Beyond Endoleaks: A Holistic Management Approach to Late Abdominal Aortic Aneurysm Ruptures After Endovascular Repair
Abstract
1. Introduction
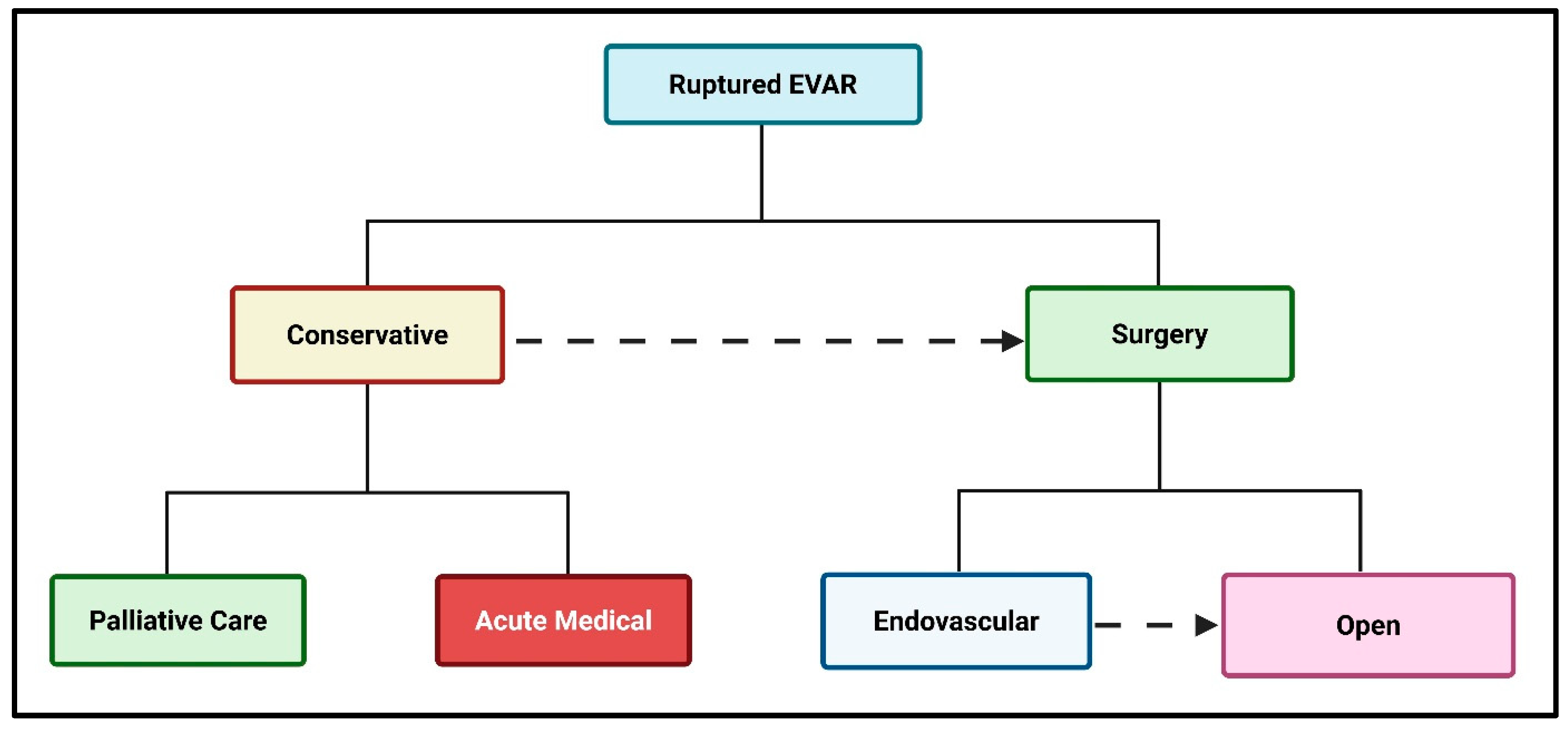
2. The Treatment Options for an AAA Rupture Post-Endovascular Repair
| Parameter | Conservative | Endovascular | Open Surgery | Notes |
|---|---|---|---|---|
| Haemodynamic Stability | Only if stable (SBP > 90 mmHg), no ongoing shock | Preferred if stable or moderately unstable (SBP 70–90 mmHg), responsive to fluids | Preferred if unstable and not unamenable to endovascular treatment | Instability and requirement for complex endovascular treatment often necessitates open repair [9] |
| Blood Pressure | SBP 90–120 mmHg, avoid hypertension and hypotension | SBP 70–90 mmHg, permissive hypotension to limit bleeding | SBP 70–90 mmHg, rapid surgical aortic cross-clamping | Maintain perfusion, avoid excessive hypertension [16] |
| Heart Rate | <100 bpm preferred, no tachycardia | <100 bpm preferred, tachycardia may indicate ongoing bleeding | Tachycardia (>100 bpm) may indicate ongoing bleeding | Tachycardia signals instability [17] |
| Time to Intervention | Considered, stable and non-surgical | <90 min from arrival is ideal | <90 min from arrival is ideal | Door-to-intervention < 90 min (30–30–30 min) [16] |
| Patient Frailty | Severe frailty (bedbound, poor baseline function) | Moderate frailty (needs some assistance) | Low/moderate frailty (independent) | Frailty increases surgical risk |
| Heart Failure | Severe (NYHA III-IV) | Mild/moderate (NYHA I-II) | No/mild heart failure | Heart failure increases perioperative risk |
| Renal Impairment | Severe (on dialysis, creatinine > 3 mg/dL) | Mild/moderate (creatinine < 2.5 mg/dL) | Mild/moderate (creatinine < 2.5 mg/dL) | Renal impairment increases risk [9] |
| Anaesthesia Suitability | Not suitable for any anaesthesia | Local anaesthesia preferred, general if needed | General anaesthesia required | Local anaesthesia lowers mortality in EVAR [18] |
| Age | Old age (>90) | Elderly (80–90) | Younger (<80) | Octogenarians more often treated with EVAR [19] |
| Gender | Either | Preferable in females | Either | EVAR reduces mortality more in females [20] |
| Previous Abdominal Surgery | Multiple prior surgeries, hostile abdomen | Feasible if access is possible | Difficult if adhesions | Adhesions complicate open repair [16] |
| Aneurysm Anatomy | Unfavourable (short neck, severe angulation) | Favourable (adequate neck, access) | Unfavourable, open surgery if EVAR not possible | Adequate neck/iliac access essential for EVAR |
| Endoleak Type | Type II, no expansion | Type I-V | Type I-V if endovascular not feasible | Types I/III especially require intervention [1] |
| Graft Infection | Present and unfit for surgery | Haemodynamic control with long-term antibiotics | Present and fit for surgery | Infection increases the risk of EVAR [21] |
| Institutional Experience | Low experience | High experience: EVAR preferred | High experience: open surgery possible | Outcomes better in high-volume centres [10] |
| Time Since Initial EVAR | Recent EVAR (<1 month), conservative if stable | Late rupture (>1 year), EVAR or open, based on anatomy | Late rupture, open surgery if EVAR not feasible | Late ruptures often endoleak-related [1] |
| Surveillance Compliance | Poor compliance, unknown anatomy | Good compliance: EVAR possible | Good compliance: endovascular not feasible | Non-compliance increases rupture risk |
| Blood Loss | Not applicable | Lower blood loss with EVAR | High blood loss with open surgery | EVAR reduces transfusion needs [9] |
| Life Expectancy | <6 months | >6 months | >6 months | Consider prognosis and quality of life |
| Access Vessel Status | Poor (occluded, small) | Good (patent, adequate size) | Good | Adequate access required for EVAR |
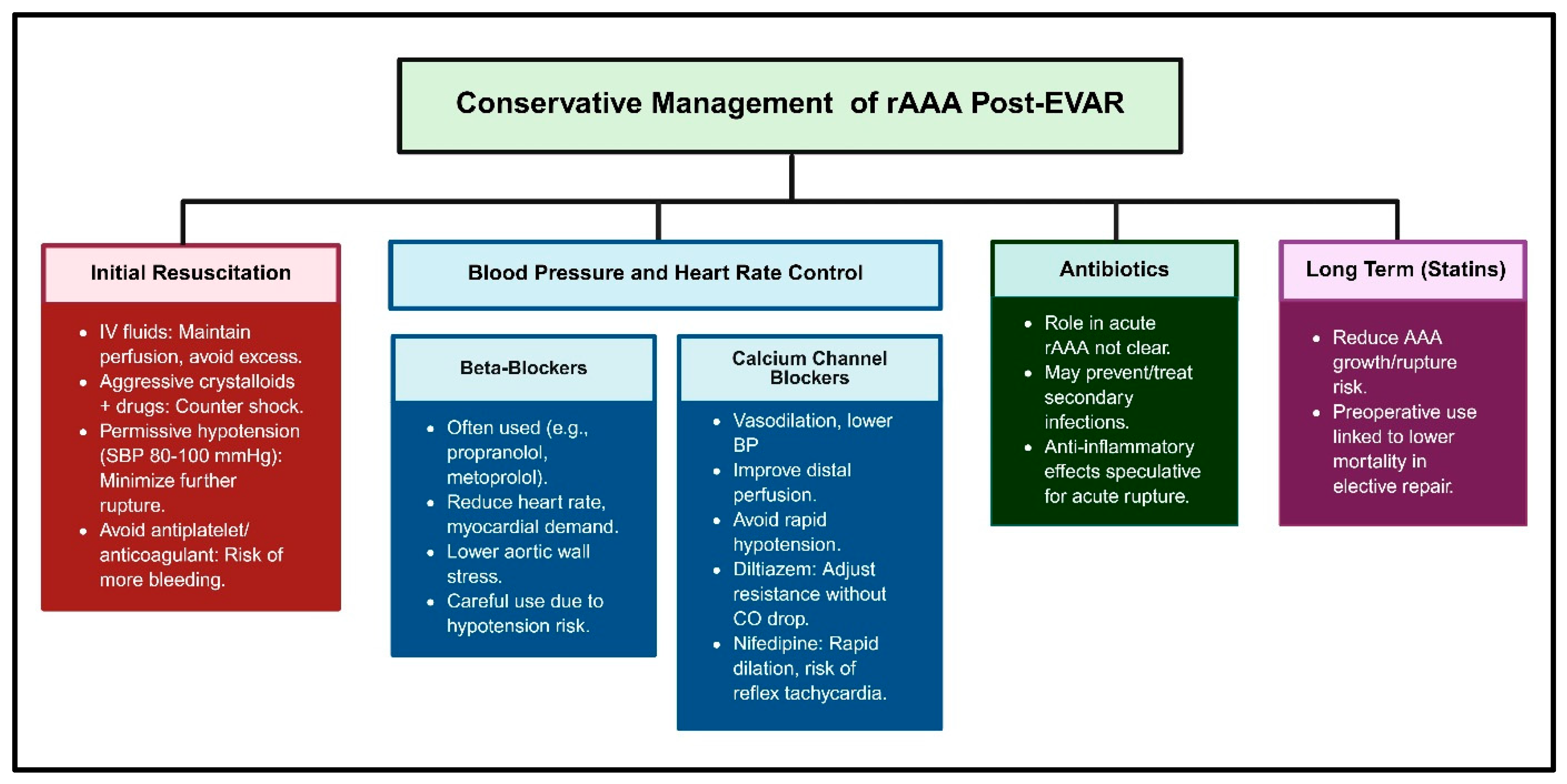
2.1. Conservative Management
2.2. Percutaneous Endovascular Repair for a Ruptured Abdominal Aortic Aneurysm (rPEVAR)
2.2.1. Fenestrated/Branched Endovascular Aneurysm Repair (F/BEVAR)
2.2.2. Parallel Graft Endovascular Aortic Repair (PGEVAR)
2.2.3. Chimney EVAR (ChEVAR) and Periscope Techniques
2.2.4. Physician-Modified Endografts (PMEGs)
2.2.5. Interposition Iliac Limb Extensions and Embolisation for Type 1B and Type 3 Endoleaks
2.3. Open Surgical Repair After Failed Endovascular Repair
3. Imaging Surveillance After EVAR
| Imaging Modality | Initial/Follow-Up Use Per Guidelines | Impact of Patient Frailty and Haemodynamics | Treatment Method’s Influence | Key Evidence/ Guideline Notes |
|---|---|---|---|---|
| CT Angiography (CTA) | Gold standard for initial and follow-up imaging; typically at 1, 6, and 12 months and then annually [65,74] | Haemodynamically unstable or frail patients may not tolerate contrast/radiation; risk of nephropathy [67] | Open or repeated endovascular repair often requires CTA for planning and post-procedure assessments [74] | High sensitivity/specificity; guidelines recommend CTA for initial assessment and when complications are suspected |
| Dual-Energy CT (DECT) and Dual-Energy CT Angiography (DECTA) | Used as an advanced alternative to CTA, especially for complex cases [75] | Similar limitations to CTA; may be less suitable for unstable/frail patients | Useful for detailed vascular assessments post-intervention | Offers improved vascular imaging; not yet standard in all guidelines |
| Duplex Ultrasound (DUS) | Increasingly used for routine follow-up, especially after first year if stable [73,76] | Well-tolerated in frail/unstable patients; no contrast/radiation | Conservative management or stable post-repair patients often monitored with DUS | Lower sensitivity than that of CTA but safe and cost-effective for long-term surveillance |
| Contrast-Enhanced Ultrasound (CEUS) | Can replace CTA for follow-up; high sensitivity for endoleak detection [67,77] | Safe for frail patients; no nephrotoxic contrast | Useful for all treatment types, especially when CTA is contraindicated | Sensitivity/specificity comparable to that of CTA; guidelines support use when CTA is risky or inconclusive |
| Abdominal X-ray | Used adjunctively for stent position; not for endoleak detection [78] | Minimal impact from frailty and haemodynamics | Used in all management types for device integrity | Not a standalone modality; complements DUS in some protocols |
| MRI | Sometimes used if CTA contraindicated (e.g., renal impairment) [79] | Preferred in patients with contrast allergies or renal dysfunction | Used for complex cases or when other imaging is inconclusive | Higher endoleak detection than that of CTA in some studies; less common in routine protocols |
4. Expert Opinion
Author Contributions
Funding
Conflicts of Interest
References
- Antoniou, G.; Georgiadis, G.; Antoniou, S.; Neequaye, S.; Brennan, J.; Torella, F.; Vallabhaneni, S. Late Rupture of Abdominal Aortic Aneurysm After Previous Endovascular Repair. J. Endovasc. Ther. 2015, 22, 734–744. [Google Scholar] [CrossRef]
- Saratzis, A.; Bown, M.; Sayers, R. Commentary: Late Rupture After Endovascular Aneurysm Repair. J. Endovasc. Ther. 2015, 22, 745–747. [Google Scholar] [CrossRef]
- Moulakakis, K.; Lazaris, A.; Georgiadis, G.; Kakkos, S.; Papavasileiou, V.G.; Antonopoulos, C.; Papapetrou, A.; Katsikas, V.; Klonaris, C.; Geroulakos, G. A Greek Multicentre Study Assessing the Outcome of Late Rupture After Endovascular Abdominal Aortic Aneurysm Repair. J. Vasc. Surg. 2024, 80, 288. [Google Scholar] [CrossRef]
- Sweeting, M.; Ulug, P.; Powell, J.; Desgranges, P.; Balm, R. Ruptured Aneurysm Trials: The Importance of Longer-term Outcomes and Meta-analysis for 1-year Mortality. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Alnefaie, S.; Alzahrani, Y.; Alzahrani, B. A Comparison of Endovascular Aneurysm Repair and Open Repair for Ruptured Aortic Abdominal Aneurysms. Cureus 2022, 14, e25672. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, P.; Kobeiter, H.; Katsahian, S.; Bouffi, M.; Gouny, P.; Favre, J.; Alsac, J.; Sobocinski, J.; Julia, P.; Alimi, Y.; et al. Editor’s Choice—ECAR (Endovasculaire ou Chirurgie dans les Anévrysmes aorto-iliaques Rompus): A French Randomized Controlled Trial of Endovascular Versus Open Surgical Repair of Ruptured Aorto-iliac Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 303–310. [Google Scholar] [CrossRef]
- Greiner, A.; Schleimer, K.; Jalaie, H.; Gombert, A.; Jacobs, M.; Kalder, J. Late rupture after EVAR: A new trend? J. Cardiovasc. Surg. 2014, 55 (Suppl. 1), 169–174. [Google Scholar]
- Yilmaz, N.; Peppelenbosch, N.; Cuypers, P.; Tielbeek, A.; Duijm, L.; Buth, J. Emergency Treatment of Symptomatic or Ruptured Abdominal Aortic Aneurysms: The Role of Endovascular Repair. J. Endovasc. Ther. 2002, 9, 449–457. [Google Scholar] [CrossRef]
- Antoniou, G.; Georgiadis, G.; Antoniou, S.; Pavlidis, P.; Maras, D.; Sfyroeras, G.; Georgakarakos, E.; Lazarides, M. Endovascular repair for ruptured abdominal aortic aneurysm confers an early survival benefit over open repair. J. Vasc. Surg. 2013, 58, 1091–1105. [Google Scholar] [CrossRef]
- Kontopodis, N.; Galanakis, N.; Antoniou, S.; Tsetis, D.; Ioannou, C.; Veith, F.; Powell, J.; Antoniou, G. Meta-Analysis and Meta-Regression Analysis of Outcomes of Endovascular and Open Repair for Ruptured Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 399–410. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Li, H.-Y.; Zhang, Y.; Lu, Q.; Jing, Z. Open surgery (OS) versus endovascular aneurysm repair (EVAR) for hemodynamically stable and unstable ruptured abdominal aortic aneurysm (rAAA). Heart Vessel. 2016, 31, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Böckler, D.; Holden, A.; Krievins, D.; De Vries, J.; Peters, A.; Geisbüsch, P.; Reijnen, M. Extended use of endovascular aneurysm sealing for ruptured abdominal aortic aneurysms. Semin. Vasc. Surg. 2016, 29, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Isogai, N.; Kume, N.; Shibutani, S.; Yashiro, H.; Takahara, M.; Fujimura, N. Evaluating the Effectiveness and Clinical Outcomes of Endovascular Aneurysm Repair-First Approach for Ruptured Abdominal Aortic Aneurysm in Japan. J. Endovasc. Ther. 2024, 15266028241248337. [Google Scholar] [CrossRef]
- McGreevy, D.; Pirouzram, A.; Gidlund, K.; Nilsson, K.; Hörer, T. A 12-year experience of endovascular repair for ruptured abdominal aortic aneurysms in all patients. J. Vasc. Surg. 2022, 77, 741–749. [Google Scholar] [CrossRef]
- Veith, F.; Veith, F.; Lachat, M.; Mayer, D.; Malina, M.; Holst, J.; Mehta, M.; Verhoeven, E.; Larzon, T.; Gennai, S.; et al. Collected World and Single Center Experience With Endovascular Treatment of Ruptured Abdominal Aortic Aneurysms. Ann. Surg. 2009, 250, 818–824. [Google Scholar] [CrossRef]
- Chaikof, E.; Dalman, R.; Eskandari, M.; Jackson, B.; Lee, A.; Mansour, A.M.H.; Mastracci, T.; Mell, M.; Murad, H.; Nguyen, L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Peppelenbosch, N.; Yilmaz, N.; Marrewijk, C.; Buth, J.; Cuypers, P.; Duijm, L.; Tielbeek, A. Emergency treatment of acute symptomatic or ruptured abdominal aortic aneurysm. Outcome of a prospective intent-to-treat by EVAR protocol. Eur. J. Vasc. Endovasc. Surg. 2003, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Cilingiroglu, M.; Inanc, H. Local or general anesthesia for EVAR in patients with ruptured AAA? Catheter. Cardiovasc. Interv. 2022, 100, 938. [Google Scholar] [CrossRef]
- Beck, A.; Sedrakyan, A.; Mao, J.; Venermo, M.; Faizer, R.; Debus, S.; Behrendt, C.; Scali, S.; Altreuther, M.; Schermerhorn, M.; et al. Variations in Abdominal Aortic Aneurysm Care: A Report From the International Consortium of Vascular Registries. Circulation 2016, 134, 1948–1958. [Google Scholar] [CrossRef]
- Png, C.; Pendleton, A.; Altreuther, M.; Budtz-Lilly, J.; Gunnarsson, K.; Kan, C.-D.; Khashram, M.; Laine, M.; Mani, K.; Pederson, C.; et al. Effect of EVAR on International Ruptured AAA Mortality—Sex and Geographic Disparities. J. Clin. Med. 2024, 13, 2464. [Google Scholar] [CrossRef]
- Shirasu, T.; Kuno, T.; Yasuhara, J.; Yokoyama, Y.; Takagi, H.; Cullen, M.; Kent, K.; Clouse, W. Recurrent infection is more common after endovascular versus open repair of infected abdominal aortic aneurysm: Systematic review and meta-analysis. J. Vasc. Surg. 2021, 75, 348–355.e10. [Google Scholar] [CrossRef]
- Grootes, I.; Barrett, J.; Ulug, P.; Rohlffs, F.; Laukontaus, S.; Tulamo, R.; Venermo, M.; Greenhalgh, R.M.; Sweeting, M. Predicting Risk of Rupture and Rupture-Preventing Reinterventions Following Endovascular Abdominal Aortic Aneurysm Repair. Br. J. Surg. 2018, 105, 1294–1304. [Google Scholar] [CrossRef]
- Troisi, N.; Bertagna, G.; Torri, L.; Canovaro, F.; D’Oria, M.; Adami, D.; Berchiolli, R. The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge. J. Clin. Med. 2023, 12, 5530. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Xie, C.; Zhao, Y.; Wang, Z. Studies Related to Ruptured Abdominal Aortic Aneurysms in the Past 10 Years (2011-2020): A Bibliometric Analysis. Med. Sci. Monit. 2021, 28, e935006. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Liu, Z.; Ren, J.; Xiang, M.-X. Pharmacological Therapy of Abdominal Aortic Aneurysm: An Update. Curr. Vasc. Pharmacol. 2018, 16, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Jiang, J. Research advances in drug therapy for abdominal aortic aneurysms over the past five years: An updated narrative review. Int. J. Cardiol. 2022, 372, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Russell, H.; Owens, A. Antithrombotic therapy in abdominal aortic aneurysm: Beneficial or detrimental? Blood 2018, 132, 2619–2628. [Google Scholar] [CrossRef]
- Siordia, J.A. Beta-Blockers and Abdominal Aortic Aneurysm Growth: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rev. 2021, 17, e230421187502. [Google Scholar] [CrossRef]
- Mieth, A.; Revermann, M.; Babelova, A.; Weigert, A.; Schermuly, R.T.; Brandes, R.P. L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension 2013, 62, 1098–1104. [Google Scholar] [CrossRef]
- Puertas-Umbert, L.; Almendra-Pegueros, R.; Jimenez-Altayo, F.; Sirvent, M.; Galán, M.; Martínez-González, J.; Rodríguez, C. Novel pharmacological approaches in abdominal aortic aneurysm. Clin. Sci. 2023, 137, 1167–1194. [Google Scholar] [CrossRef]
- Chen, J.; Hu, L.; Liu, Z. Medical treatments for abdominal aortic aneurysm: An overview of clinical trials. Expert Opin. Investig. Drugs 2024, 33, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Sahranavard, T.; Reiner, Ž.; Jamialahmadi, T.; Dhaheri, Y.; Eid, A.; Sahebkar, A. Effect of statins on abdominal aortic aneurysm. Eur. J. Pharm. Sci. 2022, 178, 106284. [Google Scholar] [CrossRef]
- Salata, K.; Syed, M.; Hussain, M.A.; de Mestral, C.; Greco, E.; Mamdani, M.; Tu, J.V.; Forbes, T.L.; Bhatt, D.L.; Verma, S.; et al. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008657. [Google Scholar] [CrossRef] [PubMed]
- Asirwatham, M.; Konanki, V.; Lucas, S.; Grundy, S.; Zwiebel, B.; Shames, M.; Arnaoutakis, D. Comparative Outcomes of Physician-Modified Fenestrated/Branched Endovascular Aortic Aneurysm Repair in the Setting of Prior Failed EVAR. J. Vasc. Surg. 2023, 78, 1153–1161. [Google Scholar] [CrossRef]
- Schanzer, A.; Beck, A.; Eagleton, M.; Farber, M.; Oderich, G.; Schneider, D.; Sweet, M.; Crawford, A.; Timaran, C. Results of fenestrated and branched endovascular aortic aneurysm repair after failed infrarenal endovascular aortic aneurysm repair. J. Vasc. Surg. 2019, 72, 849–858. [Google Scholar] [CrossRef]
- Nana, P.; Kölbel, T.; Behrendt, C.; Kouvelos, G.; Giannoukas, A.; Haulon, S.; Spanos, K. Systematic review on re-intervention with fenestrated or branched devices after failed previous endovascular aortic aneurysm repair. J. Vasc. Surg. 2022, 77, 1806–1814.e2. [Google Scholar] [CrossRef] [PubMed]
- Hostalrich, A.; Mesnard, T.; Soler, R.; Girardet, P.; Kaladji, A.; Baptiste, E.J.; Malikov, S.; Reix, T.; Ricco, J.; Chaufour, X. Prospective Multicentre Cohort Study of Fenestrated and Branched Endografts After Failed Endovascular Infrarenal Aortic Aneurysm Repair with Type Ia Endoleak. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 540–548. [Google Scholar] [CrossRef]
- Juszczak, M.; Vezzosi, M.; Nasr, H.; Claridge, M.; Adam, D. Fenestrated-Branch Endovascular Repair After Prior Abdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 728–737. [Google Scholar] [CrossRef]
- Powell, J.; Wanhainen, A. Analysis of the Differences Between the European Society for Vascular Surgery 2019 and National Institute for Health and Care Excellence 2020 Guidelines for Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 7–15. [Google Scholar] [CrossRef]
- Nana, P.; Volakakis, G.; Spanos, K.; Kouvelos, G.; Bareka, M.; Arnaoutoglou, E.; Giannoukas, A.; Matsagkas, M. Endovascular Repair of Ruptured Abdominal Aortic Aneurysms Using the Endurant™ Endograft. J. Clin. Med. 2024, 13, 5282. [Google Scholar] [CrossRef]
- Kopp, R.; Stachowski, L.; Puippe, G.; Zimmermann, A.; Menges, A. Long-Term Outcomes of Endovascular Aortic Repair with Parallel Chimney or Periscope Stent Grafts for Ruptured Complex Abdominal Aortic Aneurysms. J. Clin. Med. 2025, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Mosher, E.; Reitz, K.; Andraska, E.; Sridharan, N.; Alie-Cusson, F.; Makaroun, M.; Liang, N. Parallel grafting in ruptured aortic aneurysms with previous open infrarenal surgical repair. Ann. Vasc. Surg.—Brief Rep. Innov. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Jennings, J.; Sheahan, L.; Gloss, C.; Vogel, T.; Bath, J. Multiple Chimney Endografts (ChEVAR) for Ruptured Pararenal Aortic Aneurysm. Ann. Vasc. Surg. 2021, 75, 531.e1–531.e6. [Google Scholar] [CrossRef]
- Jernigan, E.G.; Nguyen Tran, N.; Qato, K.; Giangola, G.; Carroccio, A.; Conway, A.M. Outcomes of chimney/snorkel endovascular repair for symptomatic and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2021, 74, 1117–1124. [Google Scholar] [CrossRef]
- Touma, J.; Caradu, C.; Sylvestre, R.; Settembre, N.; Schneider, F.; Moia, A.; Ahmed, B.; Lebas, B.; Gaudric, J.; Alsac, J.; et al. Multicentre Experience with the Chimney Technique for Abdominal Aortic Aneurysms in French University Hospitals. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 776–784. [Google Scholar] [CrossRef]
- Lindblad, B.; Jabr, A.; Holst, J.; Malina, M. Chimney Grafts in Aortic Stent Grafting: Hazardous or Useful Technique? Systematic Review of Current Data. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 722–731. [Google Scholar] [CrossRef]
- Sierra-Juárez, M.; Valderrama-Treviño, A.; González-Martínez, I.; Mendoza-Barrera, G.; Ramos-Peralta, M. Initial experience of endovascular aneurysm repair at the Dr. Eduardo Liceaga General Hospital of Mexico. Int. Surg. J. 2024, 11, 697–702. [Google Scholar] [CrossRef]
- Rabellino, M.; Chiabrando, J.; Garagoli, F.; Foscolo, M.A.; Fleitas, M.; Chas, J.; Caro, V.; Bluro, I.; Shinzato, S. Incidence of endoleak type IA in patients undergoing chimney endovascular aortic repair (ChEVAR) vs. standard endovascular repair. Arch. Peru. Cardiol. Y Cirugía Cardiovasc. 2024, 5, 22–28. [Google Scholar] [CrossRef]
- Kimball, A.; Mydłowska, A.; Beck, A. Physician-modified endografts for urgent and emergent aortic pathology. Semin. Vasc. Surg. 2021, 34, 215–224. [Google Scholar] [CrossRef]
- Pyun, A.; Han, S. Contemporary indications, techniques, and outcomes of physician-modified endografts for the treatment of complex abdominal and thoracoabdominal aortic aneurysms. Semin. Vasc. Surg. 2022, 35, 364–373. [Google Scholar] [CrossRef]
- Solano, M.A.; Keller, M.M.; Colon, M.J.P.; Patel, M.R.; Timaran, M.C.; Kirkwood, M.M.; Baig, M.S. Physician Modified Endograft for Ruptured Dissecting Aortic Arch Aneurysm. Vasc. Endovasc. Surg. 2024, 58, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, J.; Panuccio, G.; Kölbel, T.; Gandet, T.; Heidemann, F.; Rohlffs, F. Physician-Modified Endograft With Inner Branches for the Treatment of Complex Aortic Urgencies. J. Endovasc. Ther. 2021, 29, 697–704. [Google Scholar] [CrossRef]
- Melo, G.; Prendes, C.; Caldeira, D.; Stana, J.; Rantner, B.; Wanhainen, A.; Oderich, G.; Tsilimparis, N. Systematic Review and Meta-analysis of Physician Modified Endografts for Treatment of Thoraco-Abdominal and Complex Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 188–199. [Google Scholar] [CrossRef]
- Oderich, G.; Ribeiro, M. Techniques of Physician-Modified Endovascular Grafts (PMEGs) for Incorporation of Renal Mesenteric Arteries. In Endovascular Aortic Repair: Current Techniques with Fenestrated, Branched and Parallel Stent-Grafts; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 671–688. [Google Scholar] [CrossRef]
- Gray, D.; Samaan, C.; Oikonomou, K.; Gruber-Rouh, T.; Schmitz-Rixen, T.; Derwich, W. Age and Oversizing Influence Iliac Dilatation after EVAR. J. Clin. Med. 2022, 11, 7113. [Google Scholar] [CrossRef] [PubMed]
- Bahroloomi, D.; Qato, K.; Nguyen, N.; Schreiber-Gregory, D.; Conway, A.; Giangola, G.; Carroccio, A. External Iliac Extension Causes Greater Aneurysm Sac Regression than Bell-bottom Technique or Iliac Branch Endoprosthesis for Repair of Concomitant Infra-renal Aortic and Iliac Artery Aneurysm. J. Vasc. Surg. 2022, 76, 132–140. [Google Scholar] [CrossRef]
- Naughton, P.; Park, M.; Kheirelseid, E.; O’Neill, S.; Rodriguez, H.; Morasch, M.; Madhavan, P.; Eskandari, M. A comparative study of the bell-bottom technique vs hypogastric exclusion for the treatment of aneurysmal extension to the iliac bifurcation. J. Vasc. Surg. 2012, 55, 956–962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mascoli, C.; Faggioli, G.; Gallitto, E.; Longhi, M.; Abualhin, M.; Pini, R.; Massoni, C.; Freyrie, A.; Stella, A.; Gargiulo, M. Planning and Endograft Related Variables Predisposing to Late Distal Type I Endoleaks. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 334–342. [Google Scholar] [CrossRef]
- Lee, C.; Dougherty, M.; Calligaro, K. Concomitant unilateral internal iliac artery embolization and endovascular infrarenal aortic aneurysm repair. J. Vasc. Surg. 2006, 43, 903–907. [Google Scholar] [CrossRef][Green Version]
- Fontana, F.; Coppola, A.; Ferrario, L.; De Marchi, G.; Macchi, E.; Zorzetto, G.; Franchin, M.; Piffaretti, G.; Tozzi, M.; Carcano, G.; et al. Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome. J. Clin. Med. 2022, 11, 7399. [Google Scholar] [CrossRef]
- Armbruster, C. Outcomes of Elective Abdominal Aortic Aneurysm Management: Open Surgical Versus Endovascular Repair. 2021. Available online: https://idun.augsburg.edu/cgi/viewcontent.cgi?article=2103&context=etd (accessed on 15 April 2025).
- Visser, J.; Bosch, J.; Hunink, M.; Van Dijk, L.; Hendriks, J.; Poldermans, D.; Van Sambeek, M. Endovascular repair versus open surgery in patients with ruptured abdominal aortic aneurysms: Clinical outcomes with 1-year follow-up. J. Vasc. Surg. 2006, 44, 1148–1155. [Google Scholar] [CrossRef][Green Version]
- Capoccia, L.; Riambau, V. Endovascular repair versus open repair for inflammatory abdominal aortic aneurysms. Cochrane Database Syst. Rev. 2015, 2015, CD010313. [Google Scholar] [CrossRef] [PubMed]
- Sommer, W.; Becker, C.; Haack, M.; Rubin, G.; Weidenhagen, R.; Schwarz, F.; Nikolaou, K.; Reiser, M.; Johnson, T.; Clevert, D. Time-resolved CT angiography for the detection and classification of endoleaks. Radiology 2012, 263, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Zaiem, F.; Almasri, J.; Tello, M.; Prokop, L.; Chaikof, E.; Murad, M. A systematic review of surveillance after endovascular aortic repair. J. Vasc. Surg. 2018, 67, 320. [Google Scholar] [CrossRef] [PubMed]
- Mirza, T.; Karthikesalingam, A.; Jackson, D.; Walsh, S.; Holt, P.; Hayes, P.; Boyle, J. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: Systematic review and bivariate meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 418–428. [Google Scholar] [CrossRef]
- Brazzelli, M.; Hernández, R.; Sharma, P.; Robertson, C.; Shimonovich, M.; Maclennan, G.; Fraser, C.; Jamieson, R.; Vallabhaneni, S. Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: A systematic review and economic evaluation. Health Technol. Assess. 2018, 22, 1–220. [Google Scholar] [CrossRef]
- Kapetanios, D.; Kontopodis, N.; Mavridis, D.; McWilliams, R.G.; Giannoukas, A.D.; Antoniou, G.A. Meta-analysis of the accuracy of contrast-enhanced ultrasound for the detection of endoleak after endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 280–294.e286. [Google Scholar] [CrossRef]
- Gürtler, V.; Sommer, W.; Meimarakis, G.; Kopp, R.; Weidenhagen, R.; Reiser, M.; Clevert, D. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. J. Vasc. Surg. 2013, 58, 340–345. [Google Scholar] [CrossRef]
- Väärämäki, S.; Uurto, I.; Hahl, T.; Suominen, V. Reliability and safety of individualized follow-up based on the 30-day CTA after endovascular aneurysm repair (EVAR). Ann. Vasc. Surg. 2022, 86, 305–312. [Google Scholar] [CrossRef]
- Grima, M.; Karthikesalingam, A.; Holt, P. Multicentre Post-EVAR Surveillance Evaluation Study (EVAR-SCREEN). Eur. J. Vasc. Endovasc. Surg. 2019, 57, 521–526. [Google Scholar] [CrossRef]
- Geraedts, A.; De Mik, S.; Ubbink, D.; Koelemay, M.; Balm, R. Postoperative surveillance and long-term outcome after endovascular aortic aneurysm repair in the Netherlands: Study protocol for the retrospective ODYSSEUS study. BMJ Open 2020, 10, e033584. [Google Scholar] [CrossRef]
- Blecha, M.; Scali, S.; Stone, D.; Mao, J.; Goodney, P.; Lemmon, G. Duplex Ultrasound Only Surveillance After Endovascular Abdominal Aortic Aneurysm Repair is Associated with Favorable Long-Term Outcomes. Ann. Vasc. Surg. 2024, 108, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, W.; Kazimierczak, N.; Serafin, Z. Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair. J. Clin. Med. 2023, 12, 7766. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, S.; Perisinakis, K.; Kontopodis, N.; Papadakis, A.E.; Maris, T.G.; Ioannou, C.V.; Karantanas, A.; Tsetis, D. Dual-energy CT angiography in imaging surveillance of endovascular aneurysm repair–preliminary study results. Eur. J. Radiol. 2022, 148, 110165. [Google Scholar] [CrossRef]
- Rakemaa, L.; Laukontaus, S.; Aho, P.; Tulamo, R.; Laine, M.; Venermo, M. Ultrasound Surveillance is Feasible After Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2019, 58, e651–e652. [Google Scholar] [CrossRef]
- Bredahl, K.; Taudorf, M.; Lönn, L.; Vogt, K.; Sillesen, H.; Eiberg, J. Contrast Enhanced Ultrasound can Replace Computed Tomography Angiography for Surveillance After Endovascular Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 729–734. [Google Scholar] [CrossRef]
- Harrison, G.; Oshin, O.; Vallabhaneni, S.; Brennan, J.; Fisher, R.; McWilliams, R. Surveillance after EVAR based on duplex ultrasound and abdominal radiography. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 187–192. [Google Scholar] [CrossRef]
- Secchi, F.; Capra, D.; Monti, C.B.; Mobini, N.; Ortiz, M.D.M.G.; Trimarchi, S.; Mazzaccaro, D.; Righini, P.; Nano, G.; Sardanelli, F. Safe follow-up after endovascular aortic repair with unenhanced MRI: The SAFEVAR study. Diagnostics 2022, 13, 20. [Google Scholar] [CrossRef]
- Andersson, M.; Talvitie, M.; Benson, L.; Roy, J.; Roos, H.; Hultgren, R. A Population Based Study of Post-EVAR Rupture during 15 years. J. Vasc. Surg. 2021, 74, 701–710.e3. [Google Scholar] [CrossRef]
- Hinchliffe, R.; Bruijstens, L.; MacSweeney, S.; Braithwaite, B. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm—Results of a pilot study and lessons learned for future studies. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 506–513. [Google Scholar] [CrossRef]
- Schlösser, F.; Gusberg, R.; Dardik, A.; Lin, P.; Verhagen, H.; Moll, F.; Muhs, B. Aneurysm rupture after EVAR: Can the ultimate failure be predicted? Eur. J. Vasc. Endovasc. Surg. 2009, 37, 15–22. [Google Scholar] [CrossRef]
- Mehta, M.; Paty, P.; Roddy, S.; Taggert, J.; Sternbach, Y.; Kreienberg, P.; Chang, B.; Darling, R. Treatment options for delayed AAA rupture following endovascular repair. J. Vasc. Surg. 2011, 53, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Criado, F.; Wilson, E.; Velazquez, O.; Carpenter, J.; Barker, C.; Wellons, E.; Abul-Khoudoud, O.; Fairman, R. Safety of coil embolization of the internal iliac artery in endovascular grafting of abdominal aortic aneurysms. J. Vasc. Surg. 2000, 32, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, K.; Sfyroeras, G.; Zambas, N.; Konstantinidis, K.; Kakkos, S.; Mitka, M. Outcomes of endovascular aneurysm repair with selective internal iliac artery coverage without coil embolization. J. Vasc. Surg. 2012, 56, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Shehata, R.; Aslam, J.; Beng, N. 276 Favorable Outcomes for Endovascular Aneurysm Repair with Aorto-Uni-Iliac (AUI) Stent Grafts for Treatment of Abdominal Aortic Aneurysms (AAA) by a Single Team in 8 Years at South Mersey Arterial (SMArt): A Tertiary Care Vascular Surgery Centre. Br. J. Surg. 2023, 110, znad258.755. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramses, R.; Agu, O. Beyond Endoleaks: A Holistic Management Approach to Late Abdominal Aortic Aneurysm Ruptures After Endovascular Repair. J. Vasc. Dis. 2025, 4, 24. https://doi.org/10.3390/jvd4030024
Ramses R, Agu O. Beyond Endoleaks: A Holistic Management Approach to Late Abdominal Aortic Aneurysm Ruptures After Endovascular Repair. Journal of Vascular Diseases. 2025; 4(3):24. https://doi.org/10.3390/jvd4030024
Chicago/Turabian StyleRamses, Rafic, and Obiekezie Agu. 2025. "Beyond Endoleaks: A Holistic Management Approach to Late Abdominal Aortic Aneurysm Ruptures After Endovascular Repair" Journal of Vascular Diseases 4, no. 3: 24. https://doi.org/10.3390/jvd4030024
APA StyleRamses, R., & Agu, O. (2025). Beyond Endoleaks: A Holistic Management Approach to Late Abdominal Aortic Aneurysm Ruptures After Endovascular Repair. Journal of Vascular Diseases, 4(3), 24. https://doi.org/10.3390/jvd4030024





