Regulation of Hippocampal GABAergic Transmission by Fluoxetine and Its Metabolite Norfluoxetine
Abstract
1. Introduction
2. Materials and Methods
2.1. Hippocampal Brain Slices
2.2. Single-Cell Electrophysiological Recordings
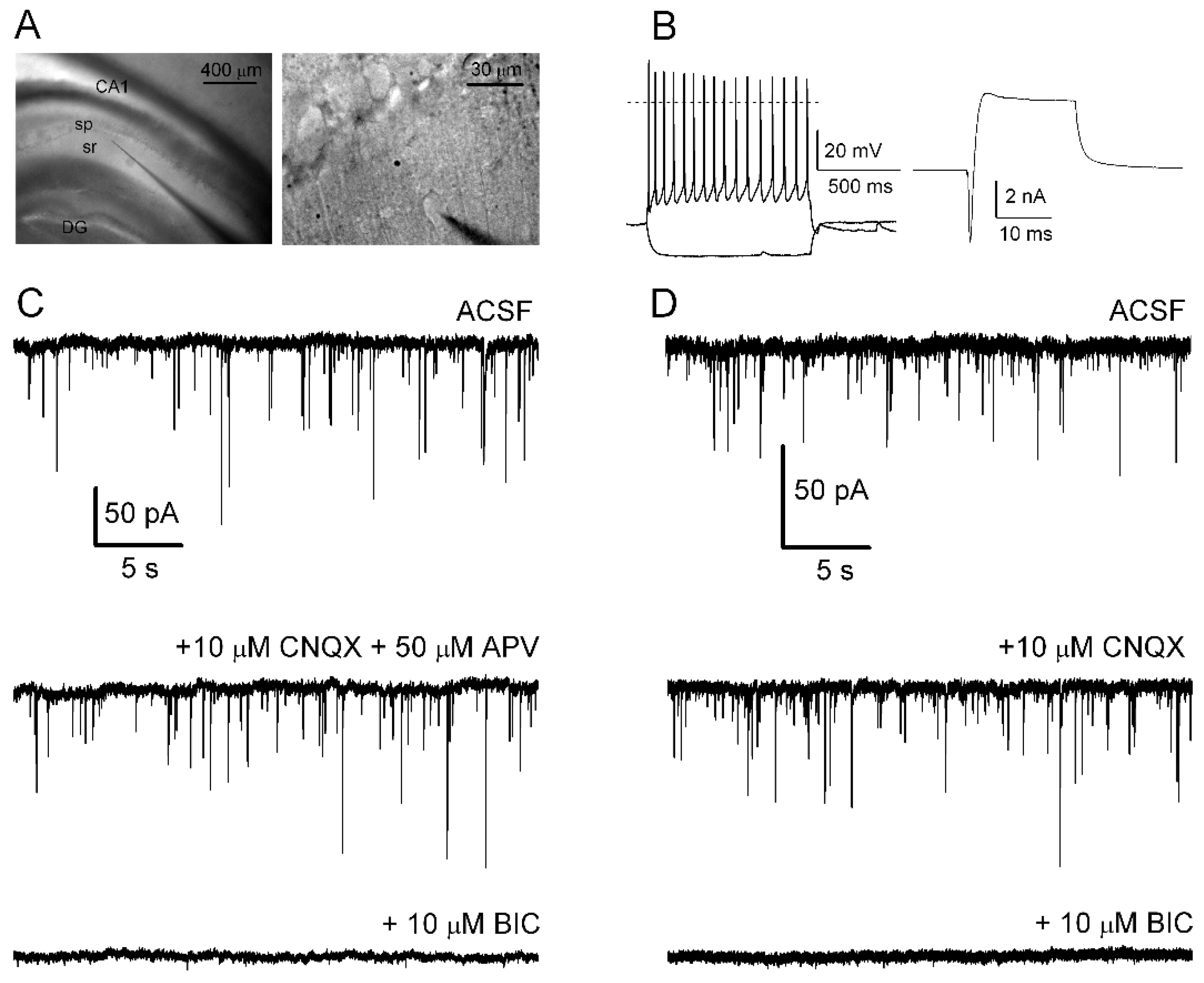
2.2.1. Recording of sIPSCs
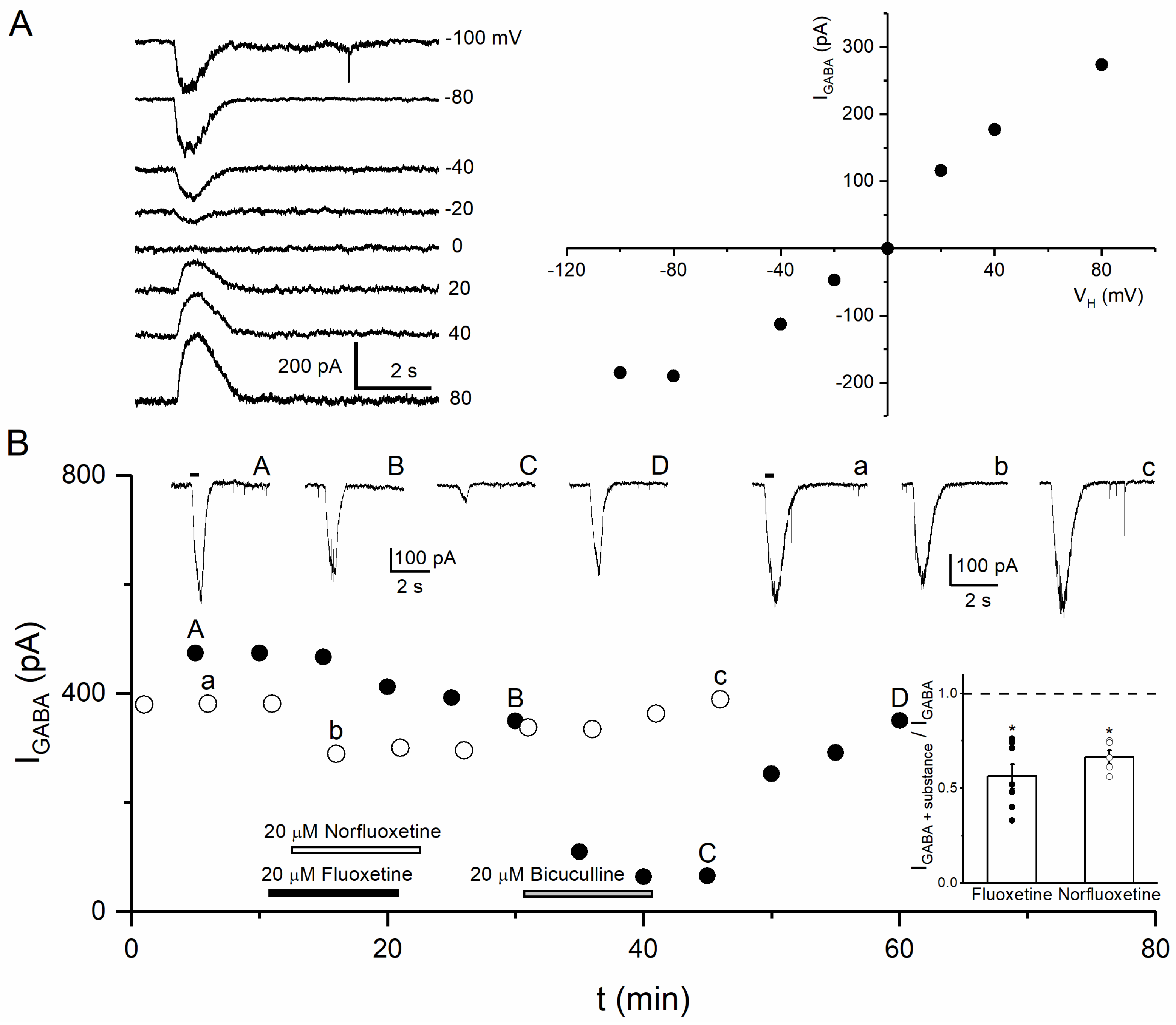
2.2.2. Recording of GABA-Induced Currents
2.3. Data Analysis and Statistics
3. Results
3.1. Spontaneous GABAergic Synaptic Activity in CA1 Interneurons
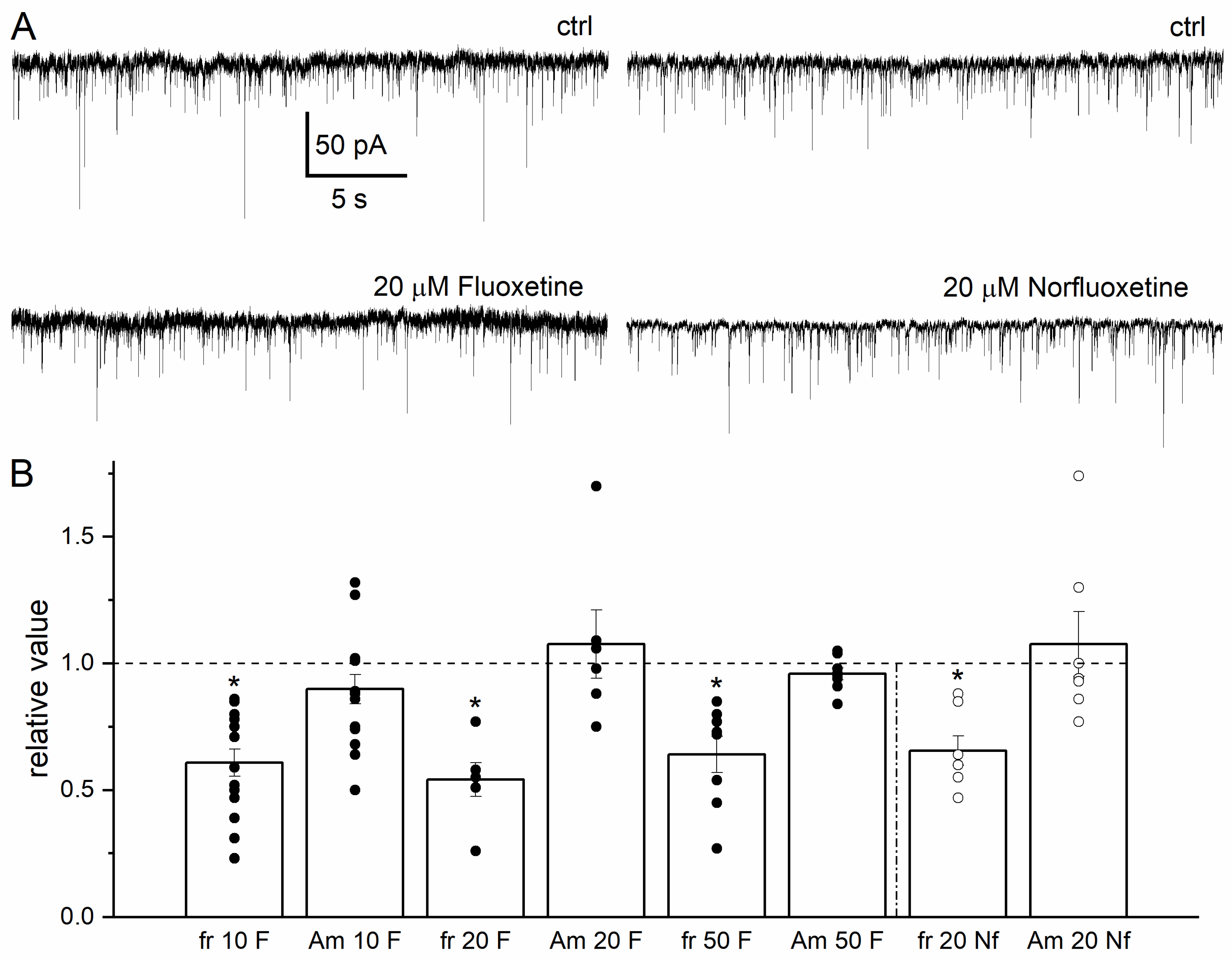
3.2. Effects of Fluoxetine and Norfluoxetine on sIPSCs in Hippocampal Interneurons
3.3. Effects of Fluoxetine and Norfluoxetine on GABA-Induced Currents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iascone, D.M.; Li, Y.; Sümbül, U.; Doron, M.; Chen, H.; Andreu, V.; Goudy, F.; Blockus, H.; Abbott, L.F.; Segev, I.; et al. Whole-neuron synaptic mapping reveals spatially precise excitatory/inhibitory balance limiting dendritic and somatic spiking. Neuron 2020, 106, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Selten, M.; Van Bokhoven, H.; Nadif Kasri, N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Research 2018, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Wang, H.L.; Sun, Y.X.; Liu, X.; Wang, H.; Ma, Y.N.; Su, Y.A.; Li, J.T.; Si, T.M. Adolescent stress increases depression-like behaviors and alters the excitatory-inhibitory balance in aged mice. Chin. Med. J. 2019, 132, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Katona, I. Perisomatic inhibition. Neuron 2007, 56, 33–42. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef] [PubMed]
- Udakis, M.; Pedrosa, V.; Chamberlain, S.E.L.; Clopath, C.; Mellor, J.R. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat. Commun. 2020, 11, 4395. [Google Scholar] [CrossRef]
- Bielau, H.; Steiner, J.; Mawrin, C.; Trübner, K.; Brisch, R.; Meyer-Lotz, G.; Brodhun, M.; Dobrowolny, H.; Baumann, B.; Gos, T.; et al. Dysregulation of GABAergic neurotransmission in mood disorders: A postmortem study. Ann. N. Y. Acad. Sci. 2007, 1096, 157–169. [Google Scholar] [CrossRef]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.C. Neural circuitry-neurogenesis coupling model of depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Chung, G.; Song, W.S.; Oh, S.P.; Kim, J.; Kim, S.J.; Kim, M.H. Altered GABAergic inhibition in CA1 pyramidal neurons modifies despair-like behavior in mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Czéh, B.; Vardya, I.; Varga, Z.; Febbraro, F.; Csabai, D.; Martis, L.S.; Højgaard, K.; Henningsen, K.; Bouzinova, E.V.; Miseta, A.; et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front. Cell. Neurosci. 2018, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Dósa, Z.; Nieto-Gonzalez, J.; Elfving, B.; Hougaard, K.; Holm, M.; Wegener, G.; Jensen, K. Reduction in hippocampal GABAergic transmission in a low birth weight rat model of depression. Acta Neuropsychiatr. 2023, 35, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef]
- Merali, Z.; Du, L.; Hrdina, P.; Palkovits, M.; Faludi, G.; Poulter, M.O.; Anisman, H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J. Neurosci. 2004, 24, 1478–1485. [Google Scholar] [CrossRef]
- Zhong, H.; Rong, J.; Zhu, C.; Liang, M.; Li, Y.; Zhou, R. Epigenetic modifications of GABAergic interneurons contribute to deficits in adult hippocampus neurogenesis and depression-like behavior in prenatally stressed mice. Int. J. Neuro-Psychopharmacol. 2020, 23, 274–285. [Google Scholar] [CrossRef]
- Judge, S.J.; Ingram, C.D.; Gartside, S.E. GABA receptor modulation of 5-HT neuronal firing: Characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem. Int. 2004, 45, 1057–1065. [Google Scholar] [CrossRef]
- Lladó-Pelfort, L.; Santana, N.; Ghisi, V.; Artigas, F.; Celada, P. 5-HT1A receptor gonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb. Cortex. 2012, 22, 1487–1497. [Google Scholar] [CrossRef]
- Filipović, D.; Stanisavljević, A.; Jasnić, N.; Bernardi, R.E.; Inta, D.; Perić, I.; Gass, P. Chronic treatment with fluoxetine or clozapine of socially isolated rats prevents subsector-specific reduction of parvalbumin immunoreactive cells in the hippocampus. Neuroscience 2018, 371, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Todorović, N.; Mićić, B.; Schwirtlich, M.; Stevanović, M.; Filipović, D. Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience 2019, 396, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Costa, E.; Guidotti, A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology 2006, 186, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Maya Vetencourt, J.F.; Sale, A.; Viegi, A.; Baroncelli, L.; De, P.R.; O’Leary, O.F.; Castren, E.; Maffei, L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008, 320, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Méndez, P.; Pazienti, A.; Szabó, G.; Bacci, A. Direct alteration of a specific inhibitory circuit of the hippocampus by antidepressants. J. Neurosci. 2013, 32, 16616–16628. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Yan, Z. Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLoS ONE 2011, 6, e16970. [Google Scholar] [CrossRef] [PubMed]
- Hannan, S.; Minere, M.; Harris, J.; Izquierdo, P.; Thomas, P.; Tench, B.; Smart, T.G. GABAAR isoform and subunit structural motifs determine synaptic and extrasynaptic receptor localisation. Neuropharmacology 2020, 169, 107540. [Google Scholar] [CrossRef] [PubMed]
- Kasaragod, V.B.; Mortensen, M.; Hardwick, S.W.; Wahid, A.A.; Dorovykh, V.; Chirgadze, D.Y.; Smart, T.G.; Miller, P.S. Mechanisms of inhibition and activation of extrasynaptic alpha beta GABAA receptors. Nature 2022, 602, 529–533. [Google Scholar] [CrossRef]
- Luscher, B.; Maguire, J.L.; Rudolph, U.; Sibille, E. GABAA receptors as targets for treating affective and cognitive symptoms of depression. Trends Pharmacol. Sci. 2023, 44, 586–600. [Google Scholar] [CrossRef]
- Thompson, S.M. Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: Past, present, and future. Neuropharmacology 2023, 49, 83–95. [Google Scholar] [CrossRef]
- Bampali, K.; Koniuszewski, F.; Silva, L.L.; Rehman, S.; Vogel, F.D.; Seidel, T.; Scholze, P.; Zirpel, F.; Garon, A.; Langer, T.; et al. Tricyclic antipsychotics and antidepressants can inhibit α5-containing GABAA receptors by two distinct mechanisms. Br. J. Pharmacol. 2022, 179, 3675–3692. [Google Scholar] [CrossRef] [PubMed]
- Goldschen-Ohm, M.P. Benzodiazepine modulation of GABAA receptors: A mechanistic perspective. Biomolecules 2022, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Balle, T. GABAA receptors as targets for anaesthetics and analgesics and promising candidates to help treat coronavirus infections: A mini-review. Basic Clin. Pharmacol. Toxicol. 2022, 131, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.T.; Drafts, B.C.; Fisher, J.L. Fluoxetine increases GABA(A) receptor activity through a novel modulatory site. J. Pharmacol. Exp. Ther. 2003, 304, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.M.; Reddy, D.S. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 2013, 230, 151–188. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Mbilinyi, R.H.; Estes, E. Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: A landmark journey from concept to clinic in neurosteroid replacement therapy. Psychopharmacology 2023, 240, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Barygin, O.I.; Nagaeva, E.I.; Tikhonov, D.B.; Belinskaya, D.A.; Vanchakova, N.P.; Shestakova, N.N. Inhibition of the NMDA and AMPA receptor channels by antidepressants and antipsychotics. Brain Res. 2017, 1660, 58–66. [Google Scholar] [CrossRef]
- Heinrich, I.A.; Freitas, A.E.; Wolin, I.A.V.; Nascimento, A.P.M.; Walz, R.; Rodrigues, A.L.S.; Leal, R.B. Neuronal activity regulated pentraxin (narp) and GluA4 subunit of AMPA receptor may be targets for fluoxetine modulation. Metab. Brain. Dis. 2021, 36, 711–722. [Google Scholar] [CrossRef]
- Duarte, Y.; Rojas, M.; Canan, J.; Pérez, E.G.; González-Nilo, F.; García-Colunga, J. Different classes of antidepressants inhibit the rat α7 nicotinic acetylcholine receptor by interacting within the ion channel: A functional and structural study. Molecules 2021, 26, 998. [Google Scholar] [CrossRef]
- García-Colunga, J.; Vázquez-Gómez, E.; Miledi, R. Combined actions of zinc and fluoxetine on nicotinic acetylcholine receptors. Pharmacogenomics J. 2004, 4, 388–393. [Google Scholar] [CrossRef]
- López-Valdés, H.E.; García-Colunga, J. Antagonism of nicotinic acetylcholine receptors by inhibitors of monoamine uptake. Mol. Psychiatry 2001, 6, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Deák, F.; Lasztóczi, B.; Pacher, P.; Petheö, G.L.; Kecskeméti, V.; Spät, A. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology 2000, 39, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Traboulsie, A.; Chemin, J.; Kupfer, E.; Nargeot, J.; Lory, P. T-type calcium channels are inhibited by fluoxetine and its metabolite norfluoxetine. Mol. Pharmacol. 2006, 69, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Abrego, A.; Vázquez-Gómez, E.; García-Colunga, J. Effects of the antidepressant mirtazapine and zinc on nicotinic acetylcholine receptors. Neurosci. Lett. 2018, 665, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- López, J.J.; Pérez, E.G.; García-Colunga, J. Dual effects of a 2-benzylquinuclidinium derivative on alpha-7-containing nicotinic acetylcholine receptors in rat hippocampal interneurons. Neurosci. Lett. 2015, 607, 35–39. [Google Scholar] [CrossRef]
- Vázquez-Gómez, E.; Arias, H.R.; Feuerbach, D.; Miranda-Morales, M.; Mihailescu, S.; Targowska-Duda, K.M.; Jozwiak, K.; García-Colunga, J. Bupropion-induced inhibition of alpha-7 nicotinic acetylcholine receptors expressed in heterologous cells and neurons from dorsal raphe nucleus and hippocampus. Eur. J. Pharmacol. 2014, 740, 103–111. [Google Scholar] [CrossRef]
- Singh, S.; Topolnik, L. Inhibitory circuits in fear memory and fear-related disorders. Front. Neural Circuits 2023, 17, 1122314. [Google Scholar] [CrossRef]
- Tzilivaki, A.; Tukker, J.J.; Maier, N.; Poirazi, P.; Sammons, R.P.; Schmitz, D. Hippocampal GABAergic interneurons and memory. Neuron 2023, 111, 3154–3175. [Google Scholar] [CrossRef]
- Hu, Y.T.; Tan, Z.L.; Hirjak, D.; Northoff, G. Brain-wide changes in excitation-inhibition balance of major depressive disorder: A systematic review of topographic patterns of GABA- and glutamatergic alterations. Mol. Psychiatry 2023, 28, 3257–3266. [Google Scholar] [CrossRef]
- Carceller, H.; Perez-Rando, M.; Castren, E.; Nacher, J.; Guirado, R. Effects of the antidepressant fluoxetine on the somatostatin interneurons in the basolateral amygdala. Neuroscience 2018, 386, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Mantas, I.; Flais, I.; Svenningsson, P. Fluoxetine suppresses glutamate- and GABA-mediated neurotransmission by altering SNARE complex. Int. J. Mol. Sci. 2019, 20, 4247. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Aluisio, L.; Lord, B.; Boggs, J.; Hoey, K.; Mazur, C.; Lovenberg, T. Pharmacokinetics and pharmacodynamics of norfluoxetine in rats: Increasing extracellular serotonin level in the frontal cortex. Pharmacol. Biochem. Behav. 2009, 92, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodkhani, M.; Ghasemi, M.; Derafshpour, L.; Amini, M.; Mehranfard, N. Long-term decreases in the expression of calcineurin and GABAA receptors induced by early maternal separation are associated with increased anxiety-like behavior in adult male rats. Dev. Neurosci. 2020, 42, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Derry, J.M.; Paulsen, I.M.; Davies, M.; Dunn, S.M. A single point mutation of the GABA(A) receptor alpha5-subunit confers fluoxetine sensitivity. Neuropharmacology 2007, 52, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Tunnicliff, G.; Schindler, N.L.; Crites, G.J.; Goldenberg, R.; Yochum, A.; Malatynska, E. The GABA(A) receptor complex as a target for fluoxetine action. Neurochem. Res. 1999, 24, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Caiati, M.D.; Cherubini, E. Fluoxetine impairs GABAergic signaling in hippocampal slices from neonatal rats. Front. Cell. Neurosci. 2013, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Shannonhouse, J.L.; DuBois, D.W.; Fincher, A.S.; Vela, A.M.; Henry, M.M.; Wellman, P.J.; Frye, G.D.; Morgan, C. Fluoxetine disrupts motivation and GABAergic signaling in adolescent female hamsters. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 19–30. [Google Scholar] [CrossRef]
- Ye, Z.Y.; Zhou, K.Q.; Xu, T.L.; Zhou, J.N. Fluoxetine potentiates GABAergic IPSCs in rat hippocampal neurons. Neurosci. Lett. 2008, 442, 24–29. [Google Scholar] [CrossRef]
- Fuchs, T.; Jefferson, S.J.; Hooper, A.; Yee, P.H.; Maguire, J.; Luscher, B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatry 2017, 22, 920–930. [Google Scholar] [CrossRef]
- Buhler, A.V.; Dunwiddie, T.V. Alpha7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J. Neurophysiol. 2002, 87, 548–557. [Google Scholar] [CrossRef]
- Massey, K.A.; Zago, W.M.; Berg, D.K. BDNF up-regulates alpha7 nicotinic acetylcholine receptor levels on subpopulations of hippocampal interneurons. Mol. Cell Neurosci. 2006, 33, 381–388. [Google Scholar] [CrossRef]
- Kennard, L.E.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.L.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef]
- Proks, P.; Schewe, M.; Conrad, L.J.; Rao, S.; Rathje, K.; Rödström, K.E.J.; Carpenter, E.P.; Baukrowitz, T.; Tucker, S.J. Norfluoxetine inhibits TREK-2 K2P channels by multiple mechanisms including state-independent effects on the selectivity filter gate. J. Gen. Physiol. 2021, 153, e202012812. [Google Scholar] [CrossRef]
- Hervieu, G.J.; Cluderay, J.E.; Gray, C.W.; Green, P.J.; Ranson, J.L.; Randall, A.D.; Meadows, H.J. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 2001, 103, 899–919. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwon, O.S.; Hur, C.G.; Nyiramana, M.M.; Lee, D.K.; Hong, S.G.; Han, J.; Kang, D. Muscimol directly activates the TREK-2 channel expressed in GABAergic neurons through its N-terminus. Int. J. Mol. Sci. 2021, 22, 9320. [Google Scholar] [CrossRef]
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Roles of TREK-1 in health and disease, focus on the central nervous system. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Noël, J.; Sandoz, G.; Lesage, F. Molecular regulations governing TREK and TRAAK channel functions. Channels 2011, 5, 402–409. [Google Scholar] [CrossRef]
- Pirker, S.; Schwarzer, C.; Wieselthaler, A.; Sieghart, W.; Sperk, G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 2000, 101, 815–850. [Google Scholar] [CrossRef]
- Sperk, G.; Schwarzer, C.; Tsunashima, K.; Fuchs, K.; Sieghart, W. GABA(A) receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience 1997, 80, 987–1000. [Google Scholar] [CrossRef]
- Wisden, W.; Laurie, D.J.; Monyer, H.; Seeburg, P.H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992, 12, 1040–1062. [Google Scholar] [CrossRef]
- Matsumoto, K.; Puia, G.; Dong, E.; Pinna, G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: Possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress 2007, 10, 3–12. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, K.; Ishima, T.; Ren, Q.; Chang, L.; Chen, J.; Hashimoto, K. Comparison of rapid and long-lasting antidepressant effects of negative modulators of α5-containing GABAA receptors and (R)-ketamine in a chronic social defeat stress model. Pharmacol. Biochem. Behav. 2018, 175, 139–145. [Google Scholar] [CrossRef]
- Brunswick, D.J.; Amsterdam, J.D.; Fawcett, J.; Quitkin, F.M.; Reimherr, F.W.; Rosenbaum, J.F.; Beasley, C.M. Fluoxetine and norfluoxetine plasma levels after discontinuing fluoxetine therapy. J. Clin. Psychopharmacol. 2001, 21, 616–618. [Google Scholar] [CrossRef]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the mechanism of fluoxetine-mediated CYP2D6 inhibition. Pharmaceutics 2021, 13, 148. [Google Scholar] [CrossRef]
- Sagahón-Azúa, J.; Medellín-Garibay, S.E.; Chávez-Castillo, C.E.; González-Salinas, C.G.; Milán-Segovia, R.D.C.; Romano-Moreno, S. Factors associated with fluoxetine and norfluoxetine plasma concentrations and clinical response in Mexican patients with mental disorders. Pharmacol. Res. Perspect. 2021, 9, e00864. [Google Scholar] [CrossRef]
- Blumberg, M.J.; Vaccarino, S.R.; McInerney, S.J. Procognitive effects of antidepressants and other therapeutic agents in major depressive disorder: A systematic review. J. Clin. Psychiatry 2020, 81, 19r13200. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Matcham, F.; Dauchy, S.; Barbui, C.; Hotopf, M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst. Rev. 2018, 4, CD011006. [Google Scholar] [CrossRef]
- An, H.; Fang, J.; Wang, M.; Lin, H.; Sun, Y.; Hu, B.; He, Z.; Ge, Z.; Wei, Y. Stereoselective study of fluoxetine and norfluoxetine across the blood-brain barrier mediated by organic cation transporter 1/3 in rats using an enantioselective UPLC-MS/MS method. Chirality 2023, 35, 983–992. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Y.; Zhang, X.; Lu, G. The metabolites could not be ignored: A comparative study of the metabolite norfluoxetine with its parent fluoxetine on zebrafish (Danio rerio). Aquat. Toxicol. 2023, 257, 106467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Gómez, E.; Hernández-Abrego, A.; Mejía-Piedras, J.; García-Colunga, J. Regulation of Hippocampal GABAergic Transmission by Fluoxetine and Its Metabolite Norfluoxetine. Receptors 2024, 3, 1-12. https://doi.org/10.3390/receptors3010001
Vázquez-Gómez E, Hernández-Abrego A, Mejía-Piedras J, García-Colunga J. Regulation of Hippocampal GABAergic Transmission by Fluoxetine and Its Metabolite Norfluoxetine. Receptors. 2024; 3(1):1-12. https://doi.org/10.3390/receptors3010001
Chicago/Turabian StyleVázquez-Gómez, Elizabeth, Andy Hernández-Abrego, Jassiel Mejía-Piedras, and Jesús García-Colunga. 2024. "Regulation of Hippocampal GABAergic Transmission by Fluoxetine and Its Metabolite Norfluoxetine" Receptors 3, no. 1: 1-12. https://doi.org/10.3390/receptors3010001
APA StyleVázquez-Gómez, E., Hernández-Abrego, A., Mejía-Piedras, J., & García-Colunga, J. (2024). Regulation of Hippocampal GABAergic Transmission by Fluoxetine and Its Metabolite Norfluoxetine. Receptors, 3(1), 1-12. https://doi.org/10.3390/receptors3010001







