Abstract
Purpose: This study investigated the impact of two different resistance training (RT) protocols on cardiac autonomic modulation during exercise recovery in trained individuals. It was hypothesized that a hypertrophic resistance training program would induce more significant stress and negatively affect cardiac autonomic modulation compared to a power/force resistance training program. Methods: Six healthy, trained participants (aged 18–40) were randomized in a crossover and controlled pilot study. Participants performed two RT protocols: (i) three sets of 10 repetitions with 85% of 10 RM, 60 s inter-set rest (3x1060s) and (ii) eight sets of three repetitions with 85% of 3 RM, 120 s inter-set rest (8x3120s). Heart rate variability (HRV) was measured before and 30 min after each RT session. Results: Significant reductions in HRV parameters (RMSSD, HF, and SD1) were observed following the 3x1060s protocol (hypertrophic design) compared to baseline. Conversely, the 8x3120s (power/force design) protocol did not show significant changes in HRV parameters. A significant interaction effect for time and RT protocol was found for all HRV measures with more significant reductions observed after 3x1060s compared to 8x3120s. Conclusions: The hypertrophic RT session (3x1060s) significantly reduced HRV parameters, suggesting higher physiological stress and potentially negative implications for cardiac autonomic recovery than the power/force RT session (8x3120s). These findings highlight the importance of considering exercise intensity and protocol design to manage cardiac autonomic stress during resistance training.
1. Introduction
Branches of the autonomic nervous system, sympathetic and parasympathetic activity, play a crucial role in modulating cardiac function [1,2]. Oscillation in the time interval between heart rate beats (R-R intervals derived from an electrocardiogram) results from complex interactions between sympathetic and parasympathetic influences on the heart [3,4]. Thus, heart rate variability can be non-invasively assessed to interrogate the autonomic response of the heart. Reduced heart rate variability (higher sympathetic activation) is linked with an inadequate adaptation of the cardiovascular system; thus, it has been interpreted as an increase in arrhythmia and sudden cardiac death [5,6]. On the other hand, higher heart rate variability reflects a good state of autonomic control and an adaptative organism [3,4].
Although a physical exercise program is recommended for most of the population to improve health parameters, a high-intensity exercise session induces sympathetic hyperactivity and reduces cardiac vagal tone (parasympathetic activity) during exercise recovery [7]. This fact can be associated with a more elevated risk of sudden cardiac death during and up to 30 min after a high-intensity exercise session [7]. In this scenario, elaborating different strategies to control exercise intensity is necessary from a perspective of cardiac autonomic recovery after exercise, presenting clinical and physiological significance.
High-intensity resistance training programs have been adopted worldwide, given their specificity in increasing skeletal muscle force, power, and endurance [8,9]. Moreover, adaptive muscle morphology, such as muscle hypertrophy, is highly induced by high-intensity resistance training programs. Typical resistance training programs involve performing multiple sets (≥3) of repetitions (≥8) for different exercises. Manipulating resistance training variables, such as intensity, volume (total number of repetitions × load), and rest intervals between sets, among others, can determine the physiological and psychological stress experienced by individuals [10], which could result in different autonomic responses after exercise.
Many resistance training strategies may be adopted to induce various muscle adaptations. For example, muscle power and force are better developed when training with a higher absolute load, fewer repetitions, and longer rest intervals between sets. On the other hand, a resistance training program focused on muscle hypertrophy typically involves a higher number of repetitions (8–12 reps), a lower absolute load, and shorter rest intervals between sets compared to a power and force resistance training program [8]. This often contributes to differences in resistance training protocols in terms of volume (total number of repetitions × load) and/or intensity, which can induce different physiological responses after exercise, including cardiac demand during exercise.
It has been shown that a resistance training program for muscle hypertrophy induces a higher physiological and metabolic stress when compared to a power/force resistance training program [10]. However, it has not been investigated if a resistance training program with equalized volume and intensity but distinct absolute load could affect cardiac autonomic response after exercise. Studies that include a non-equalized resistance training prescription make it difficult to isolate the effect of the resistance training strategy, as it is unclear whether the main cause of these changes is related to the resistance training strategy or to the higher volume performed during resistance training. Therefore, this investigation would provide valuable information for exercise physiologists to prescript resistance training programs based on autonomic cardiac modulation, which is a measure of the risk of sudden cardiac death [7]. Given that many individuals typically perform hypertrophic resistance training programs, it would be important to evaluate the cardiac autonomic modulation during exercise recovery. Therefore, the present study sought to investigate the effect of two different resistance training protocols (power/force vs. hypertrophic) on cardiac autonomic modulation during exercise recovery in resistance training-trained individuals. It was hypothesized that a hypertrophic resistance training program could induce more stress and negatively change cardiac autonomic modulation compared to power/force resistance training.
2. Materials and Methods
2.1. Participants
Six participants (three females) were recruited through announcements in flyers and social media in a university campus community in Brazil. All volunteers were young (age between 18 and 40 years old) and healthy without any comorbidities (diabetes mellitus, hypertension, overnighted or obesity, and dyslipidemia). They were engaged in a resistance training program for at least 12 months. The exclusion criteria were smoking, supplementation of psychoactive agents (caffeine, pre-workout supplement, etc.) and anabolic steroid usage for at least six months before beginning experimental procedures, cardiovascular disease, osteoarticular injuries that might impair exercising resistance training exercise for lower limbs. The women were evaluated in the follicular phase to keep the two exercise visits consistent. Moreover, exercise performance and cardiovascular parameters may vary over the menstrual cycle [11]. All experimental procedures were performed after explaining the nature of the study and obtaining written consent from participants. All study procedures were performed according to the ethical standards of the Declaration of Helsinki and approved by the institutional ethics committee of the Federal University of Rio de Janeiro, Brazil (protocol number: 55184922.5.0000.5699). Clinical Trials Registry (ReBEC) (RBR-9857xj3).
2.2. Experimental Design
This study was carried out in a crossover, randomized, and controlled pilot study. Three visits to the laboratory were necessary to complete all experimental procedures. The first and second visits to the laboratory included anthropometric measure, anamneses, blood pressure measure, and determination of repetition maximum (RM) test for leg press (Movement®, São Paulo, Brazil) and leg extension chair (Movement®, São Paulo, Brazil) equipment. During the third and fourth visits, the participants performed two different resistance training protocols, which were expected to induce distinct physiological and psychological stress [10]. Heart rate measurements were continuously monitored before and 30 min after the resistance training protocol to calculate the parameters of heart rate variability [12]. The third and fourth visits were randomized using a balanced model (1:1). The randomization was performed by a random number generator by a laboratory’s staff blinded to the participant’s code. At least 72 h intervals between the third and fourth visits were adopted to allow sufficient muscle recovery, except for women for the same phase of the menstrual cycle, which was kept for the resistance training protocol (Figure 1).
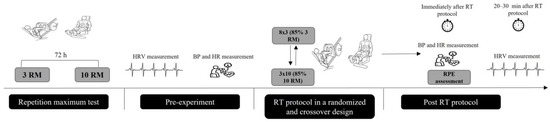
Figure 1.
Experimental design of the study. RM = repetition maximum test; HRV = heart rate variability; BP = blood pressure; HR = heart rate; RT = resistance training; RPE = rating of perceived exertion.
2.3. Resistance Training Protocol
All participants came to the laboratory twice to determine their 3RM and 10 RM in the leg press and leg extension chair equipment. The RM test was performed by adding loads in resistance training equipment until volunteers achieved muscle failure at the last repetition of a set (10 RM and 3 RM). The participants were advised to keep a constant range of motion and muscle contraction cadence during the exercise set. Muscle failure was determined as the inability to complete one repetition. The heaviest load utilized to complete 10 RM and 3 RM was determined as the maximum capacity for leg press and leg extension exercises. The 10 RM and 3 RM were determined within 3–6 attempts, which were separated by four minutes of rest. All experimental procedures were supervised by an exercise physiologist possessing expertise in resistance training programs. The resistance training protocol was designed to provide equal relative effort (85% of 10 RM or 3 RM), but the absolute load was different (higher absolute load was performed in 85% of 3 RM compared to the 10 RM), which can induce distinct physiological response [10]. The resistance training protocol was (i) 3 sets of 10 repetitions with 85% 10 RM, 60 s inter-set rest (3x1060s) and (ii) 8 sets of 3 repetitions with 85% of 3RM, 120 s inter-set rest (8x3120s). Volume load was matched as closely as possible between resistance training protocols. Before exercise protocols, individuals warmed up in leg press (the first exercise) equipment utilizing 50% of the workload. Hypertrophic and force/power resistance training protocols were 3x1060s and 8x3120s, respectively.
2.4. Heart Rate Variability Measurement
Individuals were kept in a temperature-controlled, silenced room and laid down in an exam bed, wearing a heart rate strap placed on the distal third of the sternum for 15 min. The heart rate variability data were collected with the Polar RS800CX heart rate strap, which is a validated device for heart rate evaluation [13]. The heart rate variability data were collected before and 30 min after the resistance training session. Approximately 15 min after exercise, the participants laid down again, and the heart rate variability started approximately 20 min after resistance training so that a 10-minute window of heart rate variability could be evaluated in a posterior analysis utilizing Kubios® HRV analysis software package (Kubios® HRV version 2.0, University of Kuopio, Finland). We have chosen a 20–30-minute heart rate variability analysis after resistance training protocols based on a previous study that has demonstrated a reduction in heart rate parameters after a high-intensity exercise [12]. In addition, an elevated risk of sudden cardiac death can occur up to 30 min after a high-intensity exercise session [7]. For the analysis of heart rate variability, root mean square of successive differences between adjacent normal R-R intervals (RMSSD) was adopted as time-domain acquisition, high-frequency (HF) index (in normalized units [nu] and ms2) was adopted as frequency-domain acquisition, and standard deviation of the width of the Poincaré plot (SD1) was adopted as non-linear acquisition. RMSSD, HF, and SD1 are recognized as sensitive indicators of parasympathetic activity and have been utilized in a previous study [12,13]. RMSSD, in particular, is not significantly affected by breathing frequency and can assess parasympathetic activity over short time periods, making it an appropriate marker for this research. While the frequency domain consists of various components (e.g., VLF, LF, HF, LF/HF), HF is the frequency marker widely accepted as a reliable reflection of vagal activity [12,13].
2.5. Blood Pressure Measurement
Systolic and diastolic blood pressure and heart rate were evaluated before and immediately after resistance training protocol utilizing a validated blood pressure monitor (Bp791it, Omron Co., Tokyo, Japan) with an appropriate-size upper arm cuff. The cuff was placed in the right arm, and the blood pressure and heart rate measurements were taken with the participants in the seated position [14].
2.6. Rating of Perceived Exertion
The rating of perceived exertion (RPE) for resistance training was recorded using a scale where RPE corresponds to the number of repetitions in reserve (RIR), according to [15]. Before each session, the individuals were familiarized with the scale. Data were collected only immediately after the resistance training protocol.
2.7. Volume Load and Workload
Volume load was calculated by multiplying the number of repetitions completed in the resistance training session (sum of leg press and leg extension exercises) by the actual resistance encountered (volume load: number of sets × number of repetitions × weight lifted). Volume load (kg) is an attempt to better estimate the exertion performed during a resistance training session (Haff, 2009). Workload was defined as the absolute weight lifted (in kilograms) in 3x1060s and 8x3120s protocols.
2.8. Statistical Analysis
The normality and homogeneity of variances of the data were examined with the Shapiro–Wilk and Levene tests, respectively. To identify significant differences in heart rate variability parameters (RMSSD, HF, and SD1), systolic and diastolic blood pressure, and heart rate before and after exercise protocols (3x1060s and 8x3120s), a factorial ANOVA with repeated measures (2 x 2) was performed in this study. The sphericity test was not considered in the ANOVA analysis since the study design involved repeated-measures variables that had only two levels. A dependent sample t-test was adopted to detect significant differences in the rating of perceived exertion after exercise (3x1060s and 8x3120s). For ANOVA, when a significant F was found, additional post hoc tests with Bonferroni adjustment were performed. The magnitude of the effects of the resistance training protocol was calculated by Cohen’s d with values <0.2 considered trivial, 0.2–<0.5 small effect, 0.5–<0.8 moderate effect, and ≥0.8 large effect [16]. All analyses were performed using a commercially available statistical package (IBM SPSS Statistics version 20 for Mac, Chicago, IL, USA), and the results were expressed as means ± standard deviation (SD).
3. Results
Table 1 shows the participant baseline characteristics. Table 2 shows the heart rate variability parameters of the participants.

Table 1.
Demographic characteristics of the participants.

Table 2.
Data from heart rate variability (HRV) parameters before (pre) and post-resistance training.
3.1. Heart Rate Variability Parameters
When investigating the impact of pre and post-exercise effects on RMSSD, a significant main effect for time F(1, 10) = 10.98, p = 0.008, partial η2 = 0.523 for RMSSD was observed. The post hoc test revealed a significant reduction (p < 0.001, Cohen’s d = 2.03) in RMSSD post 3x1060s protocol when compared to the baseline values. Otherwise, such a difference was not observed post 8x3120s protocol (p = 0.724). When evaluating the interaction effect (type of exercise protocol during pre and post-exercise) for RMSSD, a significant interaction effect F(1, 10) = 14.65, p = 0.003, partial η2 = 0.594) was found. The post hoc test revealed a significantly lower RMSSD (p = 0.003, Cohen’s d = 2.29) post 3x1060s compared to the post 8x3120s protocol. No significant difference (p = 0.756) in RMSSD between 3x1060s and 8x3120s protocol at the baseline was observed.
With relation to the HF index in absolute units (ms2) results, a significant main effect for time F(1, 10) = 5.86, p = 0.036, partial η2 = 0.370) was observed. The post hoc revealed a significant reduction in HF index (ms2) post 3x1060s protocol compared to the baseline value (p = 0.002, Cohen’s d = 1.85). No significant difference in HF index before and after the 8x3120s protocol was seen (p = 0.485). When investigating the interaction effect for the HF index, a significant effect F(1, 10) = 11.88, p = 0.006, partial η2 = 0.543) was found. The post hoc test revealed a significantly lower HF index post 3x1060s compared to the post 8x3120s protocol (p = 0.006), but a such differences in HF were not seen in the baseline (p = 0.602, Cohen’s d = 2.02).
Similar findings to the HF index (ms2) were observed in the HF index normalized (nu) data. A significant main effect for time F(1, 10) = 6.99, p = 0.025, partial η2 = 0.411) was observed. The post hoc revealed a significant reduction in HF index (nu) post 3x1060s protocol compared to the baseline value (p = 0.005, Cohen’s d = 1.83). There was no significant difference in the HF index before and after the 8x3120s protocol (p = 0.867). When investigating the interaction effect for HF index (nu), a significant effect F(1, 10) = 5.76, p = 0.037, partial η2 = 0.366) was found. The post hoc test revealed a significantly lower HF index (nu) post 3x1060s protocol compared to the post 8x3120s protocol (p = 0.043, Cohen’s d = 1.11), but such differences between resistance training protocols were not found in the baseline (p = 0.591).
There was a main effect for time in the SD1 index F(1, 10) = 10.98, p = 0.008, partial η2 = 0.523. The post hoc test revealed a significant reduction (p < 0.001, Cohen’s d = 2.05) in the SD1 index post 3x1060s protocol compared to the baseline value. However, no significant effect was found when comparing the post 8x3120s protocol with baseline values (p = 0.721). A significant interaction effect F(1, 10) = 14.69, p = 0.003, partial η2 = 0.595 was observed. The post hoc test revealed a significantly lower SD1 index after the 3x1060s protocol compared to the post 8x3120s protocol (p = 0.003, Cohen’s d = 2.31) but not between baseline values in 3x1060s compared to the 8x3120s protocol (p = 0.759).
No significant gender effect was observed for RMSSD (p = 0.649), HF index (ms2) (p = 0.903), HF index (nu) (p = 0.550), SD1 (p = 0.648), SBP (p = 0.454), DBP (p = 0.267), and HR (p = 0.554).
3.2. Blood Pressure and Heart Rate Data
A significant main effect for a time in systolic blood pressure F(1, 10) = 18.05, p = 0.002, partial η2 = 0.644 was observed. The post hoc test revealed a significant increase (p = 0.003, Cohen’s d = 3.64) in systolic blood pressure post 3x1060s protocol compared to the baseline value. However, no significant effect in systolic blood pressure between baseline and post 8x3120s protocol (p = 0.063) was found. No significant interaction effect F(1, 10) = 1.65, p = 0.227, partial η2 = 0.142 was observed, either.
A significant main effect for a time in diastolic blood pressure F(1, 10) = 4.75, p = 0.05, partial η2 = 0.322 was observed. The post hoc test revealed a significant increase (p = 0.044, Cohen’s d = 0.96) in diastolic blood pressure post 3x1060s protocol compared to the baseline value. However, no significant effect in diastolic blood pressure between baseline and post 8x3120s protocol (p = 0.449) was found. No significant interaction effect F(1, 10) = 1.14, p = 0.311, partial η2 = 0.102 was seen.
There was a main effect for a time in heart rate F(1, 10) = 168.99, p < 0.001, partial η2 = 0.944. The post hoc test revealed a significant increase (p < 0.001, Cohen’s d = 4.64) in heart rate post 3x1060s protocol compared to the baseline value. A significant increase in heart rate was also observed post 8x3120s protocol compared to the baseline (p = 0.016, Cohen’s d = 1.05). A significant interaction effect F(1, 10) = 79.61, p < 0.001, partial η2 = 0.888 was observed. The post hoc test revealed a significantly higher heart rate post 3x1060s protocol compared to the post 8x3120s protocol (p < 0.001, Cohen’s d = 3.95), but such differences were not observed between resistance training protocols at baseline (p = 0.818).
3.3. Volume Load, Workload, and RPE of Resistance Training Sessions
There was no significant difference for volume load t(5) = 1.22, p = 0.277 between 3x1060s (7161.6 ± 2563.3 kg) and 3x8120s (5257.8 ± 2627.07 kg). There was a significantly higher workload (t(5) = 4.72, p = 0.005) for leg press exercise in the 8x3120s protocol (227.5 ± 83.5 kg) compared to the 3x1060s (204.5 ± 78.7 kg). Similarly, a significantly higher workload for leg extension exercise (t(5) = 6.75, p = 0.001) in the 8x3120s protocol (96.2 ± 22.1 kg) compared to the 3x1060s (76.2 ± 26.4 kg)was observed. A significant lower RPE (t(5) = 2.99, p = 0.03 was observed after 8x3120s protocol (5.66 ± 2.9) compared to 3x1060s (2.83 ± 1.72).
4. Discussion
The present study sought to investigate the effect of two common resistance training protocols to induce an increase in skeletal muscle mass (muscle hypertrophy and/or power/force) on cardiac autonomic modulation. It was hypothesized that a single session of resistance training for muscle hypertrophy (3x1060s) could differently modulate cardiac autonomic parameters compared with power/force protocol (8x3120s), since increased physiological stress (heart rate, blood lactate, etc.) is observed when performing hypertrophic compared to the power/force resistance training protocol [10].
The findings of this study support our hypothesis that a single set of resistance training utilizing the 3x1060s would significantly reduce heart rate variability parameters (RMSSD, HF, and SD1) compared to the 8x3120s protocol. A previous study investigated the impact of a type of workout from CrossFit (Cindy) performed at a high intensity for 20 min on cardiac autonomic modulation. The authors observed a significant reduction in RMSSD and HF parameters over 30 min after the CrossFit exercise [12]. In addition, Heffernan et al. (2006) [7] investigated the impact of a single session of resistance training on heart rate variability parameters (i.e., HF) 30 min after exercise. A significant depression of absolute units HF (ms2) and normalized units HF (nu) was observed after the resistance training protocol, which is in line with the findings from our study.
Previous studies carried out in aerobic exercise have reported that depressed heart rate variability parameters after exercise appear to be influenced by the exercise intensity with exercise performed at a higher intensity (80% VO2reserve in treadmill running) inducing a significant reduction in heart rate variability parameter (HF index) when compared with lower intensity [17]. Similarly, Buchheit et al. (2007) [18] found that RMSSD and HF parameters presented a greater drop after high-intensity running compared to submaximal running. These data suggest that performing exercise at a higher intensity, in an acute way (after a single session), induces a greater drop in heart rate variability parameters.
Although the exercise protocol of the present study was designed to compare the impact of different resistance training protocols (3x1060s vs. 8x3120s) varying absolute load, equal volume load, and relative load (85% of 10 RM or 3 RM), the 3x1060s protocol induces a greater increase in systolic blood pressure, heart rate, and subjective perceived exertion (RPE/RIR scale) compared to the 8x3120s protocol. This fact suggests that the 3x1060s protocol induced a more robust change in physiological parameters after exercise. Such findings are in line with a previous study showing a significant increase in heart rate and subjective perceived exertion (and blood lactate concentration, which was not evaluated in the present study) after performing 3x1060s compared to the 8x3120s protocol [10].
It is likely that when performing a session of resistance training at a specific level of intensity (i.e., different rest interval periods), which is expected to disturb physiological parameters more robustly, heart rate variability parameters associated with parasympathetic activation may be depressed over 30 min after exercise. For example, Kliszczewicz et al. (2016) [12] compared the effect of a single session of high-intensity CrossFit training vs. high-intensity treadmill running on heart rate variability parameters (RMSSD and HF). The authors found a more significant reduction in RMSSD and HF after CrossFit training compared to treadmill running [12]. It was also observed that CrossFit training induced a higher subjective perceived exertion and %heart rate maximum compared to treadmill running, indicating that CrossFit training generated a higher physiological disturbance. In addition, Kliszczewicz et al. (2015) [12] also observed that CrossFit training elicited a response that was approximately twice as high in epinephrine and norepinephrine concentration when compared to treadmill running, which could explain, at least in part, the greater declines in RMSSD and HF when exercise is performed at a higher intensity [12].
Experimental Consideration
It has been mentioned that prolonged sympathetic activation and delayed parasympathetic recovery after resistance training are linked with an increased risk of acute cardiac events [19,20]. The data from this study can help exercise physiologists delineate resistance training programs for clinical populations, such as hypertensive individuals and/or those at risk for cardiovascular disease, avoiding performing certain types of resistance trading protocols (i.e., 3x1060s). However, future studies should be carried out in clinical populations to confirm such findings. Even though the finding of the present study has shown that a single session of resistance training resulted in a drop in heart rate variability parameters after exercise, it does not mean that resistance training should not be recommended to improve cardiac autonomic modulation. A previous study has shown that eight weeks of resistance training improved heart rate variability parameters in young female college students [21]. The authors observed a significant increase in SDNN and decreased LF/HF ratio, suggesting that the resistance training program reduced sympathetic activity (SDNN) and improved sympathovagal balance (LF/HF ratio). Moreover, Lin et al. (2022) [6] investigated the impact of high-intensity and low-moderate-intensity resistance training on heart rate variability parameters (HF and LF/HF ratio) during 24 weeks in middle-aged and older adults. It was shown that resistance training performed at high intensity improved heart rate variability parameters (increased HF compared to the control group). The data show that long-term resistance training programs can positively affect cardiac autonomic response; thus, the acute effects of resistance training should be interpreted with caution [6].
A limitation of this study was that the number of sets, repetition, and rest intervals between sets varied. Although the relative load (85% of 10 RM and 3 RM) and volume load of exercise were similar between 3x1060s and 8x3120s protocols, the rest interval between sets and workload varied over resistance training protocols. Rest intervals between sets can likely induce different physiological stress levels after exercise [10]. A recent study reported that 3x1060s induced a greater heart rate and subjective perceived exertion compared to the 3x10180s (2-fold longer rest interval) after performing a back squat exercise in young, healthy individuals. Such a finding is not surprising, since manipulating rest intervals between sets is a way to increase exercise intensity [22]. Thus, future studies investigating the impact of resistance training varying only absolute load (workload) are warranted. Another limitation that should be noted is the small sample size (n = 6), which can affect the findings of this study. However, the large effect size observed between the resistance training protocols in this study reinforces our findings, since the effect size calculation (Cohen’s d) is not affected by the sample size [16].
5. Conclusions
The findings of the present study showed that the hypertrophic resistance training session (3x1060s) significantly reduced HRV parameters, suggesting higher physiological stress and potential negative implications for cardiac autonomic recovery after exercise compared to the power/force resistance training session (8x3120s). These findings highlight the importance of considering exercise intensity and protocol design to manage cardiac autonomic stress during resistance training. Our finding may have clinical implications if considering applying such resistance training protocol in individuals possessing risk for cardiovascular disease.
Author Contributions
Methodology, H.R.G. and G.M.B.; Formal analysis, H.R.G., G.M.B. and L.C.d.O.; Data curation, G.M.B. and L.C.d.O.; Writing—review and editing, G.V.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (E-26/210.754/2024 and SEI-260003/006089/2024).
Institutional Review Board Statement
All study procedures were performed according to the ethical standards of the Declaration of Helsinki and approved by the institutional ethics committee of the Federal University of Rio de Janeiro, Brazil (protocol number: 55184922.5.0000.5699 date of approval: 18 March 2024). Clinical Trials Registry (ReBEC) (RBR-9857xj3).
Informed Consent Statement
All experimental procedures were performed after explaining the nature of the study and obtaining written consent from participants.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of Different Training Interventions on Heart Rate Variability and Cardiovascular Health and Risk Factors in Young and Middle-Aged Adults: A Systematic Review. Front. Physiol. 2021, 12, 657274. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.M.; O’Keefe, J.H. Autonomic Tone as a Cardiovascular Risk Factor: The Dangers of Chronic Fight or Flight. Mayo Clin. Proc. 2002, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, P.J.; Goldberger, J.J. Parasympathetic Effects on Cardiac Electrophysiology during Exercise and Recovery. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H2091–H2098. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L.; Kukielka, M.; Billman, G.E. Heart Rate Recovery after Exercise: A Predictor of Ventricular Fibrillation Susceptibility after Myocardial Infarction. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H1763–H1769. [Google Scholar] [CrossRef]
- Lin, L.L.-C.; Chen, Y.-J.; Lin, T.-Y.; Weng, T.-C. Effects of Resistance Training Intensity on Heart Rate Variability at Rest and in Response to Orthostasis in Middle-Aged and Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 10579. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Kelly, E.E.; Collier, S.R.; Fernhall, B. Cardiac Autonomic Modulation during Recovery from Acute Endurance versus Resistance Exercise. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 80–86. [Google Scholar] [CrossRef]
- McCaulley, G.O.; McBride, J.M.; Cormie, P.; Hudson, M.B.; Nuzzo, J.L.; Quindry, J.C.; Travis Triplett, N. Acute Hormonal and Neuromuscular Responses to Hypertrophy, Strength and Power Type Resistance Exercise. Eur. J. Appl. Physiol. 2009, 105, 695–704. [Google Scholar] [CrossRef]
- Behm, D.G.; Granacher, U.; Warneke, K.; Aragão-Santos, J.C.; Da Silva-Grigoletto, M.E.; Konrad, A. Minimalist Training: Is Lower Dosage or Intensity Resistance Training Effective to Improve Physical Fitness? A Narrative Review. Sports Med. 2024, 54, 289–302. [Google Scholar] [CrossRef]
- Scott, B.R.; Marston, K.J.; Teo, S.Y.M.; Forrest, M.R.L.; Jonson, A.; Walden, T.P.; Galna, B.; Peiffer, J.J. The Intensity of a Resistance Exercise Session Can Be Quantified by the Work Rate of Exercise. PLoS ONE 2023, 18, e0291857. [Google Scholar] [CrossRef]
- Giacomoni, M.; Bernard, T.; Gavarry, O.; Altare, S.; Falgairette, G. Influence of the menstrual cycle phase and menstrual symptoms on maximal anaerobic performance. Med Sci Sports Exerc. 2000, 32, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kliszczewicz, B.M.; Esco, M.R.; Quindry, J.C.; Blessing, D.L.; Oliver, G.D.; Taylor, K.J.; Price, B.M. Autonomic Responses to an Acute Bout of High-Intensity Body Weight Resistance Exercise vs. Treadmill Running. J. Strength Cond. Res. 2016, 30, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The Accuracy of Acquiring Heart Rate Variability from Portable Devices: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Vieira De Oliveira, G.; Soares, R.N.; Volino-Souza, M.; Murias, J.M.; Alvares, T.S. The Association between Near-infrared Spectroscopy Assessment of Microvascular Reactivity and Flow-mediated Dilation Is Disrupted in Individuals at High Risk for Cardiovascular Disease. Microcirculation 2019, 26, e12556. [Google Scholar] [CrossRef]
- Cavarretta, D.J.; Hall, E.E.; Bixby, W.R. The Effects of Increasing Training Load on Affect and Perceived Exertion. J. Strength Cond. Res. 2022, 36, 16–21. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Parekh, A.; Lee, C.M. Heart Rate Variability after Isocaloric Exercise Bouts of Different Intensities. Med. Sci. Sports Exerc. 2005, 37, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Laursen, P.B.; Ahmaidi, S. Parasympathetic Reactivation after Repeated Sprint Exercise. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H133–H141. [Google Scholar] [CrossRef]
- Morshedi-Meibodi, A.; Larson, M.G.; Levy, D.; O’Donnell, C.J.; Vasan, R.S. Heart Rate Recovery after Treadmill Exercise Testing and Risk of Cardiovascular Disease Events (The Framingham Heart Study). Am. J. Cardiol. 2002, 90, 848–852. [Google Scholar] [CrossRef]
- Mølgaard, H.; Sørensen, K.E.; Bjerregaard, P. Attenuated 24-h Heart Rate Variability in Apparently Healthy Subjects, Subsequently Suffering Sudden Cardiac Death. Clin. Auton. Res. 1991, 1, 233–237. [Google Scholar] [CrossRef]
- Li, R.; Yan, R.; Cheng, W.; Ren, H. Effect of Resistance Training on Heart Rate Variability of Anxious Female College Students. Front. Public Health 2022, 10, 1050469. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.; Triplett, T. Essentials of Strenght and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).