Highlights
What are the main findings?
- Dyslipidemia increases endothelial cell susceptibility to membrane injury, which is further worsened by altered shear stress patterns;
- Lipid accumulation in the vascular wall, combined with shear stress disturbances, leads to structural changes in the actin cytoskeleton;
- Excess lipids and altered shear stress disrupt lysosomal vesicle distribution in the vascular wall and endothelium.
What is the implication of the main finding?
- Chronic lipid overload and shear stress disturbances alter endothelial biomechanics, vesicle trafficking, and membrane stability, potentially impacting cell viability and atherosclerosis progression.
Abstract
Shear stress is one of the major hemodynamic forces acting on the endothelium. However, it is not well known how endothelial cells (EC) respond mechanically to these stimuli in vivo. Here we investigated whether changes in biomechanics properties and shear stress could increase cell susceptibility to injury, contributing to vascular fragility. We surgically implanted a shear stress modifier device on the carotid artery of ApoE-knockout mice (ApoE−/−), which, due to its shape, causes a gradual stenosis in the vessel, resulting in distinct shear stress patterns. Our data show actin fibers accumulation in areas with higher lipid deposition in ApoE−/−, indicating that dyslipidemia might interfere with EC actin cytoskeleton organization. We also showed that both shear stress and dyslipidemia were important for EC susceptibility to injury. Furthermore, lysosomal distribution, an important organelle for plasma membrane repair, was altered in ApoE−/−, which could compromise EC’s ability to repair from damage. Therefore, dyslipidemia and variations in shear stress patterns not only affect cellular mechanics by compromising the actin cytoskeleton organization, but also enhance cell susceptibility to injury and alter vesicle trafficking in vascular cells. This may likely contribute to vascular fragility and thus to the initial steps of atherosclerosis development.
1. Introduction
Atherosclerosis is one of the leading causes of death around the world. Besides being influenced by a variety of genetic and environmental factors, atherosclerotic lesions generally appear in predisposed areas of the artery, such as side branches and vessel bifurcations, revealing that hemodynamic forces can act locally as a risk factor [1,2].
The role of different patterns of shear stress on atherosclerotic plaque composition, as well as on biomechanical elements of the artery, have been described in the literature [3,4,5,6]. It has been demonstrated that specific patterns of wall shear stress applied in the carotid artery of apolipoprotein E-deficient (ApoE−/−) mice can determine the size and the phenotype of the lesions [3]. Indeed, atherosclerotic plaques, induced by low values of shear stress, present a vulnerable phenotype exhibiting a large lipid core, thin fibrous cap, high activation of macrophages, and metalloproteinases [3]. Meanwhile, atherosclerotic lesions induced by vortices with oscillatory shear stress display a more stable phenotype with thicker fibrous cap and increased content of collagen and smooth muscle cells [3].
In regions of vessel bifurcations and curvatures, lower values of shear stress decrease nitric oxide availability and increase the production of reactive oxygen species, adhesion molecules, and cytokines [3,4,7,8]. The variation in the level of these molecules is suggested to cause endothelium dysfunction that, consequently, becomes more sensitive to local pro-atherogenic inflammatory mediators, initiating early lesions [3,9].
Endothelial cells (EC) can sense the shear stress through several sensors and respond to these stimuli by altering their biomechanics properties. They do so by adapting their morphology and phenotype in response to flow conditions [10,11]. Additionally, exposure of EC to excess lipids can also alter their biomechanic properties. In fact, it has been shown in vitro that the exposure of EC to oxidized LDL, a key molecule during atherosclerosis development, can affect membrane rafts organization and alter the cytoskeleton arrangement [12,13,14,15]. As endothelial cells are in constant mechanical stress, generated by blood flow, it is possible that these biomechanic changes may lead to injuries and alterations in cell viability.
Recently, we demonstrated that, beyond actin reorganization and de novo polymerization, EC exposure to oxLDL results in an increase in cell rigidity, unregulated lysosomal exocytosis, and compromised constitutive endocytosis, suggesting that the reorganized cytoskeleton function is a mechanical barrier to membrane traffic. Finally, this increase in cell rigidity makes cells more prone to mechanical injury [15]. Lysosomal exocytosis is an important step in the mechanism of membrane repair [16,17,18,19], a crucial process for cell maintenance, especially for those under constant physical stress, such as endothelial cells. Possible links between disease and defects in vesicle trafficking processes or with membrane susceptibility to injury have not been explored. In this scenario, the effects of endothelial dysfunction in the first steps of atherosclerosis establishment may be directly related to the mechanical alterations and injury susceptibility due to wall shear stress.
Here, we present new evidence that EC exposure to different hemodynamic environments, combined with excess lipids, induces changes in the actin cytoskeleton, contributing to endothelium fragility. Additionally, a decrease in vascular cell lysosomal distribution is also observed, which may affect the ability of cells to repair from injury during plaque development.
2. Materials and Methods
2.1. Animal Experimentation
A total of 24 wild-type C57BL/6 (WT) and 24 ApoE−/− mice (15–20 weeks old) were used in this study, divided into 5 different feeding batches. The mice were exposed to different hemodynamic environments by using a shear stress modifier device (cast) made of polyetherketone. It consisted of 2 longitudinal halves of a cylinder with a cone-shaped lumen (Figure 1a) which causes gradual stenosis [3]. Therefore, three patterns can be identified: high shear stress (HSS; 15 N/m2) in the vessel segment inside the cast; low shear stress (LSS; 10 N/m2) in the upstream region; and oscillatory shear stress (OSS; 14 N/m2 range 60 N/m2) in the downstream region (Figure 1a,b).
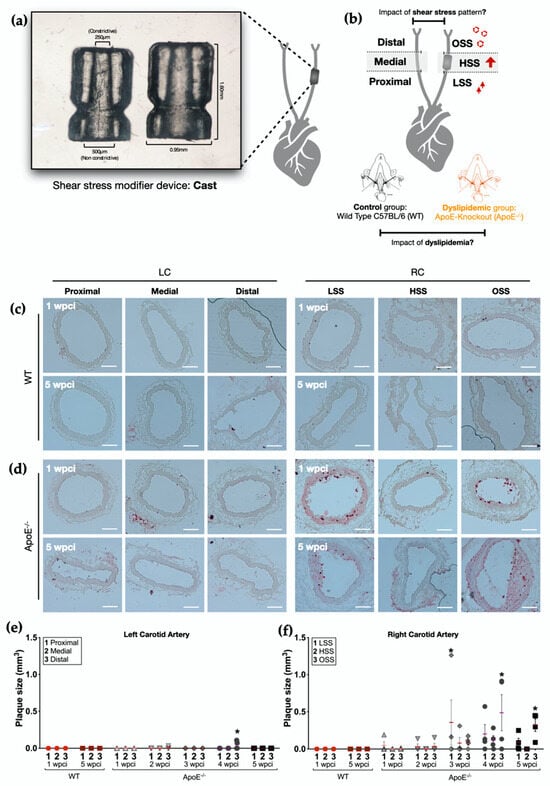
Figure 1.
Timeline of plaque formation in ApoE−/− mice. (a) Shear stress modifier device (cast). Device developed by Cheng et al. [3]; the cast consists of 2 longitudinal halves of a cylinder with a cone-shaped lumen with a gradual decline in the inner diameter (from 500 μm to 250 μm). (b) Experimental principle: the shape of the device causes gradual stenosis in the vessel, resulting in increased shear stress (HSS) in the vessel segment inside the device, a decrease in blood flow, a consequent low shear stress (LSS) region upstream, and a vortex in the downstream region (oscillatory shear stress, OSS). The left carotid artery of each animal was divided into three regions and used as an internal control. (c,d) Oil Red O staining of left and right carotids (LC and RC, respectively) of (c) WT mice (n = 4) and (d) ApoE−/− mice 1 and 5 weeks post-cast implantation (wpci) (n = 4). Scale bar, 100 µm (e). Plaque size in the left carotid artery of ApoE−/− and WT mice 1–5 wpci (n = 4). (f) Plaque size in the right carotid artery of ApoE−/− and WT mice 1–5 wpci. Asterisks indicate statistically significant differences in relation to their respective WT control (p < 0.05 using two-way ANOVA).
Both WT and ApoE−/− mice started receiving a high-fat diet (15% (w/w) cocoa butter and 1.25% (w/w) cholesterol, Research Diets D12108C) 2 weeks before cast implantation. After that, the animals continuously received the high cholesterol diet for the entire length of the experiment. The study design is described in the Supplemental Figure S1.
The device was implanted as previously described [5,20,21]. Briefly, mice were anesthetized by isoflurane inhalation (3–5% for induction and 2% for maintenance). We performed a longitudinal incision (0.8–1 cm) in the sagittal anterior area of the neck to access the right carotid artery (RC). Once the RC was localized, the cast was positioned around the artery and fixed with a layer of suture. The cast was kept for up to 5 weeks. The left carotid artery (LC) of each animal was used as an internal control. After cast implantation, the animals were treated with acetaminophen for 3 days in the drinking water (200–300 mg/kg) for analgesia.
The euthanasia was performed by anesthesia (ketamine and xylazine, 100 mg/kg and 15 mg/kg) followed by cardiac perfusion of 5 mL of PBS containing or not 500 μg/mL Propidium Iodide. Since the cell membrane is impermeable to PI, PI labelling would happen only in the presence of plasma membrane rupture. In samples exposed to PI, additional perfusion with PBS was performed to wash the excess PI.
The animal study was approved by the local ethics committee and Swiss Regulatory Authorities (license number VD3395), and it was in accordance with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
2.2. Tissue Embedding and Cryosectioning
The RC and LC of each animal were collected after PBS perfusion, included in cryomatrix, frozen, and kept at −80 °C until cryosectioning. The cryosections were performed with a thickness of 8 μm per slice, in 8 μm intervals, using the cryostat Leica CM3050S. Fresh tissue slices (Leica Cryostat, Mirabeau, France) were collected in glass slides and stored at −20 °C for later use in histology or immunostaining.
2.3. Oil-Red O Staining
Tissue samples were stained with Oil Red O (ORO) to identify atherosclerotic plaques. For this, ApoE−/− mice were divided into 5 subgroups according to the number of weeks after cast implantation (1–5 weeks post-cast implantation—wpci; n = 4 per subgroup). WT mice were separated in 2 groups, 1 and 5 wpci (n = 4 per subgroup) (Figure S1A). For that, fresh frozen tissue sections were fixed in ice-cold 10% formalin. Slides were pre-incubated in absolute propylene glycol for 2–5 min, followed by incubation with 0.5% ORO solution in absolute propylene glycol for 8–10 min in a 60 °C oven. Next, slides were differentiated in 85% propylene glycol solution, rinsed, and stained in Mayer’s hematoxylin solution. Images were acquired on a light microscope Zeiss Axiolab A1 (Carl Zeiss Microscopy GmbH, Jena, Germany) using a 10× objective. The wall area of each carotid artery section was measured using the image processing software FIJI (NIH, v. 2.0.0). After the qualitative identification of atherosclerotic plaques, the plaque size was calculated as the difference between the total wall area, including the plaque, and the mean wall area without the plaque using the LC as a control. Plaque sizes from subsequent tissue sections were added together to identify the total plaque size.
2.4. Immunofluorescence
All the immunofluorescence staining was performed in ApoE−/− (n = 20) and WT mice (n = 20), as described below (Figure S1B).
Actin cytoskeleton: Tissue sections were fixed with formaldehyde 4%, permeabilized with 0.5% Triton X-100 in PBS, and incubated with Phalloidin-Atto 565 (1:40). Slides were then mounted with Fluoroshield containing DAPI. For these slides, the endothelium was labeled (as described below) previously to the actin cytoskeleton labelling. To calculate fluorescence intensity, images of each cross-section were acquired on a Leica DM5500 fluorescence microscope (Leica Cryostat, Mirabeau, France) using a 10× objective. For the qualitative analysis of actin cytoskeleton organization, images were captured in the Zeiss Axio Imager.Z2 (ApoTome.2 structured illumination system, Carl Zeiss Microscopy GmbH, Jena, Germany) fluorescence microscope using the 63× objective.
Lysosomes: Tissue sections were fixed with PFA 4% blocked with PBS/3% BSA and incubated with rat anti-mouse LAMP-2 antibody (1:50) in PBS/1% BSA at room temperature for 2 h. After rinsing with PBS/1% BSA, the sections were incubated at room temperature for 2 h with a secondary chicken anti-rat antibody conjugated with Alexa Fluor 647, rinsed, and processed for endothelium immunostaining. To calculate fluorescence intensity, images of each cross-section were acquired on a Leica DM5500 fluorescence microscope (Leica Cryostat, Mirabeau, France) using a 10× objective.
Endothelium: To label the endothelium, the tissue samples were incubated with 0.01M Citrate Buffer for antigen unmasking, followed by permeabilization with PBS/1% BSA/0.3% Triton x-100 and blockage with PBS/3% BSA. ECs were then immunostained with rabbit anti-mouse CD31 antibody (Abcam, Cambridge, UK, 1:100) in PBS/1% BSA at room temperature for 2 h and then incubated with secondary donkey anti-rabbit antibody conjugated with Alexa Fluor 488 at room temperature for 2 h, rinsed and mounted with Fluoroshield containing DAPI.
2.5. Fluorescence Intensity Measurements
Phalloidin, LAMP-2, and PI fluorescence intensity were measured in the endothelium, according to the CD31 staining, as illustrated in Supplemental Figure S1C using FIJI (NIH, v. 2.0.0).
For all phalloidin and PI quantifications, the fluorescence intensity measurements were corrected to subtract the mean background. For LAMP-2 quantification in the vascular wall, the mean fluorescence of the artery section was measured after the autofluorescence of elastic lamella was subtracted. To do that, the elastic lamella of each artery section was segmented out via the interactive machine learning for bioimage analysis Ilastik software [22]. The resulting inverse mask was then used to calculate the mean fluorescence intensity of LAMP in the remaining regions of the vase. This quantification was done using the MATLAB Image processing toolbox. Results were normalized by the fluorescence intensity of the DAPI channel in the same selection.
2.6. Statistical Analysis
Each dataset was tested for normality and equal variance. For parametric datasets, a two-way ANOVA was performed. For all the datasets that exhibit a non-normal distribution, the non-parametric Wilcoxon Signed Rank Test or Kruskal–Wallis Test was applied. Data are presented as median ± SE, unless stated otherwise. For all tests, significance was set to p < 0.05. The statistical analysis was performed using R Statistical Software (version 4.0.0; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Disturbances on Shear Stress Patterns Induce Atherosclerotic Lesions as Early as 2 Weeks in ApoE−/− Mice
To address the role of different patterns of shear stress and dyslipidemia on cell mechanics and membrane repair in the arterial wall, we used a well-known mouse model of atherosclerosis, ApoE−/− mice fed with a high cholesterol diet. Additionally, since shear stress is an important factor for atherogenesis, we exposed the RC of these animals to different hemodynamic environments, as described in the methods. WT under the same diet and hemodynamic conditions were used as controls (Figure 1a,b).
To find a corresponding time point to our in vitro model [15] where oxLDL exposure represented the initial stages of atherosclerosis, we first performed ORO staining to label the accumulation of lipids and atherosclerotic plaques formation along a period of five weeks after cast implantation (n = 4). Representative images of both right (cast) and left (internal control) common carotid arteries from WT and ApoE−/− mice across the five weeks of the experiment are shown in Figure 1. With this approach we were able to determine both the timeline of atherosclerosis development (Figure 1c,d) and the atherosclerotic plaque size in each region of both carotid arteries (Figure 1e,f).
As expected, WT mice did not develop atherosclerotic plaques (Figure 1c,e,f) in either the RC or LC, along the entire extent of the study (5 wpci). On the other hand, ORO staining revealed an early formation of atherosclerotic plaques in the RC of ApoE−/− mice (Figure 1d,f). The accumulation of lipids and plaque formation in the RC was observed as early as 1 wpci (one mouse), and the number of ORO-positive animals increased in the following weeks of the experiment for all regions analyzed (Figure 1f).
Corroborating previous results from the literature [3,4], we showed a major role of shear stress patterns in atherosclerosis progression. Fewer and smaller plaques were observed in the contralateral carotid arteries of ApoE−/− mice (LC), which were not exposed to modified shear stress (Figure 1d,e). Based on these results, and considering the number of plaque-positive mice, we decided to continue our investigation at week one post-cast implantation.
3.2. Shear Stress and Dyslipidemia Enhance Cell Susceptibility to Injury in the Endothelium
We have shown previously that ECs exposed to excess oxLDL display increased cell susceptibility to injury, which was very likely related to changes in cell biomechanics [15]. Thus, we set out to investigate whether dyslipidemia and/or alterations in patterns of shear stress in vivo would enhance cell susceptibility to mechanical injury caused by blood flow.
For that, one week after cast implantation, ApoE−/− and WT mice were perfused with PBS containing PI. Since PI is a membrane-impermeable fluorophore, which can only enter the cell upon membrane injury.
Our data revealed that dyslipidemia alone was enough to increase susceptibility to injury as shown by the increase in PI staining of the LC of ApoE−/− mice when compared to the LC of WT (Figure 2a,b). Shear stress alone, observed when comparing the two carotids (LC and RC) of the same animal, was also able to increase injury in areas of HSS of both ApoE−/− (3.78-fold change) and WT mice (four-fold change) (Figure S2A,B, respectively). In ApoE−/− mice, however, the role of the shear stress on cell injury was more evident, once higher PI staining was observed in all regions of the RC with changes ranging from 1.46 to 8.47-fold (Figure 2e). On the other hand, dyslipidemia coupled with shear stress, observed in the RC of ApoE−/− mice, exacerbated the susceptibility to injury of shear stress alone, observed in the RC of WT mice, in HSS and OSS areas (Figure 2c,d). This reinforces the contribution of both dyslipidemia and shear stress to endothelial fragility, which favors the development of atherosclerosis.
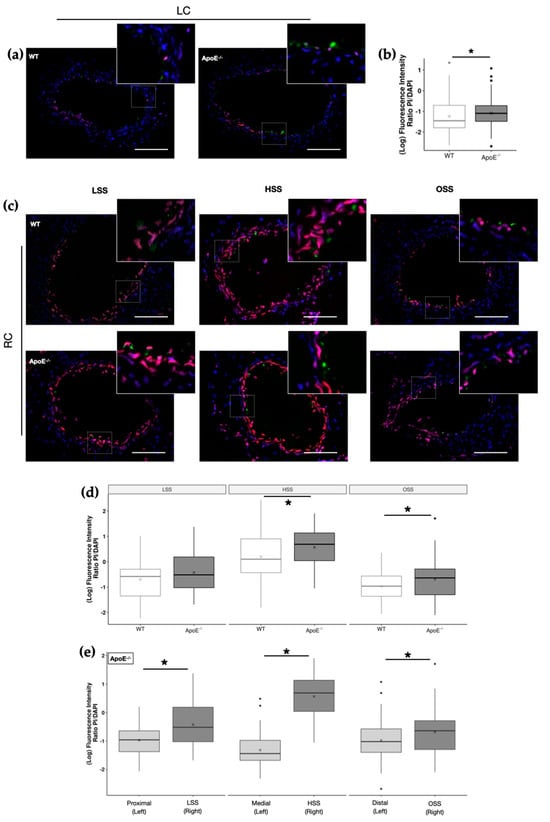
Figure 2.
Effects of dyslipidemia and shear stress on flow-induced injury in carotid arteries. (a) Representative merged images of Propidium Iodide (PI; red) staining of the left carotid artery (LC) of WT and ApoE−/− mice. The cell nuclei were stained with DAPI (blue) and endothelial cells with CD31 (green). Scale bar, 100 µm. Insets show magnification images of the boxed regions. (b) PI fluorescence intensity in LC endothelium (WT, n = 11; ApoE−/−, n = 11; * p < 0.05 using the Wilcoxon test). (c) Representative merged images of PI staining in the three distinct regions of the right carotid artery (RC; LSS, HSS, and OSS) of WT and ApoE−/− mice (PI, red; DAPI, blue). Insets show magnification images of the boxed regions. (d) PI fluorescence intensity in the endothelium (WT, n = 9; ApoE−/−, n = 11) in the three distinct regions of the RC (* p < 0.05 using the Wilcoxon test). (e) PI fluorescence intensity in the endothelium of ApoE−/− mice in the three distinct regions of the RC versus their contralateral control LC (n = 11, * p < 0.05 using the Wilcoxon test).
3.3. Accumulation of Lipids in the Vascular Wall Associated with Disturbances on Shear Stress Induces Alterations on Cell Actin Labelling
A recent in vitro study from our group revealed that EC exposure to oxLDL, a key molecule during atherosclerosis development, leads to actin reorganization and de novo polymerization, increasing cell rigidity and turning cells more prone to mechanical injury. Additionally, constitutive endocytosis was blocked upon treatment, suggesting the reorganized cytoskeleton function as a mechanical barrier to membrane trafficking [15].
However, it is not known whether the above-mentioned changes in EC mechanical properties, as well as their consequences (defects in vesicle trafficking or membrane susceptibility to injury), would also occur in vivo.
Based on that, we set out to investigate whether the accumulation of lipids in the arterial wall and/or changes in shear stress pattern were accompanied by changes in biomechanics properties of endothelial cells. For this, biomechanic properties were indirectly evaluated through the actin cytoskeleton labelling of 1-wpci ApoE−/− and WT carotid arteries using Phalloidin-Atto 565.
Representative images of LC and RC of both animal groups are shown in Figure 3a,b, respectively. Insets show magnification images of the boxed regions. It is important to state that elastic fibers are strongly evidenced in the images due to their autofluorescence, as demonstrated by the arrowheads. In both carotid arteries (LC and RC), we could observe an alteration in actin cytoskeleton organization between the WT control and ApoE−/− mice, as demonstrated by the presence of more filament-like structures (indicated by dashed arrows) in the control group, while ApoE−/− mice display a more diffuse actin labelling, with the exception of its medial-LC region.
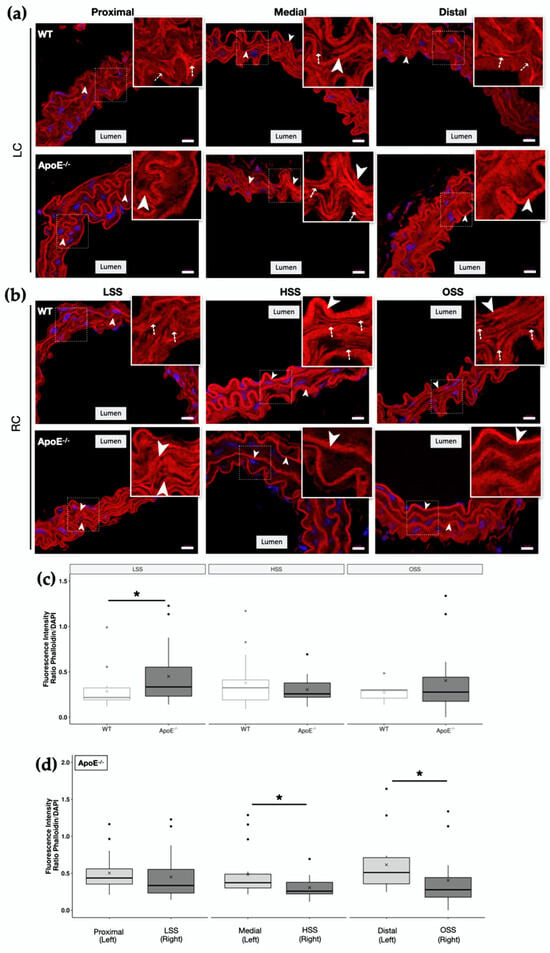
Figure 3.
Actin cytoskeleton organization in the vascular wall of ApoE−/− and WT mice. Representative merged images of Phalloidin-Atto 565 staining (red) in the different regions of (a) left carotid artery (LC) and (b) right carotid artery (RC) of WT and ApoE−/− mice. The cell nuclei were stained with DAPI (blue). Merged images are shown. Scale bar, 10 µm. Arrowheads: elastic fibers are evidenced by its autofluorescence. Dash arrows indicate filament-like structures. Insets show magnification images of the boxed regions. Images were captured in the Zeiss Axio Imager.Z2 (ApoTome.2 structured illumination system) fluorescence microscope using the 63× objective. (c) Phalloidin-Atto 565 fluorescence intensity in the endothelium of WT and ApoE−/− mice in the 3 distinct regions of the RC (LSS, HSS and OSS). (d) Phalloidin-Atto 565 fluorescence intensity in the endothelium of ApoE−/− mice in the 3 distinct regions of the RC versus their contralateral control LC (n = 5; * p < 0.05 using the Wilcoxon test).
Interestingly, despite the morphological changes observed between ApoE−/− and the WT LC carotid that suggested a rearrangement of actin fibers (Figure 3a,b), dyslipidemia alone did not alter the amount of actin cytoskeleton filaments, as no statistically significant differences were observed in Phalloidin fluorescence intensity of LC carotids (Figure S3). However, low shear stress (LSS), together with dyslipidemia in RC of ApoE−/−, showed a significant increase in Phalloidin fluorescence intensity in comparison to WT (Figure 3c). Surprisingly, we did not observe a change in actin fluorescence intensity in regions with higher injury susceptibility (HSS and OSS) (Figure 3c) as our in vitro studies suggested. In fact, HSS and OSS regions of ApoE−/− RC showed a decrease in Phalloidin fluorescence intensity in comparison with their contralateral control (Figure 3d). However, since in this condition at this time point (1 wpci) we already have plaque formation, a process posterior to the predicted actin reorganization, this decrease in actin might be associated with a disruption of the previously restructured cytoskeleton due to higher shear stress. A better detailed analysis of ApoE−/− Phalloidin staining reveals that the actin cytoskeleton structure is, indeed, compromised, with a more diffuse labelling of actin, showing less fiber-like structures when compared to the WT group (Figure 3b, insets).
3.4. Lysosomal Content Is Modulated by Dyslipidemia and Shear Stress in the Vascular Wall and in the Endothelium
In vitro alterations on cellular biomechanics (through oxLDL exposure or cholesterol depletion from plasma membrane) have been linked to altered intracellular vesicle distribution, compromised vesicle trafficking, and higher susceptibility to injury [15,23,24].
Due to the critical role of lysosomes in plasma membrane repair and the observed increase in cell injury caused by dyslipidemia and shear stress, we decided to investigate whether these conditions would also affect lysosome distribution in ApoE−/− or WT mice. To answer this question, we quantified the amount of LAMP-2 exposure to the plasma membrane in the carotid arteries of the different animals to infer lysosome exocytosis, as well as presence of lysosome pools in the cells (Figure 4).
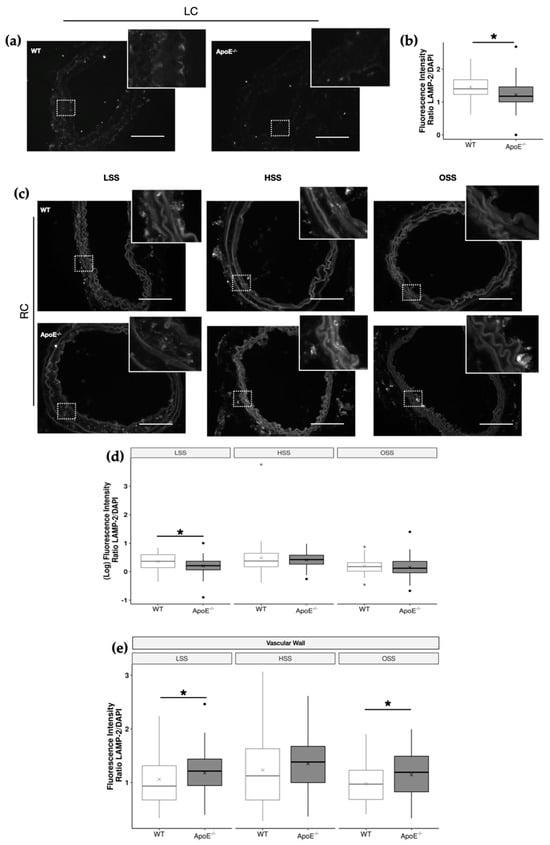
Figure 4.
Effects of dyslipidemia on LAMP−2 distribution of ApoE−/− and WT carotid arteries. (a) Representative images of LAMP−2 staining of the left carotid artery (LC) of WT and ApoE−/− mice. Scale bar, 100 µm. Insets show magnification images of the boxed regions. (b) LAMP−2 fluorescence intensity in LC endothelium (WT, n = 11; ApoE−/−, n = 11; * p < 0.05 using the Wilcoxon test). (c) Representative images of LAMP−2 staining in the 3 distinct regions of the right carotid artery (RC; LSS, HSS, and OSS) of WT and ApoE−/− mice. Scale bar, 100 µm. Insets show magnification images of the boxed regions. (d,e) LAMP−2 fluorescence intensity in the (d) endothelium (WT, n = 9; ApoE−/−, n = 11) and (e) vascular wall of WT and ApoE−/− mice in the 3 distinct regions of the RC (WT, n = 13; ApoE−/−, n = 13, * p < 0.05 using the Wilcoxon test).
In the LC, which presents a unidirectional laminar flow in physiological conditions and where no interventions were made, ApoE−/− mice displayed lower LAMP-2 fluorescence intensity in the endothelium when compared to the WT mice (Figure 4a,b). This indicates that dyslipidemia itself interferes with lysosomal vesicle trafficking in vascular cells. The reduction in LAMP-2 fluorescence intensity ranges from 12 to 15% in ApoE−/− LC arteries (Figure 4b).
When the shear stress pattern was considered (Figure 4c), ApoE−/− endothelium showed less LAMP exposure in LSS, reaching values of approximately 22% lower than the WT (Figure 4d), the same region where we observed actin cytoskeleton polymerization, lipid accumulation, and consequently plaque formation. Interestingly, when considering LAMP-2 staining in the entire vascular wall, we observed an increase in lysosomes in both LSS and OSS regions of ApoE−/− mice (Figure 4e). These results indicated a differential disturbance in vesicle trafficking in the vascular wall as compared to the endothelium of ApoE−/− mice induced by dyslipidemia and alterations in shear stress.
4. Discussion
Atherosclerosis development is influenced by a variety of genetic and environmental factors. It preferentially develops in areas of vessel bifurcation or curvature, where the blood flow tends to be slower or non-uniform, revealing that hemodynamic forces also work as local risk factors [1,2]. In fact, it has been shown in the literature that local shear stress patterns function as an important biomechanic factor for the modulation of atherogenesis, which can determine the size, composition, and phenotype of the lesions [3,4,25].
However, the contribution of cellular biomechanic alterations induced by dyslipidemia, allied to variations in shear stress and to the development of the atherosclerotic plaque in vivo, has not yet been explored. To address this issue, we used an elegant mice model of shear stress-induced atherogenesis previously described by Cheng et al. [3]. In this model, it is possible to apply specific patterns of shear stress in a straight vessel segment, using a shear-stress modifier device, combined with dyslipidemia by using ApoE−/− mice.
The data presented in this study provide evidence that dyslipidemia itself was, indeed, a primordial factor in increasing the level of cell injury, as it can be observed by the higher injury suffered by the endothelial cells of ApoE−/− mice. This injury is still aggravated by changes in shear stress patterns, which had a critical impact on the induction of mechanical injury. These findings corroborate our previous in vitro data, in which endothelial cells treated with oxLDL revealed an increase in injury. In the in vitro context, this seems to be due to an increase in membrane stiffness and cell tension (i.e., the higher the membrane rigidity, the higher the susceptibility to injury), due to actin cytoskeleton reorganization and de novo polymerization [15]. However, evidence of this process in vivo was still lacking.
Therefore, we hypothesized that dyslipidemia in the endothelium of ApoE−/− mice would induce actin cytoskeleton polymerization and consequently increase cell injury, leading to the formation of plaques. At first sight, this is not corroborated by our data. This is because, in regions of low shear stress in ApoE−/− mice, where we observe actin reorganization and plaque formation, we do not observe higher injury. On the other hand, in HSS and OSS regions that do show an increase in injury, there was a decrease in actin polymerization. However, an important factor to consider is that in the present study, the in vivo investigation was performed in dyslipidemic animals of 15–20 weeks old, which received a high fat diet for three weeks, thus presenting a chronic response and a posterior time point compared to our in vitro study. Therefore, the events described here might be a consequence of the ones described in our in vitro study. For example, we must consider possible compensatory mechanisms, since cells tend to reduce actin stress fibers to diminish subtle increases in cell tension, as the one caused by the acute exposure to oxLDL [11,13,14,15]. It has been shown that cells present acute and chronic compensatory mechanisms to readapt from sudden changes in cytoskeleton organization to buffer cell tension [26,27,28]. Sinha et al. demonstrated in EC and muscle cells a compensatory caveolae-dependent mechanism in response to acute mechanical stress [26]. They observed caveolae flattening upon cell stretching or osmotic swelling. This phenomenon would release an amount of membrane stored in the invagination, providing additional membrane that was able to restore tension homeostasis. Furthermore, they suggested the participation of other mechanisms (for instance endocytosis, exocytosis, and actin dynamics) as additional compensatory mechanisms to prolong or complete the response to chronic mechanical stress and plasma membrane repair [26].
Considering all the above, it is possible that, for ApoE−/− mice, effects of shear stress and dyslipidemia may be antagonistic. While the first (shear stress) would lead to a decrease in actin stress fiber formation, the second (dyslipidemia) would induce an increase. Corroborating this idea, LSS regions of carotid arteries are well known to accumulate lipids in the vascular wall, thus oxLDL, in vitro, induces actin rearrangement and de novo polymerization, as recently demonstrated by our group, and is now corroborated by this in vivo study [15]. In this scenario, the outcome would be a reduction in the effect of dyslipidemia in stress fiber formation due to a decrease in shear stress forces.
Nevertheless, despite any compensatory mechanism to reduce cellular tension chronically, we could observe that dyslipidemia can, indeed, impact actin dynamics also in vivo, once regions with greater injury levels presented a morphological disruption of the cytoskeleton network, where fewer stress fibers and filament-like structures were found. On the other hand, regions exposed to low shear stress display less injury, and the actin cytoskeleton is more properly arranged. Since we have previously shown through in vitro experimentation that, before inducing an acute mechanical injury, cells exposed to oxLDL are stiffer, with a reorganized actin cytoskeleton, and more prone to suffer injury [15], we hypothesize that the chronic exposure to dyslipidemia and the aggravated injury caused by the blood flow in those regions could be the major cause of damage to the actin cytoskeleton structure.
Moreover, we believe that the high increase in the level of injury suffered by the cells specifically at the HSS region of RC, in both WT and ApoE−/−, might be justified by a reduction in the distension capacity of the vessel wall, upon cast implantation. It is also worth highlighting that, in advanced stages of atherosclerotic plaque development, there is increased collagen deposition and fragmentation of elastin fibers. The last-mentioned modifications culminate in enhanced arterial stiffness and thus a reduction in the distention capacity of the vessel [29], perhaps leading to increased cell damage by mechanical injury in the region. Based on that, these data can be considered an important factor for the destabilization of the plaque and should be studied further.
Cytoskeleton reorganization and changes in biomechanics properties, induced by various conditions, have been demonstrated to play an important role in promoting disturbances in vesicle trafficking, particularly in lysosomal exocytosis [30,31,32,33,34]. For example, the existence of a docked pool of lysosomes and its interaction with actin cytoskeleton dynamics were described [33]. When inhibiting actin-myosin contractile machinery through RAB27A mutations, the secretion of a lysosome-related organelle was compromised, suggesting the crucial role of actin on vesicle fusion and lysosomal exocytosis. It has also been shown that cholesterol depletion-induced actin cytoskeleton reorganization triggers a peak of exocytosis of pre-docked lysosomes, while later exocytic events are blocked by the cytoskeleton barrier [23,24]. When this barrier was removed by disrupting the cytoskeleton with Latrunculin-A, the perinuclear pool of lysosomes was allowed to be exocytosed. Similarly, we also observed vesicle trafficking alterations in ECs upon treatment with MβCD or oxLDL, both known to affect actin cytoskeleton reorganization in vitro [15]. Interestingly, we also observed signs of lysosomal trafficking alterations in the LC endothelium of ApoE−/− mice that showed less LAMP exposure compared to WT. This indicates that dyslipidemia can impact lysosomal trafficking in vitro as well as in vivo. This was also observed when low shear stress was introduced in the context of dyslipidemia, since ApoE−/− exhibit less LAMP-2 staining in LSS, suggestive of a reduction in lysosomal vesicles when compared to the wild-type counterpart. As this is a chronic treatment, it is plausible that for ApoE−/− mice, the peak of lysosome exocytosis induced by cytoskeleton reorganization proposed by Hissa and coworkers (2013) would have already occurred, leading to the depletion of the peripheral pool of lysosomes. At the same time, the reorganized actin cytoskeleton would inhibit the access of the internal pool of lysosomes to the membrane [23]. Indeed, in this region (ApoE−/− RC LSS), we also see an increase in actin polymerization together with the low LAMP-2 exposure. In other words, while cells from WT would be capable of performing new lysosomal exocytosis events, those from ApoE−/− mice would be impaired due to the absence of the pre-docked pool of lysosomes. In HSS and OSS, however, we do not see the same effect. One hypothesis is that, due to compensatory mechanisms or the direct mechanical force of higher shear stress, the actin cytoskeleton barrier is disrupted, allowing the internal pool of lysosomes to access the membrane. In WT conditions, this exocytosis is enough to repair the injury caused by the shear stress, however, it is not in the context of dyslipidemia, where we observe more injury levels. This possible difference between injury and repair that happens in ApoE−/− might lead to the fragility of the endothelium and the progression of the atherosclerotic lesion.
It is noteworthy that lysosomal exocytosis is a crucial step for plasma membrane repair and the maintenance of cell integrity, but also might contribute to the inflammatory process by releasing lysosomal cargo in the extracellular milieu. Whether a massive and uncontrolled lysosome exocytosis is, in fact, happening in vivo in earlier time points still needs to be investigated, as the excess of lysosomal secretions delivered to the extracellular matrix could potentially disturb the extracellular environment, consequently triggering the initiation of a local inflammatory process. Interestingly, although we see a LAMP-2 staining decrease in the endothelium, we see an increase in the vessel wall of LSS and OSS. Those are the same regions where we also observe an accumulation of oxLDL and plaque formation. Additionally, the vascular wall might have less influence of shear stress compared to the endothelium, and therefore constitute a different environment with a higher influence of effects from dyslipidemia. Thus, it is possible that the increase in LAMP-2 exposure in this region indicates the increase of exocytosis induced by oxLDL observed in vitro, which might contribute to plaque formation.
Hence, the role of cell exposure to the excess of lipids, associated with the modulation of shear stress, in cell biomechanics, vesicle trafficking, and membrane injury must be considered as important factors to cell viability, endothelium integrity, and to the development of atherosclerosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jvd3040028/s1, Figure S1: experimental design; Figure S2: effects of different shear stress patterns on flow-induced injury in carotid arteries of ApoE−/− and WT mice; Figure S3: effects of dyslipidemia on the actin cytoskeleton in the LC of ApoE−/− and WT mice.
Author Contributions
Conceptualization, N.F.D.C., L.O.A. and T.C.-G.; methodology, N.F.D.C., A.M.L. and R.F.-S.; validation, N.F.D.C. and A.M.L.; formal analysis, N.F.D.C. and L.A.B.M.; investigation, N.F.D.C., A.M.L. and W.F.-B.; resources, N.F.D.C., L.O.A. and N.S.; data curation, N.F.D.C. and L.R.; writing—original draft preparation, N.F.D.C. and. L.O.A.; writing—review and editing, N.F.D.C., L.O.A., L.R., W.F.-B., T.C.-G. and A.M.L.; visualization, N.F.D.C.; supervision, L.O.A. and N.S.; project administration, N.F.D.C. and A.M.L.; funding acquisition, N.F.D.C., L.O.A. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development —CNPq (INCT-FCx 465259/2014-6), Minas Gerais State Agency for Research and Development (FAPEMIG—APQ-02974-17) and the Coordination for the Improvement of Higher Education Personnel (CAPES—PDSE 88881.189393/2018-01).
Institutional Review Board Statement
The animal study protocol was approved by the Local Ethics Committee of École Polytechnique Fédérale de Lausanne and Swiss Regulatory Authorities (license number VD3395, date of authorization 4 October 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We are especially grateful to BIOP/EPFL and CAPI/UFMG for the use of microscopes and imaging processing systems, as well as the Histology Facility/EPFL for the use of the cryostat and histological markers. Additionally, we are also grateful to Eduardo Tarazona for his helpful contribution to the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Otero-Cacho, A.; Aymerich, M.; Flores-Arias, M.T.; Abal, M.; Álvarez, E.; Pérez-Muñuzuri, V.; Muñuzuri, A.P. Determination of hemodynamic risk for vascular disease in planar artery bifurcations. Sci. Rep. 2018, 8, 2795. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2020, 17, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; van Haperen, R.; van der Baan, A.; Grosveld, F.; Daemen, M.J.; Krams, R.; de Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; van Haperen, R.; de Boer, H.C.; Segers, D.; Huisman, M.; van Zonneveld, A.J.; Leenen, P.J.; van der Steen, A.; Serruys, P.W.; et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Investig. 2007, 117, 616–626. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Savergnini, S.Q.; Montecucco, F.; Nencioni, A.; Caffa, I.; Soncini, D.; Costa-Fraga, F.P.; De Sousa, F.B.; Sinisterra, R.D.; Capettini, L.A.S.; et al. Treatment with Angiotensin-(1-7) reduces inflammation in carotid atherosclerotic plaques. Thromb. Haemost. 2014, 111, 736–747. [Google Scholar]
- De Wilde, D.; Trachet, B.; De Meyer, G.R.; Segers, P. Shear Stress Metrics and Their Relation to Atherosclerosis: An In Vivo Follow-up Study in Atherosclerotic Mice. Ann. Biomed. Eng. 2016, 44, 2327–2338. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef]
- Rodriguez, I.; Gonzalez, M. Physiological mechanisms of vascular response induced by shear stress and effect of exercise in systemic and placental circulation. Front. Pharmacol. 2014, 5, 209. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [CrossRef]
- Kowalsky, G.B.; Byfield, F.J.; Levitan, I. oxLDL facilitates flow-induced realignment of aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2008, 295, C332–C340. [Google Scholar] [CrossRef] [PubMed]
- Shentu, T.-P.; Titushkin, I.; Singh, D.K.; Gooch, K.J.; Subbaiah, P.; Cho, M.; Levitan, I. oxLDL-induced decrease in lipid order of membrane domains is inversely correlated with endothelial stiffness and network formation. Am. J. Physiol. Cell Physiol. 2010, 299, C218–C229. [Google Scholar] [CrossRef] [PubMed]
- Byfield, F.J.; Tikku, S.; Rothblat, G.H.; Gooch, K.J.; Levitan, I. OxLDL increases endothelial stiffness, force generation, and network formation. J. Lipid Res. 2006, 47, 715–723. [Google Scholar] [CrossRef]
- Chouinard, J.A.; Grenier, G.; Khalil, A.; Vermette, P. Oxidized-LDL induce morphological changes and increase stiffness of endothelial cells. Exp. Cell Res. 2008, 314, 3007–3016. [Google Scholar] [CrossRef]
- Couto, N.F.; Rezende, L.; Fernandes-Braga, W.; Alves, A.P.; Agero, U.; Alvarez-Leite, J.; Damasceno NR, T.; Castro-Gomes, T.; Andrade, L.O. OxLDL alterations in endothelial cell membrane dynamics leads to changes in vesicle trafficking and increases cell susceptibility to injury. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183139. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Caler, E.V.; Andrews, N.W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 2001, 106, 157–169. [Google Scholar] [CrossRef]
- McNeil, P.L.; Miyake, K.; Vogel, S.S. The endomembrane requirement for cell surface repair. Proc. Natl. Acad. Sci. USA 2003, 100, 4592–4597. [Google Scholar] [CrossRef]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef]
- Tam, C.; Idone, V.; Devlin, C.; Fernandes, M.C.; Flannery, A.; He, X.; Schuchman, E.; Tabas, I.; Andrews, N.W. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 2010, 189, 1027–1038. [Google Scholar] [CrossRef]
- Olivon, V.C.; Fraga-Silva, R.A.; Segers, D.; Demougeot, C.; de Oliveira, A.M.; Savergnini, S.S.; Berthelot, A.; de Crom, R.; Krams, R.; Stergiopulos, N.; et al. Arginase inhibition prevents the low shear stress-induced development of vulnerable atherosclerotic plaques in ApoE−/− mice. Atherosclerosis 2013, 227, 236–243. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Costa-Fraga, F.P.; Montecucco, F.; Sturny, M.; Faye, Y.; Mach, F.; Pelli, G.; Shenoy, V.; da Silva, R.F.; Raizada, M.K.; et al. Diminazene protects corpus cavernosum against hypercholesterolemia-induced injury. J. Sex. Med. 2015, 12, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Straehle, C.; Koethe, U.; Hamprecht, F.A. ilastik: Interactive Learning and Segmentation Toolkit. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 230–233. [Google Scholar]
- Hissa, B.; Pontes, B.; Roma, P.M.S.; Alves, A.P.; Rocha, C.D.; Valverde, T.M.; Aguiar, P.H.N.; Almeida, F.P.; Guimarães, A.J.; Guatimosim, C.; et al. Membrane cholesterol removal changes mechanical properties of cells and induces secretion of a specific pool of lysosomes. PLoS ONE 2013, 8, e82988. [Google Scholar] [CrossRef] [PubMed]
- Hissa, B.; de Oliveira Andrade, L. Trypasonoma cruzi uses a specific subset of host cell lysosomes for cell invasion. Parasitol. Int. 2015, 64, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Segers, D.; Lipton, J.A.; Leenen, P.J.M.; Cheng, C.; Tempel, D.; Pasterkamp, G.; Moll, F.L.; de Crom, R.; Krams, R. Atherosclerotic Plaque Stability Is Affected by the Chemokine CXCL10 in Both Mice and Humans. Int. J. Inflam. 2011, 2011, 936109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef]
- Batchelder, E.L.; Hollopeter, G.; Campillo, C.; Mezanges, X.; Jorgensen, E.M.; Nassoy, P.; Sens, P.; Plastino, J. Membrane tension regulates motility by controlling lamellipodium organization. Proc. Natl. Acad. Sci. USA 2011, 108, 11429–11434. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Fardin, M.A.; Roca-Cusachs, P.; Sheetz, M.P. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA 2011, 108, 14467–14472. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Aunis, D.; Bader, M.-F. The cytoskeleton as a barrier to exocytosis in secretory cells. J. Exp. Biol. 1988, 139, 253–266. [Google Scholar] [CrossRef]
- Koseoglu, S.; Love, S.A.; Haynes, C.L. Cholesterol effects on vesicle pools in chromaffin cells revealed by carbon-fiber microeouectrode amperometry. Anal. Bioanal. Chem. 2011, 400, 2963–2971. [Google Scholar] [CrossRef]
- Hissa, B.; Duarte, J.G.; Kelles, L.F.; Santos, F.P.; del Puerto, H.L.; Gazzinelli-Guimarães, P.H.; de Paula, A.M.; Agero, U.; Mesquita, O.N.; Guatimosim, C.; et al. Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells. PLOS Neglected Trop. Dis. 2012, 6, e1583. [Google Scholar] [CrossRef] [PubMed]
- Cabukusta, B.; Neefjes, J. Mechanisms of lysosomal positioning and movement. Traffic 2018, 19, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.; Nixon, R.A. Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol. Dis. 2019, 122, 94–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).