2. The Case Presentation
A 63-year-old Caucasian female with a significant past medical history in terms of hypertension and tobacco use disorder presented to the Emergency Department in an ambulance due to a syncopal event at home. The patient attested to recent alcohol and marijuana smoke exposure, after which she had laid down to rest on her couch. She stated that upon attempting to stand, her legs gave out, and she collapsed. The patient stated that she believed that she had lost consciousness; her daughter notified the emergency services, and an ambulance was dispatched.
The patient’s only complaint was that both of her legs hurt and she could not feel them. On initial examination, the patient’s initial vital signs were as follows: blood pressure: 120/44; heart rate: 53; respiratory rate: 20; rectal temperature: 93.3 F. Her lower extremities showed bilateral petechiae and molting of the feet and were cold to the touch. Dorsalis pedis pulses and posterior tibialis pulses were unable to be palpated. These absent pulses were verified using bedside Doppler and color-flow ultrasound. The patient was immediately brought to imaging, and a CT angiogram of the aorta with a contrast protocol was performed to rule out dissection. CT imaging demonstrated an occlusion of the distal abdominal aorta (without dissection), with concurrent occlusion of the bilateral common iliac arteries.
Vascular surgery was immediately discussed; however, per the evaluation, the patient’s presentation made it difficult to decipher between chronic and acute occlusion, and moreover, it was suspected that she might have developed a collateral flow. Thus, the surgical team recommended against immediate direct intervention. A heparin drip was recommended, with consideration of possible surgery later if the patient failed to demonstrate an improvement. The patient’s pain worsened over the course of the next two hours. She remained persistently hypothermic, and repeat bedside high-resolution color-flow Doppler ultrasound verified no flow in the following arteries (bilateral): the dorsalis pedis, posterior tibialis, popliteal, and femoral.
Therefore, another consultation between the vascular surgery department and the emergency physician resulted in consideration of the treatment modalities for immediate resolution of the clot, as it became clear that the patient had no functional collateral flow. Surgical intervention was deemed to be too high of a risk to the patient—and since it was decided that direct intervention was not currently an option due to her risk profile, systemic thrombolysis via tPA was decided to be a viable surrogate option. The in-patient pharmacy was consulted, and a dose of 100 mg (without a bolus) of tPA was selected (in congruence with the ‘full-dose’ PE treatment protocol/the current FDA-approved application of tPA to PE [
8]). tPA was administered at a constant rate over 2 h, and then the heparin infusion was immediately started (similar to the currently recommended PE protocol [
8]). Approximately 1 h after completion of the tPA infusion, the patient’s pain had resolved, and pulses had been restored according to palpation of the right posterior tibialis, the right dorsalis pedis, and the left dorsalis pedis (verified using bedside color-flow Doppler). Hypothermia was consequently resolved over the same one-hour period.
The patient was admitted to the ICU for monitoring. On day 2, she began to experience abdominal and flank pain. A repeat CT angiogram of the abdominal aorta and bilateral iliofemoral runoff showed complete resolution of the aortic and bilateral common iliac occlusion; however, the patient had a new retroperitoneal bleed. The heparin drip was stopped, and the patient required a transfusion of 2 units of packed red blood cells for blood-loss anemia. The patient’s hemoglobin levels stabilized without further intervention (and did not require any direct surgical or interventional radiology treatment). The patient ultimately had a full return of function of her lower extremities and was discharged, walking, from the hospital on day 6. Further anticoagulation was deferred due to the patient’s recent retroperitoneal bleed. At her 2-week follow-up appointment, she was noted to be ambulatory, without any neurodeficit.
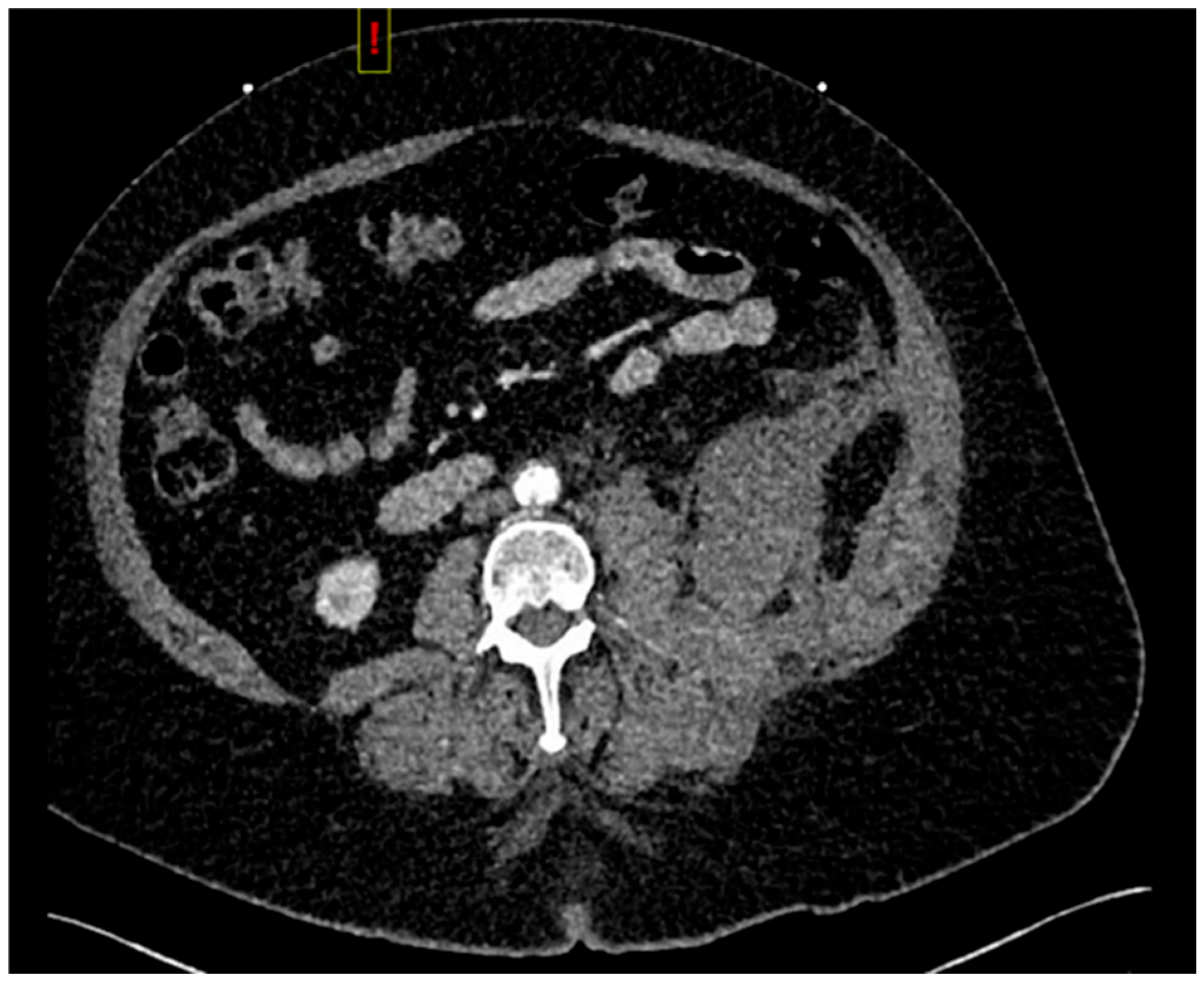
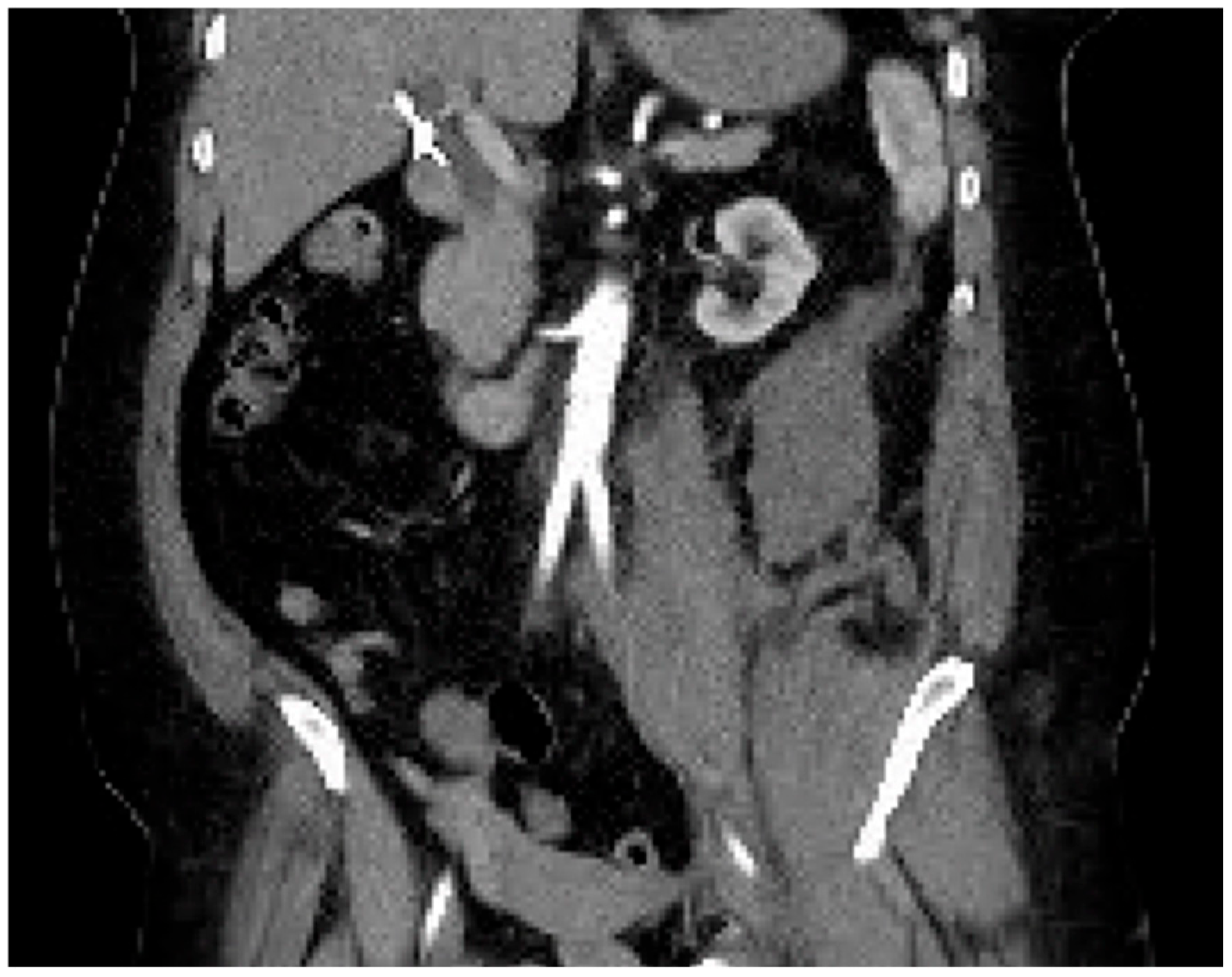
Computed tomography angiogram (CTA) protocol images are displayed below.
Figure 1 and
Figure 2 correspond to her state prior to tPA administration; notice the 100% occlusive aortic thrombus.
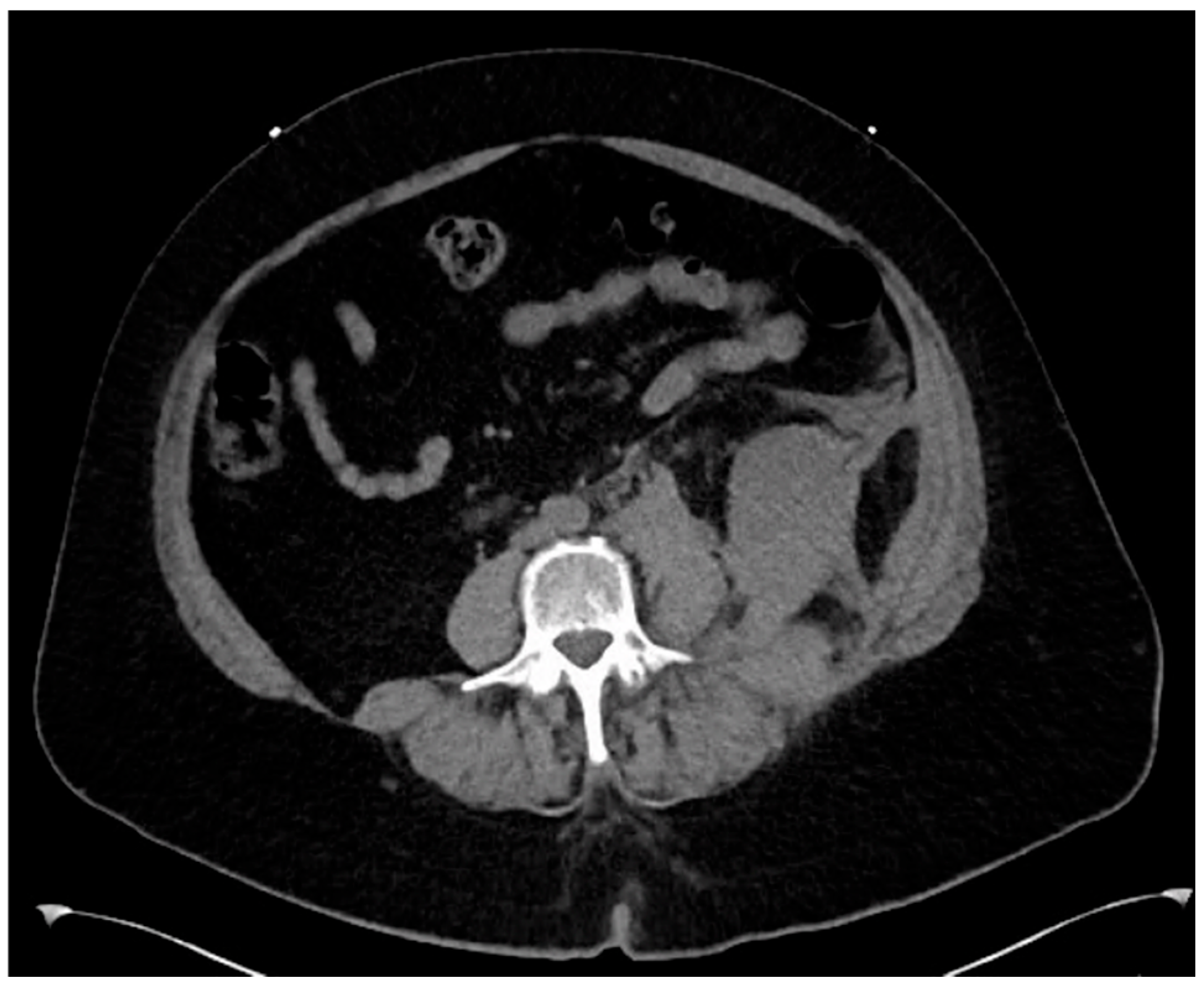
Figure 3 and
Figure 4 are post-tPA administration, demonstrating the fully restored aortic flow.
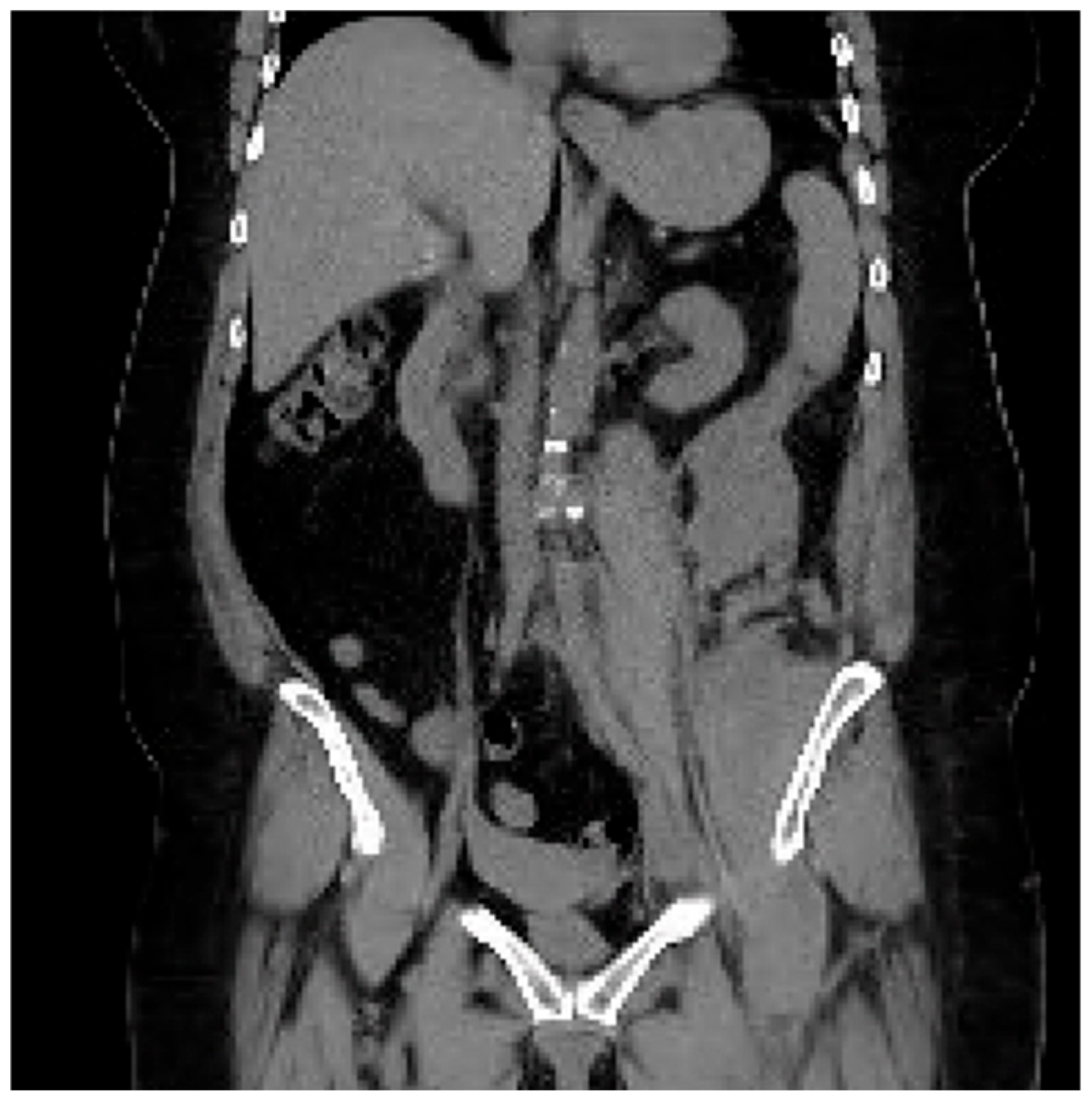
The CTA protocol images featured below (
Figure 5 and
Figure 6) demonstrate the retroperitoneal bleed noted on treatment day #2. This complication self-resolved without surgical or interventional radiological intervention. The patient required a total of 2 units of packed red blood cells.
3. Discussion
Aortic occlusions are a rare and life-threatening condition associated with high morbidity and mortality [
1,
2]. Early recognition of this pathology is crucial, though its symptoms can vary widely depending on the level of occlusion. Bilateral leg pain is the most common symptom but can be accompanied by motor and sensory loss [
4]. The symptoms of cauda equina can also be found depending on the location of the occlusion. The treatment is usually a heparin drip and then direct intervention via vascular surgery, e.g., an axillary-bifemoral bypass, an aorta-bifemoral bypass, or a thrombo-embolectomy. However, in this case, the patient had symptomatology consistent with progressive bilateral ischemic loss of the lower extremities without a foreseeable move to the operating room. Facing a possible permanent ischemic complication (including bilateral amputation), the risk of not administering tPA seemed to far outweigh any benefits of a further delay in thrombolysis. Thus, tPA was administered, though it was recognized as a non-FDA-certified application, and there was a potential risk with this action. The estimated pathophysiological resolution with tPA seemed to follow a similar mechanism to that of PE treatment; therefore, its administration reflected the PE-driven tPA protocol [
8]. The resolution interval for the AAO in the above case reflects that of tPA’s terminal half-life of 72 min [
8], indicating that it is likely appropriate to deduce that its resolution was indeed consequent to the administration of thrombolysis.
Though tPA is not authorized for ischemic limbs, this case was determined to be a lower-risk application due to the atraumatic occurrence of the AAO, no evidence of dissection, no prior anticoagulation, and no known history or evidence of associated carcinoma. This lesser risk was balanced against the well-described complications of delayed reperfusion, e.g., rhabdomyolysis, the need for hemodialysis, compartment syndrome, sustained motorsensory neurological deficits, and mesenteric ischemia [
2,
4]. Moreover, the patient was hypothermic and had worsening mottling, cold limbs, and progressively worsening pain. It was clear to all providers that she was at a significant risk of a bilateral amputation vs. death with the considerable amount of body tissue involved in the ischemia. Still, ALI requiring systemic thrombolysis of an AAO (or ALI in the setting of Leriche syndrome (Leriche syndrome (aortoiliac occlusive disease) is characterized by chronic obstruction of the abdominal aorta and the iliac arteries, resulting in claudication of the lower extremities, absent/decreased peripheral pulses, and erectile dysfunction, where risk factors include diabetes, hypertension, smoking, and hyperlipidemia [
9])) carries a risk of the above-mentioned adverse complications and also distal embolization (up to 12% for immobile thrombi [
10]). However, distal embolization can also be seen in the setting of catheter-mediated procedures (distal embolization is not merely a complication in the setting of systemic thrombolysis but rather distal embolization can occur while crossing the occlusion with a wire or catheter and may make the involved limb more severely ischemic [
11]).
While the FDA has approved tPA for myocardial infarction with ST-segment elevation, unstable pulmonary embolism, ischemic stroke for an onset within 3–4.5 h of treatment, and the re-establishment of patency in occluded IV catheters [
8], present-day off-label indications exist. Acute limb ischemia (ALI) is considered an off-label use of tPA [
7]. The most frequent serious adverse effects of tPA are associated with bleeding. Bleeding events can be divided into internal and external. Internal bleeding events include intracranial bleeding, retroperitoneal bleeding, gastrointestinal bleeding, genitourinary bleeding, and respiratory bleeding [
12]. External bleeding consists of ecchymosis, gingival bleeding, and epistaxis. This case did demonstrate a rare complication of tPA administration in the retroperitoneal bleeding observed, which presented with flank pain (with a published incidence of less than 1% [
12]); however, this resolved quickly with the discontinuation of heparin and required minimal resuscitation, without lasting complications or morbidity or requiring subsequent surgical intervention. The dose recommended in the pharmacy consultation in the involved case (due to the lack of protocols available for AAO) was congruent with the massive PE dosing recommendations: 100 mg infused intravenously over 2 h [
8]. It is possible that a lesser dose, as has been tested previously (systemic treatment with thrombolytics for nontraumatic acute limb ischemia performed by Saroukhani et al. (2015) was defined as the treatment group receiving 0.6 mg/kg (with an upper limit of 50 mg) of tPA (alteplase), with 20% administered as a bolus initially [
7]), would have facilitated clot resolution, with a lower chance of the retroperitoneal bleeding. Indeed, overall, the rate of adverse events, including bleeding, appears to correlate with the total cumulative tPA dose [
12].
Arguably, ICH remains the most severe and also the primary life-threatening complication of those against which the benefits of tPA administration must be weighed. With a published incidence of 1.3% [
7], intracranial hemorrhage represents a significant consideration; however, its incidence is up to 73% lower that the absolute risk of death in the setting of AAO [
2]. Said another way, the risk of death from AAO is up to 57 times greater than the risk of intracerebral hemorrhage due to systemic thrombolysis. In resource-limited settings (and while the current research indicates that outcomes are likely to be similar overall for the functional resolution of AAO in comparing CDT vs. systemic approaches [
7]), the decision to treat vs. observe is straightforward. Most patients are going to favor the approach of treatment in shared decision-making, rather than risking amputation or worse. We see the same in our stroke patients clinically, who consistently prefer thrombolysis in the setting of acute cerebral vascular accidents even though the risk of ICH is much higher and ranges up to 7% [
13], which is at least 5 times the rates of ICH in thrombolysis treatment of AAO.
Ultimately, this patient, who initially presented with global hypothermia, cold and mottled limbs, and severe pain, was able to walk out of the hospital with full preservation of her lower extremities. She did not require any surgical intervention throughout her stay, to include no fasciotomies and no extremity or digit amputations. Not only was her vascular flow re-established using systemic tPA dosed under a massive PE protocol but she retained intact neurological function on follow-up and was pain-free, despite her long-duration smoking status and her belonging to a community with significant social economic constraints. She was not discharged on anticoagulation (per the guideline management of AAO in the setting of ALI, there is no conclusive data on the benefit of anticoagulation (AC) for native (i.e., non-embolic/not associated with atrial fibrillation) aortic thrombosis; however, in prosthetic bypass graft thrombolysis (or thrombectomy), there is a significant improvement in secondary patency outcomes, and thus, long-term AC after thrombolysis/a thrombectomy in the setting of an occluded prosthetic bypass might be considered per the EU guidelines [
11]); remained clot-free; and had no long-duration complications. Her case represents an overall low-cost and effective treatment for AAO in resource limited settings. Such an application, especially in the setting of a likely treatment equivalence to CDT [
7], of systemic thrombolysis to nontraumatic acute aortic occlusions represents an excellent tool for rural and resource-constrained practice environments.
Limitations
The patient in the above case did not undergo subsequent anticoagulation (AC) therapy after discharge, as the decision was made to withhold AC due to the moderate risk reduction for noncompressible hemorrhage. It is possible that this decision could have impacted her outcomes. This seems likely, however, to be a null observation in that her functional and nociceptive outcomes were optimal, and thus, anticoagulation after treatment may have potentially induced unnecessary risk, which seems consistent with the current guidelines for a non-embolic AAO not associated with a prosthetic graft device [
11]. The pathophysiological mechanism of an acute nontraumatic aortic occlusion varies by risk factors and concurrent disease conditions. The primary risk factor for this patient was long-duration tobacco smoke exposure. Patients affected by other sources of occlusion risk may not respond as well as the patient above, who demonstrated functionally intact and complete nociceptive resolution by 1 h after the completion of the infusion (which is consistent with the terminal half-life of alteplase of 72 min [
8]). The dose chosen for this treatment algorithm was double that in a randomized control trial that directly compared CDT vs. systemic applications [
7], and this could logically have been associated with the retroperitoneal bleed observed. It is not known whether a lower dose would have caused such a dramatic resolution (i.e., 50 mg of alteplase) vs. that administered over 2 h (100 mg of alteplase). Additionally, the treatment of the patient described did not include any bolus thrombolysis therapy, in contrast to other protocols (10, 12), and it is unknown how this may have impacted the treatment. Ideally, while in the in-patient setting, a complete hypercoagulability work-up should be undertaken, complete with a trans-esophageal echocardiogram to rule out any ventricular clot burden/cardioembolic source. Lastly, the time intervals in the administration of systemic thrombolysis, dependent on the disease condition, are well described for indications such as myocardial infarction and acute cerebral infarction; however, they are unclear for acute limb ischemia. It is possible that with a further delay in the treatment of the patient described, the outcomes could have been worse, and the immediate complications could also have been worse.