A Comprehensive Literature Review on Diagnostic Strategies and Clinical Outcome of Intraoral Angiosarcoma and Kaposi Sarcoma
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. Angiosarcoma
3.2. Kaposi Sarcoma
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldblum, J.; Flope, A.; Weiss, S. (Eds.) Malignant Vascular Tumors. In Enzinger and Weiss’s Soft Tissue Tumors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 785–816. [Google Scholar]
- Lahat, G.; Dhuka, A.R.; Hallevi, H.; Xiao, L.; Zou, C.; Smith, K.D.; Phung, T.L.; Pollock, R.E.; Benjamin, R.; Hunt, K.K.; et al. Angiosarcoma. Ann. Surg. 2010, 251, 1098–1106. [Google Scholar] [CrossRef]
- Stănescu, L.; Foarfă, C.; Georgescu, A.C.; Georgescu, I. Kaposi’s Sarcoma Associated with AIDS. Rom. J. Morphol. Embryol. 2007, 48, 181–187. [Google Scholar]
- Jindal, J.R.; Campbell, B.H.; Ward, T.O.; Almagro, U.S. Kaposi’s Sarcoma of the Oral Cavity in a Non-aids Patient: Case Report and Review of the Literature. Head Neck 1995, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Flood, L.M. Vascular Lesions of the Head and Neck: Diagnosis and Managementm; Persky, S., Waner, M., Blei, F., Berenstein, A., Eds.; Thieme Publishers: New York, NY, USA, 2014; p. 168. ISBN 978 1 60406 059 1. EISBN 978 1 58890 282 3. [Google Scholar]
- Bishop, B.N.; Lynch, D.T. Kaposi Sarcoma; National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534839/ (accessed on 8 June 2024).
- Fatahzadeh, M. Kaposi Sarcoma: Review and Medical Management Update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 2–16. [Google Scholar] [CrossRef]
- Tounouga, D.N.; Kouotou, E.A.; Nansseu, J.R.; Zoung-Kanyi Bissek, A.-C. Epidemiological and Clinical Patterns of Kaposi Sarcoma: A 16-Year Retrospective Cross-Sectional Study from Yaoundé, Cameroon. Dermatology 2018, 234, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Lali, B.S.; Chowdhury, Z.; Gupta, M.; Mishra, A. Primary Angiosarcoma of the Oral Cavity in a Young Adult. Autops. Case Rep. 2021, 11, e2020217. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yoshitake, Y.; Nakayama, H.; Yoshida, R.; Kawahara, K.; Nakagawa, Y.; Shinohara, M. Angiosarcoma of the Oral Cavity: A Clinicopathological Study and a Review of the Literature. Int. J. Oral Maxillofac. Surg. 2014, 43, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Angiosarcoma of the Oral Cavity. Head Neck Pathol. 2011, 5, 67–70. [Google Scholar] [CrossRef]
- Kusaka, I.; Katagiri, K.; Saito, D.; Ohashi, Y.; Oikawa, S.; Tsuchida, K.; Miyaguchi, J.; Kusaka, T.; Ikeda, R.; Shiga, K. A Case Report of Angiosarcoma Originating from the Tongue. Clin. Case Rep. 2023, 11, e8330. [Google Scholar] [CrossRef]
- Komatsu, Y.; Miyamoto, I.; Ohashi, Y.; Katagiri, K.; Saito, D.; Obara, M.; Takeda, Y.; Shiga, K.; Yamada, H. Primary Epithelioid Angiosarcoma Originating from the Mandibular Gingiva: A Case Report of an Extremely Rare Oral Lesion. World J. Surg. Oncol. 2020, 18, 260. [Google Scholar] [CrossRef]
- Favia, G.; Lo Muzio, L.; Serpico, R.; Maiorano, E. Angiosarcoma of the Head and Neck with Intra-Oral Presentation. A Clinico-Pathological Study of Four Cases. Oral Oncol. 2002, 38, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Angiosarcoma of the Mandibular Gingiva. Int. J. Clin. Exp. Pathol. 2011, 4, 791–793. [Google Scholar]
- Fomete, B.; Samaila, M.; Edaigbini, S.; Agbara, R.; Okeke, U.A. Primary Oral Soft Tissue Angiosarcoma of the Cheek: A Case Report and Literature Review. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 273. [Google Scholar] [CrossRef]
- Patel, P.B.; Kuan, E.C.; Peng, K.A.; Yoo, F.; Nelson, S.D.; Abemayor, E. Angiosarcoma of the Tongue: A Case Series and Literature Review. Am. J. Otolaryngol. 2017, 38, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.H. Angiosarcoma of Tongue; Case Report. Laryngoscope 1927, 37, 869–871. [Google Scholar] [CrossRef]
- Koteshwer Rao, K.; Nanda Kumar, H.; Rao, R.V. Primary Haemangiosarcoma of the Tongue. Int. J. Oral Maxillofac. Surg. 1986, 15, 489–491. [Google Scholar] [CrossRef]
- Frick, W.G.; Keith McDaniel, R. Angiosarcoma of the Tongue: Report of a Case. J. Oral Maxillofac. Surg. 1988, 46, 496–498. [Google Scholar] [CrossRef]
- Tabata, M.; Sugihara, K.; Matsui, R.; Yonezawa, S.; Abeyama, K.; Maruyama, I. Angiosarcoma of the Tongue: Report of a Case with Immunohistochemical Findings. J. Oral Pathol. Med. 1999, 28, 92–95. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Hirakawa, N.; Tomita, K. Postradiation Angiosarcoma of the Tongue. Eur. Arch. Oto Rhino Laryngol. 2000, 257, 464–465. [Google Scholar] [CrossRef]
- Loudon, J.A.; Billy, M.L.; DeYoung, B.R.; Allen, C.M. Angiosarcoma of the Mandible. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 471–476. [Google Scholar] [CrossRef]
- Fanburg-Smith, J.C.; Furlong, M.A.; Childers, E.L.B. Oral and Salivary Gland Angiosarcoma: A Clinicopathologic Study of 29 Cases. Mod. Pathol. 2003, 16, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.J.; Radden, B.G.; Gibbons, S.D.; Busmanis, I.; Cook, R.M. Primary Angiosarcoma of the Oral Cavity. Br. J. Oral Maxillofac. Surg. 1991, 29, 38–41. [Google Scholar] [CrossRef]
- Di Battista, M.; Darling, M.R.; Scrivener, E.; Stapleford, R.; Wehrli, B.; McCord, C. Histologic and Immunopathologic Variability in Primary Intraoral Angiosarcoma: A Case Report and Review of the Literature. Head Neck Pathol. 2020, 14, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Thomas, G.; Mathew, A.; Abraham, E.; Somanathan, T.; Ramadas, K.; Iype, E.; Ahamed, I.; Sebastian, P.; Nair, M. Sarcoma of the Oral and Maxillofacial Soft Tissue in Adults. Eur. J. Surg. Oncol. 2000, 26, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.T.; Puttgen, K.B.; Westra, W.H. Angiosarcoma Arising from the Tongue of an 11-Year-Old Girl with Xeroderma Pigmentosum. Head Neck Pathol. 2012, 6, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Dutta, S.; Rout, P. Angiosarcoma of Mandible: An Uncommon Clinical Entity. Int. J. Appl. Basic Med. Res. 2015, 5, 142–144. [Google Scholar] [CrossRef]
- Hunasgi, S. Angiosarcoma of Anterior Mandibular Gingiva Showing Recurrence—A Case Report with Immunohistochemistry. J. Clin. Diagn. Res. 2016, 10, ZD01. [Google Scholar] [CrossRef]
- Henny, F.A. Angiosarcoma of the Maxilla in a 3-Month-Old Infant; Report of Case. J. Oral Surg. 1949, 7, 250–252. [Google Scholar]
- Blake, H.; Blake, F.S. Angiosarcoma. Oral Surg. Oral Med. Oral Pathol. 1956, 9, 821–825. [Google Scholar] [CrossRef]
- Quinn, J.H.; McConnell, H.A.; Leonard, G.L. Multifocal Angiosarcoma of the Gingiva: Report of Case. J. Oral Surg. 1970, 28, 215–217. [Google Scholar]
- Albright, C.R.; Shelton, D.W.; Vatral, J.J.; Hobin, F.C. Angiosarcoma of the Gingiva: Report of Case. J. Oral Surg. 1970, 28, 913–917. [Google Scholar] [PubMed]
- Piscioli, F.; Leonardi, E.; Scappini, P.; Cristofolini, M.; King, D.F. Primary Angiosarcoma of the Gingiva. Am. J. Dermatopathol. 1986, 8, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Kashima, K.; Igakura, Y.; Komura, M.; Hamada, M.; Arima, R.; Sakoda, S. Three Gingival Tumors Derived from Vascular Endothelial Cells: A Case of Hemangioendothelioma and Two Cases of Angiosarcoma. Nihon Koku Shuyo Gakkaishi 1994, 6, 251–261. [Google Scholar]
- Margiotta, V.; Florena, A.M.; Giuliana, G. Primary Angiosarcoma of the Alveolar Mucosa in a Haemodialysis Patient: Case Report and Discussion. J. Oral Pathol. Med. 1994, 23, 429–431. [Google Scholar] [CrossRef]
- Muñoz, M.; Monje, F.; del Hoyo, J.A.; Martín-Granizo, R. Oral Angiosarcoma Misdiagnosed as a Pyogenic Granuloma. J. Oral Maxillofac. Surg. 1998, 56, 488–491. [Google Scholar] [CrossRef]
- Abdullah, B.H.; Yahya, H.I.; Talabani, N.A.; Alash, N.I.; Mirza, K.B. Gingival and Cutaneous Angiosarcoma. J. Oral Pathol. Med. 2000, 29, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Simionescu, C.; Mărgăritescu, C.; Georgescu, C.V. Gingival Angiosarcoma: Histopathologic and Immunohistochemical Study. Rom. J. Morphol. Embryol. 2005, 46, 57–61. [Google Scholar]
- Uchiyama, Y.; Murakami, S.; Kishino, M.; Furukawa, S. A Case Report of Primary Gingival Angiosarcoma. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e17–e21. [Google Scholar] [CrossRef]
- Sumida, T.; Murase, R.; Fujita, Y.; Ishikawa, A.; Hamakawa, H. Epulis-like Gingival Angiosarcoma of the Mandible: A Case Report. Int. J. Clin. Exp. Pathol. 2012, 5, 830–833. [Google Scholar]
- Aditya, A.; Lele, S. A Nodular Growth on Maxillary Gingiva. Indian J. Dent. Res. 2012, 23, 116. [Google Scholar] [CrossRef]
- Tojo, M.; Yoshida, K.; Arakane, N.; Tamagawa, H.; Miyanaga, R.; Watanabe, K.; Fukuhara, S. Primary Angiosarcoma of the Ascending Colon Diagnosed after the Discovery of Intraoral Tumor. Clin. J. Gastroenterol. 2023, 16, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mei, K.; Wu, C.; Wu, Y.; Xu, Y. Angiosarcoma of the Gingiva: Metastasis from a Primary Tumor of the Scalp. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 626. [Google Scholar] [CrossRef]
- Mota, M.E.; Tomo, S.; Alves, F.D.A.; Pellissari, G.A.; do Nascimento, A.G.; Lopes, R.N. Gingival Metastasis of Angiosarcoma of the Breast as a First Manifestation of Spreading Disease: Case Report and Review of the Literature. Spec. Care Dent. 2024, 44, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Al Ali, M.M.; Al Otaibi, L.M.; Al Mohaya, M.A.; Khoja, H.A. Intraoral Angiosarcoma with Unusual Clinical Presentation: A Case Report. Heliyon 2023, 9, e17056. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.J.; Green, D.M. Oral Presentation of Disseminated Angiosarcoma. Br. J. Oral Maxillofac. Surg. 1986, 24, 277–285. [Google Scholar] [CrossRef]
- Tanaka, Y.; Seike, S.; Tomita, K.; Ikeda, J.; Morii, E.; Isomura, E.T.; Kubo, T. Possible Malignant Transformation of Arteriovenous Malformation to Angiosarcoma: Case Report and Literature Review. J. Surg. Case Rep. 2019, 2019. [Google Scholar] [CrossRef]
- Hoshimoto, Y.; Aoki, T.; Ichikawa, M.; Ishii, Y.; Kondo, Y.; Uchibori, M.; Sasaki, M.; Naito, H.; Ota, Y. Metastatic Maxillary Gingival Angiosarcoma with Aggressive Growth: A Case Report. Tokai J. Exp. Clin. Med. 2024, 49, 22–26. [Google Scholar]
- Sanchez, I.M.; DiTommaso, L.E.; Tsoukas, M.M. Oral Kaposi Sarcoma. JAMA Dermatology 2019, 155, 370. [Google Scholar] [CrossRef]
- Sakina, G.; Liew, Y.T. Kaposi’s Sarcoma of the Palate. QJM An Int. J. Med. 2022, 114, 896–897. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Goldsztein, H.; Dezube, B.J.; Pantanowitz, L. Acquired Immunodeficiency Syndrome Related Kaposi’s Sarcoma Eroding the Maxillary Bone. J. Laryngol. Otol. 2008, 122, 993–997. [Google Scholar] [CrossRef]
- Martins-de-Barros, A.V.; Carvalho, M.D.V.; Araújo, F.A.D.C. AIDS-Related Kaposi Sarcoma of the Oral Cavity. Rev. Soc. Bras. Med. Trop. 2023, 56, e0133. [Google Scholar] [CrossRef]
- Kennedy-LeJeune, E.; Cataldo, V.D. Kaposi’s Sarcoma of the Oral Cavity. N. Engl. J. Med. 2017, 376, 1268. [Google Scholar] [CrossRef]
- Wild, R.; Balmer, M.C. Have We Forgotten? Oral Manifestations of Kaposi’s Sarcoma. Sex. Transm. Infect. 2015, 91, 345. [Google Scholar] [CrossRef]
- Pugalagiri, P.; Muller, S.; Cox, D.P.; Kessler, H.P.; Wright, J.M.; Cheng, Y.-S.L. Lymphangioma-like Kaposi Sarcoma of the Oral Mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Varoni, E.; Sardella, A.; Lodi, G. Oral Kaposi’s Sarcoma in a HIV-Negative Young Patient. Oral Oncol. 2020, 103, 104567. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yang, H.; Sun, A.; Chen, H.-M. Kaposi’s Sarcoma of the Hard Palate. J. Formos. Med. Assoc. 2016, 115, 883–884. [Google Scholar] [CrossRef]
- Faden, A.; AlSheddi, M.; AlKindi, M.; Alabdulaaly, L. Oral Kaposi Sarcoma in HIV-Seronegative Saudi Patient: Literature Review and Case Report. Saudi Dent. J. 2017, 29, 129–134. [Google Scholar] [CrossRef]
- Safia, A.; Farhat, R.; Avraham, Y.; Merchavy, S. Kaposi Sarcoma at the Base of the Tongue in a Renal Transplant Patient. BMJ Case Rep. 2023, 16, e253899. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.H.; Darrow, M.; Chen, L.; Alnimri, M.; Jen, K. Tonsillar Kaposi Sarcoma in a Renal Transplant Patient. Transpl. Infect. Dis. 2020, 22, e13347. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Di Vincenzo, S.A.; Campofiorito, V.; Sireci, F.; Immordino, A.; Dispenza, F. Tonsillar Kaposi’s Sarcoma in HIV Positive Patient with Syphilis Infection. Iran. J. Otorhinolaryngol. 2024, 36, 437–441. [Google Scholar]
- Feller, L.; Anagnostopoulos, C.; Wood, N.H.; Bouckaert, M.; Raubenheimer, E.J.; Lemmer, J. Human Immunodeficiency Virus–Associated Kaposi Sarcoma as an Immune Reconstitution Inflammatory Syndrome: A Literature Review and Case Report. J. Periodontol. 2008, 79, 362–368. [Google Scholar] [CrossRef]
- Papagatsia, Z.; Jones, J.; Morgan, P.; Tappuni, A.R. Oral Kaposi Sarcoma: A Case of Immune Reconstitution Inflammatory Syndrome. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Chuang, S.-K.; Philipone, E.M.; Peters, S.M. Characteristics and Prognosis of Primary Head and Neck Angiosarcomas: A Surveillance, Epidemiology, and End Results Program (SEER) Analysis of 1250 Cases. Head Neck Pathol. 2019, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Sazumi, Y.; Mizuta, Y.; Sakae, H.; Otsuka, F. Gingival Lesion Leading to a Diagnosis of Angiosarcoma. J. Gen. Fam. Med. 2021, 22, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Miyaguchi, K.; Mogushi, K.; Watanabe, H.; Okada, N.; Shibuya, H.; Mizushima, H.; Miura, M.; Tanaka, H. PARVB Overexpression Increases Cell Migration Capability and Defines High Risk for Endophytic Growth and Metastasis in Tongue Squamous Cell Carcinoma. Br. J. Cancer 2015, 112, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Mullins, B.; Hackman, T. Angiosarcoma of the Head and Neck. Int. Arch. Otorhinolaryngol. 2015, 19, 191–195. [Google Scholar] [CrossRef]
- Mark, R.J.; Tran, L.M.; Sercarz, J.; Fu, Y.S.; Calcaterra, T.C.; Juillard, G.F. Angiosarcoma of the Head and Neck: The UCLA Experience 1955 Through 1990. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 973–978. [Google Scholar] [CrossRef]
- Lee, N.C.J.; Eskander, A.; Miccio, J.A.; Park, H.S.; Shah, C.; Rutenberg, M.; Hosni, A.; Husain, Z.A. Evaluation of Head and Neck Soft Tissue Sarcoma 8th Edition Pathologic Staging System and Proposal of a Novel Stage Grouping System. Oral Oncol. 2021, 114, 105137. [Google Scholar] [CrossRef]
- Houpe, J.E. Treatment of Angiosarcoma of the Head and Neck: A Systematic Review. Cutis 2023, 111, 247–251. [Google Scholar] [CrossRef]
- Ishida, Y.; Otsuka, A.; Kabashima, K. Cutaneous Angiosarcoma: Update on Biology and Latest Treatment. Curr. Opin. Oncol. 2018, 30, 107–112. [Google Scholar] [CrossRef]
- Nichols, C.M.; Flaitz, C.M.; Hicks, M.J. Primary Intraosseous Kaposi’s Sarcoma of the Maxilla in Human Immunodeficiency Virus Infection. J. Oral Maxillofac. Surg. 1995, 53, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.T.; Thompson, L.D.R. Kaposi Sarcoma of Major Salivary Gland Origin. Cancer 2000, 88, 15–23. [Google Scholar] [CrossRef]
- Russo, I.; Marino, D.; Cozzolino, C.; Del Fiore, P.; Nerjaku, F.; Finotto, S.; Cattelan, A.; Calabrò, M.L.; Belloni Fortina, A.; Russano, F.; et al. Kaposi’s Sarcoma: Evaluation of Clinical Features, Treatment Outcomes, and Prognosis in a Single-Center Retrospective Case Series. Cancers 2024, 16, 691. [Google Scholar] [CrossRef] [PubMed]
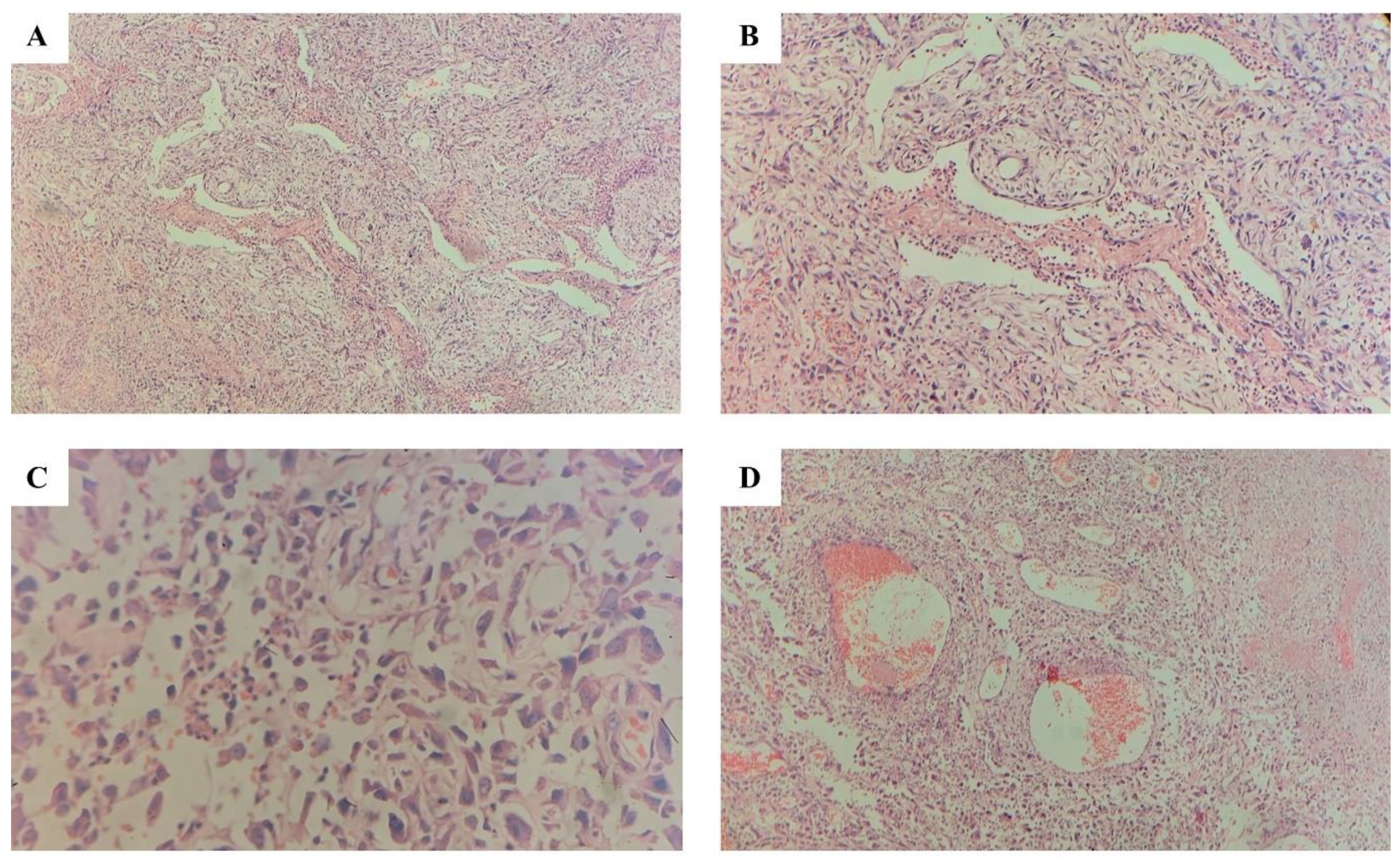

| Clinical Presentation | Histopathology | Immunohistochemistry | |
|---|---|---|---|
| Case 1 | 49-year-old male Three-month history of a growth on the right side of the cheek Intraoral examination: reddish-pink exophytic growth (6.0 × 4.0 cm2) on the right buccal mucosa Firm and non-tender lesion No palpable cervical lymph nodes | Tumor composed of anastomosing vascular spaces. Lined by atypical plump endothelial cells, predominantly epithelioid. Numerous mitoses with abnormal forms. Minimal collagenous stroma. | CD31& CD34: Strongly positive in most tumor cells. EMA & Pan CK: focally positive. S100, Desmin, SMA, Caldesmon: Negative for tumor cells |
| Case 2 | 59-year-old male Ulcerative growth over the left side floor of the mouth Impaired tongue movements Intraoral examination: 1.0 × 0.6 cm2 wedge-shaped red-colored growth with soft consistency Painful ulceration with everted edges | Unencapsulated tumor with high degree of cytological atypia. Marked cellular and nuclear pleomorphism in some tumor islands. Intracytoplasmic lumina formation in some tumor cells. | Factor VIII: Positive in tumor cells Cytokeratin: Positive in tumor cells |
| Case 3 | 61-year-old male Rapidly growing painless gingival swelling on the right side of the lower alveolar region two weeks after tooth extraction | Unencapsulated tumor composed of discohesive sheets and islands of epithelial cells. Large vesicular nuclei, eosinophilic cytoplasm, high-grade nuclear pleomorphism, increased mitotic activity. Decalcified specimen of bone margins showed evidence of the tumor. Four out of eight lymph nodes from submandibular gland specimen showed extensive tumor metastasis and necrosis with extracapsular invasion. | CK (Cytokeratin) and CD31 positive in tumor cells, MyoD1 and CK 5/6 negative |
| Feature | Primary Angiosarcoma (n = 60) | Metastatic Angiosarcoma (n = 14) |
|---|---|---|
| Age in years 0–18 19–64 65 | 11.67% (n = 7) 51.67% (n = 31) 36.66% (n = 22) | - 42.86% (n = 6) 57.14% (n = 8) |
| Sex Male Female | 56.67% (n = 34) 43.33% (n = 26) | 78.57% (n = 11) 21. 43% (n = 3) |
| Site Tongue Buccal mucosa Lip Palate Mandibular gingiva/alveolar mucosa Maxillary gingiva/alveolar mucosa Both (mandibular gingiva + maxillary gingiva) | 35.00% (n = 21) 6.67% (n = 4) 6.67% (n = 4) 6.67% (n = 4) 31.67% (n = 19) 10.00% (n = 6) 1.67% (n = 1) | 28. 57% (n = 4) 64.29% (n = 9) 7.14% (n = 1) |
| Bone involvement Yes No | 38.33% (n = 23) 61.67% (n = 37) | 71.43% (n = 10) 21.43% (n = 3) |
| Size >5 cm <5 cm Not available | 23.33% (n = 14) 26.67% (n = 16) 50.00% (n = 30) | - 42.86% (n = 6) 57.14% (n = 8) |
| Treatment Surgery only (S) Radiotherapy only (R) Chemotherapy only (C) S + R + C S + R S + C R + C | 33.33% (n = 20) 1.67% (n = 1) 1.67% (n = 1) 1.67% (n = 1) 11.67% (n = 7) 6.67% (n = 4) 5.00% (n = 3) | 7.14% (n = 1) 7.14% (n = 1) 7.14% (n = 1) 7.14% (n = 1) 7.14% (n = 1) 14.28% (n = 2) |
| Outcome NED DOD Not available DOC | 35.00% (n = 21) 36.606% (n = 22) 26.66% (n = 16) 1.67% (n = 1) | 14.28% (n = 2) 71.43% (n = 10) 14.28% (n = 2) |
| Feature | Kaposi’s Sarcoma (n = 20) |
|---|---|
| Age in years | |
| 0–18 | - |
| 19–64 | 85.00% (n = 17) |
| 65 | 10.00% (n = 2) |
| N/A | 5.00% (n = 1) |
| Sex | |
| Male | 95.00% (n = 19) |
| Female | 5.00% (n = 1) |
| Site | |
| Tongue | 5.00% (n = 1) |
| Palate | 65.00% (n = 13) |
| Mandibular gingiva | 15.00% (n = 3) |
| Maxillary gingiva | 40.00% (n = 8) |
| Tonsils | 10. 00% (n = 2) |
| Retromolar area | 10.00% (n = 2) |
| Mandible | 5.00% (n = 1) |
| Bone involvement | |
| Yes | 25.00% (n = 5) |
| No | 30.00% (n = 6) |
| Not mentioned | 45.00% (n = 9) |
| Size | |
| >5 cm | 5.00% (n = 1) |
| <5 cm | 20.00% (n = 4) |
| Not available | 75.00% (n = 15) |
| Treatment | |
| Surgery (S) | 5.00% (n = 1) |
| Radiotherapy | 15. 00% (n = 3) |
| Anti-retroviral therapy (ART) | 10.00% (n = 2) |
| ART + S | 10.00% (n = 2) |
| ART + Chemotherapy (C) | 10.00% (n = 2) |
| ART + S + C | 10.00 (n = 2) |
| R + C (palliative) | 5.00 (n = 1) |
| Outcome | |
| NED * | 45.00% (n = 9) |
| DOD ** | - |
| Partial regression | 10.00% (n = 2) |
| Not available | 45.00 (n = 9) |
| Type | |
| HIV | 65.00% (n = 13) |
| Iatrogenic | |
| Immunocompromised (DM) | 5.00% (n = 1) |
| Immunosuppressed | 10.00% (n = 2) |
| Classic | 15.00% (n = 3) |
| Not indicated | 5.00% (n = 1) |
| Clinical presentation | |
| Nodule | 65.00% (n = 13) |
| Plaque | 10.00% (n = 2) |
| Ulcerated mass | 10.00% (n = 2) |
| Soft tissue swelling | 15.00% (n = 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasooriya, P.R.; Weerasinghe, H.A.W.; Jayasinghe, L.A.H.; Peiris, P.M.; Abeyasinghe, W.A.M.U.L.; Jayasinghe, R.D. A Comprehensive Literature Review on Diagnostic Strategies and Clinical Outcome of Intraoral Angiosarcoma and Kaposi Sarcoma. J. Vasc. Dis. 2024, 3, 306-318. https://doi.org/10.3390/jvd3030024
Jayasooriya PR, Weerasinghe HAW, Jayasinghe LAH, Peiris PM, Abeyasinghe WAMUL, Jayasinghe RD. A Comprehensive Literature Review on Diagnostic Strategies and Clinical Outcome of Intraoral Angiosarcoma and Kaposi Sarcoma. Journal of Vascular Diseases. 2024; 3(3):306-318. https://doi.org/10.3390/jvd3030024
Chicago/Turabian StyleJayasooriya, Primali Rukmal, Hiruni Ashcharya Wijerathna Weerasinghe, Liyanaarachchige Anushan Hiranya Jayasinghe, Prasangi Madubhashini Peiris, Wijeyapala Abeyasinghe Mudiyanselage Udari Lakshika Abeyasinghe, and Ruwan Duminda Jayasinghe. 2024. "A Comprehensive Literature Review on Diagnostic Strategies and Clinical Outcome of Intraoral Angiosarcoma and Kaposi Sarcoma" Journal of Vascular Diseases 3, no. 3: 306-318. https://doi.org/10.3390/jvd3030024
APA StyleJayasooriya, P. R., Weerasinghe, H. A. W., Jayasinghe, L. A. H., Peiris, P. M., Abeyasinghe, W. A. M. U. L., & Jayasinghe, R. D. (2024). A Comprehensive Literature Review on Diagnostic Strategies and Clinical Outcome of Intraoral Angiosarcoma and Kaposi Sarcoma. Journal of Vascular Diseases, 3(3), 306-318. https://doi.org/10.3390/jvd3030024






