Salt Substitutes in Low-Income Settings: Blood Pressure Benefits, Cardiovascular Outcomes, and Safety Considerations: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Review
3.1. Blood Pressure–Lowering Effect of Salt Substitutes
Population-Specific Findings
3.2. Cardiovascular Outcomes
Limitations in LMICs
3.3. Major Cardiovascular Outcome Trials on Salt Substitutes
3.4. Safety Concerns: Focus on Hyperkalemia
3.4.1. Who Is at Risk: CKD, HF, RAAS Inhibitors
3.4.2. Data from Trials (Incidence and Severity of Adverse Effects)
3.4.3. Importance of Population Screening and Education
3.5. Safety of Potassium-Substituted Salts (Hyperkalemia and At-Risk Groups)
3.6. Implementation in Low-Income Settings
3.6.1. Acceptability (Taste, Habits, Marketing)
3.6.2. Lack of Regulatory Oversight and Safety Monitoring
3.6.3. Case Studies: China, Sri Lanka, and Lessons Learned
3.6.4. Equity and Feasibility Concerns
3.7. Implementation Strategies and Partnerships in Low-Income Settings
4. Discussion
4.1. Safety Challenges and Mitigation Strategies
4.2. Policy and Public Health Recommendations
4.3. LMIC-Specific Barriers and Practical Approaches
4.4. Conflicts of Interest and Transparency
4.5. Limitations
4.6. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE inhibitors | Angiotensin-Converting Enzyme Inhibitors |
| ARB | Angiotensin II Receptor Blocker |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CKD | Chronic Kidney Disease |
| CV | Cardiovascular |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| DKA | Diabetic Ketoacidosis |
| DM | Diabetes Mellitus |
| g | Gram |
| HF | Heart Failure |
| ICD | International Classification of Diseases |
| KCl | Potassium Chloride |
| Kg | Kilogram |
| LMICs | Low- and Middle-Income Countries |
| LSSS | Low-Sodium Salt Substitutes |
| MgSO4 | Magnesium Sulfate |
| mmHg | Millimeters of Mercury |
| NaCl | Sodium Chloride |
| Na+ | Sodium Ion |
| QALY | Quality-Adjusted Life Year |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RCT | Randomized Controlled Trial |
| SBP | Systolic Blood Pressure |
| SSA | Salt Substitute Adoption |
| SSaSS | Salt Substitute and Stroke Study |
| UN | United Nations |
| USA | United States of America |
| WHO | World Health Organization |
References
- Hypertension. Available online: https://perma.cc/L7TJ-8YP7 (accessed on 27 May 2025).
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer. Available online: https://www.who.int/publications/i/item/9789240081062 (accessed on 27 May 2025).
- Tsai, Y.; Tsao, Y.; Huang, C.; Tai, Y.; Su, Y.; Chiang, C.; Sung, S.; Chen, C.; Cheng, H. Effectiveness of salt substitute on cardiovascular outcomes: A systematic review and meta-analysis. J. Clin. Hypertens. 2022, 24, 1147–1160. [Google Scholar] [CrossRef]
- World Health Organization. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 27 May 2025).
- Ghimire, K.; Mishra, S.R.; Satheesh, G.; Neupane, D.; Sharma, A.; Panda, R.; Kallestrup, P.; Mclachlan, C.S. Salt intake and salt-reduction strategies in South Asia: From evidence to action. J. Clin. Hypertens. 2021, 23, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, L.; Jha, V.; Cobb, L.K.; Shibuya, K.; Appel, L.J.; Neal, B.; Schutte, A.E. Potassium-enriched salt substitutes: A review of recommendations in clinical management guidelines. Hypertension 2024, 81, 400–414. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sodium Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/sodium-reduction (accessed on 27 May 2025).
- Centers for Disease Control and Prevention. About Sodium and Health. Available online: https://www.cdc.gov/salt/about/index.html (accessed on 27 May 2025).
- United States Department of Agriculture. Dietary Guidelines for Americans 2020–2025; USDA: Washington, DC, USA, 2020.
- Food and Drug Administration. Sodium in Your Diet; FDA: Silver Spring, MD, USA, 2020.
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of salt substitution on cardiovascular events and death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Sodium Intake Reduction. Available online: https://www.who.int/publications/i/item/9789240069985 (accessed on 27 May 2025).
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary sodium and cardiovascular disease risk—Measurement matters. N. Engl. J. Med. 2016, 375, 580–586. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Brand, A.; Visser, M.E.; Schoonees, A.; Naude, C.E. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst. Rev. 2022, 8, CD015207. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Y.; Li, C.; Feng, X.; Wang, H.; Qiao, Q.; Zhang, R.; Jin, A.; Li, J.; Li, H.; et al. Effect of a salt substitute on incidence of hypertension and hypotension among normotensive adults. J. Am. Coll. Cardiol. 2024, 83, 711–722. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Witteman, J.C.M.; Bak, A.A.A.; den Breijen, J.H.; Grobbee, D.E. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ 1994, 309, 436–440. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Effect of modest salt reduction on blood pressure: A meta-analysis of randomized trials. Implications for public health. J. Hum. Hypertens. 2002, 16, 761–770. [Google Scholar] [CrossRef]
- Greer, R.C.; Marklund, M.; Anderson, C.A.M.; Cobb, L.K.; Dalcin, A.T.; Henry, M.; Appel, L.J. Potassium-enriched salt substitutes as a means to lower blood pressure. Hypertension 2020, 75, 266–274. [Google Scholar] [CrossRef]
- Yu, J.; Thout, S.R.; Li, Q.; Tian, M.; Marklund, M.; Arnott, C.; Huffman, M.D.; Praveen, D.; Johnson, C.; Huang, L.; et al. Effects of a reduced-sodium added-potassium salt substitute on blood pressure in rural Indian hypertensive patients: A randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2021, 114, 185–193. [Google Scholar] [CrossRef]
- Peng, Y.-G.; Li, W.; Wen, X.-X.; Li, Y.; Hu, J.-H.; Zhao, L.-C. Effects of salt substitutes on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jin, A.; Neal, B.; Feng, X.; Qiao, Q.; Wang, H.; Zhang, R.; Li, J.; Duan, P.; Cao, L.; et al. Salt substitution and salt-supply restriction for lowering blood pressure in elderly care facilities: A cluster-randomized trial. Nat. Med. 2023, 29, 973–981. [Google Scholar] [CrossRef]
- Bernabe-Ortiz, A.; Sal y Rosas, V.G.; Ponce-Lucero, V.; Cárdenas, M.K.; Carrillo-Larco, R.M.; Diez-Canseco, F.; Pesantes, M.A.; Sacksteder, K.A.; Gilman, R.H.; Miranda, J.J. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat. Med. 2020, 26, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, H.-L.; Wang, W.-L.; Wu, X.-M.; Fu, L.-Y.; Shi, J.-P. Long-term effects of salt substitution on blood pressure in a rural north Chinese population. J. Hum. Hypertens. 2012, 27, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-C.; Huang, L.; Tian, M.; Di Tanna, G.L.; Yu, J.; Zhang, X.; Yin, X.; Liu, Y.; Hao, Z.; Zhou, B.; et al. Cost-effectiveness of a household salt substitution intervention: Findings from 20,995 participants of the Salt Substitute and Stroke Study. Circulation 2022, 145, 1534–1541. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.; Wu, J.H.; Tian, M.; Yin, X.; Yu, J.; Liu, Y.; Zhang, X.; Wu, Y.; Paige, E.; et al. The Contribution of Sodium Reduction and Potassium Increase to the Blood Pressure Lowering Observed in the Salt Substitute and Stroke Study. J. Hum. Hypertens. 2024, 38, 1–9. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Hu, Y.-W.; Yue, C.-S.J.; Wen, Y.-W.; Yeh, W.-T.; Hsu, L.-S.; Tsai, S.-Y.; Pan, W.-H. Effect of Potassium-Enriched Salt on Cardiovascular Mortality and Medical Expenses of Elderly Men. Am. J. Clin. Nutr. 2006, 83, 1289–1296. [Google Scholar] [CrossRef]
- Lai, X.; Yuan, Y.; Wang, H.; Zhang, R.; Qiao, Q.; Feng, X.; Jin, A.; Li, H.; Li, J.; Si, L.; et al. Cost-Effectiveness of Salt Substitute and Salt Supply Restriction in Eldercare Facilities. JAMA. Netw. Open 2024, 7, e2355564. [Google Scholar] [CrossRef]
- Simon, L.V.; Farrell, M.W.; Hashmi, M.F. Hyperkalemia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470284/ (accessed on 27 May 2025).
- Sarnowski, A.; Gama, R.M.; Dawson, A.; Mason, H.; Banerjee, D. Hyperkalemia in chronic kidney disease: Links, risks and management. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 215–228. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Aeddula, N.R. Physiology, Sodium Potassium Pump (Na+ K+ Pump). Available online: https://www.ncbi.nlm.nih.gov/books/NBK537088/ (accessed on 27 May 2025).
- Doorenbos, C.J.; Vermeij, C.G. Danger of salt substitutes that contain potassium in patients with renal failure. BMJ 2003, 326, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, H.; Webster, J.; Trieu, K.; Huffman, M.D.; Miranda, J.J.; Marklund, M.; Wu, J.H.Y.; Cobb, L.K.; Li, K.C.; et al. Availability, formulation, labeling, and price of low-sodium salt worldwide: Environmental scan. JMIR Public Health Surveill. 2021, 7, e27423. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kid. Inter. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Yin, X.; Rodgers, A.; Perkovic, A.; Huang, L.; Li, K.-C.; Yu, J.; Wu, Y.; Wu, J.H.Y.; Marklund, M.; Huffman, M.D.; et al. Effects of Salt Substitutes on Clinical Outcomes: A Systematic Review and Meta-Analysis. Heart 2022, 108, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- NICE. Hypertension in Adults: Diagnosis and Hypertension in Adults: Diagnosis and Management Management NICE Guideline; NICE: London, UK, 2019.
- Chang, A.Y.; Rahman, M.; Talukder, A.; Shah, H.; Mridha, M.K.; Hasan, M.T.; Sarker, M.; Geldsetzer, P. Effectiveness of a community health worker-led low-sodium salt intervention to reduce blood pressure in rural Bangladesh: Protocol for a cluster randomized controlled trial. Trials 2023, 24, 480. [Google Scholar] [CrossRef]
- Aminde, L.N.; Nugraheni, W.P.; Mubasyiroh, R.; Rachmawati, T.; Dwirahmadi, F.; Martini, S.; Kusumawardani, N.; Veerman, J.L. Cost-effectiveness analysis of low-sodium potassium-rich salt substitutes in Indonesia: An equity modelling study. Lancet Reg. Health Southeast Asia 2024, 26, 100432. [Google Scholar] [CrossRef]
- Hendriksen, M.A.H.; van Raaij, J.M.A.; Geleijnse, J.M.; Breda, J.; Boshuizen, H.C. Health Gain by Salt Reduction in Europe: A Modelling Study. PLoS ONE 2015, 10, e0118873. [Google Scholar] [CrossRef]
- Alonso, S.; Tan, M.; Wang, C.; Kent, S.; Cobiac, L.; MacGregor, G.A.; He, F.J.; Mihaylova, B. Impact of the 2003 to 2018 Population Salt Intake Reduction Program in England. Hypertension 2021, 77, 1086–1094. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Reducing Population Salt Intake-Time for Global Action. J. Clin. Hypertens. 2014, 17, 10–13. [Google Scholar] [CrossRef]
- Finland—World Action on Salt. Sugar & Health. Available online: https://www.worldactiononsalt.com/worldaction/europe/finland/ (accessed on 10 June 2025).
- Shivashankar, R.; Sharma, M.; Sharma, M.; Bhardwaj, S.; Ide, N.; Cobb, L.; Bhargava, B. India’s Tryst with Salt: Dandi March to Low Sodium Salts. Indian J. Med. Res. 2023, 158, 233. [Google Scholar] [CrossRef]
- Action on Salt—Action on Salt. Available online: https://www.actiononsalt.org.uk/ (accessed on 10 June 2025).
- Saavedra-Garcia, L.; Bernabe-Ortiz, A.; Gilman, R.H.; Diez-Canseco, F.; Cárdenas, M.K.; Sacksteder, K.A.; Miranda, J.J. Applying the triangle taste test to assess differences between low sodium salts and common salt: Evidence from Peru. PLoS ONE 2015, 10, e0134700. [Google Scholar] [CrossRef]
- Bullen, J.; Yin, X.; Kissock, K.; Fisher, L.; Neal, B.; Trieu, K. Health claims, product features and instructions for use on the labels of potassium-enriched salt products: A content analysis. Curr. Dev. Nutr. 2024, 8, 104473. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Ajenikoko, A.; Steele, L.; Cohn, J.; Curtis, C.J.; Frieden, T.R.; Cobb, L.K. Priority actions to advance population sodium reduction. Nutrients 2020, 12, 2543. [Google Scholar] [CrossRef] [PubMed]
- Rigutto-Farebrother, J.; Zimmermann, M.B. Salt reduction and iodine fortification policies are compatible: Perspectives for public health advocacy. Nutrients 2024, 16, 2517. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, Y.; Wang, M.; Liu, J.; Bu, X.; Mu, J.; Lu, J. Global cardiovascular diseases burden attributable to high sodium intake from 1990 to 2019. J. Clin. Hypertens. 2023, 25, 868–879. [Google Scholar] [CrossRef]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic status and cardiovascular outcomes: Challenges and interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef]
- Meng, D.; Cobb, L.K.; Ide, N.; Ge, Z. The availability, price, and characteristics of low sodium salt based on an online salt market survey in China: Implications for scaling up its use. J. Clin. Hypertens. 2023, 25, 1079–1085. [Google Scholar] [CrossRef]
- Lazo-Porras, M.; Del Valle, A.; Beran, D.; Pesantes, M.A.; Perez-Leon, S.; Ponce-Lucero, V.; Bernabe-Ortiz, A.; Cárdenas, M.K.; Chappuis, F.; Perel, P.; et al. Implementation of a salt substitute intervention using social marketing in resourced-limited communities in peru: A process evaluation study. Front. Public Health 2023, 11, 1068624. [Google Scholar] [CrossRef]
- Formative Research to Inform a Behaviour Change Salt Reduction Intervention in India (SRII) Project. Who Salt Reduction. Available online: https://www.whoccsaltreduction.org/formative-research-to-inform-a-behaviour-change-salt-reduction-intervention-in-india-srii-project/ (accessed on 16 August 2025).
- Sehgal, R.; Venkateshmurthy, N.S.; Khatkar, R.; Konkati, S.P.; Jarhyan, P.; Sharma, M.; Ide, N.; Prabhakaran, D.; Mohan, S. Awareness and Availability of Low Sodium Iodized Salt: Results from Formative Research of Promoting Uptake of Low Sodium Iodized Salt by Rural and Urban Households in India—The PLURAL Study. Nutrients 2023, 16, 130. [Google Scholar] [CrossRef]
- Hu, N.; McLean, R. Targeted Approaches: Choosing Sodium Reduction Methods Based on Salt Usage Habits. Nutrients 2024, 16, 2816. [Google Scholar] [CrossRef] [PubMed]
- Kissock, K.R.; Garrett, G.S.; Mkambula, P.; Bullen, J.D.; Trieu, K.; Fisher, L.J.; Paige, E.; Gary, M.S.; Neal, B. Switching the World’s Salt Supply—Learning from Iodization to Achieve Potassium Enrichment. Adv. Nutr. 2024, 15, 100148. [Google Scholar] [CrossRef]
- Wei, F.; Liang, C.; Lin, X. Individualized Program with Iodine Supplementation. Adv. Nutr. 2024, 15, 100167. [Google Scholar] [CrossRef]
- Peru Salt Substitute Study Illustrates NHLBI’s Commitment to Global Health Research. Fogarty International Center @ NIH. Available online: https://www.fic.nih.gov/News/GlobalHealthMatters/january-february-2025/Pages/peru-salt-substitute-study.aspx (accessed on 16 August 2025).
- Liu, Y.; Chu, H.; Peng, K.; Yin, X.; Huang, L.; Wu, Y.; Pearson, S.-A.; Li, N.; Elliott, P.; Yan, L.L.; et al. Factors Associated with the Use of a Salt Substitute in Rural China. JAMA Netw. Open 2021, 4, e2137745. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Yang, S.; Long, J.; Tang, Y.; Liu, Y.; Ge, Z.; Rong, S.; Wu, Y.; Ding, G.; Yang, Y.; et al. National initiatives on salt substitutes: A scoping review. JMIR Public Health Surveill. 2023, 9, e45266. [Google Scholar] [CrossRef]
- Salt Substitutes: A Healthy Alternative to the Real Thing? Available online: https://www.uhhospitals.org/blog/articles/2023/06/salt-substitutes-a-healthy-alternative-to-the-real-thing (accessed on 27 May 2025).
- Costello, R.; Rosanoff, A.; Nielsen, F.; West, C. Perspective: Call for re-evaluation of the tolerable upper intake level for magnesium supplementation in adults. Adv. Nutr. 2023, 14, 973–982. [Google Scholar] [CrossRef]
- Wani, M.M.; Rather, J.I. Salt substitutes—Utility and safety in chronic kidney disease. J. Ren. Nutr. Metab. 2023, 8, 35–38. [Google Scholar] [CrossRef]
- Walker, M.S.; Tarasiuk, F.S.; Gustavo, A.S.; Oliveira, M.S.; Donadio, M.V.F.; Feoli, A.M.P. Lifestyle improvement reduces the consumption of ultra-processed foods in adults with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1990–1997. [Google Scholar] [CrossRef]
- Dogbe, W.; Akaichi, F.; Rungapamestry, V.; Revoredo-Giha, C. Effectiveness of implemented global dietary interventions: A scoping review of fiscal policies. BMC Public Health 2024, 24, 1–27. [Google Scholar] [CrossRef] [PubMed]
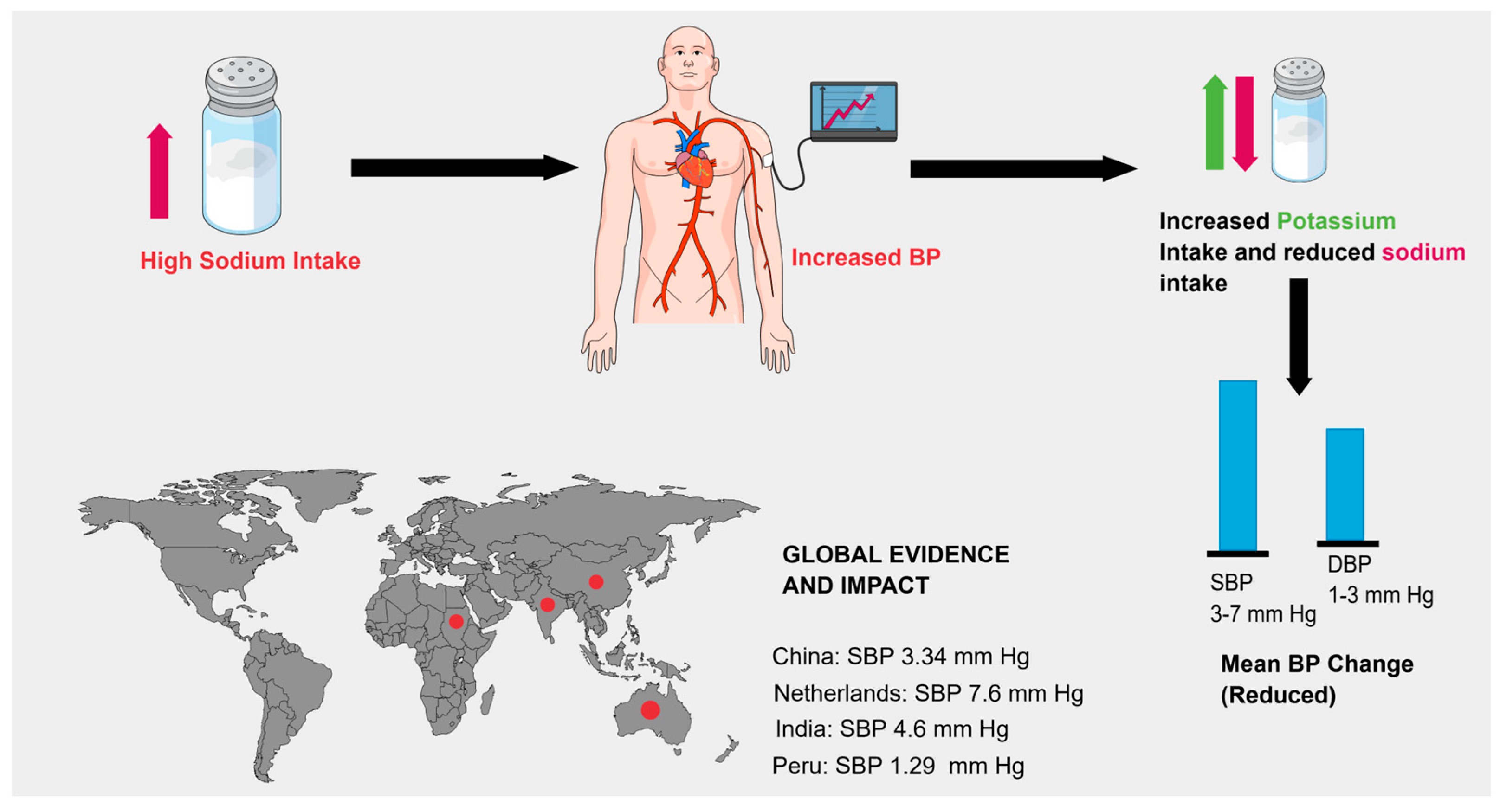

| Study | Country | Population | Duration | Salt Substitute Composition | SBP ↓ (mmHg) | DBP ↓ (mmHg) | Limitation |
|---|---|---|---|---|---|---|---|
| Neal et al. (2021) [11] | China | 600 villages (Rural China) | Variable | 75% NaCl, 25% KCl | 3.34 | 0.67 | Only included people ≥ 60 years |
| Geleijnse et al. (1994) [17] | Netherlands | 100 men and women between 55 and 75 years of age | 24 weeks | Sodium: Potassium: Magnesium: 8:6:1 | 7.6 | 3.3 | Did not Include people below <50 who also took great hit by prevalence of Hypertension |
| He et al. (2002) [18] | Global | Mixed RCTs | Variable | LSSS | 7.11 | 3.88 | Not all studies are blinded. |
| Greer et al. (2020) [19] | Global | RCTs | Variable | K-enriched LSSS | 5.58 | 2.88 | Limited evidence on effect of potassium rich salt substitute on serum potassium levels in CKD |
| Yu et al. (2021) [20] | India | 502 (7 villages in rural India) | 3 months | 70% NaCl 30% KCl | 4.6 | 1.1 | Follow up Duration is short people with CKD are not included. |
| Peng et al. (2014) [21] | Mixed Territories | Mixed RCTs (1974 participants) | Variable | Variable | 4.9 | 1.5 | None of the studies included people with CKD, Sample sizes are less for normotensive group. |
| Yuan et al. (2023) [22] | China | 1612 participants (elderly care facilities) | 6 months | 62.5% NaCl 25% KCl | 7.1 | 1.9 | - |
| Bernabe Otriz et al. (2020) [23] | Peru | 2376 | Variable | 75% NaCl, 25% KCl | 1.29 | 0.76 | Not Included people with CKD and taking Digoxin. |
| Study (Year) | Country/Setting | Population | Intervention | Duration | Main CV Outcome | Key Limitations |
|---|---|---|---|---|---|---|
| SSaSS (2021) [26] | China, rural (600 villages) | ~21,000 adults ≥ 60 year (72% with stroke/HTN) | 75% NaCl/25% KCl | 5 y | ↓ Stroke (−14%), ↓ major CV events (−13%), ↓ mortality (−12%) | Cluster design, open-label, rural only, excluded CKD |
| Chang et al. (2006) [27] | Taiwan (veterans’ homes) | 1981 older men (~75 year) | 50% NaCl/50% KCl | 2.6 y | ↓ CVD mortality (41% reduction) | Older men only, single-center, not blinded, few events |
| DECIDE-Salt (2024) [28] | China (48 eldercare facilities) | ~1600 elderly (many hypertensive) | 70% NaCl/30% KCl | 2 y | ↓ BP, fewer CV events (trend), no mortality effect | Institutionalized elderly, short follow-up, low event rates, hyperkalemia monitoring |
| Country | Strategy & Level | Key Outcomes |
|---|---|---|
| United Kingdom | Voluntary reformulation + public campaigns; government-led until ~2010, then industry-led | Salt intake dropped from ~9.4 g/day (2000) to ~7.6 g/day (2014); BP declined ~2–3 mmHg. Stroke and IHD mortality fell by ~30–40%, though progress stalled post-2014 [39,40]. |
| Finland | Legislation, labeling, reformulation, K-enriched salt (Pansalt) | Sodium intake ↓ ~40%; BP dropped >10 mmHg; stroke & IHD mortality ↓ ~75–80% [41,42]. |
| Japan | Government-led public health campaigns since the 1960s, especially in high-salt regions | Salt intake fell significantly (18 g/day → ~14 g/day); stroke mortality dropped ~80% [41]. |
| China | Mixed strategies: public awareness, community tools (e.g., salt spoons), pilot salt substitute initiatives | National intake remains high (~10.9 g/day, 2019); the SSaSS trial showed ~3 mmHg BP reduction and 14% lower stroke risk [11,41]. |
| India | Public advisories and pilot low-sodium iodized salt programs at state/local levels | Salt intake remains ~11 g/day; pilot trials show promising acceptance but limited large-scale impact [43]. |
| Peru | National front-of-pack labeling + community “Salt Liz” salt-substitute campaign | SBP reduced by ~1.3 mmHg; hypertension risk halved (51% lower incidence); high adoption rates observed [23]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younas, S.; Parvathi, H.; Sahu, S.; Rani, R.; Saher, S.; Chatzizisis, Y.S.; Delgado-Lelievre, M.C. Salt Substitutes in Low-Income Settings: Blood Pressure Benefits, Cardiovascular Outcomes, and Safety Considerations: A Narrative Review. J. Vasc. Dis. 2025, 4, 42. https://doi.org/10.3390/jvd4040042
Younas S, Parvathi H, Sahu S, Rani R, Saher S, Chatzizisis YS, Delgado-Lelievre MC. Salt Substitutes in Low-Income Settings: Blood Pressure Benefits, Cardiovascular Outcomes, and Safety Considerations: A Narrative Review. Journal of Vascular Diseases. 2025; 4(4):42. https://doi.org/10.3390/jvd4040042
Chicago/Turabian StyleYounas, Salma, Harshavardhan Parvathi, Sweta Sahu, Renu Rani, Samiya Saher, Yiannis S. Chatzizisis, and Maria Carolina Delgado-Lelievre. 2025. "Salt Substitutes in Low-Income Settings: Blood Pressure Benefits, Cardiovascular Outcomes, and Safety Considerations: A Narrative Review" Journal of Vascular Diseases 4, no. 4: 42. https://doi.org/10.3390/jvd4040042
APA StyleYounas, S., Parvathi, H., Sahu, S., Rani, R., Saher, S., Chatzizisis, Y. S., & Delgado-Lelievre, M. C. (2025). Salt Substitutes in Low-Income Settings: Blood Pressure Benefits, Cardiovascular Outcomes, and Safety Considerations: A Narrative Review. Journal of Vascular Diseases, 4(4), 42. https://doi.org/10.3390/jvd4040042






