRef]
- Clark, K.E.; Myatt, L. Uterine effects of nitric oxide. In Nitric Oxide and the Regulation of the Peripheral Circulation; Nitric Oxide in Biology and Medicine; Kadowitz, P.J., McNamara, D.B., Eds.; Birkhäuser: Boston, MA, USA, 2000; Volume 1. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; de Groat, W.C.; Apodaca, G.; et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef]
- Studeny, S.; Cheppudira, B.P.; Meyers, S.; Balestreire, E.M.; Apodaca, G.; Birder, L.A.; Braas, K.M.; Waschek, J.A.; May, V.; Vizzard, M.A. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J. Mol. Neurosci. 2008, 36, 175–187. [Google Scholar] [CrossRef]
- Majid, D.S.; Navar, L.G. Nitric oxide in the control of renal hemodynamics and excretory function. Am. J. Hypertens. 2001, 146 Pt 2, 74S–82S. [Google Scholar] [CrossRef] [PubMed]
- Majid, D.S.; Williams, D.; Navar, L.G. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am. J. Physiol. Ren. Physiol. 1993, 264, F79–F87. [Google Scholar] [CrossRef] [PubMed]
- Eppel, G.A.; Bergstrom, G.; Anderson, W.P.; Evans, R.G. Autoregulation of renal medullary blood flow in rabbits. Am. J. Physiol. Regul. Int. Reg. Comp. Physiol. 2003, 284, R233–R244. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; Wu, F. Nitric Oxide Synthase Activity and Isoforms in Rat Renal Vasculature. Hypertension 2000, 35, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Role of macula densa NOS in tubuloglomerular feedback. Curr. Opin. Nephrol. Hypertens. 1998, 7, 443–449. [Google Scholar]
- Ortiz, P.A.; Garvin, J.L. Superoxide stimulates NaCl absorption by the thick ascending limb. Am. J. Physiol. Ren. Physiol. 2002, 283, F957–F962. [Google Scholar] [CrossRef]
- Pallone, T.L.; Mattson, D.L. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr. Opin. Nephrol. Hypertens. 2002, 11, 93–98. [Google Scholar] [CrossRef]
- Locatelli, F.; Canaud, B.; Eckardt, E.U.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transpl. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Kiranmayi, V.S.; Bitla, A.R.; Krishna, G.S.; Rao, P.V.L.N.S.; Sivakumar, V. Nitric oxide status in patients with chronic kidney disease. Indian J. Nephrol. 2015, 25, 287–291. [Google Scholar]
- Martens, C.R.; Edwards, D.G. Peripheral vascular dysfunction in chronic kidney disease. Cardiol. Res. Pract. 2011, 2011, 257–267. [Google Scholar] [CrossRef]
- Zatz, R.; Baylis, C. Chronic nitric oxide inhibition model six years on. Hypertension 1998, 32, 958–964. [Google Scholar] [CrossRef]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Tomita, S.; Ishizawa, K.; Abe, S.; Ikeda, Y.; Kihira, Y.; Tamaki, T. Dietary nitrite ameliorates renal injury in L-NAME-induced hypertensive rats. Nitric Oxide 2010, 22, 98–103. [Google Scholar] [CrossRef]
- Gilchrist, M.; Shore, A.C.; Benjamin, N. Inorganic nitrate and nitrite and control of blood pressure. Cardiovasc. Res. 2011, 89, 492–498. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Steinkamp-Fenske, K.; Bollinger, L.; Völler, N.; Xu, H.; Yao, Y.; Bauer, R.; Förstermann, U.; Li, H. Ursolic acid from the Chinese herb danshen Salvia miltiorrhiza L. upregulates eNOS and downregulates Nox4 expression in human endothelial cells. Atherosclerosis 2007, 195, e104–e111. [Google Scholar] [CrossRef]
- Steinkamp-Fenske, K.; Bollinger, L.; Xu, H.; Yao, Y.; Horke, S.; Förstermann, U.; Li, H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharm. Exp. 2007, 322, 836–842. [Google Scholar] [CrossRef]
- Ovesna, Z.; Vachalkova, A.; Horvathova, K.; Tothova, D. Pentacyclic triterpenoic acids: New chemoprotective compounds. Minireview. Neoplasma 2004, 51, 327–333. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. The International Natural Product Sciences Taskforce, C.T. Supuran. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, R.; Lingaraju, M.C.; Kumar, D.; Mathesh, K.; Telang, A.G.; Singh, T.U.; Kumar, D. Betulinic acid attenuates renal fibrosis in rat chronic kidney disease model. Biomed. Pharm. 2017, 89, 796–804. [Google Scholar] [CrossRef]
- Zeng, A.Q.; Yu, Y.; Yao, Y.Q.; Yang, F.F.; Liao, M.; Song, L.J.; Li, Y.L.; Yu, Y.; Li, Y.J.; Deng, Y.L.; et al. Betulinic acid impairs metastasis and reduces immunosuppressive cells in breast cancer models. Oncotarget 2017, 9, 3794–3804. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Komers, R.; Lindsley, J.N.; Oyama, T.T.; Allison, K.M.; Anderson, S. Role of neuronal nitric oxide synthase NOS1 in the pathogenesis of renal hemodynamic changes in diabetes. Am. J. Physiol. Ren. Physiol. 2000, 279, F573–F583. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006, 187, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Oberbäumer, H. Structural and molecular dissection of the juxtaglomerular apparatus: New aspects for the role of nitric oxide. Kidney Int. 1998, 54, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Tomita, K.; Nonoguchi, H.; Yang, T.; Marumo, F. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J. Clin. Investig. 1993, 92, 2339–2345. [Google Scholar] [CrossRef]
- Datla, S.R.; Griendling, K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56, 325–330. [Google Scholar] [CrossRef]
- Jia, Y.; Kang, X.; Tan, L.; Ren, Y.; Qu, L.; Tang, J.; Liu, G.; Wang, S.; Xiong, Z.; Yang, L. Nicotinamide Mononucleotide Attenuates Renal Interstitial Fibrosis After AKI by Suppressing Tubular DNA Damage and Senescence. Front. Physiol. 2021, 12, 649547. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Perazzolli, M.; Zago, E.D.; Delledonne, M. Nitric oxide signalling functions in plant–pathogen interactions. Cell. Microbiol. 2004, 6, 795–803. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Komalavilas, P.; Boerth, N.J.; MacMillan-Crow, L.A.; Cornwell, T.L. cGMP Signaling through cAMP- and cGMP-Dependent Protein Kinases. Adv. Pharmacol. 1995, 34, 305–322. [Google Scholar]
- McKee, M.; Scavone, C.; Nathanson, J. Nitric oxide, cGMP, and hormone regulation of active sodium transport. Proc. Natl. Acad. Sci. USA 1995, 91, 12056–12060. [Google Scholar] [CrossRef]
- Snyder, S.H. Nitric oxide: First in a new class of neurotransmitters. Science 1992, 257, 494–496. [Google Scholar] [CrossRef]
- Hohmann, N.; Xia, N.; Steinkamp-Fenske, K.; Förstermann, U.; Li, H. Estrogen Receptor Signaling and the PI3K/Akt Pathway Are Involved in Betulinic Acid-Induced eNOS Activation. Molecules 2016, 21, 973. [Google Scholar] [CrossRef]
- Lee, G.H.; Park, J.S.; Jin, S.W.; Pham, T.H.; Thai, T.N.; Kim, J.Y.; Kim, C.Y.; Choi, J.H.; Han, E.H.; Jeong, H.G. Betulinic Acid Induces eNOS Expression via the AMPK-Dependent KLF2 Signaling Pathway. J. Agric. Food Chem. 2020, 68, 14523–14530. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Lu, Q.; Xia, N.; Xu, H.; Guo, L.; Wenzel, P.; Daiber, A.; Munzel, T.; Forstermann, U.; Li, H. Betulinic acid protects against cerebral ischemia-reperfusion injury in mice by reducing oxidative and nitrosative stress. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2011, 24, 132–138. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.H.; Cheong, Y.K.; Jung, H.C.; Jeong, S.O.; Park, S.H.; Pae, H.O. Piceatannol, a natural hydroxylated analog of resveratrol, promotes nitric oxide release through phosphorylation of endothelial nitric oxide synthase in human endothelial cells. Eur. Rev. Med. Pharm. Sci. 2015, 19, 3125–3132. [Google Scholar]
- Tillery, L.C.; Epperson, T.A.; Eguchi, S.; Motley, E.D. Differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells. Exp. Biol. Med. 2016, 241, 569–580. [Google Scholar] [CrossRef]
- Jin, S.W.; Choi, C.Y.; Hwang, Y.P.; Kim, H.G.; Kim, S.J.; Chung, Y.C.; Lee, K.J.; Jeong, T.C.; Jeong, H.G. Betulinic acid increases eNOS phosphorylation and no synthesis via the calcium-signaling pathway. J. Agric. Food Chem. 2016, 64, 785–791. [Google Scholar] [CrossRef]
- Koyama, T.; Hatanaka, Y.; Jin, X.; Yokomizo, A.; Fujiwara, H.; Goda, M.; Hobara, N.; Zamami, Y.; Kitamura, Y.; Kawasaki, H. Altered function of nitrergic nerves inhibiting sympathetic neurotransmission in mesenteric vascular beds of renovascular hypertensive rats. Hypertens. Res. 2020, 33, 485–491. [Google Scholar] [CrossRef]
- Shimada, S.; Todoki, K.; Omori, Y.; Toyama, T.; Matsuo, M.; Wada-Takahashi, S.; Takahashi, S.S.; Lee, M.C. Contribution of nitrergic nerve in canine gingival reactive hyperemia. J. Clin. Biochem. Nutr. 2015, 56, 98–104. [Google Scholar] [CrossRef]
- Mignini, F.; Sabbatini, M.; D’Andrea, V.; Cavallotti, C. Intrinsic innervation and dopaminergic markers after experimental denervation in rat thymus. Eur. J. Histochem. 2010, 54, e17. [Google Scholar] [CrossRef]
- Dorko, F.; Danko, J.; Flešárová, S.; Boroš, E.; Sobeková, A. Effect of pesticide bendiocar-bamate on distribution of acetylcholine- and butyrylcholine-positive nerves in rabbit’s thymus. Eur. J. Histochem. 2011, 55, e37. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Na, C.; Huh, Y. Alterations in nitric oxide synthase in the aged CNS. Oxid. Med. Cell. Longev. 2012, 2012, 718976. [Google Scholar] [CrossRef]
- Cossenza, M.; Socodato, R.; Portugal, C.C.; Domith, I.C.; Gladulich, L.F.; Encarnação, T.G.; Calaza, K.C.; Mendonça, H.R.; Campello-Costa, P.; Paes-de-Carvalho, R. Nitric oxide in the nervous system: Biochemical, developmental, and neurobiological aspects. Vitam. Horm. 2014, 96, 79–125. [Google Scholar] [PubMed]
- Liu, C.; Yang, Y.; Hu, X.; Li, J.M.; Zhang, X.M.; Cai, Y.; Li, Z.; Yan, X.X. Ontogenesis of NADPH-diaphorase positive neurons in guinea pig neocortex. Front. Neuroanat. 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.; Giulivi, C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic. Biol. Med. 2010, 49, 307–316. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.Y.; Ayesha, S.; Panneerselvam, C.; Alalawy, A.I.; Almutairi, F.M.; Seyed, M.A. Betulinic Acid: Triterpenoid Derivative Induced NADPH-d Expression in the Urinary System with a Possible Renal Protective Role of Nitric Oxide. Drugs Drug Candidates 2023, 2, 52-68. https://doi.org/10.3390/ddc2010004
Yin SY, Ayesha S, Panneerselvam C, Alalawy AI, Almutairi FM, Seyed MA. Betulinic Acid: Triterpenoid Derivative Induced NADPH-d Expression in the Urinary System with a Possible Renal Protective Role of Nitric Oxide. Drugs and Drug Candidates. 2023; 2(1):52-68. https://doi.org/10.3390/ddc2010004
Chicago/Turabian StyleYin, Soo Yue, Siddiqua Ayesha, Chellasamy Panneerselvam, Adel Ibrahim Alalawy, Fahad Mohamed Almutairi, and Mohamed Ali Seyed. 2023. "Betulinic Acid: Triterpenoid Derivative Induced NADPH-d Expression in the Urinary System with a Possible Renal Protective Role of Nitric Oxide" Drugs and Drug Candidates 2, no. 1: 52-68. https://doi.org/10.3390/ddc2010004
APA StyleYin, S. Y., Ayesha, S., Panneerselvam, C., Alalawy, A. I., Almutairi, F. M., & Seyed, M. A. (2023). Betulinic Acid: Triterpenoid Derivative Induced NADPH-d Expression in the Urinary System with a Possible Renal Protective Role of Nitric Oxide. Drugs and Drug Candidates, 2(1), 52-68. https://doi.org/10.3390/ddc2010004






