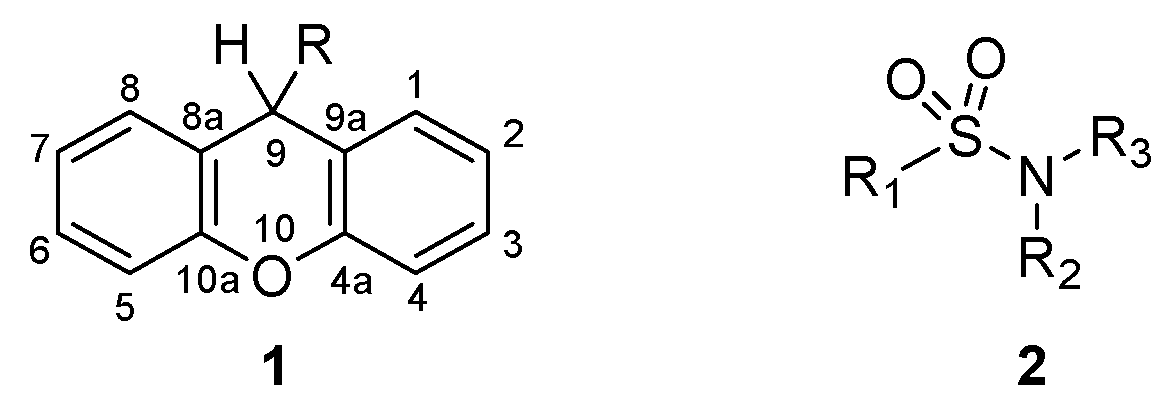
Xanthene Derivatives Targeting Bacterial Efflux Pumps, Quorum-Sensing, and Biofilm Formation
Abstract
1. Introduction
2. Results and Discussion
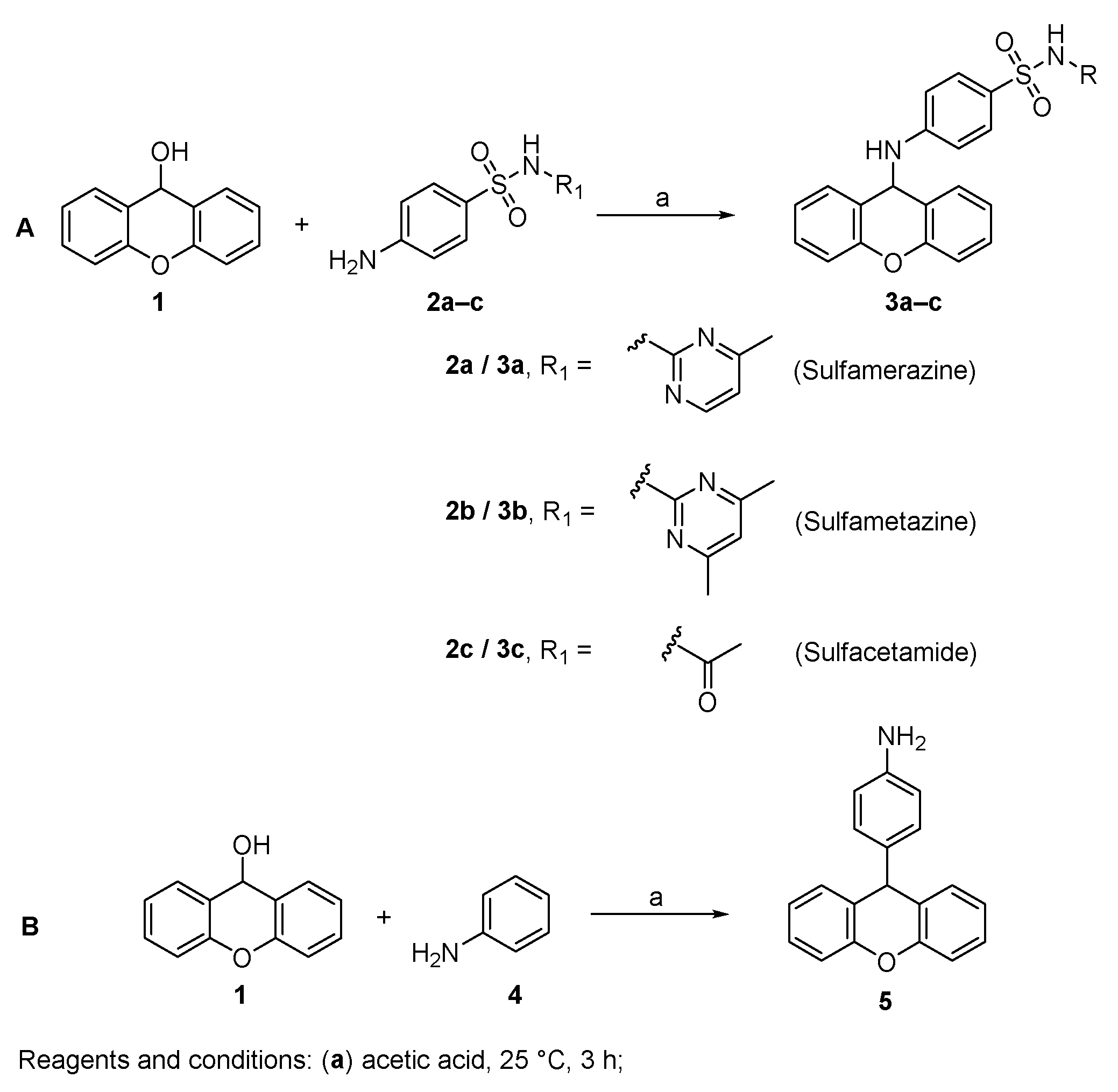
2.1. Chemistry
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Quorum-Sensing Inhibition
2.4. Biofilm Formation Inhibition
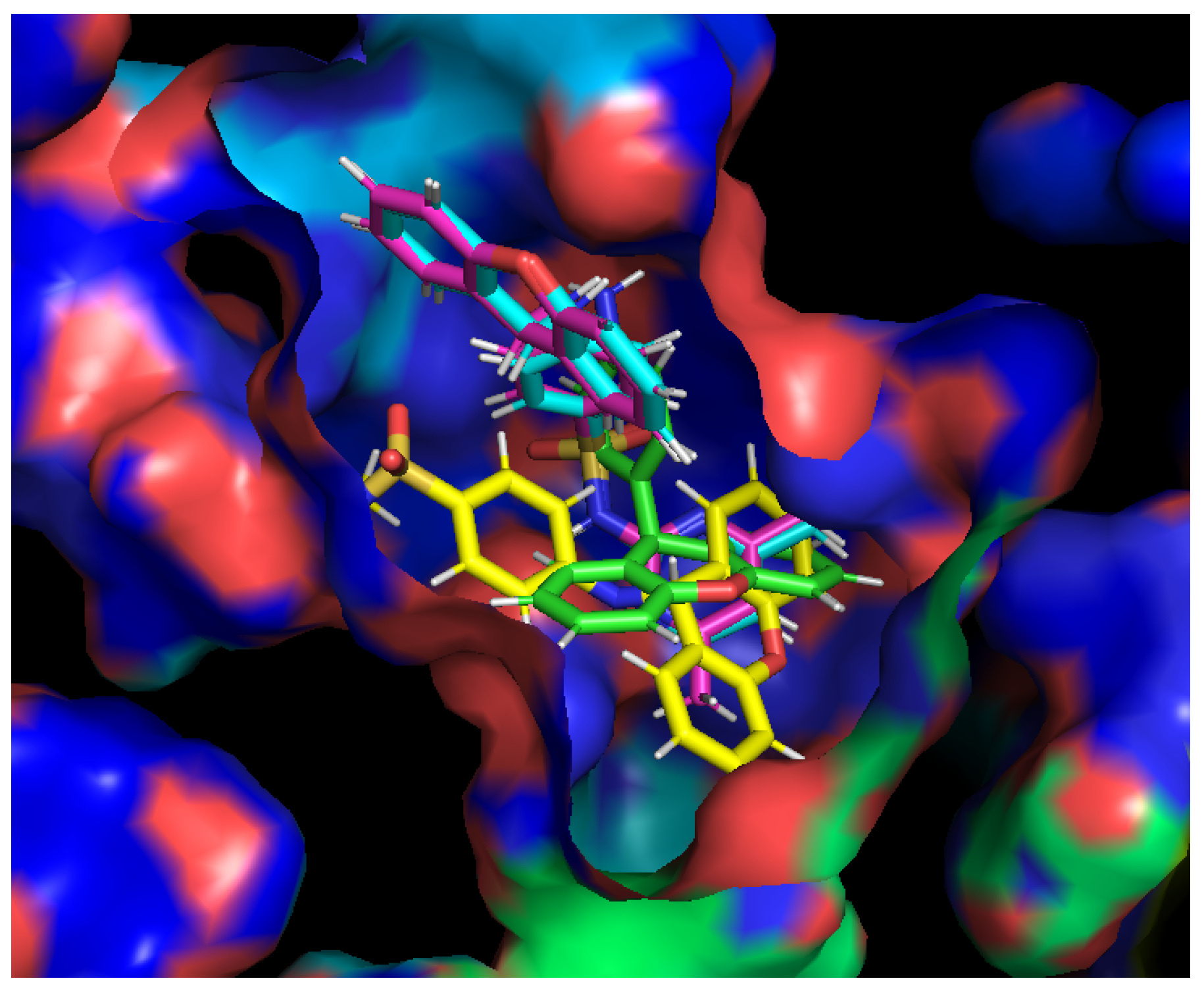
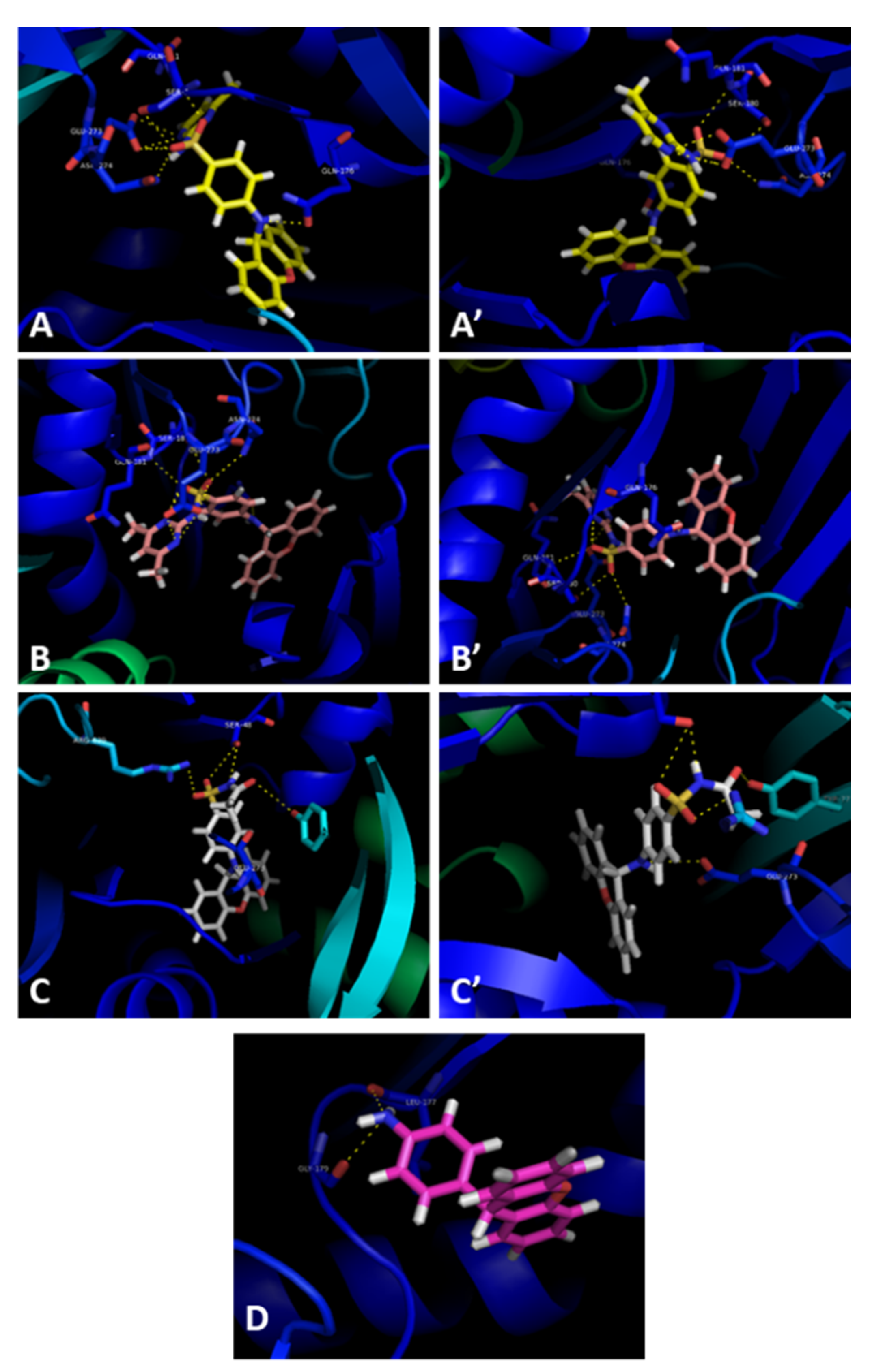
2.5. Efflux Pump Inhibition
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.3. X-ray Crystallography
3.4. Microorganisms
3.5. Antibacterial Assay
3.6. Quorum-Sensing Inhibition
3.7. Inhibition of Biofilm Formation
3.8. Efflux Pump Inhibition Assay
3.9. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations/the Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 12 April 2021).
- Kong, Q.; Yang, Y. Recent advances in antibacterial agents. Bioorganic Med. Chem. Lett. 2021, 35, 127799. [Google Scholar] [CrossRef] [PubMed]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Malhas, A.M.; Lawton, R.; Reidy, M.; Nathwani, D.; Clift, B.A. Causative organisms in revision total hip & knee arthroplasty for infection: Increasing multi-antibiotic resistance in coagulase-negative Staphylococcus and the implications for antibiotic prophylaxis. Surgeon 2015, 13, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef]
- Fuqua, C.; Greenberg, E.P. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 685–695. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Maia, M.; Resende, D.I.S.P.; Durães, F.; Pinto, M.M.M.; Sousa, E. Xanthenes in Medicinal Chemistry—Synthetic strategies and biological activities. Eur. J. Med. Chem. 2021, 210, 113085. [Google Scholar] [CrossRef]
- Barmak, A.; Niknam, K.; Mohebbi, G.; Pournabi, H. Antibacterial studies of hydroxyspiro[indoline-3,9-xanthene]trione against spiro[indoline 3,9-xanthene]trione and their use as acetyl and butyrylcholinesterase inhibitors. Microb. Pathog. 2019, 130, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Padalkar, V.S.; Phatangare, K.R.; Umape, P.G.; Borase, B.N.; Sekar, N. Synthesis, Characterization, and Antibacterial Activity of Novel (1H-Benzo[d]imidazole-2-yl)-6-(diethylamino)-3H-one-xanthene, Phenoxazine, and Oxazine. J. Heterocycl. Chem. 2015, 52, 124–129. [Google Scholar] [CrossRef]
- Kaya, M.; Demir, E.; Bekci, H. Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Khaki, D.; Amininasab, S.M.; Namazi, H. Novel poly(imide-ether)s based on xanthene and a corresponding composite reinforced with a GO grafted hyperbranched polymer: Fabrication, characterization, and thermal, photophysical, antibacterial and chromium adsorption properties. New J. Chem. 2020, 44, 17346–17359. [Google Scholar] [CrossRef]
- Gorokhov, V.Y.; Makhova, T.V. Synthesis and Antibactericidal Activities of Amines and Imines Containing (Aza, Thio) Xanthene Rings. Pharm. Chem. J. 2016, 50, 530–533. [Google Scholar] [CrossRef]
- Amininasab, S.M.; Esmaili, S.; Shami, Z. Synthesis of polyamides contains pyridine and xanthene pendant group: Study of optical, thermal, antibacterial activity and hexavalent chromium ion adsorption. J. Macromol. Sci. A 2020, 57, 35–45. [Google Scholar] [CrossRef]
- Gabor Varga, Z.; Agnes Szabo, M.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnar, J. Quorum Sensing Inhibition by Phenothiazines and Related Compounds. Lett. Drug Des. Discov. 2011, 8, 133–137. [Google Scholar] [CrossRef]
- Durães, F.; Palmeira, A.; Cruz, B.; Freitas-Silva, J.; Szemerédi, N.; Gales, L.; da Costa, P.M.; Remião, F.; Silva, R.; Pinto, M.; et al. Antimicrobial Activity of a Library of Thioxanthones and Their Potential as Efflux Pump Inhibitors. Pharmaceuticals 2021, 14, 572. [Google Scholar] [CrossRef]
- Kristiansen, M.M.; Leandro, C.; Ordway, D.; Martins, M.; Viveiros, M.; Pacheco, T.; Kristiansen, J.E.; Amaral, L. Phenothiazines alter resistance of methicillin-resistant strains of Staphylococcus aureus (MRSA) to oxacillin in vitro. Int. J. Antimicrob. Agents 2003, 22, 250–253. [Google Scholar] [CrossRef]
- Kristiansen, M.M.; Leandro, C.; Ordway, D.; Martins, M.; Viveiros, M.; Pacheco, T.; Molnar, J.; Kristiansen, J.E.; Amaral, L. Thioridazine reduces resistance of methicillin-resistant staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. Vivo 2006, 20, 361. [Google Scholar]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L. Sulphonamides: Updates on use in veterinary medicine. Vet. Dermatol. 1999, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Kristiansen, J.E. On the 75th anniversary of Prontosil. Dye. Pigment. 2011, 88, 231–234. [Google Scholar] [CrossRef]
- al-Rashida, M.; Hussain, S.; Hamayoun, M.; Altaf, A.; Iqbal, J. Sulfa Drugs as Inhibitors of Carbonic Anhydrase: New Targets for the Old Drugs. BioMed Res. Int. 2014, 2014, 162928. [Google Scholar] [CrossRef]
- Loubatières-Mariani, M.-M. La découverte des sulfamides hypoglycémiants. J. Soc. Biol. 2007, 201, 121–125. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pandeya, S.N.; Khan, S.A.; Stables, J.; Rana, A.; Alam, M.; Arshad, M.F.; Bhat, M.A. Synthesis and anticonvulsant activity of sulfonamide derivatives-hydrophobic domain. Bioorganic Med. Chem. Lett. 2007, 17, 255–259. [Google Scholar] [CrossRef]
- Okolotowicz, K.J.; Dwyer, M.; Ryan, D.; Cheng, J.; Cashman, E.A.; Moore, S.; Mercola, M.; Cashman, J.R. Novel tertiary sulfonamides as potent anti-cancer agents. Bioorganic Med. Chem. 2018, 26, 4441–4451. [Google Scholar] [CrossRef]
- Andrea, S.; Takashi, O.; Antonio, M.; Claudiu, T.S. Anticancer and Antiviral Sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef]
- Phillips, R.F.; Frank, V.S. Xanthydrol as a reagent for the identification of sulfonamides. J. Org. Chem. 1944, 9, 9–12. [Google Scholar] [CrossRef]
- Moskalyk, R.E.; Chatten, L.G. Alkylation by secondary alcohols. I. The reaction of xanthydrol with some N1-monosubstituted sulfanilamides and related compounds. Can. J. Chem. 1967, 45, 1411–1424. [Google Scholar] [CrossRef]
- Westcott, W.L. Reactions of Xanthydrol with the Amino Acids and Cytochrome C; University of Utah: Salt Lake City, UT, USA, 1953. [Google Scholar]
- Tacic, A.; Nikolic, V.; Nikolic, L.; Savic, I. Antimicrobial sulfonamide drugs. Adv. Technol. 2017, 6, 58–71. [Google Scholar] [CrossRef]
- Connor, E.E. Sulfonamide antibiotics. Prim. Care Update OB/GYNS 1998, 5, 32–35. [Google Scholar] [CrossRef]
- Seydel, J.K. Sulfonamides, Structure-Activity Relationship, and Mode of Action: Structural Problems of the Antibacterial Action of 4-Aminobenzoic Acid (PABA) Antagonists. J. Pharm. Sci. 1968, 57, 1455–1478. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Spengler, G. Standard operating procedure (SOP) for disk diffusion-based quorum sensing inhibition assays. Acta Pharm. Hung. 2020, 89, 117–125. [Google Scholar] [CrossRef][Green Version]
- Gajdács, M.; Spengler, G. The Role of Drug Repurposing in the Development of Novel Antimicrobial Drugs: Non-Antibiotic Pharmacological Agents as Quorum Sensing-Inhibitors. Antibiotics 2019, 8, 270. [Google Scholar] [CrossRef]
- Durães, F.; Resende, D.I.S.P.; Palmeira, A.; Szemerédi, N.; Pinto, M.M.M.; Spengler, G.; Sousa, E. Xanthones Active against Multidrug Resistance and Virulence Mechanisms of Bacteria. Antibiotics 2021, 10, 600. [Google Scholar] [CrossRef]
- Durães, F.; Cravo, S.; Freitas-Silva, J.; Szemerédi, N.; Martins-da-Costa, P.; Pinto, E.; Tiritan, M.E.; Spengler, G.; Fernandes, C.; Sousa, E.; et al. Enantioselectivity of Chiral Derivatives of Xanthones in Virulence Effects of Resistant Bacteria. Pharmaceuticals 2021, 14, 1141. [Google Scholar] [CrossRef]
- Eicher, T.; Cha, H.; Seeger, M.A.; Brandstaetter, L.; El-Delik, J.; Bohnert, J.A.; Kern, W.V.; Verrey, F.; Gruetter, M.G.; Diederichs, K.; et al. 4DX5. Available online: https://www.rcsb.org/structure/4DX5 (accessed on 14 April 2021).
- Aron, Z.; Opperman, T.J. The hydrophobic trap-the Achilles heel of RND efflux pumps. Res. Microbiol. 2018, 169, 393–400. [Google Scholar] [CrossRef]
- Zhu, Z.; Espenson, J.H. Organic Reactions Catalyzed by Methylrhenium Trioxide: Dehydration, Amination, and Disproportionation of Alcohols. J. Org. Chem. 1996, 61, 324–328. [Google Scholar] [CrossRef]
- Shen, G.; Zhao, L.; Wang, Y.; Xia, W.; Yang, M.; Zhang, T. Palladium–copper catalyzed C(sp3)–C(sp2) bond C–H activation cross-coupling reaction: Selective arylation to synthesize 9-aryl-9H-xanthene and 9,9-diaryl-xanthene derivatives. RSC Adv. 2016, 6, 84748–84751. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Nové, M.; Kincses, A.; Szalontai, B.; Rácz, B.; Blair, J.M.A.; González-Prádena, A.; Benito-Lama, M.; Domínguez-Álvarez, E.; Spengler, G. Biofilm Eradication by Symmetrical Selenoesters for Food-Borne Pathogens. Microorganisms 2020, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.K. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef]
- Sussman, J.L.; Lin, D.; Jiang, J.; Manning, N.O.; Prilusky, J.; Ritter, O.; Abola, E.E. Protein Data Bank (PDB): Database of three-dimensional structural information of biological macromolecules. Acta Cryst. D Biol. Cryst. 1998, 54, 1078–1084. [Google Scholar] [CrossRef]
- Mikolosko, J.; Bobyk, K.; Zgurskaya, H.I.; Ghosh, P. Conformational Flexibility in the Multidrug Efflux System Protein AcrA. Structure 2006, 14, 577–587. [Google Scholar] [CrossRef]
- Koronakis, V.; Sharff, A.; Koronakis, E.; Luisi, B.; Hughes, C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 2000, 405, 914–919. [Google Scholar] [CrossRef]
- Shi, X.; Chen, M.; Yu, Z.; Bell, J.M.; Wang, H.; Forrester, I.; Villarreal, H.; Jakana, J.; Du, D.; Luisi, B.F.; et al. In situ structure and assembly of the multidrug efflux pump AcrAB-TolC. Nat. Commun. 2019, 10, 2635. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]

| Compound | Quorum Sensing Inhibition ± SD (mm) | ||
|---|---|---|---|
| S. marcescens | wt85 | EZF + CV026 | |
| Promethazine a | 18 ± 0.8 | 40 ± 0.1 | 41 ± 0.5 |
| 3a | 0 | 0 | 0 |
| 3b | 0 | 0 | 48 ± 0.1 |
| 3c | 0 | 0 | 41 ± 0.8 |
| 5 | 0 | 0 | 0 |
| Compound | Inhibition of Biofilm Formation (%) ± SD | |
|---|---|---|
| S. aureus ATCC 25923 | S. aureus 272123 | |
| Reserpine a | 22.29 ± 2.09 * | 77.62 ± 1.44 *** |
| 3a | 4.49 ± 0.27 * | 18.16 ± 0.27 *** |
| 3b | 1.91 ± 1.74 | 78.29 ± 9.27 |
| 3c | 0.06 ± 0.99 * | 79.22 ± 1.12 ** |
| 5 | 1.08 ± 1.33 | 16.07 ± 0.72 ** |
| Compound | Relative Fluorescence Index (RFI) ± SD | |
|---|---|---|
| S. aureus MRSA 272123 | S. typhimurium SL1344 | |
| 3a | 0.63 ± 0.09 | 2.75 ± 0.11 |
| 3b | 0.33 ± 0.04 | 2.18 ± 0.04 |
| 3c | 0.14 ± 0.10 | 2.94 ± 0.23 |
| 5 | 0.14 ± 0.05 | 1.66 ± 0.01 |
| Positive control | ||
| Reserpine | 0.56 ± 0.04 | --- |
| CCCP | --- | 0.34 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, M.; Durães, F.; Resende, D.I.S.P.; Szemerédi, N.; Gales, L.; Martins-da-Costa, P.; Pinto, M.; Spengler, G.; Sousa, E. Xanthene Derivatives Targeting Bacterial Efflux Pumps, Quorum-Sensing, and Biofilm Formation. Drugs Drug Candidates 2022, 1, 29-42. https://doi.org/10.3390/ddc1010004
Maia M, Durães F, Resende DISP, Szemerédi N, Gales L, Martins-da-Costa P, Pinto M, Spengler G, Sousa E. Xanthene Derivatives Targeting Bacterial Efflux Pumps, Quorum-Sensing, and Biofilm Formation. Drugs and Drug Candidates. 2022; 1(1):29-42. https://doi.org/10.3390/ddc1010004
Chicago/Turabian StyleMaia, Miguel, Fernando Durães, Diana I. S. P. Resende, Nikoletta Szemerédi, Luís Gales, Paulo Martins-da-Costa, Madalena Pinto, Gabriella Spengler, and Emília Sousa. 2022. "Xanthene Derivatives Targeting Bacterial Efflux Pumps, Quorum-Sensing, and Biofilm Formation" Drugs and Drug Candidates 1, no. 1: 29-42. https://doi.org/10.3390/ddc1010004
APA StyleMaia, M., Durães, F., Resende, D. I. S. P., Szemerédi, N., Gales, L., Martins-da-Costa, P., Pinto, M., Spengler, G., & Sousa, E. (2022). Xanthene Derivatives Targeting Bacterial Efflux Pumps, Quorum-Sensing, and Biofilm Formation. Drugs and Drug Candidates, 1(1), 29-42. https://doi.org/10.3390/ddc1010004










