1. Introduction
Mycotic aneurysms, which are arterial dilatations resulting from infection, represent rare but potentially devastating vascular lesions [
1].
Staphylococcus aureus is among the most commonly implicated pathogens, with the risk of development heightened in individuals experiencing recurrent bacteremia or possessing compromised immune systems [
2]. In the case of this patient, repeated episodes of methicillin-resistant
Staphylococcus aureus (MRSA) bacteremia raised suspicions that previously implanted subclavian artery stent grafts may have become infected, leading to further aneurysmal degeneration and ultimately resulting in contained ruptures of both the right and left subclavian arteries [
3].
Upper extremity deep vein thrombosis (UEDVT) is relatively uncommon compared to lower extremity deep vein thrombosis (DVT); however, it is increasingly observed in patients with long-term indwelling venous catheters, malignancies, or conditions that induce a hypercoagulable state [
4]. The presence of DVT in proximity to major arterial pathologies, such as mycotic aneurysms, complicates standard clinical management pathways significantly. While systemic anticoagulation is typically recommended for the prevention and treatment of DVT [
5,
6], in cases involving a partially ruptured aneurysm or infection of an endovascular stent, the risk of catastrophic hemorrhage may outweigh the benefits of thrombus prevention [
7].
We describe a 75-year-old immunocompromised male with lung adenocarcinoma and cutaneous squamous cell carcinoma on immunotherapy, who developed recurrent MRSA bacteremia in the setting of bilateral subclavian artery stent grafts. His course was complicated by UEDVT ipsilateral to the vascular pathology, contained ruptures of bilateral subclavian artery mycotic aneurysms, and persistent concerns for infected stent grafts with endoleak. This case highlights the diagnostic and therapeutic challenges of managing concurrent arterial infections, venous thrombosis, and iatrogenic vascular hardware in immunocompromised hosts. It underscores the necessity of multidisciplinary care to balance anticoagulation for thrombotic complications against the hemorrhage risk of mycotic aneurysms, while emphasizing the urgency of timely surgical intervention in graft infections.
2. Case Report
A 75-year-old Caucasian male presented to the Emergency Department (ED) with complaints of persistent swelling and pain in the right arm, persisting for over 1 week. He reported a sensation of a tight, swollen knot in the axillary region. His medical history is notable for lung cancer and squamous cell carcinoma of the skin, both of which required immunotherapy administered through long-term venous access devices. Additionally, the patient suffers from chronic hypoxic respiratory failure, necessitating the use of 2 L of home oxygen. The patient had no known drug allergies. Of particular concern is his history of two recurrent episodes of MRSA bacteremia within the past 6 months, which required multiple courses of intravenous antibiotic therapy. Furthermore, he underwent bilateral subclavian artery stent placement 7 months prior for aneurysmal disease, and he had a peripherally inserted central catheter (PICC) line in place for several months for long-term immunotherapy access, which was removed 2 days ago.
On arrival, the patient’s blood pressure measured 80/51 mm Hg, heart rate was 110 beats per minute, respiratory rate was 20 breaths per minute, temperature was 36.8 °C, and oxygen saturation was 95% on room air. He appeared systemically unwell, with acute distress stemming from the pain and swelling in his right arm. Laboratory data revealed a leukocytosis of 19.3 × 10
3/µL with significant bandemia (16%,
Table 1), an initial hemoglobin concentration of 9.1 g/dL, and a platelet count of 520 × 10
3/µL suggestive of reactive thrombocytosis. Blood cultures drawn in the ED eventually grew MRSA, consistent with his prior episodes of bacteremia 1 month and 3 months ago. Chest X-ray showed pulmonary fibrosis with some asymmetric increased opacity in the left lung base and possibly superimposed pneumonia or pulmonary edema (
Supplementary Figure S1).
For the antibiotics regimen, the patient received initial antibiotic treatment via a peripheral IV line in the ER, including cefazolin (Ancef) and cefepime (Maxipime). In addition, the patient was initiated on intravenous vancomycin on hospital day 0. Vancomycin was administered exclusively via a peripheral IV line throughout hospitalization. Renal function remained stable, and serum creatinine levels were closely monitored (as shown in
Table 1).
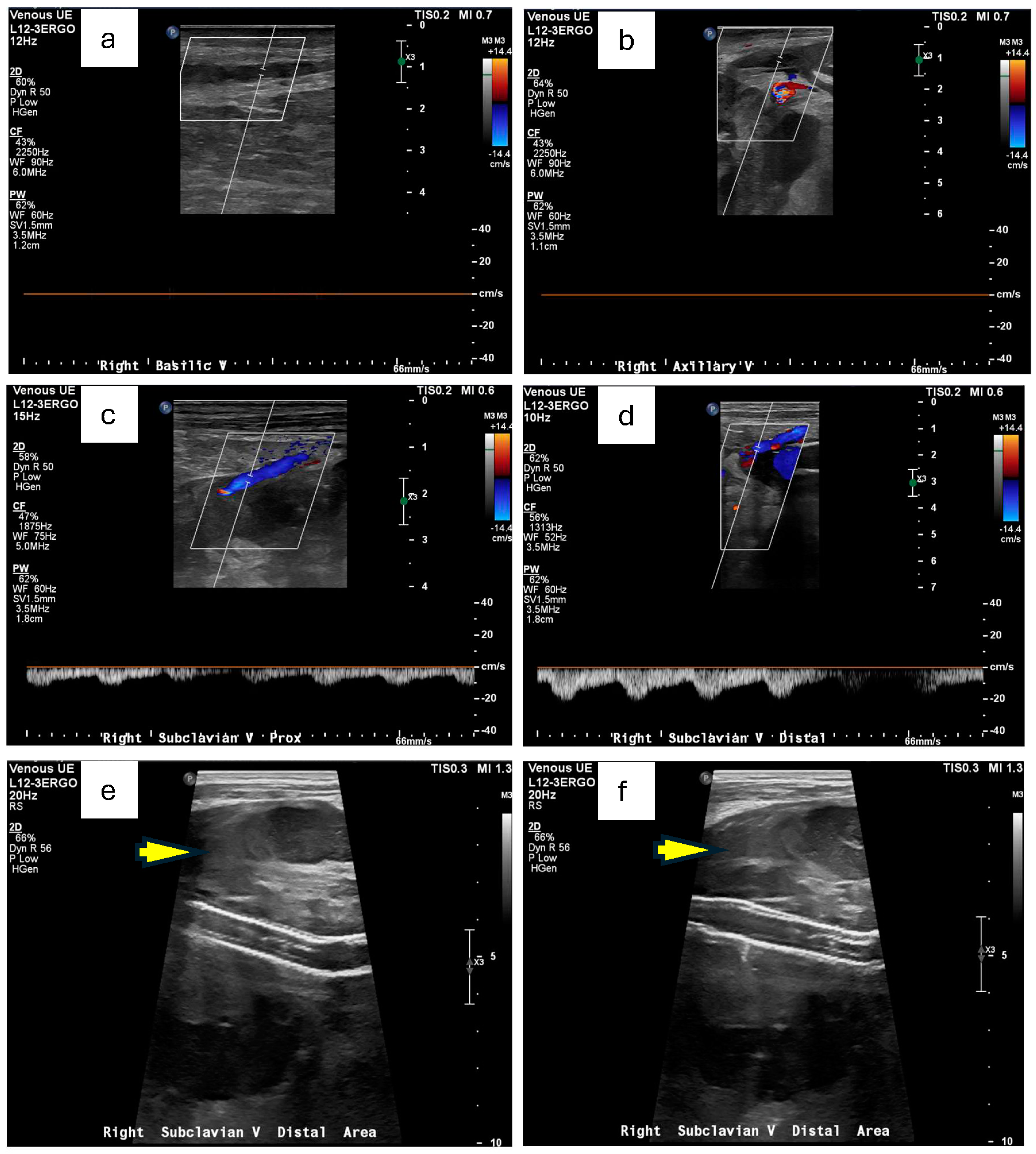
Doppler ultrasound of the right upper extremity confirmed an occlusive deep vein thrombosis of the right axillary vein and a concurrent occlusive superficial thrombosis in the basilic vein (
Figure 1a,b). There was also the suggestion of a perivascular hematoma in the region of his subclavian artery stent (
Figure 1e,f). Concerned about a possible arterial origin for the hematoma, Vascular Surgery was consulted. A subsequent CT angiogram of the right extremities identified contained ruptures in the right subclavian arteries near the existing stent-grafts, explaining his acute upper extremity swelling and pain (
Figure 2a,b). Baseline serum creatinine on admission was approximately 0.84 mg/dL and remained stable (0.71 mg/dL the next day) after this contrast-enhanced imaging, indicating preserved renal function post-CTA (
Table 1).
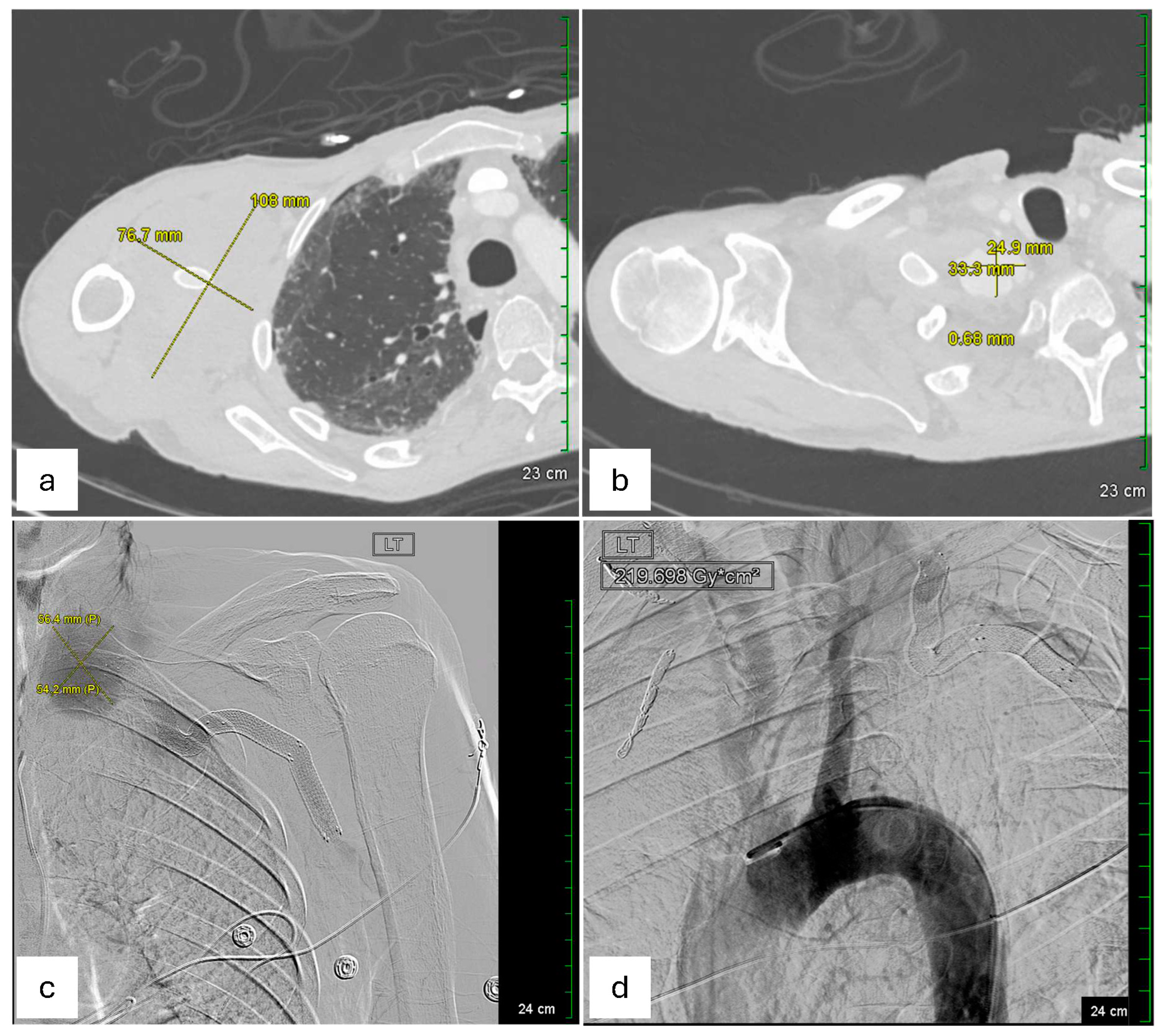
Given the symptomatic presentation and partial rupture of the right subclavian artery aneurysm, an emergent endovascular intervention was performed on the day of admission after obtaining informed consent. The procedure was performed under moderate sedation for 3 h. Vascular access was obtained via ultrasound-guided retrograde right common femoral artery access, and a pigtail catheter was advanced into the proximal ascending aorta for arch and thoracic aorta angiography. Selective catheterization of the right subclavian artery confirmed a large, contained rupture in the mid-subclavian segment, approximately 1 cm distal to the vertebral artery takeoff. Intravascular ultrasound (IVUS) was performed to delineate vessel dimensions and the aneurysm’s relation to the vertebral artery. The intervention involved embolization of multiple collateral branches (right transverse cervical, suprascapular, and internal mammary arteries) using Ruby coils to minimize endoleak risk. Subsequently, an 11 × 59 mm VBX stent (Flagstaff, AZ, USA) was deployed in the mid-subclavian artery with post-stent balloon angioplasty using a 12 × 40 mm balloon. A completion angiogram confirmed successful exclusion of the aneurysm without evidence of endoleak, with brisk antegrade flow into the vertebral artery and upper extremity vasculature. The procedure concluded with percutaneous closure of the right femoral artery using ProGlide Perclose. This procedure succeeded in restoring normal arterial flow and preventing further bleeding. Notably, pre-procedural IR imaging also identified a large left subclavian artery aneurysm, measured to be 56.4 mm * 54.2 mm in dimension, with a contained rupture at its mid-segment, approximately 1.5–2 cm distal to the vertebral artery origin (
Figure 2c). The left-sided aneurysm was not well visualized on CTA of the right extremities but was confirmed on angiography.
On the next day (hospital day 1), a similar endovascular approach was used, again via ultrasound-guided retrograde right common femoral artery access. The procedure lasted 2 h and 28 min under moderate sedation with a total of 2 mg of midazolam and 100 mcg of fentanyl administered. Arch angiography and IVUS confirmed a large, contained rupture in the mid-segment of the left subclavian artery, approximately 1.5–2 cm distal to the vertebral artery takeoff. The placement of the stent was complicated by an area of stenosis distal to the aneurysm, which impeded stent advancement. Multiple sheath exchanges and pre-dilation balloon angioplasty with a 4 × 40 mm balloon were required, adding an additional 60 min to the case. Once adequate luminal diameter was achieved, an 11 × 59 mm VBX stent (Gore Medical) was successfully deployed, followed by post-stent balloon angioplasty to 11 mm. The final angiogram confirmed brisk flow through the left subclavian artery, with complete aneurysm exclusion and preserved antegrade flow into the vertebral artery and left internal mammary artery (LIMA). The procedures were well tolerated, with minimal estimated blood loss. The patient remained hemodynamically stable throughout and was transferred to the ICU in stable condition postoperatively.
Despite these technically successful procedures, the patient’s blood cultures remained positive for MRSA until day 4 of hospitalization, raising ongoing concern for an infected stent graft. The Infectious Disease service was consulted and recommended repeated blood cultures to assess for persistent bacteremia every other day and intravenous vancomycin for at least 6 weeks once repeat blood cultures are negative for 72 h. Due to the concern about cardiac vegetation, transthoracic echocardiography was performed on day 3 of hospitalization, which demonstrated the normal size of ventricles and upper chambers with normal valvular structure and function for his age. Management of the coexisting axillary DVT posed a considerable challenge. Standard therapy would involve anticoagulation to mitigate the risk of thrombus propagation or pulmonary embolism, yet newly placed stent-grafts in arteries prone to re-rupture meant that aggressive anticoagulation carried a prohibitively high risk of bleeding.
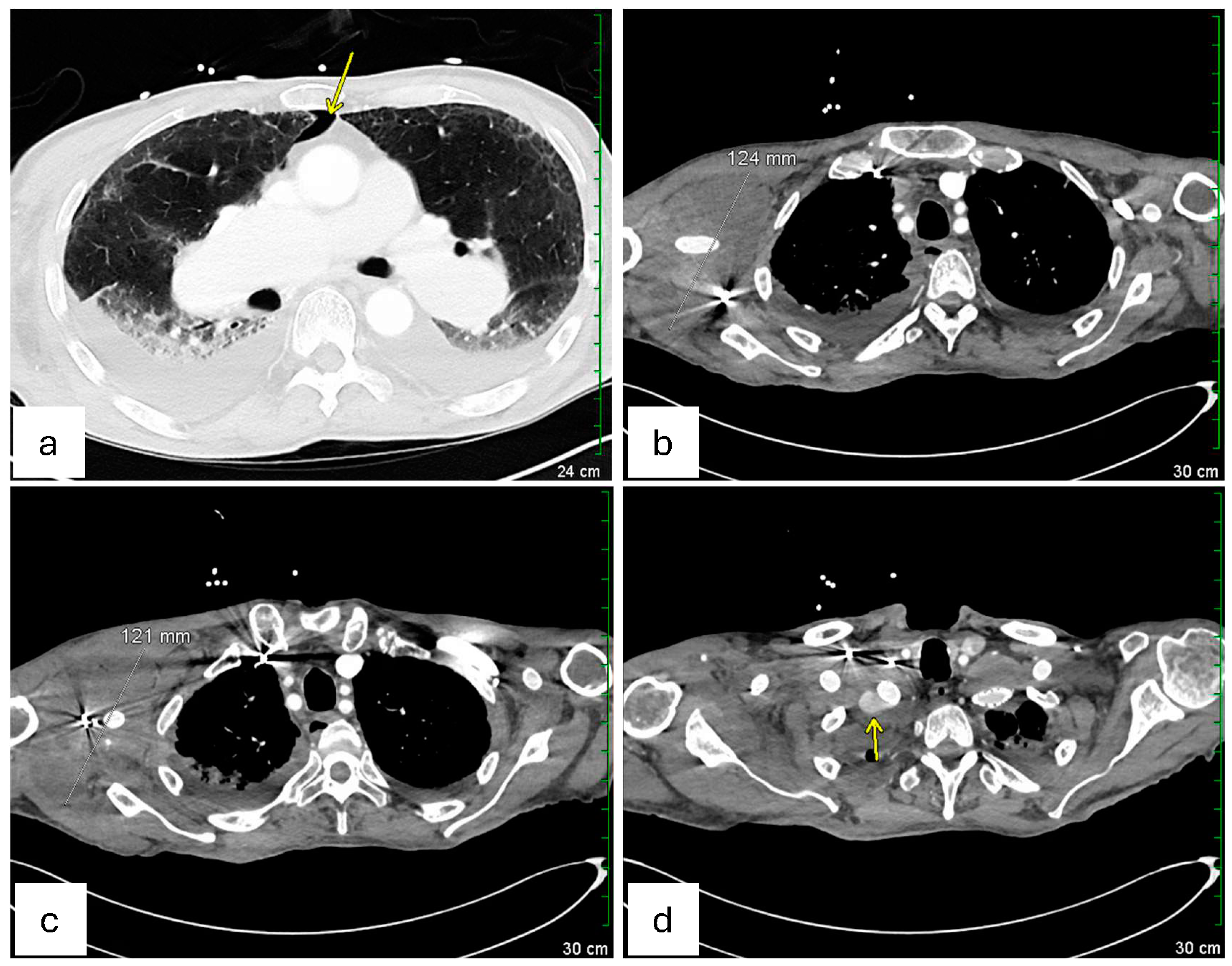
On hospital day 4 (3 days after endovascular repair of the left subclavian artery aneurysm), a repeat CT scan (
Figure 3) was performed. Axial chest images (
Figure 3a) showed a trace right pneumothorax (yellow arrow), unchanged since the day of admission. Sequential axial views (
Figure 3b,c) demonstrated the right axillary hematoma, stable in size since hospital day 2 and without imaging findings suggestive of superinfection. Finally, an axial view of the proximal right subclavian artery (
Figure 3d) revealed a type 1A endoleak in the right subclavian artery stent, indicating a possible proximal seal failure or ongoing infection that prevented full exclusion of the aneurysm (as pointed by the yellow arrow in
Figure 3d). Although the patient’s white blood cell count gradually improved, and follow-up blood cultures eventually turned negative on hospital day 4 (as shown in
Table 1), the possibility of a mycotic stent graft persisted. Vascular Surgery concluded that definitive source control might necessitate surgical graft explanation with extensive debridement, a high-risk procedure best conducted in a tertiary or quaternary center.
On hospital day 9, the patient was transferred to a specialized facility capable of advanced cardiothoracic and vascular reconstructive surgery, with a plan to continue intravenous antibiotics and closely monitor his condition. Plans included continued intravenous antibiotics, close hemodynamic and infectious monitoring, and readiness for possible open surgical intervention if imaging or clinical status suggested persistent graft infection or inadequate stent exclusion of the aneurysms. A summary of the Laboratory values, blood cultures, and treatment timeline over hospitalization is present in
Table 1.
3. Discussion
The case of bilateral mycotic subclavian artery aneurysms (SAAs) in a background of recurrent MRSA bacteremia and concomitant right UEDVT highlights the complexity of balancing hemorrhagic and thrombotic risks in a critically ill patient. Although occlusive deep vein thrombosis typically calls for anti-coagulation, the looming threat of subclavian artery rupture, especially in stent grafts that may harbor persistent infection, forces a more cautious, individualized approach to management [
8]. The presence of persistent MRSA bacteremia further complicates the scenario, as infected vascular grafts rarely respond to antibiotics alone, often requiring surgical debridement or graft explantation for definitive source control [
9]. For instance, antibiotics therapy alone for a mycotic aneurysm carries a high failure rate, with reported mortality around 50% [
10]. Therefore, while prolonged antimicrobial therapy (a minimum of 6–8 weeks intravenously) is essential, it is usually insufficient by itself; most patients ultimately require surgical excision of the infected tissue for cure [
10]. In cases where surgery is not possible, an indefinite suppressive antibiotic regimen may be attempted, but this is considered a last resort and is associated with a substantial risk of recurrent infection [
10].
Traditionally, the management of SAA has involved open surgical repair, with the surgical approach determined by the aneurysm’s anatomical location. Extrathoracic aneurysms are generally accessed via infra- or supraclavicular incisions, whereas intrathoracic aneurysms necessitate thoracotomy or sternotomy for adequate exposure [
11,
12,
13]. These surgical interventions, however, are associated with significant risks, including ischemic complications, laryngeal nerve injury, and chylothorax [
14]. In recent years, endovascular repair has emerged as a viable alternative, characterized by minimally invasive stent placement through femoral or brachial arterial access, thereby obviating the need for surgical incisions in the neck or chest [
12,
13]. For patients with challenging access due to aortic or arterial tortuosity, the “through-and-through” technique, which involves passing a stiff wire from one arterial access site to another, has been described to facilitate device delivery [
12]. Although long-term comparative data between endovascular and open repair remain limited, emerging evidence indicates that endovascular approaches may offer reduced recurrence, and fewer complications, particularly in suitable surgical candidates [
11,
14,
15,
16,
17]. One study noted zero 30-day mortality in both open and endovascular treatments of subclavian artery aneurysms, with long-term primary patency rates of 91% for open repair and 100% for endovascular repair [
18]. Likewise, a single-center experience reported a high technical success rate and favorable mid-term outcomes for endovascular stent grafting of SAAs [
13].
However, endovascular management of mycotic aneurysms presents distinct challenges. Although covered stents are frequently utilized to exclude the aneurysm, they are prone to complications such as thrombosis, stenosis, endoleaks, and embolization. Additionally, infection of the prosthetic stent, particularly by virulent pathogens like MRSA, poses a significant risk and often necessitates explantation and revascularization [
19]. One systemic review reported that although EVAR demonstrated lower intraoperative (1.0% vs. 1.8%) and early mortality rates (6.5% vs. 15.9%) compared to OSR, it was associated with higher late sepsis rates (5.7% vs. 0.9%) and reintervention rates (17.6% vs. 7.3%) [
20]. Another study indicates that MRSA infection portends a poor prognosis after EVAR of mycotic aortic aneurysms and aortic graft infections, suggesting that OSR may be more appropriate in patients with MRSA bacteremia [
21].
In this instance, an emergent endovascular repair was conducted as a life-saving intervention to avert imminent exsanguination. Nevertheless, persistent MRSA bacteremia despite antimicrobial therapy raised concerns about ongoing graft infection, which is rarely resolved with antibiotics alone [
19]. The gold standard for definitive treatment in such scenarios remains the surgical removal of the infected stent, accompanied by extensive debridement and followed by either in situ or extra-anatomic revascularization [
22]. Given the considerable morbidity associated with these procedures, especially in elderly patients or those with multiple comorbidities, the decision to pursue open surgical intervention must be carefully considered in light of the patient’s overall prognosis. In choosing the method of reconstruction after infected graft removal, surgeons often favor graft materials that resist infection, such as autologous vein grafts (for example, a great saphenous vein or femoral vein harvest) or cryopreserved arterial allografts. These conduits provide durable revascularization while minimizing reinfection risk [
23]. In some cases, an extra-anatomic bypass (e.g., a carotid-to-axillary bypass) may be performed to restore blood flow without placing graft material in a contaminated field [
24].
This case underscores the critical need for maintaining a high degree of clinical suspicion for arterial pathology in patients who present with unilateral extremity swelling, particularly when this is coupled with recurrent bloodstream infections and a history of vascular interventions. Prompt diagnostic imaging, such as Doppler ultrasound and CT angiography, is essential for the early detection of vascular abnormalities. Although endovascular repair provides a swift and minimally invasive option for acute cases, the long-term management hinged upon effective infection control–either by eradicating the infection with antibiotics or by removing the infected graft. In our patient’s case, transfer to a tertiary center allowed for definitive surgical management if needed. Had there been evidence of persistent infection, the plan was to explant the stent grafts and reconstruct the subclavian arteries with an appropriate graft, as outlined above. Such an open surgical approach carries substantial risk but offers the best chance of cure when a prosthetic graft is infected [
10]. If the infection had been completely controlled with antibiotics alone, surgery could potentially be avoided; however, that strategy would require prolonged suppressive antibiotics and vigilant follow-up given the high risk of relapse. Reported experiences in infected aneurysms emphasize that timely surgical intervention, when feasible, significantly improves outcomes over medical therapy alone [
10]. Thus, at the specialized center, the team’s plan was to continue IV antibiotics and proceed with open surgical debridement and graft replacement if any sign of ongoing infection or aneurysm expansion was noted. In complex and high-risk scenarios, referral to a tertiary care center with specialized expertise in advanced vascular reconstruction is often the most effective approach to restoring vascular integrity and optimizing patient outcomes.
4. Conclusions
This case exemplifies the intricate interplay among vascular infection, thrombosis, and aneurysmal pathology in an immunocompromised patient experiencing recurrent MRSA bacteremia. Although emergent endovascular repair effectively reduced the immediate risk of hemorrhage, the persistence of bacteremia and the development of an endoleak raised concerns regarding graft infection, highlighting the limitations of stent-based interventions in the management of mycotic aneurysms. While endovascular techniques present a minimally invasive alternative to open repair, they may not ensure definitive infection control, often necessitating subsequent surgical explantation and revascularization.
Given the high morbidity associated with open surgical procedures, treatment strategies should be individualized and informed by a multidisciplinary team comprising vascular surgeons, infectious disease specialists, and internal medicine practitioners. Early recognition, prompt imaging, and coordinated management are crucial for optimizing outcomes in such high-risk cases. For patients with infected vascular grafts, referral to a specialized center with advanced surgical expertise should be considered to achieve definitive infection control and restore vascular integrity.