G-Protein-Coupled Receptor Kinase 2 Limits CCL21-Induced T Cell Migration via Phospholipase Cγ1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
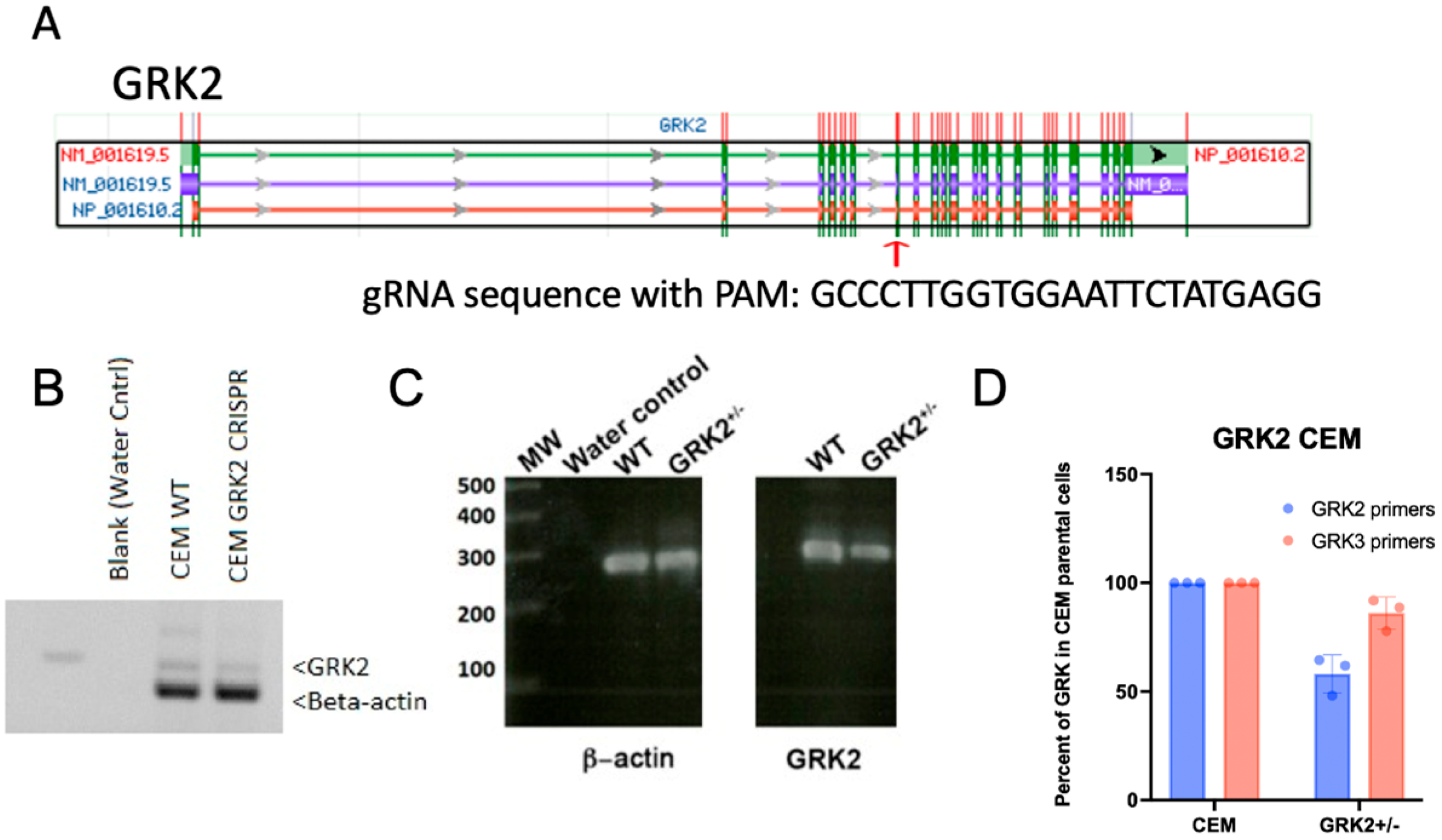
2.2. Construct CRISPR and Target Sites for GRK2
2.3. Cell Transfections
2.4. Lentiviral CRISPR-Cas9 Knockdown of GRK2
2.5. RT-PCR
2.6. Western Blots
2.7. Receptor Internalization Assays
2.8. Phosphorylation Assay
2.9. Immunofluorescence
2.10. Nucleofections
2.11. Fluorescence Resonance Energy Transfer (FRET) Measurements
2.12. Mice
2.13. Splenic Isolation of T Cells
2.14. Chemotaxis Assays
2.15. Calcium Mobilization Assay
3. Results
3.1. GRK2 Promotes Receptor Phosphorylation and Recruitment of Arrestin-3 to CCL19 but Not CCL21 Stimulated CCR7
3.2. GRK2 Kinase Activity Mediates Recruitment of Arrestin3 to CCR7
3.3. GRK2 Limits Recruitment of GαI Subunits to Ligand-Bound CCR7
3.4. GRK2 Kinase Activity Promotes the Extent of CCR7 Internalization of CCL19 and Increases the Rate of CCR7 Internalization of CCL21
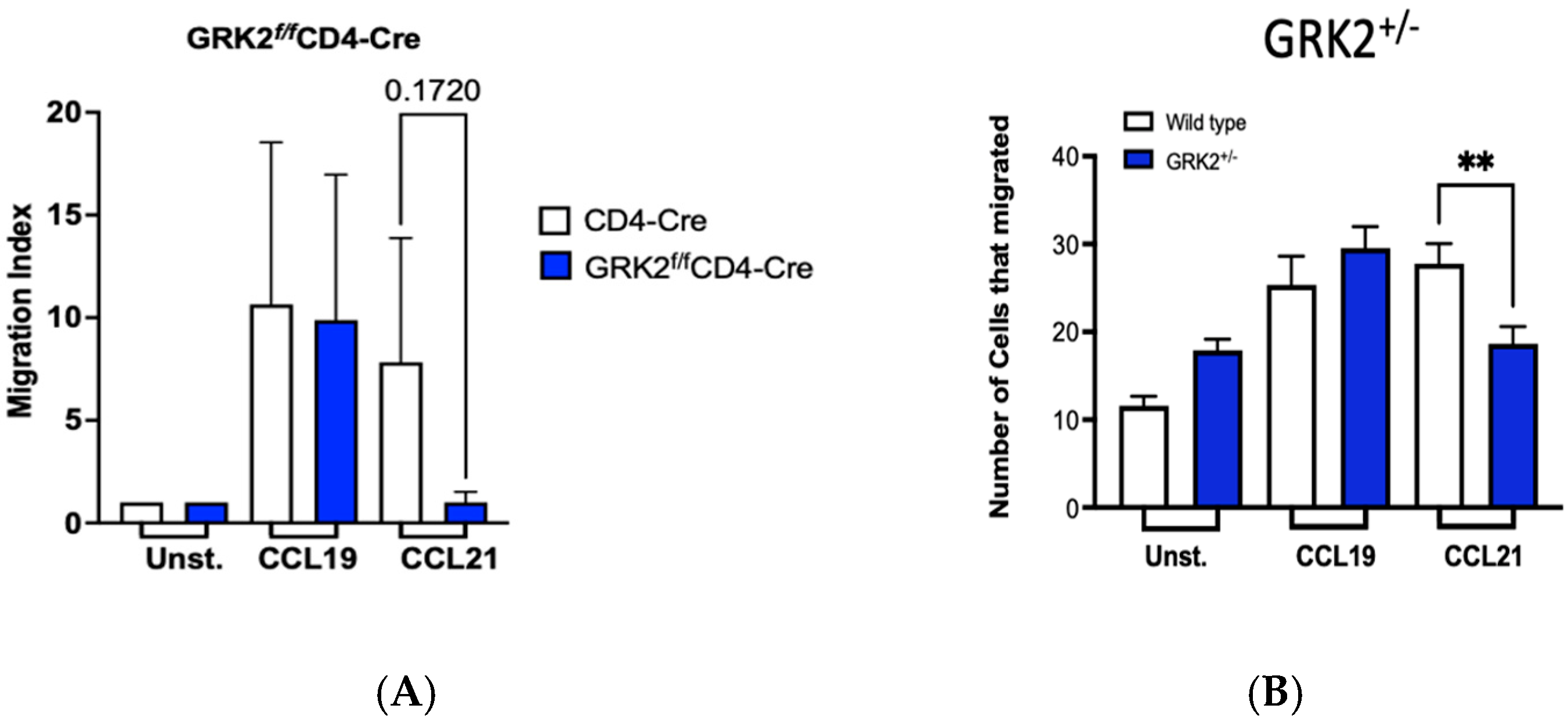
3.5. GRK2 Promotes Migration of T Cells to CCL21 but Not CCL19
3.6. GRK2 Promotes Recruitment of Phospholipase Cγ1 to the Membrane Downstream of/CLL19/CCR7
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| CCR7 | C-C Chemokine Receptor 7 |
| CCL19 | C-C Chemokine Ligand 19 |
| CCL21 | C-C Chemokine Ligand 21 |
| CNS | Central Nervous System |
| CpVenus | Circularly permutated Venus fluorescent protein |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EAE | Experimental Autoimmune Encephalomyelitis |
| FACS | Fluorescence Activated Cell Sorting |
| FRET | Fluorescence Resonance Energy Transfer |
| GPCR | G Protein-Coupled Receptor |
| GRK | G protein-coupled Receptor Kinase |
| gRNA | Guide RNA |
| HBSS | Hanks Balanced Saline Solution |
| HEK293T | Human Embryonic Kidney cells 293 large T antigen |
| IACUC | Institutional Animal Care and Use Committee |
| JLAT-WT | Jurkat e6 LAT CRISPR Deletion; reconstituted with wildtype LAT |
| IMEM | Iscove’s Modified Dulbecco’s Medium |
| MMLV | Moloney Murine Leukemia Virus |
| MS | Multiple Sclerosis |
| PAM | Protospacer Adjacent Motif |
| PLCγ1 | Phospholipase Cγ1 |
| RPMI | Roswell Park Memorial Institute (Media) |
| TBST | Tris Buffered Saline Tween |
| WT | Wild Type |
References
- Gruber, C.W.; Muttenthaler, M.; Freissmuth, M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr. Pharm. Des. 2010, 16, 3071–3088. [Google Scholar] [CrossRef]
- Palczewski, K. GTP-binding-protein-coupled receptor kinases—Two mechanistic models. Eur. J. Biochem. 1997, 248, 261–269. [Google Scholar] [CrossRef]
- Kohout, T.A.; Nicholas, S.L.; Perry, S.J.; Reinhart, G.; Junger, S.; Struthers, R.S. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 2004, 279, 23214–23222. [Google Scholar] [CrossRef]
- Penela, P.; Ribas, C.; Mayor, F., Jr. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal 2003, 15, 973–981. [Google Scholar] [CrossRef]
- Premont, R.T.; Macrae, A.D.; Aparicio, S.A.; Kendall, H.E.; Welch, J.E.; Lefkowitz, R.J. The GRK4 subfamily of G protein-coupled receptor kinases. Alternative splicing, gene organization, and sequence conservation. J. Biol. Chem. 1999, 274, 29381–29389. [Google Scholar] [CrossRef]
- Sallese, M.; Iacovelli, L.; Cumashi, A.; Capobianco, L.; Cuomo, L.; De Blasi, A. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim. Biophys. Acta 2000, 1498, 112–121. [Google Scholar] [CrossRef]
- Gurevich, E.V.; Tesmer, J.J.; Mushegian, A.; Gurevich, V.V. G protein-coupled receptor kinases: More than just kinases and not only for GPCRs. Pharmacol. Ther. 2012, 133, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S.; Barak, L.S.; Zhang, J.; Caron, M.G. G-protein-coupled receptor regulation: Role of G-protein-coupled receptor kinases and arrestins. Can. J. Physiol. Pharmacol. 1996, 74, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. Overview of different mechanisms of arrestin-mediated signaling. Curr. Protoc. Pharmacol. 2014, 67, 2–10. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. Extensive shape shifting underlies functional versatility of arrestins. Curr. Opin. Cell Biol. 2014, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef]
- Reiter, E.; Lefkowitz, R.J. GRKs and beta-arrestins: Roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. TEM 2006, 17, 159–165. [Google Scholar] [CrossRef]
- Ren, X.R.; Reiter, E.; Ahn, S.; Kim, J.; Chen, W.; Lefkowitz, R.J. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.K.; Lefkowitz, R.J. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 2003, 375, 503–515. [Google Scholar] [CrossRef]
- Shukla, A.K.; Xiao, K.; Lefkowitz, R.J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 2011, 36, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. Molecular Mechanisms of GPCR Signaling: A Structural Perspective. Int. J. Mol. Sci. 2017, 18, 2519. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006, 110, 465–502. [Google Scholar] [CrossRef]
- Campbell, J.J.; Bowman, E.P.; Murphy, K.; Youngman, K.R.; Siani, M.A.; Thompson, D.A.; Wu, L.; Zlotnik, A.; Butcher, E.C. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J. Cell Biol. 1998, 141, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef]
- Gunn, M.D.; Kyuwa, S.; Tam, C.; Kakiuchi, T.; Matsuzawa, A.; Williams, L.T.; Nakano, H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999, 189, 451–460. [Google Scholar] [CrossRef]
- Carlsen, H.S.; Haraldsen, G.; Brandtzaeg, P.; Baekkevold, E.S. Disparate lymphoid chemokine expression in mice and men: No evidence of CCL21 synthesis by human high endothelial venules. Blood 2005, 106, 444–446. [Google Scholar] [CrossRef]
- Gunn, M.D. Chemokine mediated control of dendritic cell migration and function. Semin. Immunol. 2003, 15, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nakano, H.; Aritomi, K.; Wang, C.R.; Gunn, M.D.; Kakiuchi, T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 2001, 193, 207–218. [Google Scholar] [CrossRef]
- Ohl, L.; Henning, G.; Krautwald, S.; Lipp, M.; Hardtke, S.; Bernhardt, G.; Pabst, O.; Forster, R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 2003, 197, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Cyster, J.G. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J. Immunol. 2007, 178, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Sandberg, J.L.; Ziarek, J.J.; Gerarden, K.P.; Rode, R.R.; Jensen, D.R.; McCaslin, D.R.; Peterson, F.C.; Veldkamp, C.T. Solution structure of CCL21 and identification of a putative CCR7 binding site. Biochemistry 2012, 51, 733–735. [Google Scholar] [CrossRef]
- Hromas, R.; Gray, P.W.; Chantry, D.; Godiska, R.; Krathwohl, M.; Fife, K.; Bell, G.I.; Takeda, J.; Aronica, S.; Gordon, M.; et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood 1997, 89, 3315–3322. [Google Scholar]
- Hromas, R.; Kim, C.H.; Klemsz, M.; Krathwohl, M.; Fife, K.; Cooper, S.; Schnizlein-Bick, C.; Broxmeyer, H.E. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J. Immunol. 1997, 159, 2554–2558. [Google Scholar] [CrossRef]
- Jorgensen, A.S.; Brandum, E.P.; Mikkelsen, J.M.; Orfin, K.A.; Boilesen, D.R.; Egerod, K.L.; Moussouras, N.A.; Vilhardt, F.; Kalinski, P.; Basse, P.; et al. The C-terminal peptide of CCL21 drastically augments CCL21 activity through the dendritic cell lymph node homing receptor CCR7 by interaction with the receptor N-terminus. Cell Mol. Life Sci. 2021, 78, 6963–6978. [Google Scholar] [CrossRef]
- Zidar, D.A.; Violin, J.D.; Whalen, E.J.; Lefkowitz, R.J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 9649–9654. [Google Scholar] [CrossRef]
- Byers, M.A.; Calloway, P.A.; Shannon, L.; Cunningham, H.D.; Smith, S.; Li, F.; Fassold, B.C.; Vines, C.M. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J. Immunol. 2008, 181, 4723–4732. [Google Scholar] [CrossRef]
- Hjortø, G.M.; Larsen, O.; Steen, A.; Daugvilaite, V.; Berg, C.; Fares, S.; Hansen, M.; Ali, S.; Rosenkilde, M.M. Differential CCR7 Targeting in Dendritic Cells by Three Naturally Occurring CC-Chemokines. Front. Immunol. 2016, 7, 568. [Google Scholar] [CrossRef]
- Hauser, M.A.; Legler, D.F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 2016, 99, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Shannon, L.A.; McBurney, T.M.; Wells, M.A.; Roth, M.E.; Calloway, P.A.; Bill, C.A.; Islam, S.; Vines, C.M. CCR7/CCL19 controls expression of EDG-1 in T cells. J. Biol. Chem. 2012, 287, 11656–11664. [Google Scholar] [CrossRef]
- Glaser, K.M.; Tarrant, T.K.; Lammermann, T. Combinatorial depletions of G-protein coupled receptor kinases in immune cells identify pleiotropic and cell type-specific functions. Front. Immunol. 2022, 13, 1039803. [Google Scholar] [CrossRef]
- Vroon, A.; Kavelaars, A.; Limmroth, V.; Lombardi, M.S.; Goebel, M.U.; Van Dam, A.M.; Caron, M.G.; Schedlowski, M.; Heijnen, C.J. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Immunol. 2005, 174, 4400–4406. [Google Scholar] [CrossRef]
- Penela, P.; Barradas, M.; Alvarez-Dolado, M.; Munoz, A.; Mayor, F., Jr. Effect of hypothyroidism on G protein-coupled receptor kinase 2 expression levels in rat liver, lung, and heart. Endocrinology 2001, 142, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Penela, P.; Elorza, A.; Sarnago, S.; Mayor, F., Jr. Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 2001, 20, 5129–5138. [Google Scholar] [CrossRef]
- Reinkober, J.; Tscheschner, H.; Pleger, S.T.; Most, P.; Katus, H.A.; Koch, W.J.; Raake, P.W. Targeting GRK2 by gene therapy for heart failure: Benefits above beta-blockade. Gene Ther. 2012, 19, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.; Bohm, M.; Elce, J.S.; Erdmann, E.; Lohse, M.J. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 1993, 87, 454–463. [Google Scholar] [CrossRef]
- Vroon, A.; Heijnen, C.J.; Kavelaars, A. GRKs and arrestins: Regulators of migration and inflammation. J. Leukoc. Biol. 2006, 80, 1214–1221. [Google Scholar] [CrossRef]
- Violin, J.D.; Crombie, A.L.; Soergel, D.G.; Lark, M.W. Biased ligands at G-protein-coupled receptors: Promise and progress. Trends Pharmacol. Sci. 2014, 35, 308–316. [Google Scholar] [CrossRef]
- Lo, W.L.; Shah, N.H.; Rubin, S.A.; Zhang, W.; Horkova, V.; Fallahee, I.R.; Stepanek, O.; Zon, L.I.; Kuriyan, J.; Weiss, A. Slow phosphorylation of a tyrosine residue in LAT optimizes T cell ligand discrimination. Nat. Immunol. 2019, 20, 1481–1493. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef]
- Wagener, B.M.; Marjon, N.A.; Revankar, C.M.; Prossnitz, E.R. Adaptor protein-2 interaction with arrestin regulates GPCR recycling and apoptosis. Traffic 2009, 10, 1286–1300. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Zhan, X.; Song, X.; Kook, S.; Gurevich, V.V.; Gurevich, E.V. Ubiquitin ligase parkin promotes Mdm2-arrestin interaction but inhibits arrestin ubiquitination. Biochemistry 2011, 50, 3749–3763. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chiang, S.C.; Ye, R.D.; Prossnitz, E.R. Phosphorylation of the N-formyl peptide receptor is required for receptor internalization but not chemotaxis. J. Biol. Chem. 1997, 272, 29426–29429. [Google Scholar] [CrossRef] [PubMed]
- van Unen, J.; Stumpf, A.D.; Schmid, B.; Reinhard, N.R.; Hordijk, P.L.; Hoffmann, C.; Gadella, T.W.; Goedhart, J. A New Generation of FRET Sensors for Robust Measurement of Gαi1, Gαi2 and Gαi3 Activation Kinetics in Single Cells. PLoS ONE 2016, 11, e0146789. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Koch, W.J.; Rockman, H.; Smith, B.; Bond, R.A.; Sulik, K.K.; Ross, J., Jr.; Lefkowitz, R.J.; Caron, M.G.; Giros, B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. USA 1996, 93, 12974–12979. [Google Scholar] [CrossRef]
- Peppel, K.; Boekhoff, I.; McDonald, P.; Breer, H.; Caron, M.G.; Lefkowitz, R.J. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J. Biol. Chem. 1997, 272, 25425–25428. [Google Scholar] [CrossRef] [PubMed]
- Matkovich, S.J.; Diwan, A.; Klanke, J.L.; Hammer, D.J.; Marreez, Y.; Odley, A.M.; Brunskill, E.W.; Koch, W.J.; Schwartz, R.J.; Dorn, G.W., 2nd. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ. Res. 2006, 99, 996–1003. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10, 125. [Google Scholar] [CrossRef]
- Cardona, C.I.; Rodriguez, A.; Torres, V.C.; Sanchez, A.; Torres, A.; Vazquez, A.E.; Wagler, A.E.; Brissette, M.A.; Bill, C.A.; Vines, C.M. C-C Chemokine Receptor 7 Promotes T-Cell Acute Lymphoblastic Leukemia Invasion of the Central Nervous System via beta2-Integrins. Int. J. Mol. Sci. 2024, 25, 9649. [Google Scholar] [CrossRef]
- Shannon, L.A.; Calloway, P.A.; Welch, T.P.; Vines, C.M. CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J. Biol. Chem. 2010, 285, 38781–38787. [Google Scholar] [CrossRef]
- Beals, C.R.; Wilson, C.B.; Perlmutter, R.M. A small multigene family encodes Gi signal-transduction proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 7886–7890. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ang, S.L.; Bloch, D.B.; Bloch, K.D.; Kawahara, Y.; Tolman, C.; Lee, R.; Seidman, J.G.; Neer, E.J. Identification of cDNA encoding an additional alpha subunit of a human GTP-binding protein: Expression of three alpha i subtypes in human tissues and cell lines. Proc. Natl. Acad. Sci. USA 1988, 85, 4153–4157. [Google Scholar] [CrossRef]
- Mastop, M.; Bindels, D.S.; Shaner, N.C.; Postma, M.; Gadella, T.W.J.; Goedhart, J. Characterization of a spectrally diverse set of fluorescent proteins as FRET acceptors for mTurquoise2. Sci. Rep. 2017, 7, 11999. [Google Scholar] [CrossRef]
- Tesmer, V.M.; Kawano, T.; Shankaranarayanan, A.; Kozasa, T.; Tesmer, J.J. Snapshot of activated G proteins at the membrane: The Galphaq-GRK2-Gbetagamma complex. Science 2005, 310, 1686–1690. [Google Scholar] [CrossRef]
- Dhami, G.K.; Ferguson, S.S. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 2006, 111, 260–271. [Google Scholar] [CrossRef]
- Campbell, J.J.; Hedrick, J.; Zlotnik, A.; Siani, M.A.; Thompson, D.A.; Butcher, E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 1998, 279, 381–384. [Google Scholar] [CrossRef]
- Arnon, T.I.; Xu, Y.; Lo, C.; Pham, T.; An, J.; Coughlin, S.; Dorn, G.W.; Cyster, J.G. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 2011, 333, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Laufer, J.M.; Hauser, M.A.; Kindinger, I.; Purvanov, V.; Pauli, A.; Legler, D.F. Chemokine Receptor CCR7 Triggers an Endomembrane Signaling Complex for Spatial Rac Activation. Cell Rep. 2019, 29, 995–1009.e6. [Google Scholar] [CrossRef]
- Penela, P.; Ribas, C.; Aymerich, I.; Mayor, F., Jr. New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. Cell Adh Migr. 2009, 3, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, T.K.; Billard, M.J.; Timoshchenko, R.G.; McGinnis, M.W.; Serafin, D.S.; Foreman, O.; Esserman, D.A.; Chao, N.J.; Lento, W.E.; Lee, D.M.; et al. G protein-coupled receptor kinase-3-deficient mice exhibit WHIM syndrome features and attenuated inflammatory responses. J. Leukoc. Biol. 2013, 94, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, Y.; Wang, Y.; Cui, D.; Luo, T.; Zhang, Y.; Ma, Y.; Wei, W. Development of Inflammatory Immune Response-Related Drugs Based on G Protein-Coupled Receptor Kinase 2. Cell Physiol. Biochem. 2018, 51, 729–745. [Google Scholar] [CrossRef]
- Fredericks, Z.L.; Pitcher, J.A.; Lefkowitz, R.J. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J. Biol. Chem. 1996, 271, 13796–13803. [Google Scholar] [CrossRef]
- Freeman, J.L.; Gonzalo, P.; Pitcher, J.A.; Claing, A.; Lavergne, J.P.; Reboud, J.P.; Lefkowitz, R.J. Beta 2-adrenergic receptor stimulated, G protein-coupled receptor kinase 2 mediated, phosphorylation of ribosomal protein P2. Biochemistry 2002, 41, 12850–12857. [Google Scholar] [CrossRef]
- Onorato, J.J.; Gillis, M.E.; Liu, Y.; Benovic, J.L.; Ruoho, A.E. The beta-adrenergic receptor kinase (GRK2) is regulated by phospholipids. J. Biol. Chem. 1995, 270, 21346–21353. [Google Scholar] [CrossRef]
- Tsuga, H.; Kameyama, K.; Haga, T.; Kurose, H.; Nagao, T. Sequestration of muscarinic acetylcholine receptor m2 subtypes. Facilitation by G protein-coupled receptor kinase (GRK2) and attenuation by a dominant-negative mutant of GRK2. J. Biol. Chem. 1994, 269, 32522–32527. [Google Scholar] [CrossRef]
- Wu, G.; Bogatkevich, G.S.; Mukhin, Y.V.; Benovic, J.L.; Hildebrandt, J.D.; Lanier, S.M. Identification of Gbetagamma binding sites in the third intracellular loop of the M(3)-muscarinic receptor and their role in receptor regulation. J. Biol. Chem. 2000, 275, 9026–9034. [Google Scholar] [CrossRef]
- Kammermeier, P.J.; Ruiz-Velasco, V.; Ikeda, S.R. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Galpha q/11 and Gbeta gamma. J. Neurosci. 2000, 20, 5623–5629. [Google Scholar] [CrossRef]
- Luo, J.; Busillo, J.M.; Benovic, J.L. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol. Pharmacol. 2008, 74, 338–347. [Google Scholar] [CrossRef]
- Obara, K.; Arai, K.; Tomita, Y.; Hatano, A.; Takahashi, K. G-protein coupled receptor kinase 2 and 3 expression in human detrusor cultured smooth muscle cells. Urol. Res. 2001, 29, 325–329. [Google Scholar] [CrossRef]
- DebBurman, S.K.; Ptasienski, J.; Boetticher, E.; Lomasney, J.W.; Benovic, J.L.; Hosey, M.M. Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. J. Biol. Chem. 1995, 270, 5742–5747. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, L.; Geng, B. Muscarinic M1 and M2 receptor subtypes play opposite roles in LPS-induced septic shock. Pharmacol. Rep. 2019, 71, 1108–1114. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, L.-D.; Ma, T.-M.; Song, S.-T.; Liu, H.-T.; Wang, X.; Li, N.; Yang, C.; Yu, S. Feishu Acupuncture Inhibits Acetylcholine Synthesis and Restores Muscarinic Acetylcholine Receptor M2 Expression in the Lung When Treating Allergic Asthma. Inflammation 2018, 41, 741–750. [Google Scholar] [CrossRef]
- Braza, F.; Dirou, S.; Forest, V.; Sauzeau, V.; Hassoun, D.; Chesné, J.; Cheminant-Muller, M.-A.; Sagan, C.; Magnan, A.; Lemarchand, P. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells 2016, 34, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Schaller, B. Central Noradrenergic Agonists in the Treatment of Ischemic Stroke-an Overview. Transl. Stroke Res. 2019, 11, 165–184. [Google Scholar] [CrossRef]
- Ahmari, N.; Schmidt, J.T.; Krane, G.A.; Malphurs, W.; Cunningham, B.E.; Owen, J.L.; Martyniuk, C.J.; Zubcevic, J. Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiol. Genom. 2016, 48, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sainz, M.C.; Murga, C.; Kavelaars, A.; Jurado-Pueyo, M.; Krakstad, B.F.; Heijnen, C.J.; Mayor, F.; Aragay, A.M. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol. Biol. Cell 2006, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Premont, R.T.; Kontos, C.D.; Zhu, S.; Rockey, D.C. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat. Med. 2005, 11, 952–958. [Google Scholar] [CrossRef]
- Vroon, A.; Heijnen, C.J.; Lombardi, M.S.; Cobelens, P.M.; Mayor, F.; Caron, M.G.; Kavelaars, A. Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. J. Leukoc. Biol. 2004, 75, 901–909. [Google Scholar] [CrossRef]
- Chuang, T.T.; Paolucci, L.; De Blasi, A. Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J. Biol. Chem. 1996, 271, 28691–28696. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Parameswaran, N. G-protein-coupled receptor kinases in inflammation and disease. Genes. Immun. 2015, 16, 367–377. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Otero, C.; Eisele, P.S.; Schaeuble, K.; Groettrup, M.; Legler, D.F. Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking and chemotaxis. J. Cell Sci. 2008, 121 Pt 16, 2759–2767. [Google Scholar] [CrossRef]
- de Paz, J.L.; Moseman, E.A.; Noti, C.; Polito, L.; von Andrian, U.H.; Seeberger, P.H. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem. Biol. 2007, 2, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Kiermaier, E.; Moussion, C.; Veldkamp, C.T.; Gerardy-Schahn, R.; de Vries, I.; Williams, L.G.; Chaffee, G.R.; Phillips, A.J.; Freiberger, F.; Imre, R.; et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 2016, 351, 186–190. [Google Scholar] [CrossRef]
- Hauser, M.A.; Kindinger, I.; Laufer, J.M.; Spate, A.K.; Bucher, D.; Vanes, S.L.; Krueger, W.A.; Wittmann, V.; Legler, D.F. Distinct CCR7 glycosylation pattern shapes receptor signaling and endocytosis to modulate chemotactic responses. J. Leukoc. Biol. 2016, 99, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Loef, E.J.; Kelch, I.D.; Verdon, D.J.; Black, M.M.; Middleditch, M.J.; Greenwood, D.R.; Graham, E.S.; Brooks, A.E.; Dunbar, P.R.; et al. Plasmin and regulators of plasmin activity control the migratory capacity and adhesion of human T cells and dendritic cells by regulating cleavage of the chemokine CCL21. Immunol. Cell Biol. 2016, 94, 955–963. [Google Scholar] [CrossRef]
- Schumann, K.; Lammermann, T.; Bruckner, M.; Legler, D.F.; Polleux, J.; Spatz, J.P.; Schuler, G.; Forster, R.; Lutz, M.B.; Sorokin, L.; et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 2010, 32, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.W.; Frimurer, T.M.; Holst, B.; Rosenkilde, M.M.; Elling, C.E. Molecular mechanism of 7TM receptor activation—A global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 481–519. [Google Scholar] [CrossRef] [PubMed]
- Giorelli, M.; Livrea, P.; Trojano, M. Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. J. Neuroimmunol. 2004, 155, 143–149. [Google Scholar] [CrossRef]
- Han, C.C.; Ma, Y.; Li, Y.; Wang, Y.; Wei, W. Regulatory effects of GRK2 on GPCRs and non-GPCRs and possible use as a drug target (Review). Int. J. Mol. Med. 2016, 38, 987–994. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, A.; Winebrenner, C.T.; Garcia, N.; Kaiser, B.; Kilgore, L.; Cardona, C.I.; Bassuk, D.W.; Miller, M.E.; Bill, C.A.; Shannon, L.A.; et al. G-Protein-Coupled Receptor Kinase 2 Limits CCL21-Induced T Cell Migration via Phospholipase Cγ1. Receptors 2025, 4, 17. https://doi.org/10.3390/receptors4030017
Sanchez A, Winebrenner CT, Garcia N, Kaiser B, Kilgore L, Cardona CI, Bassuk DW, Miller ME, Bill CA, Shannon LA, et al. G-Protein-Coupled Receptor Kinase 2 Limits CCL21-Induced T Cell Migration via Phospholipase Cγ1. Receptors. 2025; 4(3):17. https://doi.org/10.3390/receptors4030017
Chicago/Turabian StyleSanchez, Anahi, Caitlin T. Winebrenner, Natalia Garcia, Brian Kaiser, Lyndsey Kilgore, Cesar I. Cardona, Daniel W. Bassuk, Mary E. Miller, Charles A. Bill, Laura A. Shannon, and et al. 2025. "G-Protein-Coupled Receptor Kinase 2 Limits CCL21-Induced T Cell Migration via Phospholipase Cγ1" Receptors 4, no. 3: 17. https://doi.org/10.3390/receptors4030017
APA StyleSanchez, A., Winebrenner, C. T., Garcia, N., Kaiser, B., Kilgore, L., Cardona, C. I., Bassuk, D. W., Miller, M. E., Bill, C. A., Shannon, L. A., Wagener, B. M., Wagler, A., Llano, M., Bill, C. A., & Vines, C. M. (2025). G-Protein-Coupled Receptor Kinase 2 Limits CCL21-Induced T Cell Migration via Phospholipase Cγ1. Receptors, 4(3), 17. https://doi.org/10.3390/receptors4030017









