Angiogenesis under Opioids Preconditioning in Renal Ischemia Reperfusion
Abstract
:1. Introduction
2. Kidney Physiology
3. Renal Ischemia-Reperfusion Injury
4. Renal Endothelial Damage Associated with IR
5. Angiogenesis and Renal IR
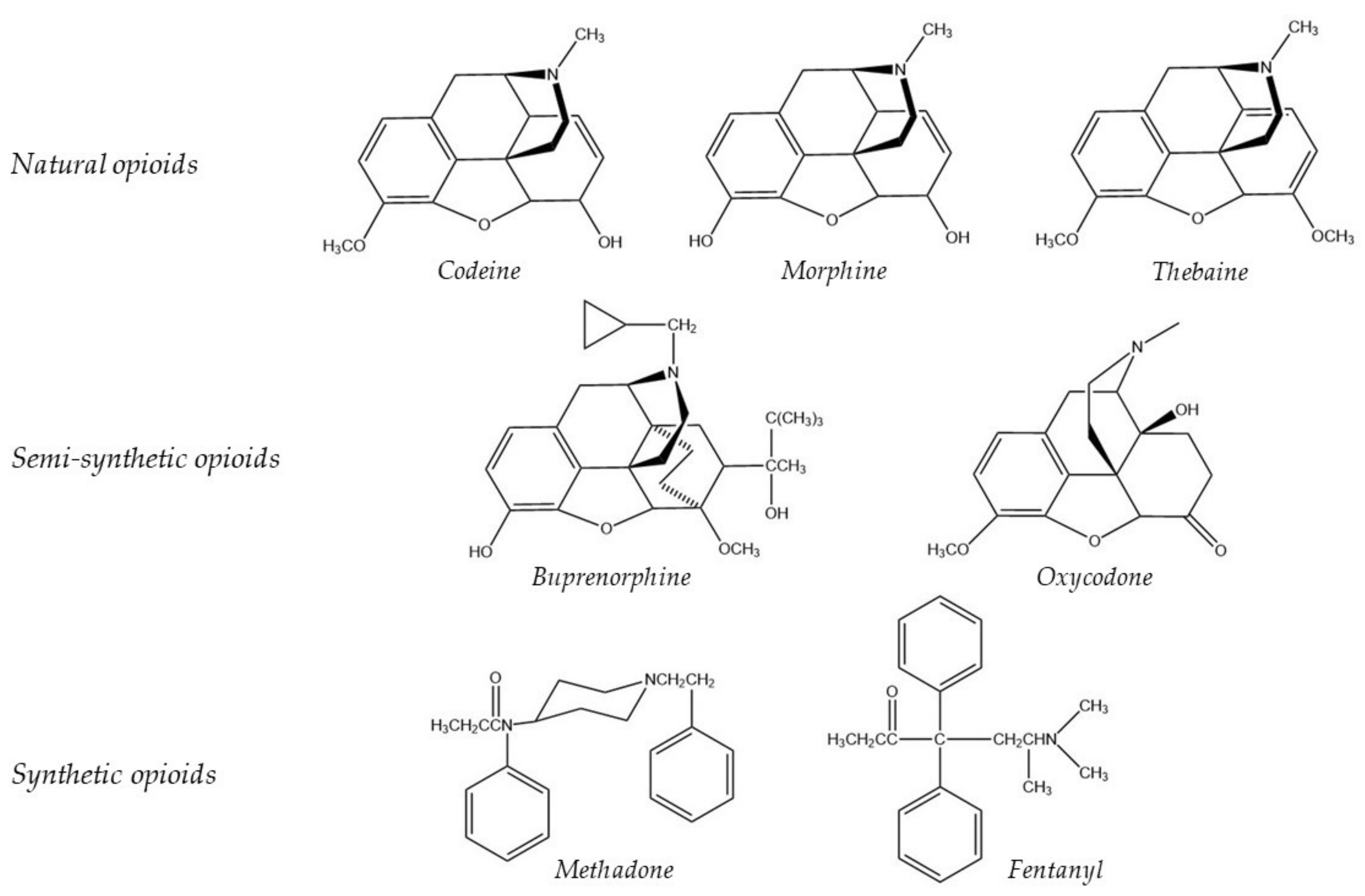
6. Opioids
7. Opioids and Kidney Preconditioning
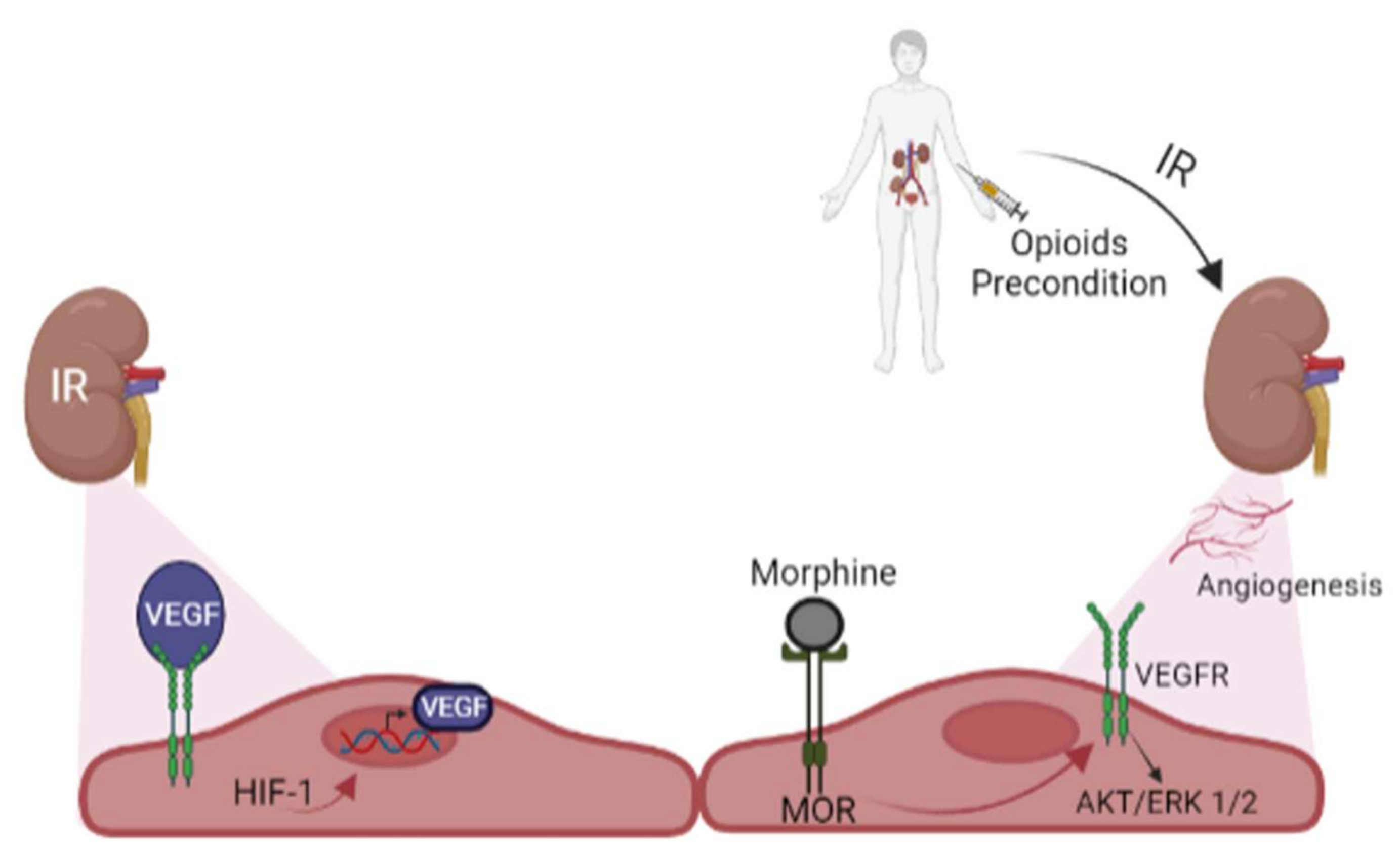
8. Opioids Preconditioning and Angiogenesis in Kidney
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Evans, R.G.; Smith, D.W.; Lee, C.J.; Ngo, J.P.; Gardiner, B.S. What Makes the Kidney Susceptible to Hypoxia? Anat. Rec. 2020, 303, 2544–2552. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Nadarajah, L.; Yaqoob, M.M.; McCafferty, K. Ischemic conditioning in solid organ transplantation: Is it worth giving your right arm for? Curr. Opin. Nephrol. Hypertens 2017, 26, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, K.; Tympa, A.; Karmaniolou, I.; Tsaroucha, A.; Arkadopoulos, N.; Smyrniotis, V. Ischemia/reperfusion injury in liver resection: A review of precondittioning methods. Surg. Today 2011, 41, 620–629. [Google Scholar] [CrossRef]
- Iliodromitis, E.K.; Lazou, A.; Kremastinos, D.T. Ischemic preconditioning: Protection against myocardial necrosis and apoptosis. Vasc. Health Risk Manag. 2007, 3, 629–637. [Google Scholar]
- Das, M.; Das, D.K. Molecular mechanism of preconditioning. IUBMB Life 2008, 60, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N. Ischemic preconditioning mediated angiogenic response in the heart. Antioxid. Redox Signal. 2004, 6, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, K. Improved Cardiac Function Following Ischemia Reperfusion Injury Using Exercise Preconditioning and L-Arginine Supplementation via Oxidative Stress Mitigation and Angiogenesis Amelioration. Cardiovasc. Toxicol. 2022, 22, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, A.; Marsch, L.A.; Joseph, H.; Portenoy, R.K. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008, 16, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.; Echavarria, R.; Franco-Acevedo, A.; Moreno-Carranza, B.; Melo, Z. Opioids Preconditioning Upon Renal Function and Ischemia-Reperfusion Injury: A Narrative Review. Medicina 2019, 55, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A. Kidney Pathophysiology, Toxicology, and Drug-Induced Injury in Drug Development. Int. J. Toxicol. 2019, 38, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H.; Boivin, F.J.; Schmidt-Ott, K.M.; Bachmann, S.; Eckardt, K.U.; Scholl, U.I.; Persson, P.B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021, 17, 335–349. [Google Scholar] [CrossRef]
- Mian, A.N.; Schwartz, G.J. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv. Chronic. Kidney Dis. 2017, 24, 348–356. [Google Scholar] [CrossRef]
- Brenner, B.M.; Chertow, G.M. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am. J. Kidney Dis. 1994, 23, 171–175. [Google Scholar] [CrossRef]
- Nourbakhsh, N.; Singh, P. Role of renal oxygenation and mitochondrial function in the pathophysiology of acute kidney injury. Nephron Clin. Pract. 2014, 127, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal oxygenation: Unique challenges and the biophysical basis of homeostasis. Am. J. Physiol. Renal. Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.C.; Mason, J.; O’Connor, P.M. Ischemic Renal Injury: Can Renal Anatomy and Associated Vascular Congestion Explain Why the Medulla and Not the Cortex Is Where the Trouble Starts? Semin. Nephrol. 2019, 39, 520–529. [Google Scholar] [CrossRef]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Chatauret, N.; Badet, L.; Barrou, B.; Hauet, T. Ischemia-reperfusion: From cell biology to acute kidney injury. Progrès Urol. 2014, 24, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, M.R.; Castle, E.P.; Lohse, C.M.; Sebo, T.J.; Leslie, K.O.; Andrews, P.E. Renal ischemia time in laparoscopic surgery: An experimental study in a porcine model. Int. J. Urol. 2009, 16, 105–109. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Dahl, R.; Geerdes, A. Cytoskeleton disruption and apical redistribution of proximal tubule Na+-K+-ATPase during ischemia. Am. J. Physiol. 1992, 263, F488–F495. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A.; Falk, S.A.; Dahl, R.H. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J. Clin. Investig. 1989, 84, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Kellerman, P.S.; Clark, R.A.; Hoilien, C.A.; Linas, S.L.; Molitoris, B.A. Role of microfilaments in maintenance of proximal tubule structural and functional integrity. Am. J. Physiol. 1990, 259, F279–F285. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [Green Version]
- Piper, H.M.; Balser, C.; Ladilov, Y.V.; Schafer, M.; Siegmund, B.; Ruiz-Meana, M.; Garcia-Dorado, D. The role of Na+/H+ exchange in ischemia-reperfusion. Basic Res. Cardiol. 1996, 91, 191–202. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Roberts, B.N.; Christini, D.J. NHE inhibition does not improve Na+ or Ca2+ overload during reperfusion: Using modeling to illuminate the mechanisms underlying a therapeutic failure. PLoS Comput. Biol. 2011, 7, e1002241. [Google Scholar] [CrossRef] [Green Version]
- Malis, C.D.; Bonventre, J.V. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for post-ischemic and toxic mitochondrial damage. J. Biol. Chem. 1986, 261, 14201–14208. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Melo, Z.; Gutierrez-Mercado, Y.K.; Garcia-Martinez, D.; Portilla-de-Buen, E.; Canales-Aguirre, A.A.; Gonzalez-Gonzalez, R.; Franco-Acevedo, A.; Palomino, J.; Echavarria, R. Sex-dependent mechanisms involved in renal tolerance to ischemia-reperfusion: Role of inflammation and histone H3 citrullination. Transpl. Immunol. 2020, 63, 101331. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Sutton, T.A.; Fisher, C.J.; Molitoris, B.A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002, 62, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Tada, T.; Brodsky, S.V.; Tanaka, H.; Noiri, E.; Kajiya, F.; Goligorsky, M.S. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am. J. Physiol. Renal. Physiol. 2002, 282, F1150–F1155. [Google Scholar] [CrossRef] [Green Version]
- Jankauskas, S.S.; Andrianova, N.V.; Alieva, I.B.; Prusov, A.N.; Matsievsky, D.D.; Zorova, L.D.; Pevzner, I.B.; Savchenko, E.S.; Pirogov, Y.A.; Silachev, D.N.; et al. Dysfunction of Kidney Endothelium after Ischemia/Reperfusion and Its Prevention by Mitochondria-Targeted Antioxidant. Biochemistry 2016, 81, 1538–1548. [Google Scholar] [CrossRef]
- Seron, D.; Cameron, J.S.; Haskard, D.O. Expression of VCAM-1 in the normal and diseased kidney. Nephrol. Dial. Transplant. 1991, 6, 917–922. [Google Scholar] [CrossRef]
- Takada, M.; Nadeau, K.C.; Shaw, G.D.; Marquette, K.A.; Tilney, N.L. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J. Clin. Investig. 1997, 99, 2682–2690. [Google Scholar] [CrossRef] [Green Version]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Horbelt, M.; Lee, S.Y.; Mang, H.E.; Knipe, N.L.; Sado, Y.; Kribben, A.; Sutton, T.A. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Renal. Physiol. 2007, 293, F688–F695. [Google Scholar] [CrossRef]
- Menshikh, A.; Scarfe, L.; Delgado, R.; Finney, C.; Zhu, Y.; Yang, H.; de Caestecker, M.P. Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am. J. Physiol. Renal. Physiol. 2019, 317, F1383–F1397. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, B.; Lehir, M.; Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 2013, 1832, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballermann, B.J.; Obeidat, M. Tipping the balance from angiogenesis to fibrosis in CKD. Kidney Int. Suppl. 2014, 4, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chade, A.R. Renovascular disease, microcirculation, and the progression of renal injury: Role of angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R783–R790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolte, D.; McClung, J.A.; Aronow, W.S. Chapter 6—Vasculogenesis and Angiogenesis. In Translational Research in Coronary Artery Disease; Aronow, W.S., McClung, J.A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 49–65. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef] [Green Version]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Lerman, L.O.; Chade, A.R. Angiogenesis in the kidney: A new therapeutic target? Curr. Opin. Nephrol. Hypertens. 2009, 18, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Freeburg, P.B.; Abrahamson, D.R. Hypoxia-inducible factors and kidney vascular development. J. Am. Soc. Nephrol. 2003, 14, 2723–2730. [Google Scholar] [CrossRef]
- Nordquist, L.; Friederich-Persson, M.; Fasching, A.; Liss, P.; Shoji, K.; Nangaku, M.; Hansell, P.; Palm, F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallet, N.; Thervet, E.; Timsit, M.O. Angiogenic response following renal ischemia reperfusion injury: New players. Prog. Urol. 2014, 24 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Ryan, H.E.; Lo, J.; Johnson, R.S. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998, 17, 3005–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, D.D.; Zaleski, J.K.; Liu, S.; Brock, T.A. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am. J. Physiol. 1993, 265, H586–H592. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Kang, D.H.; Hughes, J.; Mazzali, M.; Schreiner, G.F.; Johnson, R.J. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 2001, 12, 1448–1457. [Google Scholar] [CrossRef]
- Basile, D.P.; Fredrich, K.; Chelladurai, B.; Leonard, E.C.; Parrish, A.R. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am. J. Physiol. Renal. Physiol. 2008, 294, F928–F936. [Google Scholar] [CrossRef]
- Kanellis, J.; Mudge, S.J.; Fraser, S.; Katerelos, M.; Power, D.A. Redistribution of cytoplasmic VEGF to the basolateral aspect of renal tubular cells in ischemia-reperfusion injury. Kidney Int. 2000, 57, 2445–2456. [Google Scholar] [CrossRef]
- Vannay, A.; Fekete, A.; Adori, C.; Toth, T.; Losonczy, G.; Laszlo, L.; Vasarhelyi, B.; Tulassay, T.; Szabo, A. Divergence of renal vascular endothelial growth factor mRNA expression and protein level in post-ischaemic rat kidneys. Exp. Physiol. 2004, 89, 435–444. [Google Scholar] [CrossRef]
- Kramer, B.K.; Bucher, M.; Sandner, P.; Ittner, K.P.; Riegger, G.A.; Ritthaler, T.; Kurtz, A. Effects of hypoxia on growth factor expression in the rat kidney in vivo. Kidney Int. 1997, 51, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Chapal, M.; Neel, M.; Le Borgne, F.; Meffray, E.; Carceles, O.; Hourmant, M.; Giral, M.; Foucher, Y.; Moreau, A.; Fakhouri, F. Increased soluble Flt-1 correlates with delayed graft function and early loss of peritubular capillaries in the kidney graft. Transplantation 2013, 96, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Wewers, T.M.; Mayer, A.B.; Pfleiderer, A.; Beul, K.; Schmidt, R.; Heitplatz, B.; Van Marck, V.; Nolte, I.; Pavenstadt, H.; Reuter, S.; et al. Increased soluble fms-like tyrosine kinase 1 after ischemia reperfusion contributes to adverse clinical outcomes following kidney transplantation. Kidney Int. 2019, 95, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Paizis, K.; Cox, A.J.; Stacker, S.A.; Gilbert, R.E.; Cooper, M.E.; Power, D.A. Renal ischemia-reperfusion increases endothelial VEGFR-2 without increasing VEGF or VEGFR-1 expression. Kidney Int. 2002, 61, 1696–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia and Dysregulated Angiogenesis in Kidney Disease. Kidney Dis. 2015, 1, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Mahbuba, W.; Lambert, D.G. Opioids and neovascularization; pro or anti? Br. J. Anaesth. 2015, 115, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.P. Opioids in Cancer Pain, 2nd ed.; Oxford University Press: New York, NY, USA, 2009; 487p. [Google Scholar]
- Cruz, S.L. (Ed.) Opioids: Pharmacology, Abuse, and Addiction; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Abrimian, A.; Kraft, T.; Pan, Y.X. Endogenous Opioid Peptides and Alternatively Spliced Mu Opioid Receptor Seven Transmembrane Carboxyl-Terminal Variants. Int. J. Mol. Sci. 2021, 22, 3779. [Google Scholar] [CrossRef]
- Gomes, I.; Sierra, S.; Lueptow, L.; Gupta, A.; Gouty, S.; Margolis, E.B.; Cox, B.M.; Devi, L.A. Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. USA 2020, 117, 11820–11828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.T.; Hang, L.; Liu, T. Mu Opioid Receptor Heterodimers Emerge as Novel Therapeutic Targets: Recent Progress and Future Perspective. Front. Pharmacol. 2020, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Hartman, A.; Bilfinger, T.V.; Magazine, H.I.; Liu, Y.; Casares, F.; Goligorsky, M.S. Presence of the μ3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J. Biol. Chem. 1995, 270, 30290–30293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Sarkar, S.; Chang, S.L. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012, 124, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Hu, C.; Han, F.; Cai, F.; Wang, J.; Chen, J. Preconditioning is an effective strategy for improving the efficiency of mesenchymal stem cells in kidney transplantation. Stem Cell Res. Ther. 2020, 11, 197. [Google Scholar] [CrossRef]
- Ha, H.; Park, J.; Kim, Y.S.; Endou, H. Oxidative stress and chronic allograft nephropathy. Yonsei Med. J. 2004, 45, 1049–1052. [Google Scholar] [CrossRef]
- Schultz, J.E.; Gross, G.J. Opioids and cardioprotection. Pharmacol. Ther. 2001, 89, 123–137. [Google Scholar] [CrossRef]
- Murphy, G.S.; Szokol, J.W.; Marymont, J.H.; Avram, M.J.; Vender, J.S. Opioids and cardioprotection: The impact of morphine and fentanyl on recovery of ventricular function after cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2006, 20, 493–502. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. Opioids in Remote Ischemic Preconditioning-Induced Cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 112–121. [Google Scholar] [CrossRef]
- Echavarria, R.; Garcia, D.; Figueroa, F.; Franco-Acevedo, A.; Palomino, J.; Portilla-Debuen, E.; Goldaraz-Monraz, M.P.; Moreno-Carranza, B.; Melo, Z. Anesthetic preconditioning increases sirtuin 2 gene expression in a renal ischemia reperfusion injury model. Minerva Urol. Nefrol. 2020, 72, 243–249. [Google Scholar] [CrossRef]
- Coupe, N.; O’Brien, M.; Gibson, P.; de Lima, J. Anesthesia for pediatric renal transplantation with and without epidural analgesia—A review of 7 years experience. Paediatr. Anaesth. 2005, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kirvela, M.; Lindgren, L.; Seppala, T.; Olkkola, K.T. The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J. Clin. Anesth. 1996, 8, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Freir, N.M.; Murphy, C.; Mugawar, M.; Linnane, A.; Cunningham, A.J. Transversus abdominis plane block for analgesia in renal transplantation: A randomized controlled trial. Anesth. Analg. 2012, 115, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Guirguis, M.N.; Helou, M.; Dalton, J.E.; Ngo, F.; Ghobrial, M.; O’Hara, J.; Seif, J.; Krishnamurthi, V.; Goldfarb, D. Continuous transversus abdominis plane block catheter analgesia for postoperative pain control in renal transplant. J. Anesth. 2015, 29, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Soltani Mohammadi, S.; Dabir, A.; Shoeibi, G. Efficacy of transversus abdominis plane block for acute postoperative pain relief in kidney recipients: A double-blinded clinical trial. Pain Med. 2014, 15, 460–464. [Google Scholar] [CrossRef]
- Feng, Y.; He, X.; Yang, Y.; Chao, D.; Lazarus, L.H.; Xia, Y. Current research on opioid receptor function. Curr. Drug Targets 2012, 13, 230–246. [Google Scholar] [CrossRef] [Green Version]
- Ondrovics, M.; Hoelbl-Kovacic, A.; Fux, D.A. Opioids: Modulators of angiogenesis in wound healing and cancer. Oncotarget 2017, 8, 25783–25796. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Zeng, S.; Ding, J.; Chen, G.; Wang, B.; Wang, D.; Li, X.; Wang, K. Comparative analysis of the effects of opioids in angiogenesis. BMC Anesthesiol. 2021, 21, 257. [Google Scholar] [CrossRef]
- Leo, S.; Nuydens, R.; Meert, T.F. Opioid-induced proliferation of vascular endothelial cells. J. Pain Res. 2009, 2, 59–66. [Google Scholar] [CrossRef]
- Tuerxun, H.; Cui, J. The dual effect of morphine on tumor development. Clin. Transl. Oncol. 2019, 21, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Novy, D.M.; Nelson, D.V.; Koyyalagunta, D.; Cata, J.P.; Gupta, P.; Gupta, K. Pain, opioid therapy, and survival: A needed discussion. Pain 2020, 161, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. Does Opioid Use Cause Angiogenesis and Metastasis? Pain Med. 2017, 18, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapusta, D.R.; Kenigs, V.A. Cardiovascular and renal responses produced by central orphanin FQ/nociceptin occur independent of renal nerves. Am. J. Physiol. 1999, 277, R987–R995. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, D.R.; Dzialowski, E.M. Central mu opioids mediate differential control of urine flow rate and urinary sodium excretion in conscious rats. Life Sci. 1995, 56, PL243-8. [Google Scholar] [CrossRef] [PubMed]
- Shweta, A.; Malpas, S.C.; Anderson, W.P.; Evans, R.G. Effects of naloxone on the haemodynamic and renal functional responses to plasma volume expansion in conscious rabbits. Pflügers Arch. 1999, 439, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Z.; Long, S.; Li, W.; Wang, B.; Liang, N. Opioids in cancer: The kappaopioid receptor (Review). Mol. Med. Rep. 2022, 25, 44. [Google Scholar] [CrossRef] [PubMed]
- Franco-Acevedo, A.; Echavarria, R.; Moreno-Carranza, B.; Ortiz, C.I.; Garcia, D.; Gonzalez-Gonzalez, R.; Bitzer-Quintero, O.K.; Portilla-De Buen, E.; Melo, Z. Opioid Preconditioning Modulates Repair Responses to Prevent Renal Ischemia-Reperfusion Injury. Pharmaceuticals 2020, 13, 387. [Google Scholar] [CrossRef] [PubMed]


| Reference | Opioid | Effect | Recipients |
|---|---|---|---|
| Coupe et al. [84] | Morphine, bupivacaine, and fentanyl | Improve intraoperative hemodynamic stability | Fifty-three pediatrics |
| Kirvela et al. [85] | Oxycodone | Opioid metabolites accumulation | Ten uremic transplant recipients |
| Freir et al. [86] | Morphine and Levobupivacaine | Risk of nausea | Sixty-five adults |
| Farag et al. [87] | Morphine and Ropivacaine | Nausea, hospital stay | Sixty-three adults |
| Mohammadi et al. [88] | Fentanyl and bupivacaine | Postrenal transplantation pain and amount of opioids consumption | Sixty-seven adults |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdugo-Molinares, M.G.; Franco-Acevedo, A.; Ortiz, C.I.; Cerino-Recinos, J.L.; Moreno-Carranza, B.; Melo, Z. Angiogenesis under Opioids Preconditioning in Renal Ischemia Reperfusion. Drugs Drug Candidates 2023, 2, 1-13. https://doi.org/10.3390/ddc2010001
Verdugo-Molinares MG, Franco-Acevedo A, Ortiz CI, Cerino-Recinos JL, Moreno-Carranza B, Melo Z. Angiogenesis under Opioids Preconditioning in Renal Ischemia Reperfusion. Drugs and Drug Candidates. 2023; 2(1):1-13. https://doi.org/10.3390/ddc2010001
Chicago/Turabian StyleVerdugo-Molinares, Maritza G., Adriana Franco-Acevedo, Cesar I. Ortiz, José L. Cerino-Recinos, Bibiana Moreno-Carranza, and Zesergio Melo. 2023. "Angiogenesis under Opioids Preconditioning in Renal Ischemia Reperfusion" Drugs and Drug Candidates 2, no. 1: 1-13. https://doi.org/10.3390/ddc2010001
APA StyleVerdugo-Molinares, M. G., Franco-Acevedo, A., Ortiz, C. I., Cerino-Recinos, J. L., Moreno-Carranza, B., & Melo, Z. (2023). Angiogenesis under Opioids Preconditioning in Renal Ischemia Reperfusion. Drugs and Drug Candidates, 2(1), 1-13. https://doi.org/10.3390/ddc2010001








