The HEART-FGF Study: Cardiovascular Remodeling and Risk Stratification by FGF-23 in Patients with CKD: An Integrative Cross-Sectional Study of Cardiac, Renal, and Mineral Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Sample Size Estimation
2.4. Data Collection Procedures
2.5. Laboratory Assays
2.6. Cardiovascular Imaging
2.7. Cardiovascular Risk Stratification
2.8. Ethical Considerations
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cardiovascular Structural Abnormalities
- LA/LV changes were seen in 73%, predominantly left ventricular hypertrophy (LVH).
- Diastolic dysfunction (Grade 1) was observed in 45%.
- Valvular abnormalities were noted in 87%, including mitral regurgitation (67%) and tricuspid regurgitation (47%).
- Pulmonary hypertension (PAP ≥ 30 mmHg) was present in 25%.
- Carotid intima abnormalities (thickening or plaques) were present in 43%.
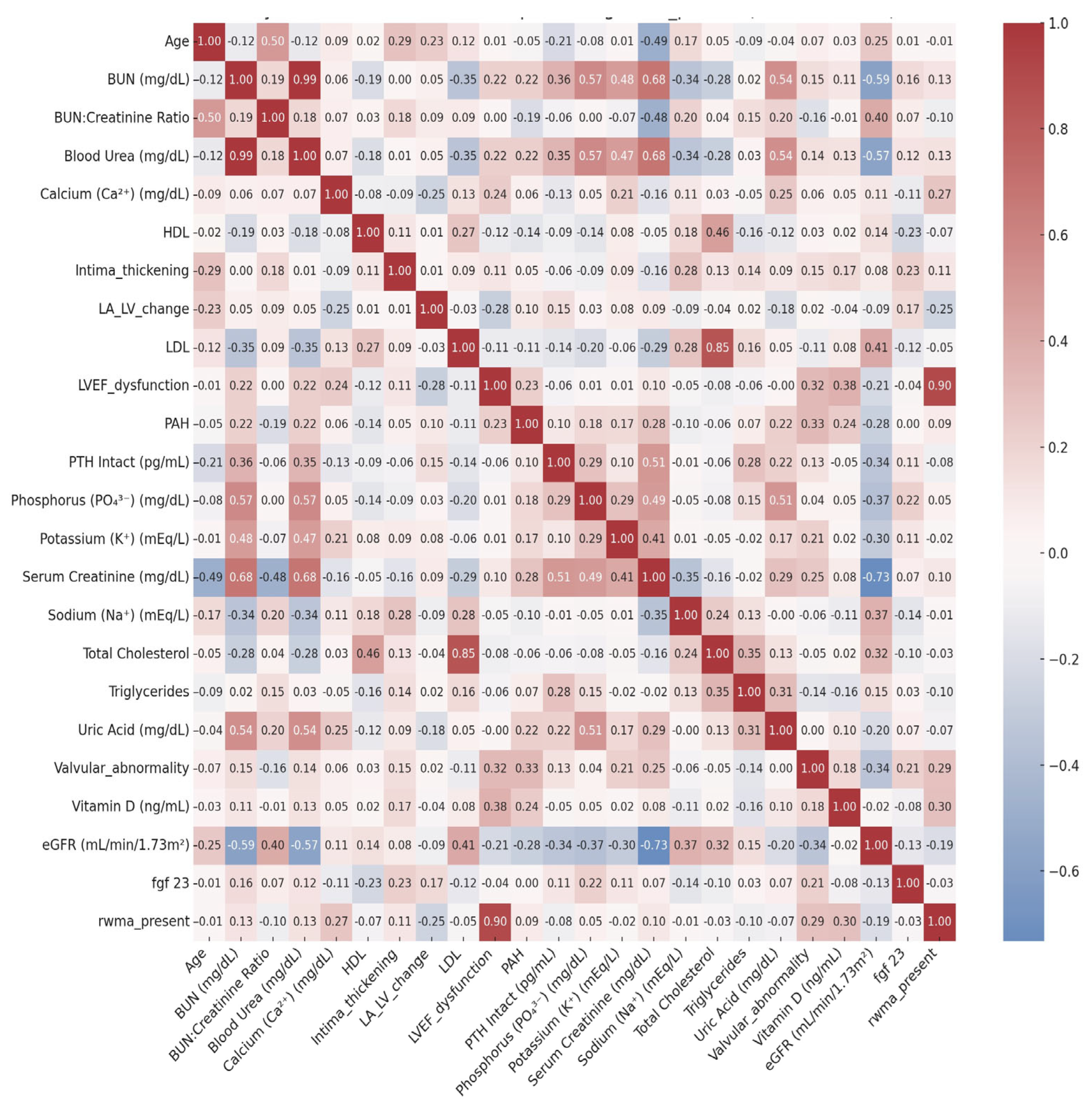
3.3. Correlations of FGF-23 with Renal, Mineral, and Cardiovascular Parameters
- Mineral–Bone–Cardiac Overlap: Higher serum vitamin D levels showed moderate positive correlations with preserved left ventricular ejection fraction (LVEF) and fewer regional wall motion abnormalities (RWMA), underscoring vitamin D’s potential cardioprotective role in modulating myocardial structure and contractility. A similar trend was observed with serum calcium, likely reflecting the delicate interplay between calcium homeostasis, myocardial excitability, and ischemic burden.
- Cardiorenal Axis: Declining renal function—as indexed by lower eGFR and elevated creatinine—was consistently associated with structural and hemodynamic cardiac abnormalities, including valvular pathology and pulmonary artery hypertension (PAH). These findings reinforce the concept of bidirectional cardiorenal interactions, where renal dysfunction contributes to cardiac remodeling and vice versa.
- Vascular, Lipid, and Age-Related Dynamics: Advancing age and elevated serum sodium correlated with increased carotid intima-media thickness, indicative of progressive vascular stiffening and atherosclerosis. Traditional lipid relationships (e.g., HDL vs. total cholesterol, triglycerides vs. uric acid) remained intact, reflecting the additive burden of dysmetabolic states in CKD.
- FGF23, iPTH, and eGFR Correlations with Cardiac–Vascular Markers: Among the bone-mineral axis markers, eGFR consistently showed inverse correlations with key cardiac abnormalities, most notably valvular disease and LVEF dysfunction—reaffirming the central role of declining renal function in promoting myocardial remodeling. FGF23 showed a weak but directionally relevant correlation with vascular intima thickening, suggesting potential early involvement in CKD-associated arteriosclerosis. iPTH exhibited minimal associations, likely reflecting the complexity and stage-dependence of its cardiovascular effects.
3.4. FGF-23 Levels Across Cardiovascular Risk Categories
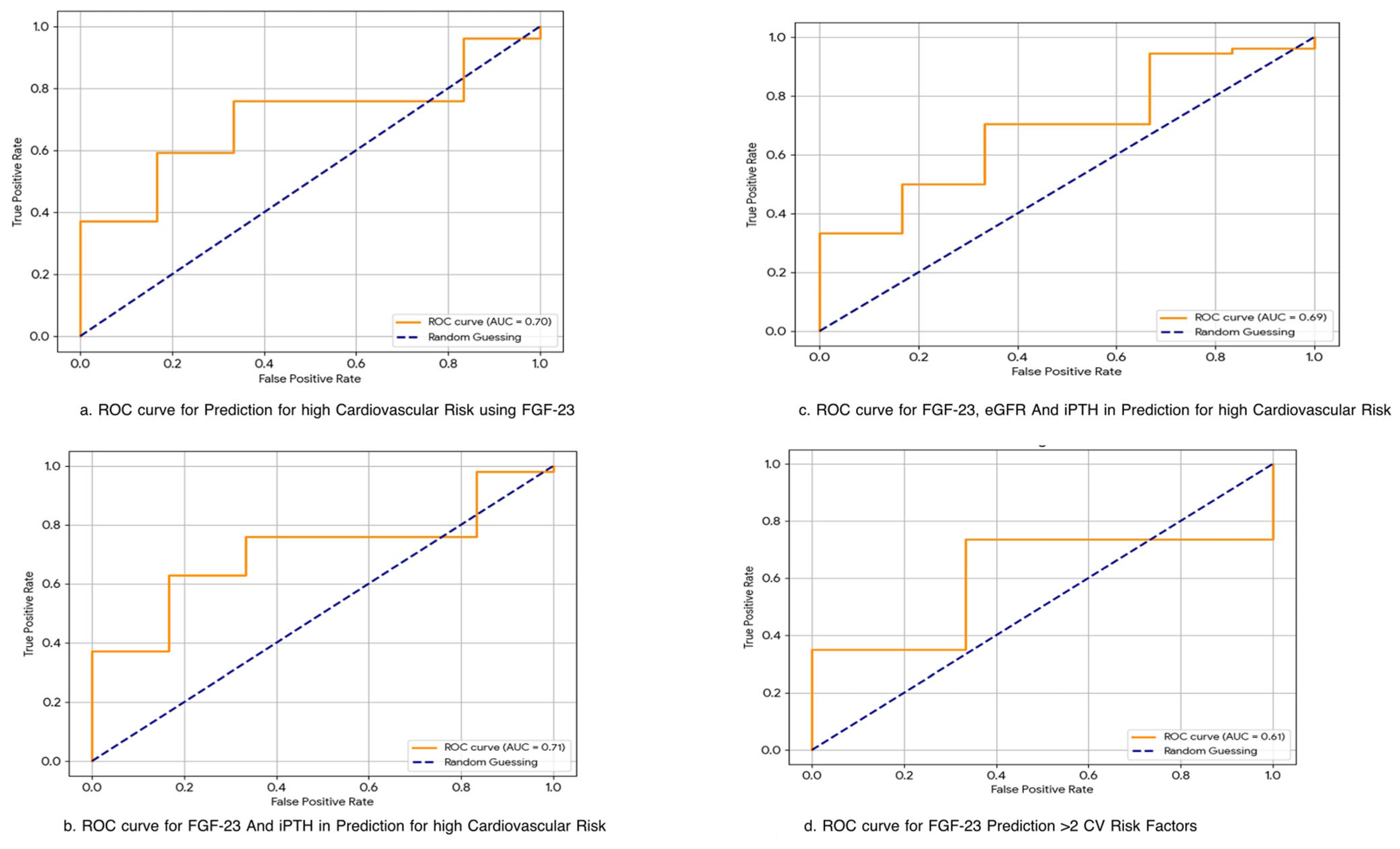
3.5. Predictive Performance of FGF-23 for Cardiovascular Risk
4. Discussion
4.1. Study Strengths and Limitations
4.2. Future Directions
5. Conclusions
- FGF-23 is a phosphaturic hormone elevated early in chronic kidney disease (CKD) and has been implicated in left ventricular hypertrophy (LVH) and vascular dysfunction.
- Mechanistic studies have shown that FGF-23 can induce myocardial remodeling via FGFR4-mediated pathways, independent of Klotho.
- Large cohort studies (e.g., CRIC) have associated high FGF-23 with increased cardiovascular morbidity, especially in dialysis-dependent populations.
- Demonstrates strong correlations between FGF-23 and early structural cardiovascular changes, including LA/LV remodeling and diastolic dysfunction, even in non-dialysis CKD.
- Proposes a novel multi-marker model (FGF-23 + iPTH + eGFR) with improved predictive power for cardiovascular risk (AUC = 0.76).
- Introduces a stratified FGF-23-based cardiac risk phenotype, showing a dose–response relationship between FGF-23 levels and cardiac/vascular abnormalities.
- Provides first-of-its-kind data from a South Asian CKD cohort, addressing a major gap in the global literature on cardiorenal risk profiling.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. No | Abbreviation | Full Form |
| 1 | BUN | Blood Urea Nitrogen |
| 2 | CKD | Chronic Kidney Disease |
| 3 | eGFR | Estimated Glomerular Filtration Rate |
| 4 | FGF-23 | Fibroblast Growth Factor 23 |
| 5 | HDL | High-Density Lipoprotein |
| 6 | iPTH | Intact Parathyroid Hormone |
| 7 | LA/LV | Left Atrial-to-Left Ventricular Ratio |
| 8 | LDL | Low-Density Lipoprotein |
| 9 | LVEF | Left Ventricular Ejection Fraction |
| 10 | PAH | Pulmonary Artery Hypertension |
| 11 | PTH | Parathyroid Hormone |
| 12 | RWMA | Regional Wall Motion Abnormality |
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Li, P.K.; Garcia-Garcia, G.; Lui, S.F.; Andreoli, S.; Fung, W.W.-S.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney health for everyone everywhere—From prevention to detection and equitable access to care. Braz. J. Med. Biol. Res. 2020, 53, e9614. [Google Scholar] [CrossRef]
- International Society of Nephrology. More Than 850 Million Worldwide Have Some Form of Kidney Disease: Help Raise Awareness. Available online: https://www.theisn.org/more-than-850-million-worldwide-have-some-form-of-kidney-disease-help-raise-awareness/ (accessed on 19 July 2025).
- Shah, A.; Hashmi, M.F.; Aeddula, N.R. Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560742/ (accessed on 19 July 2025).
- Rodrigues, F.G.; Ormanji, M.S.; Heilberg, I.P.; Bakker, S.J.L.; de Borst, M.H. Interplay between gut microbiota, bone health and vascular calcification in chronic kidney disease. Eur. J. Clin. Investig. 2021, 51, e13588. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A.; Coresh, J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105 (Suppl. S4), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yun, H.R.; Joo, Y.S.; Lee, S.; Kim, J.; Nam, K.H.; Jhee, J.H.; Park, J.T.; Yoo, T.-H.; Kang, S.-W.; et al. Framingham risk score and risk of incident chronic kidney disease: A community-based prospective cohort study. Kidney Res. Clin. Pract. 2019, 38, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF-23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef]
- Gutierrez, O.M.; Januzzi, J.L.; Isakova, T.; Laliberte, K.; Smith, K.; Collerone, G.; Sarwar, A.; Hoffmann, U.; Coglianese, E.; Christenson, R.; et al. Fibroblast growth factor-23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009, 119, 2545–2552. [Google Scholar] [CrossRef]
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 2014, 25, 349–360. [Google Scholar] [CrossRef]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutiérrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef]
- Ix, J.H.; Katz, R.; Kestenbaum, B.; de Boer, J.H.; Chonchol, M.; Mukamal, K.J.; Rifkin, D.; Siscovick, D.S.; Sarnak, M.J.; Shlipak, M.G. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals (CHS). J. Am. Coll. Cardiol. 2012, 60, 200–207. [Google Scholar] [CrossRef]
- Kendrick, J.; Cheung, A.K.; Kaufman, J.S.; Greene, T.; Roberts, W.L.; Smits, G.; Chonchol, M. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J. Am. Soc. Nephrol. 2011, 22, 1913–1922. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, S.; Poveda, J.; Navarro-García, J.A.; González-Lafuente, L.; Rodríguez-Sánchez, E.; Ruilope, L.M.; Ruiz-Hurtado, G. An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk. Front. Physiol. 2021, 12, 632260. [Google Scholar] [CrossRef]
- Heine, G.H.; Seiler, S.; Fliser, D. FGF-23: The rise of a novel cardiovascular risk marker in CKD. Nephrol. Dial. Transplant. 2012, 27, 3072–3081. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sachs, M.C.; Hoofnagle, A.N.; Siscovick, D.S.; Ix, J.H.; Robinson-Cohen, C.; Lima, J.A.C.; Polak, J.F.; Blondon, M.; Ruzinski, J.; et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: The Multi-Ethnic Study of Atherosclerosis. Circ. Heart Fail. 2014, 7, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate–Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Leidner, A.S.; Cai, X.; Zelnick, L.R.; Lee, J.; Bansal, N.; Pasch, A.; Nahsal, M.; Chen, J.; Anderson, A.H.; Sondheimer, J.H.; et al. Fibroblast Growth Factor 23 and Risk of Heart Failure Subtype: The CRIC (Chronic Renal Insufficiency Cohort) Study. Kidney Med. 2023, 5, 100723. [Google Scholar] [CrossRef]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef]
- Richter, B.; Haller, J.; Haffner, D.; Leifheit-Nestler, M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflug. Arch. 2016, 468, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Vergara, N.; de Mier, M.V.P.; Rodelo-Haad, C.; Revilla-Gonzáles, G.; Membrives, C.; Díaz-Tocados, J.M.; Martínez-Moreno, J.M.; Torralbo, A.I.; Herencia, C.; Rodríguez-Ortiz, M.E.; et al. The direct effect of fibroblast growth factor 23 on vascular smooth muscle cell phenotype and function. Nephrol. Dial. Transplant. 2023, 38, 322–343. [Google Scholar] [CrossRef]
- Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012, 82, 737–747. [Google Scholar] [CrossRef]
- Yildirim, M.; Salbach, C.; Mueller-Hennessen, M.; Frey, N.; Giannitsis, E. Diagnostic and Prognostic Evaluation of Novel Biomarkers Compared to ESC 0/1 h and 0/3 h Algorithms in Patients with Suspected Non-ST-Elevation Myocardial Infarction. J. Clin. Med. 2025, 14, 2957. [Google Scholar] [CrossRef]
| Variable/Parameter | Value | Units |
|---|---|---|
| Demographics | ||
| Age | 56.9 ± 16.0 | years |
| Male sex | 31 (52%) | — |
| CKD Stage, n (%) | — | |
| Stage 1–2 | 12 (20%) | — |
| Stage 3 | 18 (30%) | — |
| Stage 4 | 10 (17%) | — |
| Stage 5 | 20 (33%) | — |
| Renal Parameters | ||
| eGFR | 27.2 ± 25.5 | mL/min/1.73 m2 |
| Serum Creatinine | 4.93 ± 3.51 | mg/dL |
| Blood Urea | 111.45 ± 76.51 | mg/dL |
| BUN | 51.92 ± 35.66 | mg/dL |
| BUN–Creatinine Ratio | 11.45 ± 5.56 | ratio (unitless) |
| Uric Acid | 6.81 ± 2.45 | mg/dL |
| Mineral Metabolism | ||
| FGF-23 | 152.9 ± 105.7 | RU/mL |
| Intact PTH (iPTH) | 386.2 ± 370.8 | pg/mL |
| Vitamin D | 19.3 ± 11.2 | ng/mL |
| Calcium | 8.28 ± 0.94 | mg/dL |
| Phosphorus | 5.1 ± 2.5 | mg/dL |
| Electrolytes | ||
| Sodium | 139.75 ± 5.15 | mmol/L |
| Potassium | 4.71 ± 0.86 | mmol/L |
| Lipid Profile | ||
| Total Cholesterol | 141.5 ± 40.9 | mg/dL |
| LDL | 77.2 ± 31.9 | mg/dL |
| HDL | 35.8 ± 12.9 | mg/dL |
| Triglycerides | 148.6 ± 85.8 | mg/dL |
| Cardiac Parameters | ||
| LVEF | 56.4 ± 9.5 | % |
| Pulmonary Artery Pressure | 31.8 ± 10.4 | mmHg |
| Parameter | Correlation (r) | p-Value |
|---|---|---|
| eGFR | −0.288 | 0.028 |
| iPTH | 0.361 | 0.006 |
| Phosphorus | 0.335 | 0.011 |
| Creatinine | 0.271 | 0.042 |
| Thematic Domain | Variable Pair | Correlation Coefficient (r) | p-Value | Interpretation |
|---|---|---|---|---|
| Mineral–Bone + Cardiac Interplay | Vitamin D vs. LVEF Dysfunction | 0.38 | 0.0032 | Suggests the protective role of vitamin D in preserving systolic function |
| Vitamin D vs. RWMA Present | 0.3 | 0.0202 | Higher vitamin D may be associated with reduced myocardial damage | |
| Calcium vs. RWMA Present | 0.27 | 0.0406 | Possible role of calcium in myocardial excitability or ischemic susceptibility | |
| Cardiorenal Axis | Valvular Abnormality vs. eGFR | −0.34 | 0.007 | Declining GFR correlates with structural heart changes |
| PAH vs. Creatinine | 0.28 | 0.0292 | Worsening renal function linked with pulmonary hypertension | |
| PAH vs. eGFR | −0.28 | 0.0315 | Supports the presence of cardiorenal interaction | |
| Vascular/Lipid/Age-Related Parameters | Age vs. Intima Thickening | 0.29 | 0.0249 | Vascular aging evident through intimal changes |
| Sodium vs. Intima Thickening | 0.28 | 0.0324 | Electrolyte shifts may reflect vascular stiffness | |
| HDL vs. Total Cholesterol | 0.46 | 0.0002 | Expected inverse pattern in lipid metabolism | |
| Total Cholesterol vs. Triglycerides | 0.35 | 0.0067 | Co-association in dysmetabolism | |
| Triglycerides vs. Uric Acid | 0.31 | 0.0145 | May indicate shared metabolic and oxidative stress pathways | |
| FGF23/iPTH/eGFR vs. Cardiac–Vascular Correlates | Valvular Abnormality vs. eGFR | −0.345 | — | Moderate inverse correlation; valvular disease may worsen with kidney function decline |
| PAH vs. eGFR | −0.278 | — | Mild link of renal impairment with elevated pulmonary pressures | |
| LA/LV Change vs. FGF23 | 0.168 | — | Weak positive association; possible early remodeling | |
| LA/LV Change vs. iPTH | 0.149 | — | Weak trend | |
| LA/LV Change vs. eGFR | −0.086 | — | Minimal negative trend | |
| LVEF Dysfunction vs. eGFR | −0.213 | — | Mild inverse relationship, reinforcing cardiorenal link | |
| Intima Thickening vs. FGF23 | 0.227 | — | Weak correlation; may reflect vascular remodeling in the CKD milieu |
| Cardiovascular/Vascular Marker | Correlation with FGF-23 (r) | Interpretation |
|---|---|---|
| Valvular Abnormality | 0.211 | Mild positive correlation; may reflect FGF-23’s role in valvular calcification in CKD |
| Pulmonary Arterial Hypertension (PAH) | 0.002 | No association; PAH likely driven by volume overload or diastolic dysfunction rather than FGF-23 |
| LA/LV Structural Change | 0.168 | Weak trend; aligns with literature on FGF-23 promoting LV hypertrophy and remodeling |
| LVEF Dysfunction | −0.036 | No significant correlation; FGF-23 appears unrelated to systolic dysfunction severity |
| Carotid Intima Thickening/Plaque | 0.227 | Mild positive correlation; suggests possible involvement in early vascular stiffness or subclinical atherosclerosis |
| Predictor(s) | Target Outcome | AUC (Sensitivity, Specificity) | Interpretation |
|---|---|---|---|
| FGF-23 alone | High Cardiovascular Risk * | 0.70 (76%, 67%) | Moderate predictive value; may aid early screening |
| FGF-23 + iPTH | High Cardiovascular Risk | 0.71 | Slightly improved discrimination compared to FGF-23 alone |
| FGF-23 + eGFR + iPTH | High Cardiovascular Risk | 0.69 | No significant added value over FGF-23 alone |
| FGF-23 alone | >2 Cardiovascular Risk Markers ** | 0.61 | Low-to-moderate discriminatory power |
| FGF-23 + iPTH + eGFR | >2 Cardiovascular Risk Markers | 0.76 | Best predictive model; supports multivariable biomarker approach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, D.; Prasad, A.; Shahi, H.; Wadhera, N.; Goel, A.; Sethi, Y. The HEART-FGF Study: Cardiovascular Remodeling and Risk Stratification by FGF-23 in Patients with CKD: An Integrative Cross-Sectional Study of Cardiac, Renal, and Mineral Parameters. J. Vasc. Dis. 2025, 4, 39. https://doi.org/10.3390/jvd4040039
Jain D, Prasad A, Shahi H, Wadhera N, Goel A, Sethi Y. The HEART-FGF Study: Cardiovascular Remodeling and Risk Stratification by FGF-23 in Patients with CKD: An Integrative Cross-Sectional Study of Cardiac, Renal, and Mineral Parameters. Journal of Vascular Diseases. 2025; 4(4):39. https://doi.org/10.3390/jvd4040039
Chicago/Turabian StyleJain, Dhruv, Anand Prasad, Harsha Shahi, Nishant Wadhera, Ashish Goel, and Yashendra Sethi. 2025. "The HEART-FGF Study: Cardiovascular Remodeling and Risk Stratification by FGF-23 in Patients with CKD: An Integrative Cross-Sectional Study of Cardiac, Renal, and Mineral Parameters" Journal of Vascular Diseases 4, no. 4: 39. https://doi.org/10.3390/jvd4040039
APA StyleJain, D., Prasad, A., Shahi, H., Wadhera, N., Goel, A., & Sethi, Y. (2025). The HEART-FGF Study: Cardiovascular Remodeling and Risk Stratification by FGF-23 in Patients with CKD: An Integrative Cross-Sectional Study of Cardiac, Renal, and Mineral Parameters. Journal of Vascular Diseases, 4(4), 39. https://doi.org/10.3390/jvd4040039







