The Comorbidity and Associations between Depression, Cognitive Impairment, and Sleep after Stroke and How They Affect Outcomes: A Scoping Review of the Literature
Abstract
1. Introduction
2. Objectives
- How often do studies in stroke populations include these three variables in their study design?
- What are the rates of occurrences of these sequelae in post-stroke studies that included them as variables?
- How are depression, cognitive impairment, and sleep disturbances associated with one another in post-stroke populations?
- How do depression, cognitive impairment, and sleep disturbances interact with each other to influence stroke outcomes?
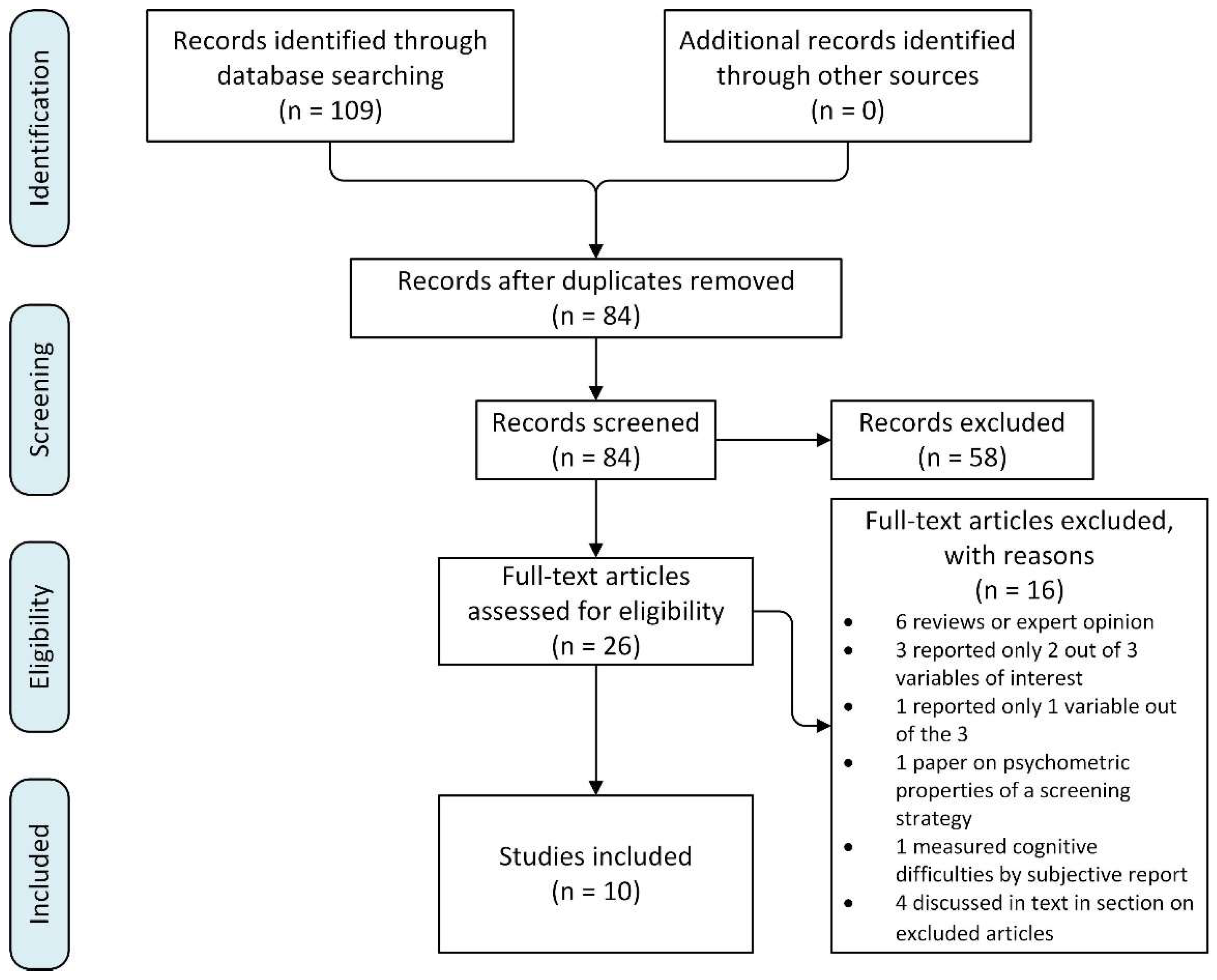
3. Method
4. Search Strategy
5. Inclusion Criteria
- Studies that included post-stroke adult individuals;
- Studies that included variables of post-stroke depression, sleep, and objectively measured cognitive function in the data collection as well as in the results (all three must be present);
- Research in the form of observational or epidemiological quantitative studies.
6. Exclusion Criteria
- Animal studies;
- Studies that specified including children or people aged < 18 with stroke (to reduce heterogeneity);
- Studies reporting the psychometric properties of tools that screen for depression, cognitive impairment, and/or sleep;
- Studies that only included two out of the three variables of interest (to limit heterogeneity);
- Studies that used depression, cognitive impairment, or sleep as a subject exclusion criterion;
- Studies that measured depression, sleep, or cognitive impairment pre-stroke;
- Interventional trials (study samples and methods do not meet the review objectives);
- Study protocols;
- Qualitative studies;
- Studies that measured cognitive impairment only using subjective reports;
- Expert opinion and narrative review articles.
7. Study Selection
8. Results
8.1. Rates of Occurrence
8.2. Inter-Relationships
8.3. Impact on Stroke Outcomes
9. Excluded Articles
10. Discussion
- What are the trajectories of different non-motor stroke outcomes?
- How does the inter-relationship between these outcomes change over time?
- What is the magnitude of the impact of each outcome on the global health outcomes, and does the relative magnitude change over time?
- What stroke characteristics are predictive of these non-motor outcomes?
- Do hyperacute stroke treatments influence the answers to the above questions?
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Sheth, B.; Gill, J.; Yadegarfar, M.; Stubbs, B.; Yadegarfar, M.; Meader, N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry 2017, 47, 48–60. [Google Scholar] [CrossRef] [PubMed]
- de Man-van Ginkel, J.M.; Hafsteinsdóttir, T.B.; Lindeman, E.; Geerlings, M.I.; Grobbee, D.E.; Schuurmans, M.J. Clinical Manifestation of Depression after Stroke: Is It Different from Depression in Other Patient Populations? PLoS ONE 2015, 10, e0144450. [Google Scholar] [CrossRef]
- Cumming, T.B.; Churilov, L.; Skoog, I.; Blomstrand, C.; Linden, T. Little evidence for different phenomenology in poststroke depression. Acta Psychiatr. Scand. 2010, 121, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Sexton, E.; McLoughlin, A.; Williams, D.J.; Merriman, N.A.; Donnelly, N.; Rohde, D.; Hickey, A.; Wren, M.-A.; Bennett, K. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur. Stroke J. 2019, 4, 160–171. [Google Scholar] [CrossRef]
- Baylan, S.; Griffiths, S.; Grant, N.; Broomfield, N.M.; Evans, J.J.; Gardani, M. Incidence and prevalence of post-stroke insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101222. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Zhang, Y.-H.; Qin, L.-Q. Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis 2013, 229, 489–495. [Google Scholar] [CrossRef]
- Johnson, K.G.; Johnson, D.C. Frequency of Sleep Apnea in Stroke and TIA Patients: A Meta-analysis. J. Clin. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Taylor-Piliae, R.E.; Hepworth, J.T.; Coull, B.M. Predictors of depressive symptoms among community-dwelling stroke survivors. J. Cardiovasc. Nurs. 2013, 28, 460–467. [Google Scholar] [CrossRef]
- Davis, J.C.; Falck, R.S.; Best, J.R.; Chan, P.; Doherty, S.; Liu-Ambrose, T. Examining the Inter-relations of Depression, Physical Function, and Cognition with Subjective Sleep Parameters among Stroke Survivors: A Cross-sectional Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, T.S. The Effect of Stroke on Selected Characteristics of Depression in Elderly Nursing Home Residents; Texas A&M University: College Station, TX, USA, 1992. [Google Scholar]
- Katzan, I.L.; Thompson, N.R.; Walia, H.K.; Moul, D.E.; Foldvary-Schaefer, N. Sleep disturbance predicts future health status after stroke. J. Clin. Sleep Med. 2020, 16, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Chun, M.H.; Kang, S.H.; Lee, J.A.; Kim, B.R.; Shin, M.J. Functional outcome in poststroke patients with or without fatigue. Am. J. Phys. Med. Rehabil. 2009, 88, 554–558. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum kynurenines correlate with depressive symptoms and disability in poststroke patients: A cross-sectional study. Neurorehabilit. Neural Repair. 2020, 34, 936–944. [Google Scholar] [CrossRef]
- Rabat, Y.; Houeze, R.; Sagnier, S.; Olindo, S.; Poli, M.; Debruxelles, S.; Renou, P.; Rouanet, F.; Berthoz, S.; Sibon, I. Association between neurological outcome and poststroke comorbid mood and anxiety disorders: A real-life experience. Brain Behav. 2021, 11, e02158. [Google Scholar] [CrossRef]
- Mandzia, J.L.; Smith, E.E.; Horton, M.; Hanly, P.; Barber, P.A.; Godzwon, C.; Asdaghi, N.; Patel, S.; Coutts, S.B. Imaging and baseline predictors of cognitive performance in minor ischemic stroke and patients with transient ischemic attack at 90 days. Stroke 2016, 47, 726–731. [Google Scholar] [CrossRef]
- Sandberg, O.; Franklin, K.A.; Bucht, G.; Gustafson, Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J. Am. Geriatr. Soc. 2001, 49, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q. Relationship between obstructive sleep apnea syndrome and cognitive impairment and functional status after stroke. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 1017–1021. [Google Scholar]
- Lisan, Z.; Yi, S.; Tiantian, W.; Yu, P.; Ying, Y.; Liuqing, P.; Qinglin, X.; Wenying, Z.; Jiahui, X.; Yingxue, H. Restless legs syndrome in ischemic stroke patients: Clinical features and significance. Zhejiang Da Xue Xue Bao Yi Xue Ban = J. Zhejiang Univ. Med. Sci. 2019, 48, 275–281. [Google Scholar]
- Kim, C.R.; Chun, M.H.; Han, E.Y. Effects of hypnotics on sleep patterns and functional recovery of patients with subacute stroke. Am. J. Phys. Med. Rehabil. 2010, 89, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Cosin, C.; Sibon, I.; Poli, M.; Allard, M.; Debruxelles, S.; Renou, P.; Rouanet, F.; Mayo, W. Circadian sleep/wake rhythm abnormalities as a risk factor of a poststroke apathy. Int. J. Stroke 2015, 10, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.; Hawkins, L.; Sprigg, N.; Ward, N.S.; Mistri, A.; Tyrrell, P.; Mead, G.E.; Worthington, E.; Lincoln, N.B. The Nottingham Fatigue after Stroke (NotFAST) study: Factors associated with severity of fatigue in stroke patients without depression. Clin. Rehabil. 2017, 31, 1406–1415. [Google Scholar] [CrossRef]
- Brunner, H. Success and failure of mirtazapine as alternative treatment in elderly stroke patients with sleep apnea—A preliminary open trial. Sleep Breath. 2008, 12, 281–285. [Google Scholar] [CrossRef]
| MEDLINE Advanced Search Parameter | No. of Returned Citations |
|---|---|
| “stroke (mesh term) AND depression (title/abstract)” | 3974 |
| “stroke (mesh term) AND cognitive (title/abstract)” | 7041 |
| “stroke (mesh term) AND sleep (title/abstract)” | 1446 |
| “stroke (mesh term) AND depression (title/abstract) AND cognitive (title/abstract)” | 893 |
| Author/ Year | Study Title | Study Design/Main Objective | Inclusion/Exclusion Criteria | Primary Outcome Measure(s) | Other Measurements Used | Study Findings | |
|---|---|---|---|---|---|---|---|
| 1. | Chadwick 1992 (unpublished) | The effect of stroke on selected characteristics of depression in elderly nursing home residents | Observational (cross-sectional); To examine how selected characteristics of depression in elderly nursing home residents are affected by the presence of stroke | Inclusion: Aged > 65 Residents of 3 identified nursing homes Able to respond meaningfully to 75% of study instruments Exclusion: Dementia or too medically ill to respond Roommates of enrolled study subjects | Depression as defined by score of ≥11 on the Geriatric Depression Scale/Amended | Cognitive Impairment: MMSE Sleep: 4 questions developed by the author (Do you have problems sleeping? Going to sleep? Waking in the middle of the night? Waking up too early?) | n = 44 (23 with stroke, of which 13 were depressed; 21 non-stroke of which 11 were depressed). Residents with stroke and depression had worse MMSE scores than residents with stroke and no depression (mean 15.39 ± 6.63 vs. 19.30 ± 5.66, p < 0.05). No difference in reported sleep problems regardless of presence of stroke and depression. |
| 2. | Taylor-Piliae et al., 2013 | Predictors of Depressive Symptoms Among Community-Dwelling Stroke Survivors | Observational (cross-sectional); To examine the prevalence of depressive symptoms among community-dwelling chronic stroke survivors, and to examine potential independent predictors of depressive symptoms | Inclusion: Community-dwelling stroke survivors aged 50 years or older with mild to moderate disabilities Exclusion: Stroke survivors with no disability or severe disability, or a serious medical condition that interferes with study participation | Depression: CES-D ≥ 16 | Sleep: PSQI > 5 Cognitive Impairment: MMSE | n = 100 Prevalence of depressive symptoms was 35% Depressive symptoms correlated with poor sleep quality (r = 0.40, p < 0.01) but not cognitive score. 64% of the variance in depressive symptoms could be explained by a model of 12 independent variables: quality of life physical and mental health, sleep quality, social support, cognitive function, functional disability, time since stroke, age, gender, history of major depression, and lesion location, but sleep quality and cognitive function were significant independent predictors. |
| 3. | Carrilo-Mora et al. 2020 | Serum Kynurenines Correlate With Depressive Symptoms and Disability in Poststroke Patients: A Cross-Sectional Study | Observational (cross-sectional); To investigate if there is a correlation between serum kynurenines levels with poststroke anxiety and depression and disability scales | Inclusion: Patients aged at least 18 years with first ischemic or haemorrhagic stroke corroborated by neuroimaging, no history of neurological or psychiatric disorders, stroke at >1 month but <1 year, able to answer the evaluations of mood and cognition Exclusion: Patients with severe aphasia or acute complications that prevent objective assessment (e.g., delirium), current consumption of antidepressants, drugs of abuse, immunosuppressants, immunomodulators, or other drugs that affect mood or cognitive performance | Significant depressive and/or anxiety symptoms as measured by HADS (>6 on Anxiety or Depression subscales) | Cognitive impairment: MOCA < 24 Sleep: PSQI > 5 | n = 60 55% male, mean age 57.3 ± 14 years, mean time since stroke 5.2 ± 3.5 months, 82% ischemic stroke, 55% on the right. 63% significant depressive symptoms. 53% significant anxiety symptoms. 45% had both. 50% had poor sleep quality. 68% cognitively impaired. PSQI score significantly higher in the depressed group (11.7 ± 10.7 vs. 7.8 ± 9.8, p = 0.0172). PSQI scores significantly correlated with HADS subscale (HADS-A, r = 0.6030, p < 0.0001; HADS-D, r = 0.4378, p = 0.0023) and total scores (r = 0.5789, p < 0.0001). Significant positive correlation between 3HK levels with HADS-Total (r = 0.30, p = 0.025) and HADS-D (r = 0.28, p = 0.039). Significantly higher levels of 3HK (p = 0.048) and KYNA (p = 0.0271) in depressed patients Significantly higher 3HK levels in patients poor sleep (p = 0.019). |
| 4. | Rabat et al. 2021 | Association between neurological outcome and post-stroke comorbid mood and anxiety disorders: A real-life experience | Observational (retrospective chart review of cross-sectional data); To investigate the association between post-stroke mood and anxiety disorders and 3-month stroke outcome | Inclusion: Consecutive stroke patients who attended poststroke follow-up visit 3 to 4 months from time of stroke hospitalization. Age over 18. History of ischemic or haemorrhagic stroke confirmed on MRI or CT performed at symptoms onset. Exclusion: Missing data in the electronic health record for the variables of NIHSS, mRS, HADS or MOCA | Comorbid mood and anxiety disorder defined as Poststroke Emotional Distress (PSED): >7 on both the anxiety and depression subscales of HADS | Cognitive Impairment: MOCA Sleep: Documented presence of sleep problems (Yes/No) | n = 2300 28% met threshold for HADS-D 34.7% met threshold for HADS-A 19% had PSED. 26.7% had sleep problems. PSED subjects scored lower for MOCA (24.92 ± 4.62 vs. 26.17 ± 4.02, p < 0.001), and larger proportion of PSED reported sleep problems (42.6% vs. 21.4%, p < 0.001). Multivariate analysis showed that lower cognitive abilities (OR 0.953, p < 0.001) and experiencing sleep problems (OR 2.334, p < 0.001) were independently associated with presence of PSED on follow-up. Other significant factors include younger age, female gender, smoking, higher functional disability, pain, fatigue, and abnormal movements. |
| 5. | Mandzia et al. 2016 | Imaging and Baseline Predictors of Cognitive Performance in Minor Ischemic Stroke and Patients with Transient Ischemic Attack at 90 Days | Observational study (cross-sectional); To examine cognitive performance in minor stroke and TIA patients at 90 days and identify factors associated with cognitive dysfunction | Inclusion: Consecutive patients aged at least 18 years presenting with high-risk TIA or minor ischemic stroke (NIHSS ≤ 3). Exclusion: If received thrombolysis, had premorbid mRS score of ≥2, serious comorbidity with life expectancy <3 months, baseline dementia, could not complete neuropsychological testing, could not complete home sleep apnea testing. | Mild cognitive impairment as defined by ≥1 SD below normal composite score for executive function (EF), psychomotor processing speed (PS), and verbal memory-memory | Depression: CES-D ≥ 16 Sleep: Diagnosis of sleep apnea based on overnight home monitoring of oximetry | n = 92 76% male, 54% TIA, mean age 65.1 ± 12.0. 73% had OSA. 20% impaired on memory, 16% on PS, 17% on EF. Cognitive scores did not differ by presence of OSA. In multivariable analysis, lower EF was associated with depression CES-D ≥ 16 (p = 0.0003), recurrent cortical stroke and greater disability. Lower PS scores were associated with depression CES-D ≥ 16 (p = 0.03), recurrent cortical stroke and greater disability. |
| 6. | Feng 2017 (published in Chinese, full text not available) | Relationship between sleep apnea syndrome and cognitive impairment and functional status after stroke | Observational (case-control); To analyse the correlation between obstructive sleep apnea syndrome and cognitive impairment and functional status after stroke | Inclusion and exclusion criteria not available in abstract | Cognitive performance in vigilance, attention, memory, working memory, executive, language, insight, mental activity, psychomotor and intelligence | Depression and anxiety: Not specified Sleep quality and sleepiness: Not specified | OSA cases = 86, controls = 70 Cognitive performance of OSA cases significantly worse than control group (t = 9.276, p = 0.012), specifically worse in attention, executive, insight, mental adjustment, and intelligence (p < 0.05). Functional status worse for cases (t = 38.094, p < 0.001). No significant differences between groups for sleepiness, sleep quality, anxiety, and depression. |
| 7. | Sandberg et al., 2001 | Sleep Apnea, Delirium, Depressed Mood, Cognition, and ADL Ability After Stroke | Observational (cross-sectional); To investigate the presence of sleep apnea after stroke and its relationship to delirium, depressed mood, cognitive functioning, ability to perform activities of daily living (ADLs), and psychiatric and behavior symptoms | Inclusion: Consecutive stroke admissions to a geriatric stroke rehabilitation unit. Exclusion (not stated a priori): Refusal to participate. Failed study procedure due to delirium. | Sleep: Overnight sleep apnea recording in hospital through monitoring of nasal and oral air flow, abdominal movements, respiratory and body movements, oxygen saturation and heart rate, body position and snoring | Cognitive Impairment: MMSE Depression: MADRS | n = 133 59% fulfilled criteria for sleep apnea with no difference between those with and without for age, gender, BMI, type of stroke and lesion location. Those with sleep apnea had higher MADRS scores (19.2 ± 11.3 vs. 14.0 ± 10.7, p = 0.018), and more ADL dependent, but no difference in MMSE (15.9 ± 8.5 vs. 17.8 ± 8.3, p = 0.206). Larger proportion of sleep apnea patients were delirious (75% vs. 56%, p = 0.018). Logistic regression shows depressed mood to be significantly associated with sleep apnea, together with high BMI, ADL dependency and presence if ischemic heart disease (OR 1.74, 95% CI 1.02-2.94, p < 0.001). Central sleep apnea patients (26%) also had higher MADRS scores (19.9 vs. 14.0, p = 0.013). |
| 8. | Davis et al., 2019 | Examining the Inter-relations of Depression, Physical Function, and Cognition with Subjective Sleep Parameters among Stroke Survivors: A Cross-Sectional Analysis | Observational (cross-sectional); To examine the association of subjective sleep parameters with depression, health related quality of life, physical function, and cognition among stroke survivors. | Inclusion: Community-dwelling survivors of ischemic or haemorrhagic stroke confirmed by CT or MRI. Aged 55 years and over. History of single stroke of at least 1 year prior to study enrolment. MMSE score of greater than or equal to 20/30 on screening. Living in Greater Vancouver area. Able to comply with study procedures. Read, write, and speak English with acceptable visual and auditory acuity. Not expected to start or are stable on a fixed dose of cognitive medications. Able to walk for a minimum of 6 m with rest intervals with or without assistive devices. Have an activity tolerance of at least 60 min with rest intervals. Not currently participating in any regular therapy or progressive exercise. Exclusion: Diagnosed dementia. Diagnosis of another neurodegenerative or neurological condition that affects cognitive function and mobility. At high risk for cardiac complications during exercise and/or unable to self-regulate activity or to understand recommended activity level. Have clinically significant peripheral neuropathy or severe musculoskeletal or joint disease that impairs mobility. Taking medications that may negatively affect cognitive function. Aphasia. | Sleep: PSQI ≥ 5 | Cognitive Impairment: MOCA MMSE ADAS-Cog Trail Making Tests A and B Digits Forward minus Backwards Depression: CES-D ≥ 4 | n = 72 68% rated as having depressive symptoms (CES-D ≥ 4). 47% rated significant poor sleep quality (PSQI ≥ 5). Global PSQI associated with depression (r2 = 0.21, p < 0.001) but not with cognition or physical function. In bivariate analysis, subjective sleep quality (r2 = 0.06, p = 0.038), sleep latency (r2 = 0.10, p = 0.007), daytime dysfunction (r2 = 0.18, p < 0.001) associated with depression. In multivariate analysis, daytime dysfunction was associated with depression after adjusting for age and Functional Comorbidities Index. Sleep quality was associated with depression after adjusting for age. No significant predictors of cognition were identified. |
| 9. | Katzan et al., 2020 | Sleep disturbance predicts future health status after stroke | Observational (retrospective cohort, analysis of extracted electronic health data); To evaluate factors associated with the presence of sleep disturbances in stroke patients, and to determine the role of patient-reported sleep disturbances in patient-reported outcomes after stroke | Inclusion: Age > 18 years, clinical diagnosis of ischemic stroke, intracranial haemorrhage (ICH), subarachnoid haemorrhage (SAH, or transient ischemic attack (TIA), completion of Patient-Reported Outcomes Information Measurement System (PROMIS) sleep disturbance scale at 1 or more ambulatory visits during the study period Exclusion: Nil | Sleep: PROMIS sleep disturbance scale by computer adaptive testing | Depression: PHQ-9 and an equivalent cross-linked PROMIS depression score Cognitive Impairment: Computer adaptive testing version of the Quality of Life in Neurological Disorders (NeuroQoL) cognitive function v1.0 scale | n = 2190, of which 476 had follow-up data. 14.5% had depression. Sleep score highly correlated with depression score (r = 0.57;95% CI 0.50, 0.63). In separate multivariate models examining the effect of outcomes of physical function, anxiety, fatigue, pain interference, social role satisfaction and cognitive function on sleep score as the dependent variable and adjusting for depression score and other clinical and demographic variables, poorer outcomes were significantly associated with worse sleep scores even after adjusting for depression. Depression, pain, and fatigue were associated with sleep disturbance in all stroke types. Cognitive function and anxiety were associated with sleep disturbance in all stroke types except SAH. Social role satisfaction was associated with sleep disturbance in all stroke types except ICH. Physical function was associated with sleep disturbance in the TIA group only. Younger age was associated with worse sleep disturbance in ischemic stroke and TIA. In ICH group, men had more sleep disturbance than women after adjusting for depression. Worse baseline sleep scores were associated with worse follow-up scores for depression, fatigue, social role satisfaction and physical function after adjusting for each outcome’s baseline score, baseline depression score and other clinical variables. |
| 10. | Park et al., 2009 | Functional Outcome in Poststroke Patients With or Without Fatigue | Observational (cross-sectional); To evaluate the influence of fatigue on functional outcomes after stroke | Inclusion: Consecutive stroke patients receiving outpatient rehabilitation at study site Exclusion: <3 months after stroke onset. <18 years old. Previous history of stroke. Multiple or bilateral lesions. Communication problems due to aphasia or dementia. History of diagnosed depression. Rapidly progressive medical disease. | Fatigue: Fatigue Severity Scale score > 4 | Cognitive Impairment: MMSE Depression: BDI > 15 Sleep: Single question asking whether study subjects experience any sleep disturbances | n = 40 30% had FSS score ≥ 4. 55% had BDI > 15. 30% had sleeping problems. No difference in MMSE score between fatigued group and non-fatigued group. More fatigued stroke patients had sleep problems (50% vs. 17.9%, p < 0.05). BDI score was significantly correlated with FSS score (r = 0.47, p < 0.05) even though the mean BDI score was not significantly different between groups (22.4 ± 12.08 vs. 12.9 ± 9.2). No difference in scores of ADL independence and motor function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.G. The Comorbidity and Associations between Depression, Cognitive Impairment, and Sleep after Stroke and How They Affect Outcomes: A Scoping Review of the Literature. J. Vasc. Dis. 2024, 3, 134-151. https://doi.org/10.3390/jvd3020012
Chan LG. The Comorbidity and Associations between Depression, Cognitive Impairment, and Sleep after Stroke and How They Affect Outcomes: A Scoping Review of the Literature. Journal of Vascular Diseases. 2024; 3(2):134-151. https://doi.org/10.3390/jvd3020012
Chicago/Turabian StyleChan, Lai Gwen. 2024. "The Comorbidity and Associations between Depression, Cognitive Impairment, and Sleep after Stroke and How They Affect Outcomes: A Scoping Review of the Literature" Journal of Vascular Diseases 3, no. 2: 134-151. https://doi.org/10.3390/jvd3020012
APA StyleChan, L. G. (2024). The Comorbidity and Associations between Depression, Cognitive Impairment, and Sleep after Stroke and How They Affect Outcomes: A Scoping Review of the Literature. Journal of Vascular Diseases, 3(2), 134-151. https://doi.org/10.3390/jvd3020012





