2. Materials and Methods
This is a multicentric retrospective observational study conducted over a period of 2 years between August 2020 and September 2022, including 49 patients presented with chronic limb-threatening ischemia (CLTI) and preprocedural investigations revealed restenosis of previously applied peripheral stent for which a special pre-determined protocol was applied in all patients aiming to perform endovascular procedures to treat in-stent restenosis (ISR) using percutaneous mechanical rotational atherectomy combined with drug-coated balloon versus drug-coated balloon alone, all interventions were performed at (name deleted to maintain the integrity of the review process) (30 patients) and (name deleted to maintain the integrity of the review process) (19 patients) by interventionalists share the same high learning curve and experience in both hospitals.
All patients were meticulously assessed by clinical examination, color-coded duplex ultrasonography (US) and computed tomography angiography (CTA). Study approval was obtained from the medical ethical committee, and informed consent was obtained from all patients.
Inclusion criteria included patients with CLTI, and investigations revealed in-stent restenosis/occlusion (thrombotic or intimal hyperplasia) with vessel diameter of more than 3 mm and the onset of ischemia either acute (less than 14 days), subacute (less than 3 months) or chronic (more than 3 months). Patients who refused intervention, patients with extensive gangrene (Rutherford 6) or in case of subintimal lesion crossing by the wire were excluded from the study.
Data regarding each patient at the time of the intervention and details about the procedure performed were entered retrospectively in the peripheral intervention report. The data were recorded using standardized forms and entered into a computerized database.
Full history was taken from all patients including name, gender, age, occupation, risk factors (smoking, diabetes, hypertension, +ve family history), affected lower limb, clinical presentation, duration of symptoms, past history (coronary disease, cardiomyopathy, congestive heart failure, stroke) and history of lower limb revascularization surgeries.
Clinical examinations with blood pressure, pulse, temperature, respiratory rate and local examination of the affected lower extremity (affected side, skin temperature, peripheral pulsations, trophic changes, ischemic ulcers, gangrene and previous amputation) were performed on all patients. Preoperative blood samples for serum creatinine level, fasting blood sugar, lipid profile (Cholesterol, LDL, HDL, TG) and CBC (Hemoglobin, WBC, PLT) were collected from all patients.
All patients were transferred to (Siemens Artis Zee and Philips FD20) machine catheter labs. After local anesthesia, we used US guidance (9-MHz linear probe, GE Logic E9; General Electric Medical Systems, Milwaukee, WI, USA) for access puncture to minimize the risk of bleeding.
2.1. Technique
All patients were loaded with Clopidogrel (300 mg), and an ipsilateral antegrade approach via femoral access was used as standard access. However, brachial and contralateral retrograde cross-over femoral accesses could be used in certain cases. A 6-F end-hole introducer sheath or a 7-F end-hole long cross-over sheath (Radifocus; Terumo, Tokyo, Japan) was advanced. After obtaining access, patients were anticoagulated with a 5000-unit bolus of intravenous unfractionated heparin.
Ultravist (Bayer Healthcare, Leverkusen, Germany) was used as a contrast medium for image acquisition during the endovascular intervention. Delayed imaging up to 30 s and large contrast volumes may be needed to opacify the tibial runoff vessels. In patients with impaired kidney functions, a CO2-guided endovascular procedure was performed with the aid of an automated injector system (Angiodroid ©, San Lazzaro di Savena, Italy).
After lesion localization, lesion crossing should be carefully performed trans-luminally, and subintimal passage should be avoided at all means. We used Command wire (Abbott Park, IL, USA) or V-18 standard wire (Watertown, MA, USA) with supporting catheter Trail-Blazer (Medtronic, Dublin, Ireland) or Renegade (Watertown, MA, USA) to achieve transluminal guidance of the wire through the lesion.
According to the lesion nature, morphology and length, the decision was tailored for the treatment plan, as we used a wire traversal test through the re-stenosed or occluded segment to raise the suspicion of thrombosis on top of hyperplasia, we tried to confirm the presence of thrombus by either obtaining different angiographic images in different projections or by applying suction to the thrombotic materials. If the diagnosis of thrombosis is confirmed, Rotarex® is preferred to clear the thrombus load and the underlying hyperplastic lesion. DCB is usually not preferred to avoid distal embolization and trashing with balloon inflation.
Regarding the hyperplastic lesions, the decision was variable; for single or multiple focal stenotic lesions, paclitaxel drug-coated balloon application was considered, while for long, diffuse and calcific lesions, percutaneous mechanical debulking using Rotarex in conjunction with paclitaxel drug-coated balloon was chosen. So, we can classify our treated patients into two groups:
Group 1: Patients were treated with an atherectomy and adjunctive drug-coated balloon.
Group 2: patients were treated with a drug-coated balloon only.
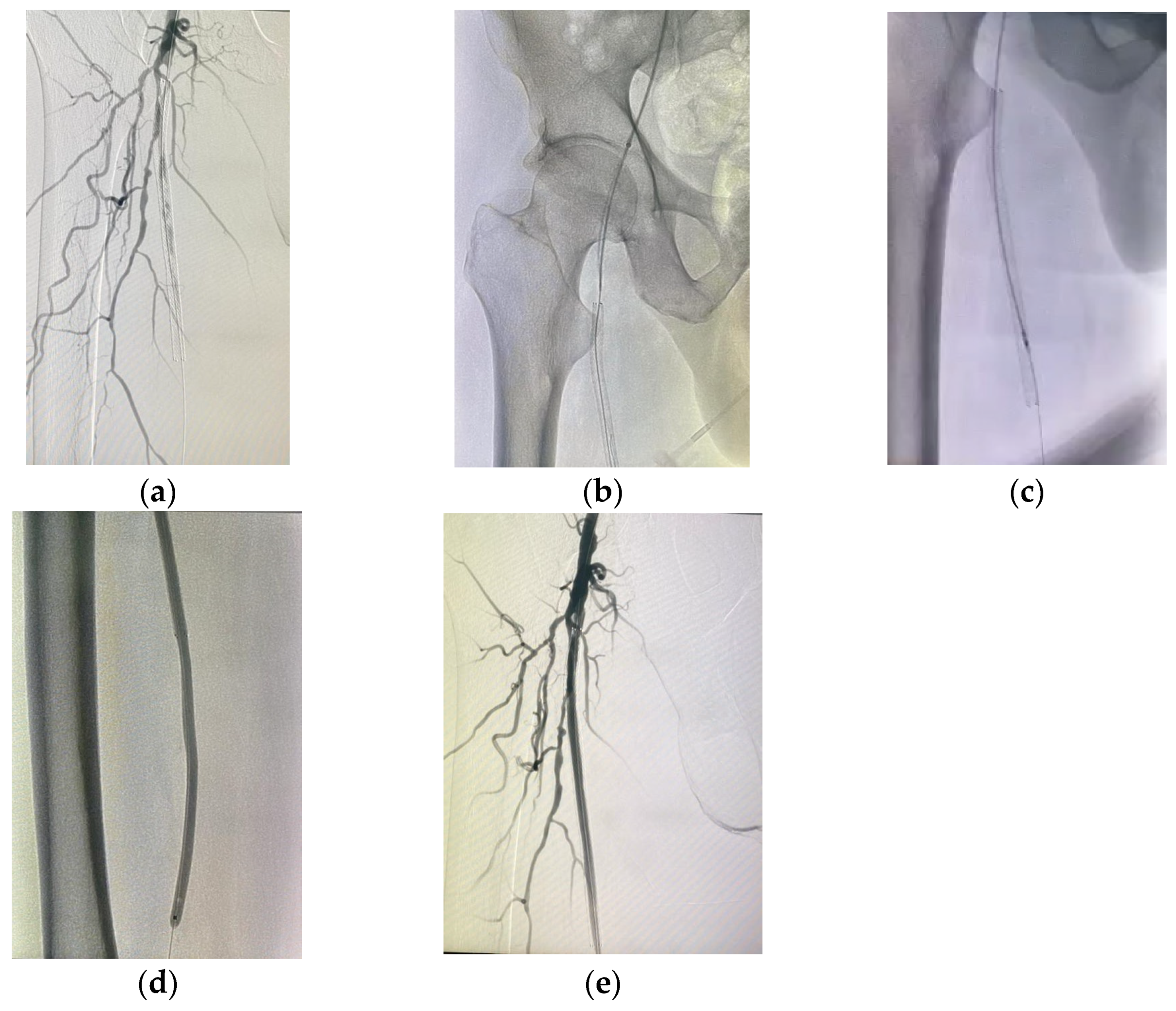
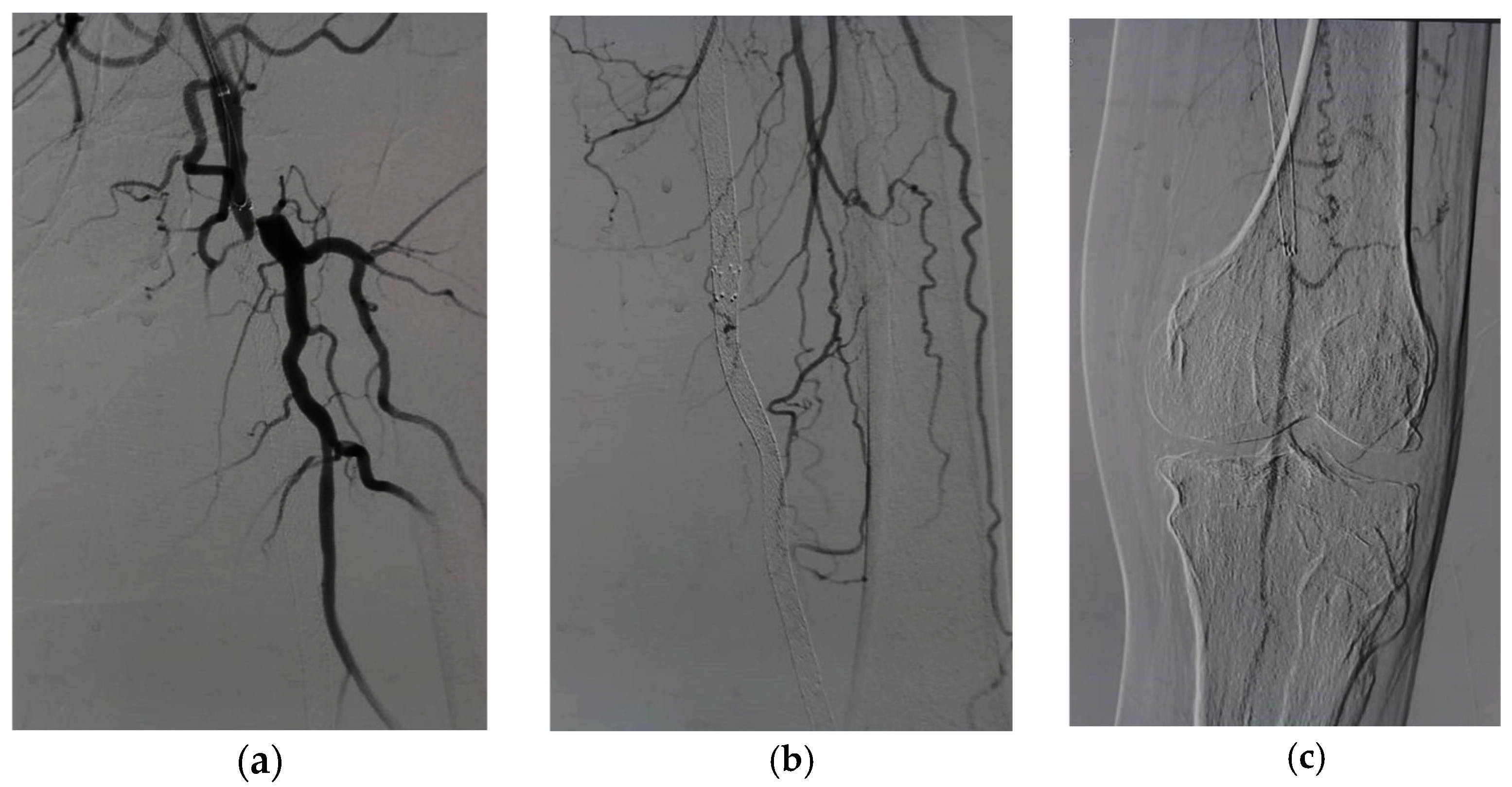
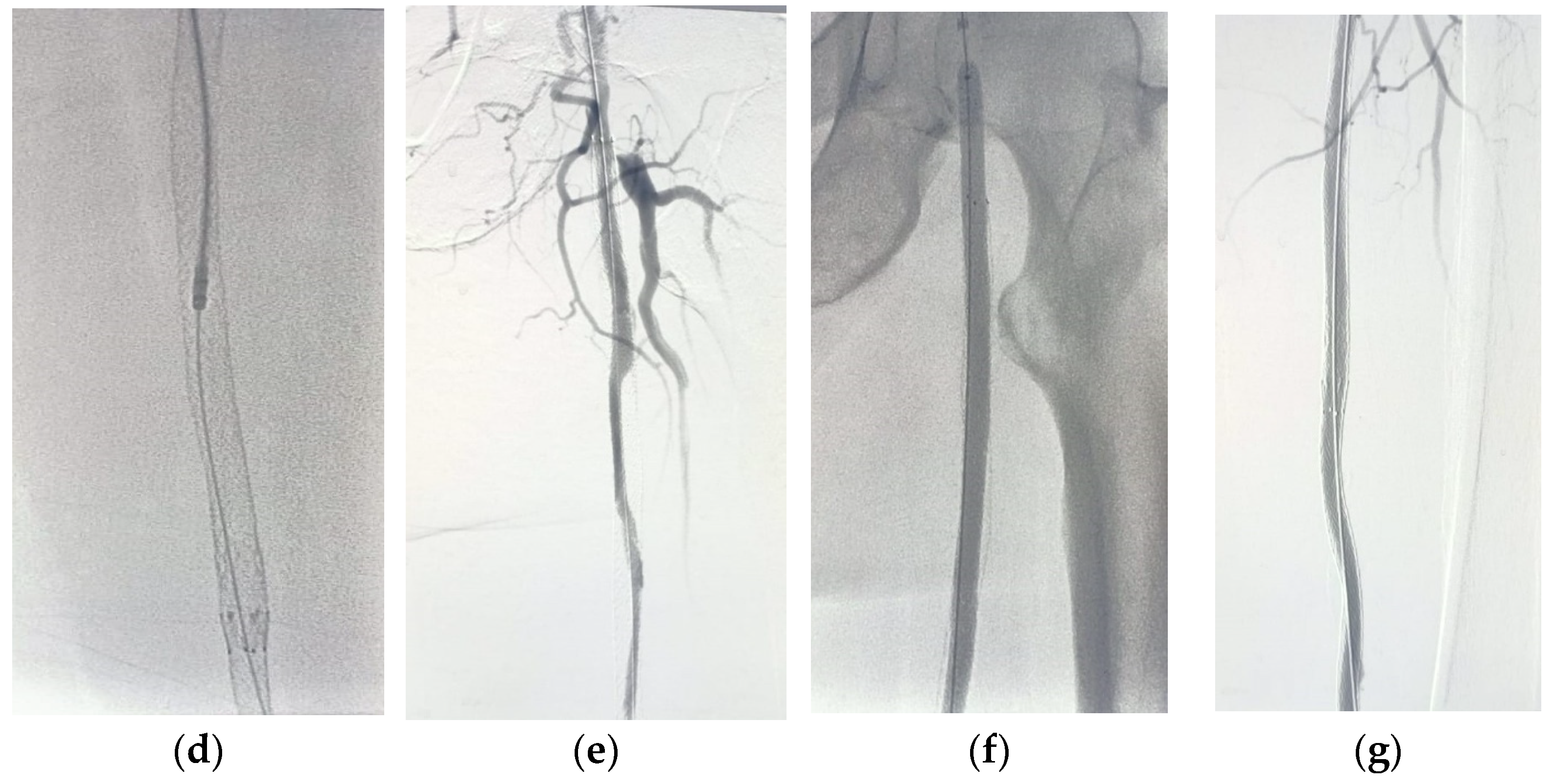
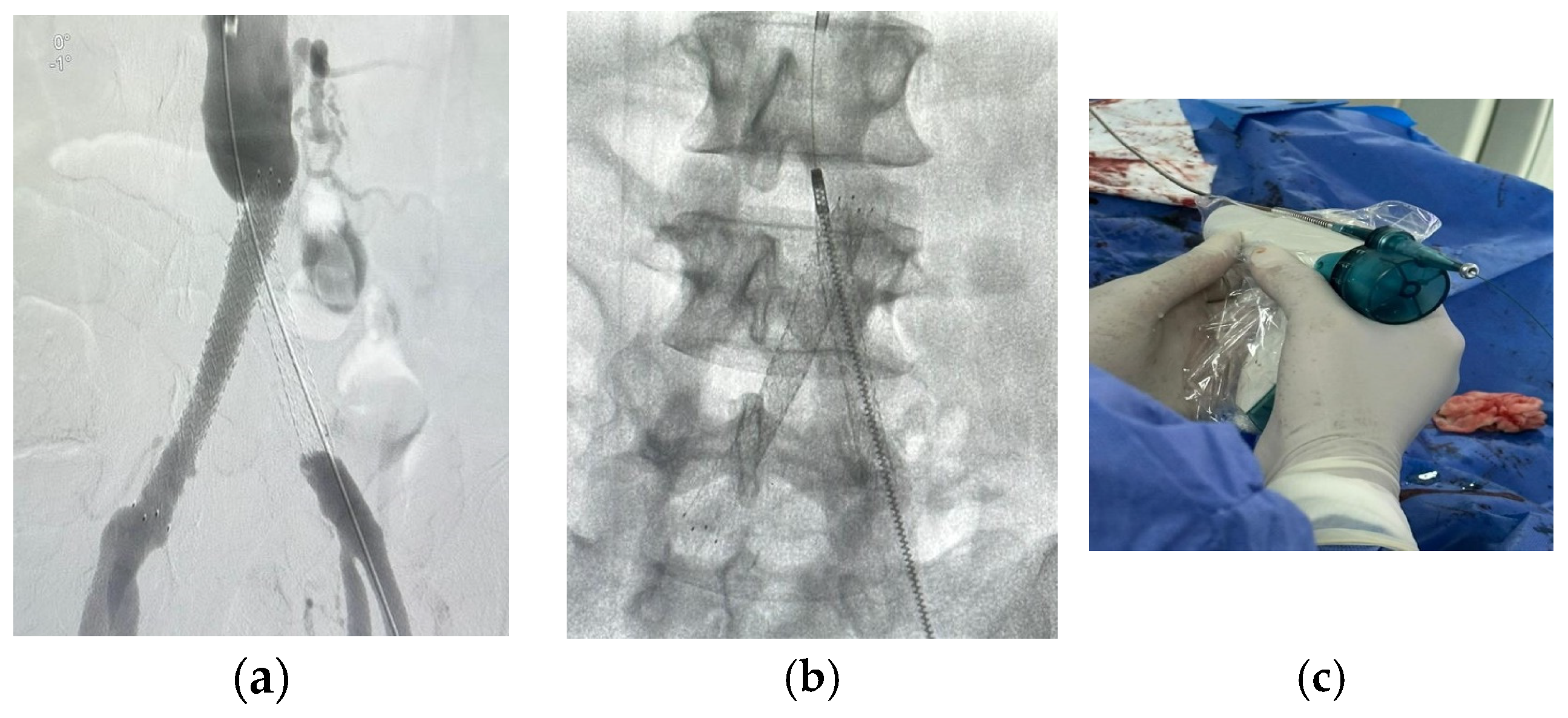
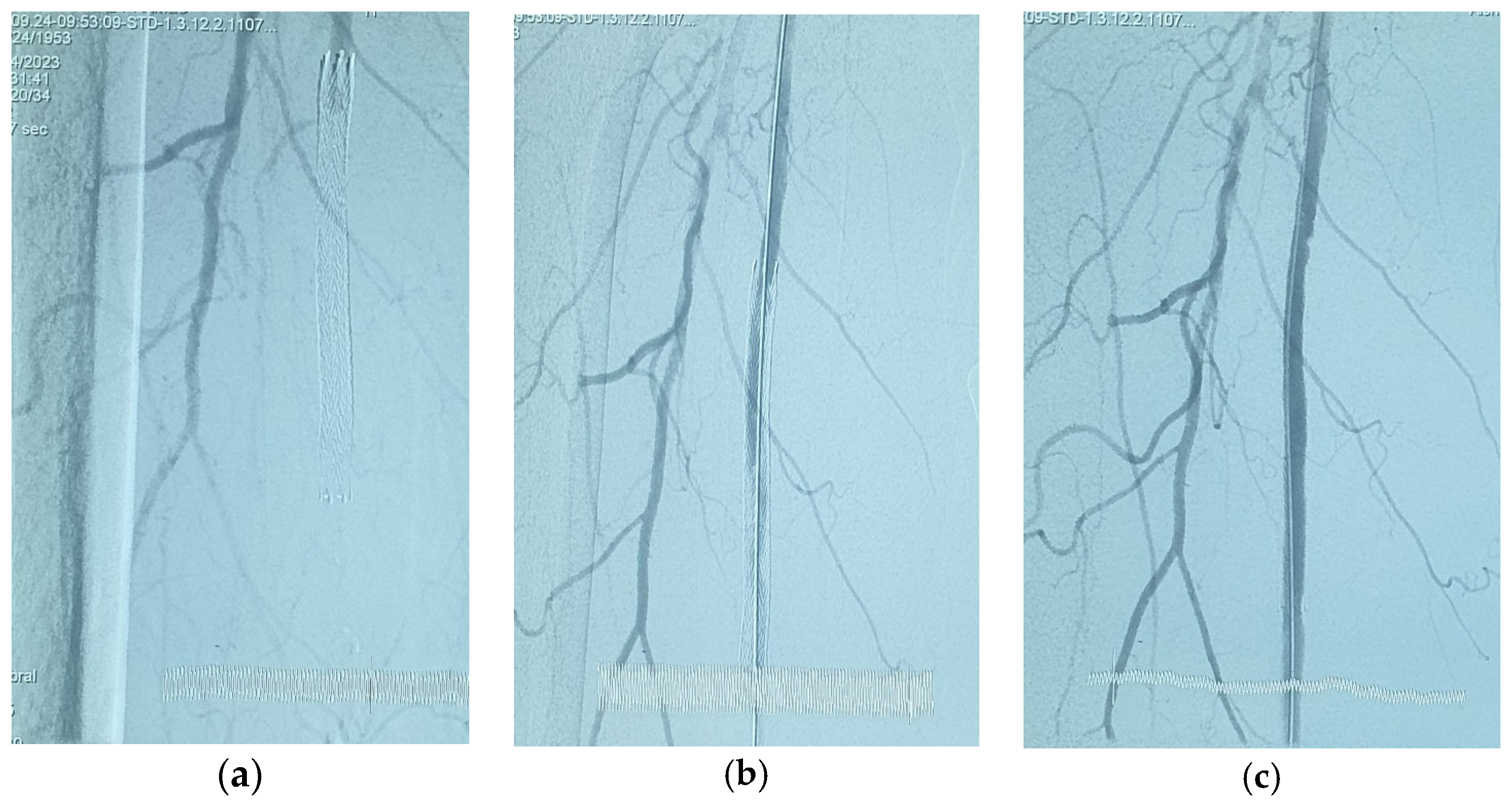
For patients in group 1, after securing the wire passage through the lesion, the mechanical rotational atherectomy device for percutaneous mechanical debulking (PMD) Rotarex® System (Straub Medical, Wangs, Switzerland), with 6-, 8- or 10-French (Fr) sheath compatible devices, depending on the vessel diameter to be treated, and variable length 85, 110 and 185 cm, was advanced over 0.018 wire.
The rotational thrombectomy device was slowly advanced and retracted in order to avoid embolic complications downstream of the occlusion. The rotations produce a continuous vacuum inside the catheter, which leads to aspiration of the thrombus into the catheter and transportation into the collection bag. The suction performance is about 0.66 mL/s with the 6-Fr system and 1.5 mL/s with the 8- and 10-Fr system. If necessary, the procedure was repeated until the blood flow was fully restored.
The Rotarex® catheter was then withdrawn while keeping the wire in place, a drug-coated balloon, IN.PACT Admiral paclitaxel-eluting balloon (Medtronic Cardiovascular, Santa Rosa, CA, USA) or Lutonix (Bard Peripheral Vascular, Tempe, AZ, USA), which were the FDA approved DCB, was advanced over the 0.018 wire with inflation time for 3 min at 8–12 atm pressure.
For patients in group 2, pre-dilatation with a plain balloon (1 mm smaller than the reference diameter) was performed first for optimal wall preparation, followed by using a paclitaxel drug-coated balloon, as previously mentioned.
All candidates were followed up clinically and by duplex ultrasonography after one, three and six months after the intervention.
2.2. Study Endpoints
Primary endpoints include safety and efficacy. The safety endpoint was procedure-related complications, while efficacy was based on procedure success (successful access and ≤30% residual stenosis by quantitative angiography with evidence of at least one patent tibial artery to the foot). Other efficacy outcomes included immediate hemodynamic success (restored pulse), defined as (ABI) improvement ≥ 0.15 compared to baseline, except for patients with falsely elevated ABI (defined as ABI ≥ 1.3).
Secondary endpoints include freedom from clinically driven target lesion revascularization CD-TLR, major limb adverse event (MALE) and thirty-day mortality.
2.3. Statistical Analysis
The collected data were coded, tabulated and statistically analyzed using the statistical package SPSS (Statistical Package for the Social Sciences) version 28. Continuous data were presented as mean ± SD (standard deviation) and minimum and maximum of the range, while categorical data were expressed as numbers and percentages. Comparisons between groups were performed using an unpaired t test. For comparing categorical data, a Chi-square (χ2) test was performed. The exact test was used instead when the expected frequency was less than 5. A level of significance of p value < 0.05 was considered significant; otherwise, it was non-significant.
4. Discussion
In the last few years, ISR has become a frequently faced endovascular challenge, which raises the requirement for developing a modality of treatment [
1,
6]. Reduction in the bulk of atheromatous plaque with subsequent modification of its nature through the endovascular atherectomy devices can allow better outcomes and lower rate of procedural complications [
4,
7]. Particularly, debulking of heavily calcified atheromatous plaques using mechanical rotational atherectomy devices showed improved lesion response to endovascular balloon dilation and subsequently provided better delivery of antiproliferative agents to the wall [
5,
8].
Several devices and techniques can be used to treat femoropopliteal segments in stent stenotic and/or occlusive lesions. Bare metal stent implantation is one of the most popular and commonly used bail-out solutions in these lesions, as stent application can resolve intra-procedural problems induced by elastic recoil, residual stenosis or dissection. However, ISR stays high, with an incidence of 15% to 32% in 12 months [
9,
10].
Recorded risk factors for ISR in many published literature include male gender, patients on regular hemodialysis, severe lesion calcifications, lesion length and hypercholesterolemia [
11]. Surprisingly, diabetes was not found to be associated with an increased risk of ISR. On the other hand, insulin-dependent diabetes was reported as a predictor of CD-TLR [
12].
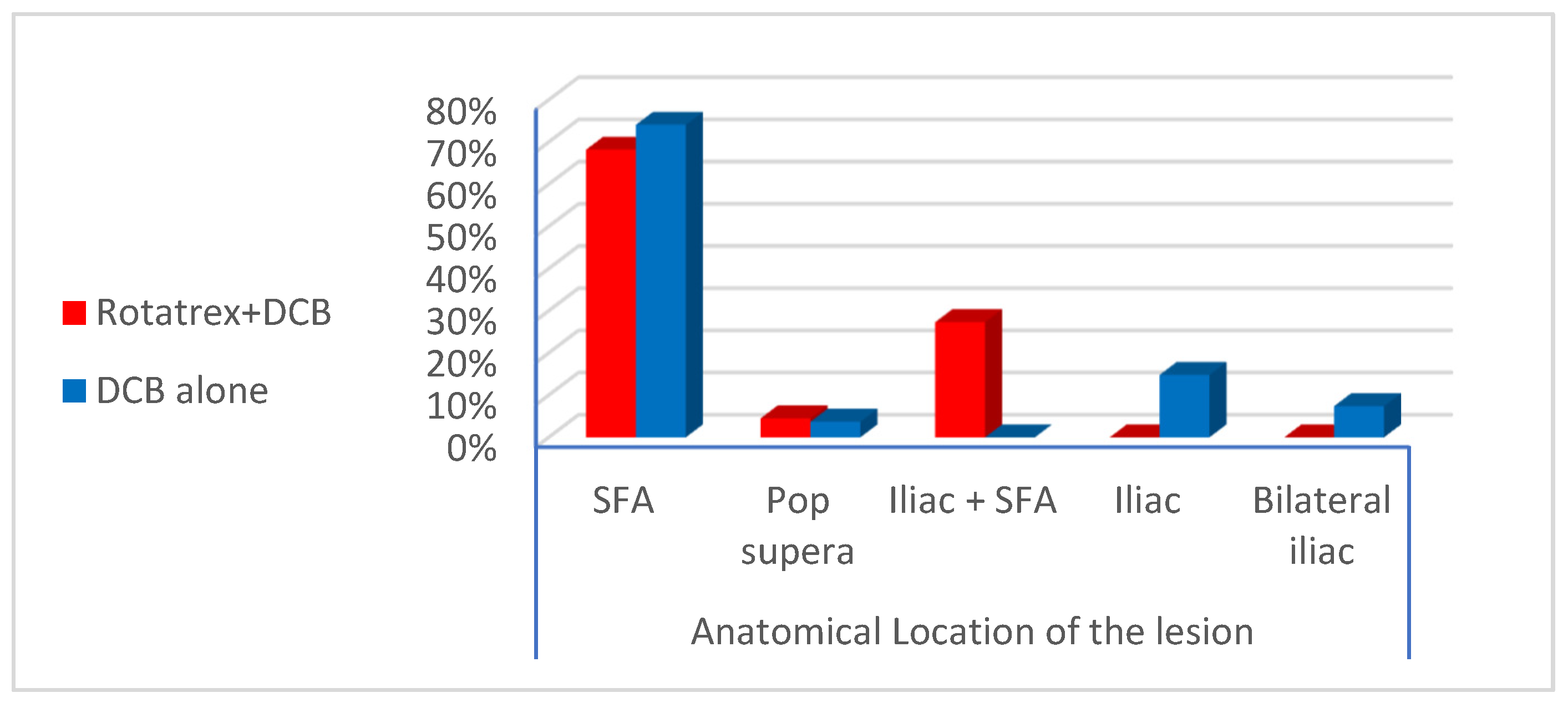
For most of the published studies and trials, the femoropopliteal segment is the arterial territory most frequently involved by ISR following previous endovascular stenting. The lesion distribution observed in the enrolled candidates revealed restenosis of previously deployed SFA stents in (71.4%) of patients while combined restenosis in iliac + SFA stents in (12.2%) of patients [
1].
In our study, ISR lesions within the femoropopliteal segment were found to be focal single stenosis in 6.1% of patients, multiple stenoses in 32.7% of patients, total occlusion in 22.4% of patients, and combined segmental stenosis and occlusion in 10.2% patients. For the patients with combined iliac + SFA stents, all of them showed stenosis of the iliac stent accompanied by occlusion of the SFA stent. The mean length of the lesion was 141.8 mm for Rotarex + DCB patients and 92.9 mm for DCB alone patients. Most of these criteria were similar to published studies and trials by Tepe et al. and Kokkinidis et al. [
10,
13].
Zeller T et al. documented 78.8% in-stent stenosis and 30% in-stent occlusion at 12 months follow-up for lesions with a mean length of 133 ± 91.7 mm [
14].
The outcome of several published studies and trials for the treatment modalities and techniques used for the treatment of femoropopliteal segment ISR is shown in
Table 7 and
Table S7. Also, multiple studies have documented more fruitful outcomes of hybrid treatment using the combination of atherectomy (laser, directional) with DCB angioplasty [
15].
The EXCITE ISR trial is the first randomized prospective study that highlighted the superiority of laser atherectomy plus PTA versus PTA alone, giving procedural success (93.5% vs. 82.7%,
p = 0.01) and few complications. Also, 6 months follow-up revealed freedom from CD-TLR (73.5% versus 51.8%,
p < 0.005) [
16].
The Rotarex
® system is a percutaneous mechanical rotational endovascular atherectomy in combination with a thrombectomy device, which provides safety and effectiveness in eliminating large thrombus load [
17,
18].
A comparative study between PMD and local thrombolysis for the treatment of acute and subacute in-stent thrombotic occlusion was conducted by Kronlage et al. The results showed technical success, reaching up to 98% for both modalities. However, one year of primary and secondary patency after PMD documented significantly better results compared to local thrombolysis (63% and 85%,
p < 0.05) [
19].
Also, successful recanalization was reported in (95%) of performed interventions by Stanek et al. for patients presenting with acute and subacute thrombotic and/or atherosclerotic in-stent occlusion in the peripheral limb arteries using Rotarex
® catheter system for PMD [
20].
According to multiple published studies, the Rotarex
® catheter system can be used for short or long occlusions with almost the same success rate, especially if combined with other adjunctive measures, particularly DCB, preferably in heavily calcified vessels and keeping in consideration guidewire lesion traversal should pass transluminal at all possible means [
20,
21,
22].
This study included 49 patients with ISR in a previously deployed peripheral stent, conducted on a heterogeneous patch of the population with different target vessels (iliac, SFA, popliteal or combined lesions) and with different types of in-stent lesions (stenosis, occlusion or combined), (thrombotic or hyperplastic) and the use of different techniques (PMD + DCB or DCB alone).
Much recent literature has documented that pre-treatment with atherectomy devices prior to balloon dilatation and drug-coated balloon showed a significant reduction in dissection within the offending lesion with subsequent need for endovascular stenting. Despite these superior early and mid-term results, these studies did not support more favorable outcomes of combined use of atherectomy and drug-coated balloon when compared to the use of drug-coated balloon alone regarding CD-TLR. Also, a meta-analysis of five clinical studies revealed no statistically significant privilege of combined use of atherectomy and DCB in terms of the primary patency or TLR compared to DCB alone [
23].
One of the major limitations of these published trials was the small study sample, giving rise to insufficient statistical power, making clear data on whether atherectomy with adjunctive DCB has clinical benefit in the treatment of ISR of the femoropopliteal segment inconclusive. Other explanations were concluded for these limitations as multiple types of atherectomy devices were used for the treatment of ISR or occlusion. It is still also unclear how much volume of the offending plaque should be debulked in order to obtain optimal early and late outcomes. In addition, too much lesion debulking can induce medial and adventitial injuries, which might promote subsequent restenosis [
24].
A study by Fanelli et al. concluded that severe lesion calcification was considered a significant risk factor for losing patency after DCB alone [
5]. Severe lesion calcification can easily limit the lesion response to balloon dilation and also impede the delivery of antiproliferative drugs. A trial published by Cioppa et al. has reported a 12-month primary patency rate of 90% after combined atherectomy and DCB in severely calcified ISR lesions [
8] while in the long-lesion subgroups of the Lutonix and IN.PACT global registries, dissection of the lesion happened in 34.3% and 62.1%, and bail-out stenting was needed in 35.7% and 39.4%, respectively [
25,
26].
Drawbacks related to the use of atherectomy devices have been encountered in many studies, especially intra-procedural distal embolization (advocating the use of embolic protection devices) [
27,
28] and vessel wall perforation in 2.6% of cases, as presented by Zhen Y et al. in a meta-analysis study [
23]. Meanwhile, a study by Boitet et al. suggested an association between the different types of DCB profiles and the occurrence of distal embolic events [
29].
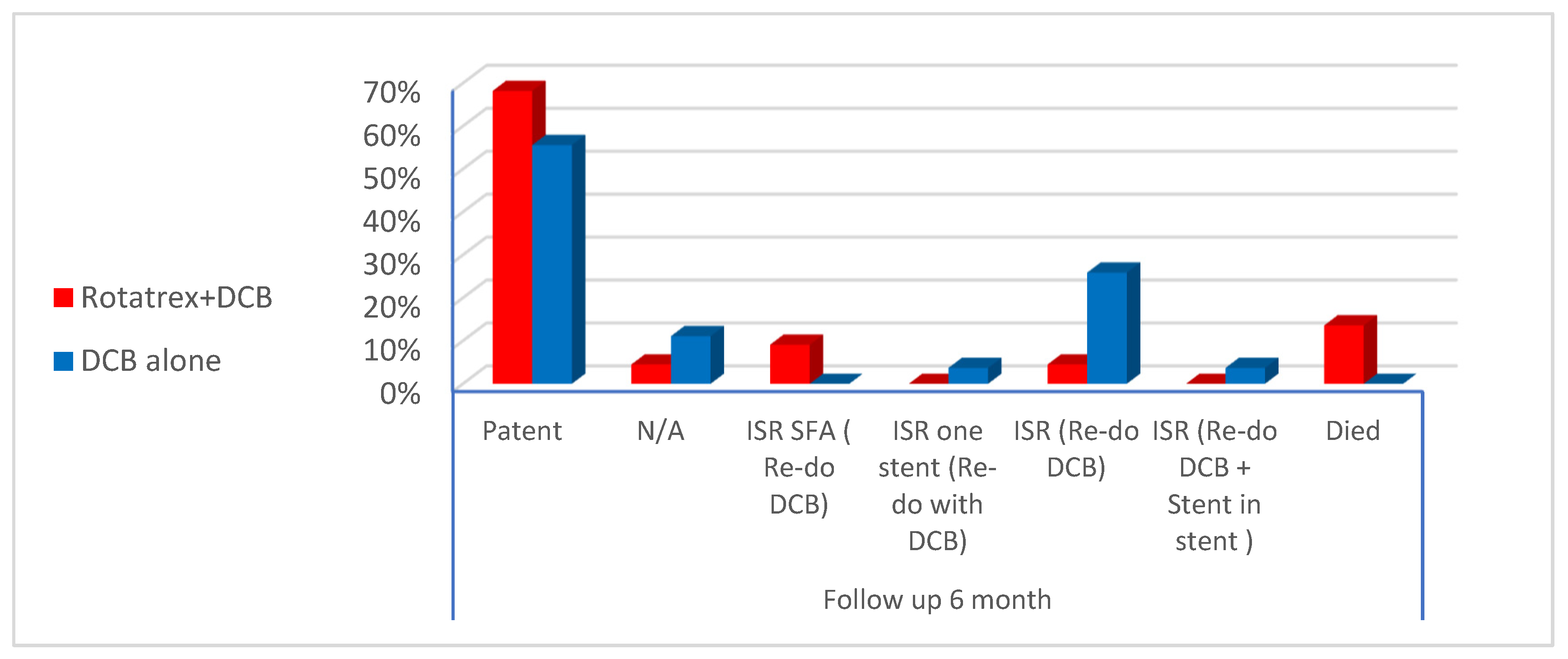
The current study showed intra-procedural distal embolization in (9%) of cases who were treated with Rotarex. Also, there was no statistical difference in distal embolization regarding the profile of DCB in both the Rotarex + DCB and DCB alone. To be noted, in the current study, we did not encounter vessel wall perforation while using Rotarex [
21].
There is a paucity of studies comparing lower extremity bypass and different types of peripheral atherectomy devices. However, in 2024, Van Leeuwen G. et al. published a single-center retrospective study and discussed the technical success and only short-term outcomes of the BYCROSS
® atherectomy device used for the management of long-segment femoropopliteal lesions (including both atherosclerotic disease and hyperplastic lesions of previously deployed stents) compared to standard lower extremity surgical bypass regarding both safety, feasibility and possible complications. They showed a technical success rate of 100%, a 30-day mortality of 5%, a 30-day MACE rate of 11%, and a 30-day MALE rate of 0% in a cohort of patients with significant comorbidities (i.e., 75% with American Society of Anesthesiologists Physical Status Classification (ASA) III and IV). The patency rate at 30 days was acceptable at 83% [
30].