Abstract
Vascular dementia (VaD), characterized by cognitive decline attributable to cerebrovascular disease, is the second most common type of dementia after Alzheimer’s disease. This review aims to explore the prevalent risk factors, pharmacological interventions, and non-pharmacotherapeutic strategies associated with the condition. Recognized risk factors include advanced age, hypertension, diabetes mellitus, obesity, and hyperlipidemia with emerging evidence implicating additional lifestyle and genetic factors. Pharmacotherapy for VaD mainly focuses on managing these underlying risk factors, coupled with symptomatic treatments. Therapeutic agents commonly used include antihypertensives, statins, antiplatelet drugs, antidiabetic agents, and specific cognitive enhancers like cholinesterase inhibitors. However, the effectiveness of these treatments remains under continuous study, underscoring the need for comprehensive, individualized treatment plans. Non-pharmacotherapeutic strategies, encompassing lifestyle modifications such as diet and exercise have gained considerable attention. They have shown promise in improving cognitive function and enhancing the quality of life in patients with VaD. The application of a multi-domain intervention approach may provide a more holistic management strategy for VaD. Further research is needed to define the best practices in both pharmacotherapy and non-pharmacotherapy treatments, considering the multifactorial and heterogeneous nature of this condition.
1. Introduction
The increasing global population of older adults has led to a growing focus on age-related concerns. Dementia is a prevalent and enduring health condition that mostly impacts the elderly demographic [1]. It has garnered significant attention as a pressing global health issue, leading to substantial socioeconomic and familial consequences [2]. Alzheimer’s disease (AD) and vascular dementia (VaD) have historically been recognized as the two most prominent forms of dementia, with AD being the primary variety and VaD following closely behind [3]. VaD is a neurocognitive disorder characterized by a decline in cognitive functioning caused by diminished cerebral blood flow. This reduction in blood flow is frequently attributed to cerebrovascular incidents, such as stroke or a succession of minor strokes, which subsequently result in neuronal damage [4]. Cerebral small vessel disease (SVD) is a commonly observed vascular aetiology of dementia among individuals with cardiovascular disease (CVD). It comprises a range of clinical, radiological, and pathological features [5]. The brain regions that are frequently impacted by this condition encompass the frontal lobes, hippocampus, and basal ganglia [6]. At the cellular level, both macrovascular and microvascular routes including big and small cause abrupt cognitive deficits through events such as strokes, but a more gradual decline may be observed as a result of microvascular pathology [7]. The pathogenesis of VaD is also influenced by inflammatory processes. The occurrence of ischemia injury can be intensified and result in the long-term impairment of neurological function due to the release of pro-inflammatory cytokines [8]. Extensive research has been conducted on the involvement of oxidative stress in the process of neurodegeneration in VaD. The production of free radicals during ischemia episodes has the potential to cause oxidative harm and ultimately result in the demise of neurons [9]. In contrast to the gradual progression typically observed in AD, VaD is characterized by the quick onset of symptoms and the potential for stepwise deterioration.
The prevalence of vascular abnormalities tends to be higher in older individuals due to the increased susceptibility of their brains to age-related degenerative changes and other pathological conditions. The VaD condition primarily encompasses behavioural symptoms, locomotion abnormalities, and autonomic dysfunction, which can manifest as early, late, or severe dementia syndromes [4]. The new discovery that vascular-related cognitive loss occurs more frequently than previously believed, either independently or in combination with neurodegenerative illnesses, has raised doubts about this notion.
The diagnostic procedure for vascular dementia often encompasses several sequential stages, including clinical assessment, neuroimaging analysis, and cognitive evaluation. The initial stages of the diagnostic process often involve conducting a comprehensive history and physical examination, which are afterward followed by the utilization of imaging modalities such as magnetic resonance imaging (MRI) or computed tomography (CT) scans in order to detect any cerebrovascular abnormalities [10]. The evaluation of cognitive function is frequently conducted using assessment tools such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) [11]. This concise review has provided an overview of the growing body of research and potential risk factors, blood biomarkers, diagnostic methods, and treatment options pertaining to VaD.
2. Epidemiology
2.1. Prevalence and Incidence
The prevalence of VaD exhibits significant variability, contingent upon the specific population under study and the diagnostic criteria employed. Nevertheless, it is commonly believed that the prevalence of this condition impacts approximately 1.5–4.2% of the population aged 65 and above [12,13]. According to the cited source [14], the frequency of AD increases two-fold every 4.3 years, and the frequency of VaD doubles every 5.3 years. The prevalence of dementia is approximately 50 times higher in patients within the first year following a significant stroke compared to the general population [15]. VaD constitutes approximately 15% to 20% of cases in North America and Europe, as reported by previous studies [3]. However, in Asia and emerging economies, the prevalence of VaD is significantly greater, with estimates reaching over 30% [16]. According to a study referenced as [17], the incidence of the condition appears to increase two-fold every 5–10 years after individuals reach the age of 65. The precise prevalence rate of people with VaD remains uncertain; however, it is associated with an increased risk of developing dementia and mortality.
2.2. Risk Factors
According to epidemiological data, ageing is the major contributor to dementia risk, where advanced age [3] is the strongest risk factor for VaD (Table 1). Similar to AD, the incidence of VaD increases exponentially with age, contributing to the prevalence of dementia in aging populations worldwide. The major vascular risk factors include hypertension, diabetes mellitus, cigarette use, atrial fibrillation, and stroke history [18]. It has been reported that VaD and cerebrovascular disease share common risk factors such as hypertension, insulin resistance, diabetes, obesity, hyperhomocystinemia, and hyperlipidemia [19]. The nine potentially modifiable risk factors for dementia identified by the 2017 Lancet Commission on dementia prevention, intervention, and care are less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and low social contact [20]. The modifiable risk factors, especially obesity and hypertension, have been found to have a particularly negative impact on brain health in middle age and beyond. Elevated systolic and diastolic blood pressure values are associated with an increased risk of VaD. It was suggested that the diabetes risk factor causes approximately double the risk of dementia. Therefore, diabetes increases the incidence of VaD, especially in middle-aged adults [20]. Indeed, it was found that modifiable risk factors such as obesity, hypertension, and diabetes mellitus account for ~25% of dementia in the population [21]. It was suggested that homocysteine levels in plasma appear to be closely connected to Aβ42 levels. Hyperhomocysteinaemia inhibits neurogenesis via a fibroblast growth factor-dependent mechanism. Homocysteine levels that are moderately high are a risk factor for VaD and AD; a considerable rise in plasma homocysteine levels has been seen in AD [22,23]. Systematic and quantitative studies suggest that atrial fibrillation (AF) associations exist between AF and cognitive impairment, especially in VaD [24]. The genetic factors include familial vascular encephalopathies, such as autosomal dominant cerebral artery disease with subcortical infarction and leukoencephalopathy (CADASIL), and possibly apolipoprotein (apoE) ε4, which is implicated in increased VaD risk [25]. A history of stroke was recognized as a key chance figure for incorporation in dementia hazard scores, particularly for mature people 70–80 [26]. Overall, the body of evidence supporting these risk factors is growing.

Table 1.
Vascular dementia and associated risk factors (modified from Livingston et al. 2020) [20].
2.3. Gender Differences
Research indicates that men seem to exhibit a marginally increased susceptibility to developing VaD compared to women [27]. This observed difference in risk has perplexed the medical community as the exact reasons behind this discrepancy remain somewhat elusive. Several studies have shown a gender difference in the prevalence of VaD; this disparity may be attributed to several potential reasons:
- ▪
- Men are generally at a higher risk of certain CVDs compared to women. Studies conducted around the world show that the incidence of stroke, whether ischemic or hemorrhagic, is 44% higher in men than in women. Research findings indicate that there is a tendency for men to encounter their first stroke at a comparatively younger age, typically around 68.6 years. However, it has been suggested that men who reach the age of 72.9 years may potentially be more susceptible to developing vascular dementia (VaD), therefore contributing to a higher susceptibility in this population [28].
- ▪
- Historically, certain lifestyle factors associated with vascular health, such as smoking and high alcohol consumption, have been more prevalent among men than women. The World Health Organization (WHO) has noted that men, especially in certain regions, smoke and consume alcohol at higher rates than women [29], increasing their risk of vascular problems which might in turn affect their dementia risk.
- ▪
- Women generally have a longer life expectancy than men. Since age is a significant risk factor for most types of dementia, including AD, women might be more likely to develop other types of dementia at an older age [30]. On the other hand, because VaD can occur earlier than Alzheimer’s, the shorter average lifespan of men might result in a higher observed prevalence of VaD during their lifetime [31].
However, it is important to note that while these factors might influence the differences observed between men and women, the exact reasons for the gender disparity in VaD prevalence remain an active area of research. A combination of genetic, biological, environmental, and lifestyle factors is likely at play.
2.4. Ethnicity Differences
A few studies have indicated that VaD might be more prevalent in certain ethnic groups [32]. Individuals belonging to the Black ethnic group exhibit an approximately 30% higher prevalence of dementia compared to individuals from the White ethnic group. Conversely, individuals from the Asian ethnic group demonstrate a similar incidence rate of dementia when compared to their White counterparts [33]. Specifically, research has highlighted a potentially elevated risk among African Americans and Hispanics, groups that have historically displayed a higher prevalence of CVD [34]. For instance, older African Americans are about twice as likely as older white Americans to have Alzheimer’s or other dementias, with VaD being a contributing factor. Moreover, Hispanics also face higher risks of developing VaD, with cardiovascular risk factors like diabetes and hypertension being more common in this demographic [35]. These findings underscore the importance of addressing cardiovascular health disparities among ethnic groups to reduce the burden of VaD.
3. Biomarkers in VaD
To date, no blood biomarker has proven useful in clinical practice for the diagnosis of VaD [36]. Complicating the clinical use of blood biomarkers is the BBB, which limits the leakage of proteins from the brain parenchyma into the blood, and the presence of these markers in other pathological conditions such as epilepsy, encephalitis, and myocardial infarction [37]. However, blood biomarkers may be the best candidates for identifying VaD in terms of accessibility, minimal invasiveness, and cost effectiveness. A few blood biomarkers in VaD are available; however, future studies are warranted to validate these promising biomarkers.
3.1. Oxidative Biomarkers
Membrane phospholipid peroxidation causes oxidized low-density lipoprotein (oxLDL) and multiple pathological events such as endothelial cell damage, enhanced foam cell formation, vascular smooth muscle cell proliferation, and platelet adhesion aggregation [38]. Elevated oxLDL levels in acute ischemic stroke (AIS) patients in a large cohort (VaD group, 2711; control group, 1015) were reported to be associated with lower MMSE scores [39]. The endothelium cells asymmetric dimethylarginine (ADMA) is an inhibitor of endogenous nitric oxide (NOS) synthesis, where L-arginine serves as NOS substrate to produce NO (nitric oxide). Regarding blood biomarkers of AIS, ADMA levels were significantly higher in patients with AIS than in controls [40]. These may be promising blood biomarkers for VaD, but further studies are needed to confirm and validate them.
3.2. Hormonal Biomarkers
Thyroid hormones play crucial roles in a range of physiological processes, including metabolism, growth, and development, as well as brain function. Cognitive dysfunction and mood disturbances are among the well-known clinical manifestations of hypothyroidism. The central nervous system (CNS) is a major target for thyroid hormones, and the influence of thyroid hormones on the CNS is evident from fetal life to old age. Both excess and deficiency of these hormones can cause neurological and psychiatric disorders because of the following reasons:
- ▪
- During the early stages of fetal development, thyroid hormones play a role in neuronal migration, differentiation, myelination, and synaptogenesis. Thus, congenital hypothyroidism can lead to severe and irreversible intellectual disability, highlighting the importance of thyroid hormones for brain function [41].
- ▪
- Thyroid hormones can influence the concentrations and turnover rates of various neurotransmitters, including norepinephrine and serotonin. Changes in these neurotransmitters have been implicated in mood and cognitive disturbances [42].
- ▪
- In adults, severe and chronic thyroid hormone deficiencies (like in untreated hypothyroidism) are associated with cognitive impairments. Multiple studies have documented deficits in memory, attention, and executive function in hypothyroid patients. Correcting the hormone deficiency often results in significant cognitive improvements, although not always to the levels of healthy controls [43].
- ▪
- Elevated thyroid stimulating hormone (TSH), even in subclinical hypothyroidism, has been associated with cognitive deficits, particularly in elderly populations [44]. However, the direct influence of TSH on cognition remains a topic of investigation. A few studies have been suggested that elevated TSH itself might be a marker for other factors that influence cognition, rather than a direct contributor [45].
- ▪
- Total triiodothyronine (T3) is the active form of thyroid hormone and has been directly associated with various physiological processes in the brain. Decreased levels of total T3 and free triiodothyronine (fT3) in the blood can indicate reduced thyroid function, potentially leading to the cognitive deficits observed in hypothyroid individuals [46]. Further, there is a correlation between cognitive decline and reduced concentrations of total T3 and fT3 in the bloodstream, and elevated levels of serum TSH [47].
In addition, community-based trial showed that plasma parathyroid (PTH) predicted clinically diagnosed VaD as well as neuroimaging markers of cerebral small artery disease and their findings suggested that PTH has a role in the development of VaD [48]. Therefore, thyroid and parathyroid hormone profiles could be a promising blood biomarker for VaD; however, further randomized trial warrants confirmation of its importance in VaD patients.
Furthermore, multiple lines of evidence imply that oestrogen deficiency in the ageing brains of both men and women may have a role in the cognitive impairments associated with AD [49]. Studies have showed that hormone replacement therapy use is related with enhanced delayed memory and greater entorhinal and amygdala sizes only among APOE4 carriers [50]. This might be an effective targeted therapy to reduce the increased lifetime risk of AD in this big at-risk demographic subset. To demonstrate causation, findings in a fit-for-purpose randomized control trial with prospective enrolment based on APOE genotype must be confirmed.
3.3. Amyloid-Beta (Aβ) and Tau Proteins
Amyloid-beta (Aβ), a peptide found in the brain, can aggregate and form plaques, which is one of the pathological hallmarks of AD [51]. Unlike Alzheimer’s, which involves Aβ plaques and tau tangles, VaD does not have specific protein biomarkers. It has also been found that the accumulation of these plaques can cause cerebrovascular dysfunction, leading to reduced blood flow to the brain, which could contribute to the development of VaD [52]. The plasma and serum levels of Aβ proteins were found to be substantially lower than those in cerebrospinal fluid. Because plasma and serum contain large quantities of assay-interfering components, this makes singulex or multiplex ELISA platforms challenging to utilize [53] and unable to detect AD in investigation mainly in the early stages of AD. However, Aβ plaques are absent in individuals diagnosed with frontotemporal dementia or pure VaD [54]. Hence, the utilization of amyloid imaging will encompass the verification or negation of AD, the differentiation of dementia, specifically the separation between AD and frontotemporal dementia, and the early detection of AD. A few studies have reported that plasma Aβ levels significantly rise post-stroke and could be a potential biomarker for VaD following a stroke [55]. The ratio of plasma Aβ1–38/Aβ1–40 peptides could be a potential blood-based biomarker candidate for VaD [56]. Although amyloid plaques are not typically observed in cases of VaD, there exists a potential for intricate interactions between the two conditions that may contribute to the worsening of cognitive loss. Due to the intricate nature of VaD and AD, it is probable that comprehensive investigation of their link will require the integration of multiple disciplines such as neurology, cardiology, radiology, biochemistry, and pharmacology.
Tau proteins are primarily known for their role in neurodegenerative diseases, particularly in AD. They are microtubule-associated proteins that stabilize microtubules in neurons, allowing for normal cellular function. When tau proteins become abnormally phosphorylated, they aggregate and form neurofibrillary tangles (NFTs), one of the hallmark pathological features of AD. In the context of VaD, evidence has shown that tau proteins can exacerbate cerebrovascular injury and may thus participate in VaD pathology [57]. In a pilot study, cerebrospinal fluid in patients with AD, and high levels of the T-tau and P-tau were found in cerebrospinal fluid (CSF); T-tau and P-tau231 levels were significantly higher in AD patients than in VaD patients [58]. There is considerable overlap between AD and VaD, with many patients showing mixed features of the individual diseases; some individuals have been found to have both Aβ plaques and phosphorylated tau in their brains. These findings suggest that Aβ and tau may be involved in the pathogenesis of VaD, although more research is needed to better understand the precise relationships. In brief, it is imperative to use comprehensive, multi-faceted methodology encompassing fundamental scientific research, clinical observation, and interventional investigations in order to conclusively ascertain the association between neurofibrillary tangles and VaD.
While the primary pathological feature of VaD is related to vascular lesions, there is increasing evidence that tau pathology plays a role in the disease’s progression and presentation. It highlights the interconnected nature of different neurodegenerative processes and the importance of understanding the multifactorial nature of dementia syndromes. Therefore, it may be possible that Aβ and tau may serve as blood biomarkers for VaD, especially in the cases with overlap with AD, but this is an area that needs further exploration.
4. Diagnosis of VaD
An unequivocal conclusion of immaculate VaD can, as it were, be performed histologically to avoid the nearness of other neurodegenerative conditions such as AD. Diagnosis of VaD can use the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV or the ICD-10 criteria of the International Statistical Classification of Diseases and Related Health Problems (Figure 1). Memory impairment is an imperative highlight of these investigative criteria. In any case, this may be an afterward indication of vascular cognitive impedance observed before by cognitive shortage in other spaces. In the DSM-V, the criteria for both midlife and late-life strokes were more common among African Americans than White populations, suggesting that a potential pathway to the development of VaD has been updated and classified as follows: mild and severe vascular cognitive impairment with less emphasis on memory impairment alone. These criteria also emphasize the need to collect an accompanying medical history and establish a temporal relationship between symptoms and vascular injury. The Mini Mental State Exam (MMSE) is the most widely used diagnostic and screening tool for dementia and is recommended by the NICE guidelines [59]. It was estimated that the whole investigation task takes approximately 10–15 min. However, it is insensitive to executive detection dysfunction and visuospatial impairment, limiting its use in VaD.
Furthermore, Montreal cognitive evaluation (MOCA) is free to utilize and can be more delicate within the diagnosis of VaD because it contains tests of official work, such as the trail-making test, word affiliation, and category naming. The MOCA takes around 15–25 min in total [60]. However, a 5 min MOCA subtest was proposed by Hachinski et al. [61], which will be valuable in an active GP clinic. This incorporates a five-word quick and deferred (after 30 s) word review, a six-item introduction errand, and a one-letter phonemic familiarity test (Figure 1). The NICE Dementia Assessment and Diagnosis Roadmap recommends the following blood tests prior to referral to secondary memory assessment services: routine hematology and biochemistry, including calcium and glucose, thyroid function tests, and B12 and folate levels [59].
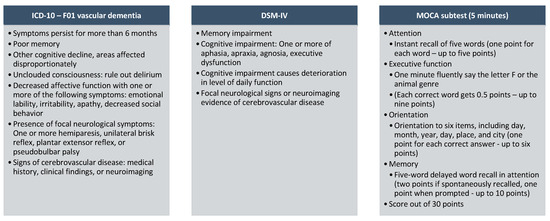
Figure 1.
Suggested diagnostic criteria for VaD [62,63].
Figure 1.
Suggested diagnostic criteria for VaD [62,63].
5. Treatments
VaD is a complex and still poorly understood condition that involves multiple pathophysiological mechanisms and presents significant challenges for effective treatment. There are currently no US Food and Drug Administration (FDA) approved treatments for VaD. Therefore, there are therapeutic strategies (Figure 2) primarily focused on controlling underlying vascular risk factors and related symptoms [64].
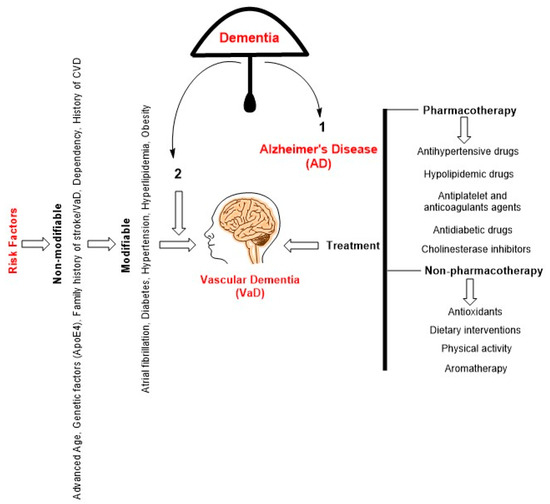
Figure 2.
A schematic diagram depicting the hierarchical relationship within the category of dementia reveals that AD occupies the foremost position, with VaD ranking second. VaD can be caused by several risk factors, which can be classified into two categories: non-modifiable and modifiable ones. The present approach to treating VaD is not yet definitive and primarily centers around pharmacology, which has demonstrated efficacy in mitigating cardiovascular problems. Non-pharmacological interventions have demonstrated favorable outcomes in both the prevention and mitigation of VaD.
5.1. Pharmacotherapy
Currently, there is a lack of exact pharmacological interventions that are endorsed for enhancing cognitive abilities or functional outcomes in individuals diagnosed with Vascular Cognitive Impairment (VCI). In persons with VaD, a few drugs may help prevent future brain damage and may halt its course. The primary emphasis of treatment frequently revolves around the effective management of the various health conditions and risk factors that are known to contribute to the development and progression of VaD. The implementation of measures to regulate factors that impact the fundamental well-being of the cardiovascular system has the potential to decelerate the progression of VaD, and in certain instances, may even impede further deterioration. Studies suggest that after a serious vascular event such as a stroke, preventive measures are needed to minimize the risk of developing VaD [65]. A few studies have shown that antihypertensives and statins might reduce the incidence of dementia and AD [66]. In a 5-year follow-up study of a community sample of 1,617 African Americans, the use of drugs that mediate vascular risk factors (antihypertensive, antihyperlipidemics, and antidiabetics) reduced the risk of developing dementia by 40% [67]. Depending on an individual’s specific circumstances, the following drugs may be prescribed.
5.1.1. Antihypertensive Drugs
The relationship between antihypertensive drugs and VaD is complex but generally favorable when considering the prevention or management of the disease. Since hypertension is a major risk factor for vascular diseases like stroke and small-vessel disease, which can contribute to or exacerbate VaD, antihypertensive drugs can play a role in mitigating this risk. The efficacy of anti-hypertensive medications in providing protection seems to be contingent upon the particular medicine employed. Favorable outcomes have been documented with the utilization of calcium channel blockers (CCBs), including lercanidipine and nitrendipine, as well as the combination therapy of perindopril-indapamide and telmisartan [68]. A systematic review study conducted in 2015 revealed that four randomized controlled trials reported the potentially preventive effect of antihypertensive drugs with a 41% reduction in cognitive decline associated with stroke [69]. Further, an analysis incorporated a total of 39 pertinent publications, comprising 20 longitudinal investigations, 10 randomized-controlled trials, and 9 meta-analyses [70]. The findings indicate that a majority of observational studies have indicated a potential protective impact of antihypertensive treatments on cognitive decline and various forms of dementia, such as VaD and AD. Specifically, calcium channel blockers and renin-angiotensin system blockers appear to be particularly effective in this regard [70].
The current study endeavors to determine the most efficacious antihypertensive drugs for the prevention or deceleration of VaD. The implementation of personalized antihypertensive medication based on the individual’s particular condition has the potential to become a widely adopted approach in medical practice.
5.1.2. Hypolipidemic Drugs
The intricate relationships between cholesterol and small vascular disease (SVD), stroke, cognitive impairment, and later dementia remain multifaceted and as of now lack complete comprehension. The current understanding of the impact of lipids and lipid-lowering medication on the prevention and treatment of dementia is limited, with a lack of conclusive evidence. Existing trials investigating the effects of lipid-lowering therapy have shown a notable favorable association between statin treatment and a reduced incidence of VaD [71]. To prevent or delay the onset of VaD, it may be necessary to initiate interventional trials targeting long-term vascular risk markers during midlife. A recent study has presented findings suggesting that the occurrence of VaD may lead to a decrease in the levels of circulating high-density lipoprotein cholesterol (HDL-C) [72]. These findings also support the concept that the use of statins to lower low-density lipoprotein cholesterol (LDL-C) levels could be an effective strategy for preventing the development of VaD.
5.1.3. Antiplatelet and Anticoagulants Agents
Ischemia can manifest as a consequence of atherosclerosis, thrombosis, or vasculopathy. The cerebral blood flow might be diminished due to a partial obstruction or may be entirely obstructed as a result of the presence of a thrombus. One of the major risk factors for VaD condition arises due to the occurrence of brain tissue ischemia, leading to the development of gliosis and demyelination. A few studies supported the administration of antiplatelet therapy, such as aspirin and cilostazol, as well as anticoagulant therapy using warfarin, which may have the potential to impede and decelerate the advancement of VaD subsequent to an accurate diagnosis [73]. Additional research utilizing extended randomized control trials is necessary in order to gain a more comprehensive understanding of the potential advantages associated with the use of antiplatelet and anticoagulant medications for the prevention and treatment of VaD.
5.1.4. Antidiabetic Drugs
The elderly population afflicted with diabetes exhibits a heightened prevalence of vascular pathology, which may, independently or in conjunction with AD-type pathology, contribute to an increased susceptibility to VaD [74]. The presence of diabetes may contribute to an elevated risk of VaD due to the accumulation of reactive oxygen species (ROS) caused by high blood sugar levels, which further exacerbates the progression of this pathological condition. Further, the association between diabetes and VaD is not unexpected, as diabetes mellitus is known to elevate the likelihood of stroke, lacunar infarcts, and vascular impairment, consequently augmenting the risk of developing VaD.
Several studies have demonstrated that the utilization of metformin is associated with a 24% decrease in the likelihood of developing dementia when compared to individuals who do not employ any antidiabetic medication [75]. An observational study showed that the utilization of pioglitazone over an extended period of time exhibited a correlation with a reduced occurrence of dementia. Conversely, the employment of pioglitazone for a shorter duration was found to be linked to a dementia risk similar to that observed in individuals without diabetes [76]. Based on the shared pathophysiological mechanisms, the utilization of antidiabetic therapy may offer potential benefits in both the prevention and treatment of vascular dementia (VaD).
5.1.5. Cholinesterase Inhibitors
Cholinesterase inhibitors are a class of drugs often used to treat symptoms of dementia. They work by increasing the amount of acetylcholine, a neurotransmitter that helps with memory and cognition, in the brain. The efficacy of cholinesterase inhibitors in VaD is not as well established as it is in AD. Studies have revealed that donepezil at a dosage of 10 mg had the highest level of efficacy, although it ranked third in terms of adverse effects [77]. In terms of both benefit and harm, Galantamine achieved a second-place ranking [77]. In terms of both benefit and damage, Rivastigmine exhibited the least favorable score among the cholinesterase inhibitors. Donepezil, galantamine, and rivastigmine have been broadly considered in patients with VaD over the past decade [78]. A study reported that the cholinesterase inhibitors might have some beneficial effects on cognition and global function in people with VaD [79], while the authors noted that the quality of the evidence was low and that more research was needed. These studies suggest there may be some benefit to using cholinesterase inhibitors in VaD; however, the evidence is not strong and more studies are needed to examine the effects of individual anticholinergics drug classes and regimens in VaD.
5.2. Non-Pharmacotherapy
5.2.1. Antioxidants
Accumulating evidence suggests that mitochondrial dysfunction and increased ROS mainly superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (HO) are implicated in the formation to the development of AD [80]. Antioxidants have shown several benefits limited to in vitro and in vivo studies. The antioxidant capacity of tetramethylpyrazine nitrone (TBN) has been demonstrated to exhibit neuroprotective qualities in rats with vascular dementia (VaD) [81]. Research findings have indicated that the treatment of alpha-lipoic acid significantly decreases the generation of malondialdehyde (MDA) and ROS in the hippocampus of rats with VaD generated by chronic cerebral hypoperfusion (CCH). Furthermore, alpha-lipoic acid (ALA) administration has been shown to enhance cognitive functions related to learning and memory. Hence, it has been proposed that ALA enhances cognitive impairments to some extent by virtue of its antioxidant properties [82]. Edaravone demonstrates a significant inhibitory effect on oxidative stress in the hippocampus of rats with VaD, as evidenced by its ability to increase superoxide dismutase (SOD) activity and decrease MDA and ROS levels. Furthermore, the injection of edaravone has been found to effectively mitigate the cognitive impairments observed in rats with VaD [83]. Sailuotong (SLT), a formulation consisting of Ginkgo biloba, ginsenosides, and saffron in a ratio of 5:5:1 per capsule, was utilized in a clinical trial involving a total of 227 participants [84]. These individuals were randomly assigned to different experimental groups, with 114 patients receiving SLT at a dosage of 360 mg and 113 patients receiving SLT at a dosage of 240 mg for a duration of 52 weeks. Additionally, control groups were established, with 113 patients in each group receiving either SLT at a dosage of 360 mg or SLT at a dosage of 240 mg, but only during weeks 27 to 52. Following the administration of this therapy, it was observed that the experimental groups had enhanced scores in comparison to the control groups [84].
Early clinical trial results with antioxidants in mild cognitive impairment (MCI) and AD are not believed to support the use of antioxidants in AD, they are characterized by extremely poor comparability and a lack of benefits [85]. The antioxidants proposed to combat oxidative damage in AD or VaD have limited efficacy in part due to unbalanced dose, duration, monotherapy, and the presence of a blood–brain barrier that does not permit small amounts of antioxidants to enter the brain. Therefore, a balanced diet and lifestyle modifications may be the only long-term solution to preventing or reversing the cognitive decline in AD or VaD.
5.2.2. Dietary Interventions
Certain dietary interventions can help manage symptoms, prevent further brain damage, and improve quality of life. These interventions are often centered on promoting overall vascular health and limiting dietary factors associated with CVD (Table 2).

Table 2.
Dietary intervention and its beneficial effects.
- How will the Mediterranean Diet be beneficial in the development of VaD?
The Mediterranean diet (MedDi) has shown potential in managing VaD due to its heart-healthy components. This diet is rich in fruits, vegetables, whole grains, beans, nuts and seeds, olive oil, and lean proteins like fish and poultry. The cardiovascular benefits of this phenomenon have been extensively acknowledged, potentially exerting an indirect influence on the onset or advancement of VaD.
- ▪
- Reduction in stroke risk: VaD may also manifest gradually as a result of the accumulation of minor obstructions inside the blood arteries. VaD frequently manifests as a consequence of diseases, such as strokes, which lead to cerebral oxygen deprivation. The MedDi has the potential to mitigate the likelihood of stroke occurrence as a result of its focus on promoting the consumption of foods that are beneficial for cardiovascular health. Multiple research studies have provided evidence suggesting that adherence to a MedDi can substantially decrease the likelihood of experiencing a stroke. The study findings revealed that individuals who maintained adherence to a MedDi supplemented with either extra-virgin olive oil or nuts had a 30% decreased likelihood of experiencing significant cardiovascular complications, such as stroke, in comparison to those who adhered to a low-fat diet [86]. In a similar vein, a further study documented a reduction of 20% in the likelihood of experiencing a stroke among those who adhered to a MedDi [87]. A further study documented a reduction of 20% in the likelihood of experiencing a stroke among those who adhered to a MedDi [88]. Undoubtedly, the implementation of the MedDi can contribute to the mitigation of stroke risk, thereby serving as a preventive measure against one of the prevailing aetiologies of VaD. Simultaneously, it provides support for the overall well-being of the brain and potentially contributes to a decrease in the likelihood of developing dementia.
- ▪
- Improvement of vascular health: Atherosclerosis is the primary causative factor in approximately 90% of cases of acute coronary syndromes, 60% of strokes, chronic heart failure, peripheral arterial disease, and the majority of VaD cases. Several extensive intervention studies have provided compelling evidence of the considerable impact of the MedDi as a key preventative measure against various cardiovascular events. These events encompass stroke, atrial fibrillation, peripheral vascular disease, and myocardial infarction. [89,90]. The MedDi has the potential to increase vascular health as it prioritizes the consumption of unsaturated fats, such as olive oil, lean proteins, fruits, and vegetables. These dietary components have been recognized for their ability to mitigate inflammation, optimize lipid profiles, and promote improved vascular function. This has the potential to reduce the probability of VaD.
- ▪
- Lowering hypertension: The reduction in hypertension, or elevated blood pressure, can confer advantages for VaD by mitigating the potential for further cerebrovascular injury, a common etiological factor in this kind of cognitive decline. The MedDi dietary pattern has been found to have a beneficial effect on reducing blood pressure levels, which are considered a prominent risk factor for VaD. According to a meta-analysis undertaken by Ndanuko et al. [91], there is substantial evidence indicating a noteworthy decrease in blood pressure among individuals who adhere to a MedDi. Additional comprehensive investigation further corroborated and demonstrated that the use of a MedDi is frequently characterized as a measure for the prevention or management of hypertension in adult individuals [92]. Although the aforementioned studies indicate a potential association, it is crucial to acknowledge that further investigation is required to examine the precise influence of the MedDi on VaD. Nevertheless, considering the diet’s favourable impact on cardiovascular well-being, it is plausible to deduce that it may have the potential to postpone the initiation or decelerate the advancement of VaD by preserving the health of blood vessels and mitigating hypertension. It is advisable to get guidance from a healthcare practitioner prior to implementing substantial modifications to one’s diet, particularly for individuals who have pre-existing medical issues such as hypertension or VaD.
- ▪
- Improvement of cognitive function: Research has shown that adherence to the MedDi can improve cognitive function and potentially slow cognitive decline, which may delay the onset of VaD or slow its progression. Studies have shown that greater adherence to the MedDi has been associated with a reduction in cardiovascular and neurological disorders, including AD, related cognitive decline, and improved longevity in individuals with VaD [93,94]. The implementation of a dietary regimen aimed at mitigating oxidative stress, such as the MedDi, has the potential to contribute to the preservation of cognitive function [95]. According to a recent study, it was shown that the MedDi had a high content of biophenols while being low in red and processed meat [96]. These dietary characteristics are believed to potentially contribute to a neuroprotective impact against age-related brain shrinkage. The results of a comprehensive, population-based, prospective cohort study revealed a significant correlation between increased adherence to the MedDi and a reduced incidence of dementia [97]. Overall, adopting a MedDi could be an effective strategy for preventing or slowing the progression of VaD. However, further research is needed to fully understand the mechanisms behind these effects and to determine the optimal dietary patterns for individuals with this condition.
- ▪
- Reduction in neuroinflammation and oxidative stress: The MedDi includes a high intake of antioxidants from fruits and vegetables, which can reduce oxidative stress and neuroinflammation. Both are related to the pathogenesis of dementia, including VaD. A study has showed the effectiveness of the MedDi in reducing both the prevalence of metabolic syndrome and the associated cardiovascular risks by reducing the mild inflammatory conditions associated with metabolic syndrome [98]. The MedDi downregulates cellular and circulating inflammatory biomarkers associated with atherogenesis in individuals at high cardiovascular risk, which supported the recommendation of the MedDi [99]. The study found that patients with metabolic syndrome and high adherence to the MedDi were shown to have less changes in anthropometric parameters, less changes in blood biochemical profiles, and improved oxidative and inflammatory status [100]. Further, long-term studies will be required to determine the beneficial effects of this pro-inflammatory state produced by increased MedDi adherence.
- b.
- How effective is the DASH (Dietary Approaches to Stop Hypertension) Diet against the development of VaD?
The DASH diet, designed to reduce blood pressure, may also be beneficial in the development or progression of VaD. This diet promotes fruits, vegetables, lean protein, whole grains, and low-fat dairy, and limits foods high in saturated fats and sugars. Stroke is one of the major risk factors for developing VaD, and its reduction could be beneficial as shown by a study where high adherence to the DASH diet showed association with a reduction in ischemic stroke risk [101]. There is limited evidence from intervention studies for the beneficial effects of DASH in the development of VaD. Further prospective studies in different population groups are recommended to determine associations between DASH dietary habits and clinically relevant measures of cognitive decline and VaD.
- c.
- How effective is the MIND Diet against the development of VaD?
The MIND (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay) diet combines elements of the DASH and Mediterranean diets and may offer particular benefits for individuals with VaD. The MIND diet includes specific food groups beneficial for brain health, including green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil, and wine. A study showed that the MIND diet slowed cognitive decline in stroke survivors, potentially indicating its usefulness for VaD [102]. High adherence to the MIND diet is associated with reduced risk of dementia in the first year after follow-up, which can be partially explained by reverse causation and residual lifestyle disruption [103]. The MIND dietary intervention can reverse the devastating effects of obesity on cognition and brain structure that can be exacerbated by moderate caloric restriction [104]. A recent study showed high levels of MIND diet adherence were associated with better brain and cognitive outcomes when physical activity was low [105]. Substantial evidence suggested that the MIND diet has been associated with slower cognitive decline and reduced AD risk; however, more research is needed to determine its specific effects on VaD.
5.2.3. How Physical Activities Would Be Effective against the Development of VaD?
Engaging in physical activity is widely recognized as beneficial for general health and particularly for cognitive function. This includes individuals with VaD, a form of dementia caused by reduced blood flow to the brain, typically due to stroke or transient ischemic attack.
Improvement in Cognitive Function: Regular physical activity may improve cognitive function in individuals with VaD. Physical activity may improve cognitive function compared to inactive people with cognitive impairment [106]. A systematic review analysis has shown that 6–12 months of physical activity in people with MCI or dementia leads to better cognitive function than in sedentary controls [107]. A meta-analysis found that aerobic exercise in older adults with AD and non-Alzheimer’s dementia helped improve cognitive functioning when combined with other standard dementia treatments, and that more frequent interventions improved cognitive functioning shown to have no additional effect [108]. Another study demonstrates that a six-month aerobic exercise program improved cognitive function and reduced the rate of cognitive decline in older adults with mild vascular cognitive impairment, a common precursor to VaD [109]. Though studies have shown supporting evidence of physical activity benefits, each individual’s condition and capabilities are different. Therefore, it is important for each exercise regimen to be tailored to the individual’s needs and capacities, preferably under the guidance of healthcare professionals. Additionally, more research is needed to understand the precise relationship between physical activity and cognitive function in those with VaD.
5.2.4. How effective Is Aroma Therapy in VaD Patients?
The utilization of aromatherapy has been extensively employed as a therapeutic intervention for managing agitation in individuals diagnosed with dementia or VaD. The autonomic nervous system may be activated, and the limbic system and hypothalamus may be induced to react through the absorption of essential oil via transdermal administration or inhalation [110]. Therefore, this therapeutic intervention is expected to promote a sense of calmness and reduce restlessness in individuals diagnosed with dementia [111]. Lavender and lemon balms are frequently employed in aromatherapy as essential oils. Research has indicated that both botanical species exhibit properties conducive to tranquility and relaxation, as well as the facilitation of restful sleep [112]. Furthermore, these plants have been seen to alleviate agitation and enhance the overall well-being of individuals affected by dementia. Nevertheless, the available research does not provide strong and persuasive support for the beneficial effects of aromatherapy, namely the exposure to fragrant plant oils, in individuals with dementia or VaD. To ensure the establishment of unambiguous conclusions, future studies necessitate improved design and reporting, as well as enhanced uniformity in outcome measurement.
6. Conclusions
In conclusion, the role of vascular pathologies in the emergence of VaD is unmistakable. Various vascular diseases, whether systemic or local, contribute significantly to the pathophysiology of VaD.
Managing VaD requires a comprehensive approach that integrates pharmacotherapy, non-pharmacotherapy, and dietary modifications. Current evidence supports the use of a range of interventions to slow disease progression and improve quality of life. However, due to the heterogeneity of the disease, a tailored approach is necessary, taking into account the patient’s clinical condition, lifestyle, preferences, and risk factors. Therefore, further work is needed to determine the effects of aerobic exercise on cerebrovascular function and potential benefits in delaying the development or progression of VaD and implement exercise interventions in the early stages of treatment or prevention of cognitive decline.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank The Library, Charles Sturt University, Building 1005, Leeds Parade, Orange, NSW 2800, Australia.
Conflicts of Interest
The author declares no conflict of interest.
References
- Collaborators, G.B.D.D.F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar]
- The Lancet Public, H. Will dementia hamper healthy ageing? Lancet Public Health 2022, 7, e93. [Google Scholar] [CrossRef]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018, 134 Pt B, 226–239. [Google Scholar] [CrossRef]
- Deramecourt, V.; Slade, J.Y.; Oakley, A.E.; Perry, R.H.; Ince, P.G.; Maurage, C.A.; Kalaria, R.N. Staging and natural history of cerebrovascular pathology in dementia. Neurology 2012, 78, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Lecordier, S.; Manrique-Castano, D.; El Moghrabi, Y.; ElAli, A. Neurovascular Alterations in Vascular Dementia: Emphasis on Risk Factors. Front. Aging Neurosci. 2021, 13, 727590. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shue, F.; Bu, G.; Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 46. [Google Scholar] [CrossRef]
- Health Quality, O. The appropriate use of neuroimaging in the diagnostic work-up of dementia: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2014, 14, 1–64. [Google Scholar]
- Ghafar, M.; Miptah, H.N.; O’Caoimh, R. Cognitive screening instruments to identify vascular cognitive impairment: A systematic review. Int. J. Geriatr. Psychiatry 2019, 34, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Hoo, Z.L.; Yan, T.Z.; Wardlaw, J.; Quinn, T.J. Prevalence of dementia in ischaemic or mixed stroke populations: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M.; Oxford Vascular, S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019, 18, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. 2017, 131, 1059–1068. [Google Scholar] [CrossRef]
- Barbay, M.; Taillia, H.; Nedelec-Ciceri, C.; Arnoux, A.; Puy, L.; Wiener, E.; Canaple, S.; Lamy, C.; Godefroy, O.; Roussel, M.; et al. Vascular cognitive impairment: Advances and trends. Rev. Neurol. 2017, 173, 473–480. [Google Scholar] [CrossRef]
- Song, J.; Lee, W.T.; Park, K.A.; Lee, J.E. Association between risk factors for vascular dementia and adiponectin. Biomed. Res. Int. 2014, 2014, 261672. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Janel, N.; Sarazin, M.; Corlier, F.; Corne, H.; de Souza, L.C.; Hamelin, L.; Aka, A.; Lagarde, J.; Blehaut, H.; Hindie, V.; et al. Plasma DYRK1A as a novel risk factor for Alzheimer’s disease. Transl. Psychiatry 2014, 4, e425. [Google Scholar] [CrossRef] [PubMed]
- Janel, N.; Alexopoulos, P.; Badel, A.; Lamari, F.; Camproux, A.C.; Lagarde, J.; Simon, S.; Feraudet-Tarisse, C.; Lamourette, P.; Arbones, M.; et al. Combined assessment of DYRK1A, BDNF and homocysteine levels as diagnostic marker for Alzheimer’s disease. Transl. Psychiatry 2017, 7, e1154. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, C.A.; Theochari, C.A.; Zareifopoulos, N.; Arfaras-Melainis, A.; Giannakoulas, G.; Karamitsos, T.D.; Palaiodimos, L.; Ntaios, G.; Avgerinos, K.I.; Kapogiannis, D.; et al. Atrial Fibrillation Is Associated with Cognitive Impairment, All-Cause Dementia, Vascular Dementia, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ko, K.H.; Oh, J.H.; Kim, J.G.; Kang, C.H.; Song, S.K.; Kang, S.Y.; Kang, J.H.; Park, J.H.; Koh, M.J.; et al. Apolipoprotein E epsilon4 Is Associated With the Development of Incident Dementia in Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy Patients With p.Arg544Cys Mutation. Front. Aging Neurosci. 2020, 12, 591879. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.R.; Beiser, A.S.; O’Donnell, A.; Himali, J.J.; Pase, M.P.; Satizabal, C.L.; Seshadri, S. Determining Vascular Risk Factors for Dementia and Dementia Risk Prediction Across Mid- to Later-Life: The Framingham Heart Study. Neurology 2022, 99, e142–e153. [Google Scholar] [CrossRef] [PubMed]
- Akhter, F.; Persaud, A.; Zaokari, Y.; Zhao, Z.; Zhu, D. Vascular Dementia and Underlying Sex Differences. Front. Aging Neurosci. 2021, 13, 720715. [Google Scholar] [CrossRef]
- Appelros, P.; Stegmayr, B.; Terent, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Mielke, M.M. Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Cross, P.; Andrews, H.; Jacobs, D.M.; Small, S.; Bell, K.; Merchant, C.; Lantigua, R.; Costa, R.; Stern, Y.; et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001, 56, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, S.I.; Cadogan, S.L.; Lin, L.Y.; Mathur, R.; Smeeth, L.; Warren-Gash, C. Ethnic Differences in Dementia Risk: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2021, 80, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Dilworth-Anderson, P.; Hendrie, H.C.; Manly, J.J.; Khachaturian, A.S.; Fazio, S.; Social, B.; Diversity Research Workgroup of the Alzheimer’s Association. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008, 4, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.L.; Kuller, L.H.; Ives, D.G.; Lopez, O.L.; Jagust, W.; Breitner, J.C.; Jones, B.; Lyketsos, C.; Dulberg, C. Incidence and prevalence of dementia in the Cardiovascular Health Study. J. Am. Geriatr. Soc. 2004, 52, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mijajlovic, M.D.; Pavlovic, A.; Brainin, M.; Heiss, W.D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A.; et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Kumar, A.; Kumar, P.; Yadav, A.K.; Mohania, D.; Pandit, A.K.; Prasad, K.; Vibha, D. Blood-based protein biomarkers for stroke differentiation: A systematic review. Proteomics Clin. Appl. 2017, 11, 1700007. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, Y.; Su, Z.; Yue, W.; Hao, H.; Ren, L.; Wang, Y.; Cao, Y.; Wang, Y. Association of Oxidized Low-Density Lipoprotein With Prognosis of Stroke and Stroke Subtypes. Stroke 2017, 48, 91–97. [Google Scholar] [CrossRef]
- Wang, A.; Liu, J.; Meng, X.; Li, J.; Wang, H.; Wang, Y.; Su, Z.; Zhang, N.; Dai, L.; Wang, Y.; et al. Association between oxidized low-density lipoprotein and cognitive impairment in patients with ischemic stroke. Eur. J. Neurol. 2018, 25, 185–191. [Google Scholar] [CrossRef]
- Ercan, M.; Mungan, S.; Guzel, I.; Celik, H.T.; Bal, C.; Abusoglu, S.; Akbulut, D.; Oguz, E.F.; Yilmaz, F.M. Serum asymmetric dimethylarginine and nitric oxide levels in Turkish patients with acute ischemic stroke. Adv. Clin. Exp. Med. 2019, 28, 693–698. [Google Scholar] [CrossRef]
- Prezioso, G.; Giannini, C.; Chiarelli, F. Effect of Thyroid Hormones on Neurons and Neurodevelopment. Horm. Res. Paediatr. 2018, 90, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pilhatsch, M.; Marxen, M.; Winter, C.; Smolka, M.N.; Bauer, M. Hypothyroidism and mood disorders: Integrating novel insights from brain imaging techniques. Thyroid. Res. 2011, 4 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Mulat, B.; Ambelu, A.; Yitayih, S.; Gela, Y.Y.; Adera, A.; Yeshaw, Y.; Akalu, Y. Cognitive Impairment and Associated Factors Among Adult Hypothyroid Patients in Referral Hospitals, Amhara Region, Ethiopia: Multicenter Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2021, 17, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Forti, P.; Olivelli, V.; Rietti, E.; Maltoni, B.; Pirazzoli, G.; Gatti, R.; Gioia, M.G.; Ravaglia, G. Serum thyroid-stimulating hormone as a predictor of cognitive impairment in an elderly cohort. Gerontology 2012, 58, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Akintola, A.A.; Jansen, S.W.; van Bodegom, D.; van der Grond, J.; Westendorp, R.G.; de Craen, A.J.; van Heemst, D. Subclinical hypothyroidism and cognitive function in people over 60 years: A systematic review and meta-analysis. Front. Aging Neurosci. 2015, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Eslami-Amirabadi, M.; Sajjadi, S.A. The relation between thyroid dysregulation and impaired cognition/behaviour: An integrative review. J. Neuroendocrinol. 2021, 33, e12948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, X.; Zhang, C.; Wang, J.; Chen, G.; Zhang, H.; Sun, Z. Correlation of thyroid dysfunction and cognitive impairments induced by subcortical ischemic vascular disease. Brain Behav. 2016, 6, e00452. [Google Scholar] [CrossRef]
- Hagstrom, E.; Kilander, L.; Nylander, R.; Larsson, E.M.; Michaelsson, K.; Melhus, H.; Ahlstrom, H.; Johansson, L.; Lind, L.; Arnlov, J. Plasma parathyroid hormone is associated with vascular dementia and cerebral hyperintensities in two community-based cohorts. J. Clin. Endocrinol. Metab. 2014, 99, 4181–4189. [Google Scholar] [CrossRef]
- Janicki, S.C.; Schupf, N. Hormonal influences on cognition and risk for Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 359–366. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Hornberger, M.; Ritchie, C.W.; Minihane, A.M. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: Results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res. Ther. 2023, 15, 10. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mims, P.N.; Roman, R.J.; Fan, F. Is Beta-Amyloid Accumulation a Cause or Consequence of Alzheimer’s Disease? J. Alzheimers Parkinsonism Dement. 2016, 1, 007. [Google Scholar] [PubMed]
- Lue, L.F.; Guerra, A.; Walker, D.G. Amyloid Beta and Tau as Alzheimer’s Disease Blood Biomarkers: Promise From New Technologies. Neurol. Ther. 2017, 6 (Suppl. S1), 25–36. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.C.; Villemagne, V.L. Brain amyloid imaging. J. Nucl. Med. Technol. 2013, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Yang, K.C.; Chen, C.H.; Yang, S.Y.; Chiu, M.J.; Wu, C.C.; Jeng, J.S. Plasma beta-Amyloids and Tau Proteins in Patients with Vascular Cognitive Impairment. Neuromolecular Med. 2018, 20, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bibl, M.; Esselmann, H.; Mollenhauer, B.; Weniger, G.; Welge, V.; Liess, M.; Lewczuk, P.; Otto, M.; Schulz, J.B.; Trenkwalder, C.; et al. Blood-based neurochemical diagnosis of vascular dementia: A pilot study. J. Neurochem. 2007, 103, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.; Zilkova, M.; Deli, M.A.; Zilka, N.; Novak, M. Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J. Alzheimers Dis. 2009, 18, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Qian, S.; Ba, L.; Lin, Z.; Xiao, S. A Pilot Longitudinal Study on Cerebrospinal Fluid (CSF) Tau Protein in Alzheimer’s Disease and Vascular Dementia. Shanghai Arch. Psychiatry 2016, 28, 271–279. [Google Scholar]
- NICE. Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers. 2018. Available online: https://www.nice.org.uk/guidance/ng97/resources/dementia-assessment-management-and-support-for-people-living-with-dementia-and-their-carers-pdf-1837760199109 (accessed on 4 July 2023).
- Feldman, H.H.; Jacova, C.; Robillard, A.; Garcia, A.; Chow, T.; Borrie, M.; Schipper, H.M.; Blair, M.; Kertesz, A.; Chertkow, H. Diagnosis and treatment of dementia: 2. Diagnosis. CMAJ 2008, 178, 825–836. [Google Scholar] [CrossRef]
- Hachinski, V.; Iadecola, C.; Petersen, R.C.; Breteler, M.M.; Nyenhuis, D.L.; Black, S.E.; Powers, W.J.; DeCarli, C.; Merino, J.G.; Kalaria, R.N.; et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006, 37, 2220–2241. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Wong, A.; Nyenhuis, D.; Black, S.E.; Law, L.S.; Lo, E.S.; Kwan, P.W.; Au, L.; Chan, A.Y.; Wong, L.K.; Nasreddine, Z.; et al. Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke 2015, 46, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Gong, M.; Zhang, Y.; Lyu, X. Effects of different kinds of anti-Alzheimer’s disease drugs on cognitive improvement: Protocol for a systematic review and network meta-analysis of phase III clinical trials. Syst. Rev. 2022, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; Einhaupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.M.; Sweeney, M.D.; Zlokovic, B.; Iturria-Medina, Y.; Iadecola, C.; et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimers Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef]
- Larsson, S.C.; Markus, H.S. Does Treating Vascular Risk Factors Prevent Dementia and Alzheimer’s Disease? A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2018, 64, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.D.; Lane, K.A.; Gao, S.; Evans, R.M.; Unverzagt, F.W.; Hall, K.S.; Hendrie, H. Preservation of cognitive function with antihypertensive medications: A longitudinal analysis of a community-based sample of African Americans. Arch. Intern. Med. 2002, 162, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Coca, A. Hypertension and vascular dementia in the elderly: The potential role of anti-hypertensive agents. Curr. Med. Res. Opin. 2013, 29, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Rouch, L.; Cestac, P.; Hanon, O.; Cool, C.; Helmer, C.; Bouhanick, B.; Chamontin, B.; Dartigues, J.F.; Vellas, B.; Andrieu, S. Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 2015, 29, 113–130. [Google Scholar] [CrossRef]
- Hernandorena, I.; Duron, E.; Vidal, J.S.; Hanon, O. Treatment options and considerations for hypertensive patients to prevent dementia. Expert. Opin. Pharmacother. 2017, 18, 989–1000. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Katsanos, A.H.; Kosmidou, M.; Tsivgoulis, G. Statins and vascular dementia: A review. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S315–S320. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, T.; Li, N.; Wu, L.; Wang, Y.; Zheng, D.; Guo, B.; Wang, B. Associations of Lipids and Lipid-Lowering Drugs with Risk of Vascular Dementia: A Mendelian Randomization Study. Nutrients 2022, 15, 69. [Google Scholar] [CrossRef]
- Alexander, P.; Visagan, S.; Jawhar, S.; Kare, A.; Issa, N.; Issa, R.; Jawhar, A.; Thomas, S.; Gorantla, V. Antiplatelets and Vascular Dementia: A Systematic Review. J. Aging Res. 2022, 2022, 9780067. [Google Scholar] [CrossRef]
- Ahtiluoto, S.; Polvikoski, T.; Peltonen, M.; Solomon, A.; Tuomilehto, J.; Winblad, B.; Sulkava, R.; Kivipelto, M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 2010, 75, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Fink, A.; Doblhammer, G. Effect of pioglitazone medication on the incidence of dementia. Ann. Neurol. 2015, 78, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Battle, C.E.; Abdul-Rahim, A.H.; Shenkin, S.D.; Hewitt, J.; Quinn, T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2, CD013306. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Pai, M.C.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson’s disease dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Kavirajan, H.; Schneider, L.S. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: A meta-analysis of randomised controlled trials. Lancet Neurol. 2007, 6, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, J.; Wu, L.; Li, N.; Sun, Y.; Yu, P.; Wang, Y.; Zhang, G.; Zhang, Z. Neuroprotective and axonal outgrowth-promoting effects of tetramethylpyrazine nitrone in chronic cerebral hypoperfusion rats and primary hippocampal neurons exposed to hypoxia. Neuropharmacology 2017, 118, 137–147. [Google Scholar] [CrossRef]
- Zhao, R.R.; Xu, F.; Xu, X.C.; Tan, G.J.; Liu, L.M.; Wu, N.; Zhang, W.Z.; Liu, J.X. Effects of alpha-lipoic acid on spatial learning and memory, oxidative stress, and central cholinergic system in a rat model of vascular dementia. Neurosci. Lett. 2015, 587, 113–119. [Google Scholar] [CrossRef]
- Li, X.; Lu, F.; Li, W.; Qin, L.; Yao, Y.; Ge, X.; Yu, Q.; Liang, X.; Zhao, D.; Li, X.; et al. Edaravone injection reverses learning and memory deficits in a rat model of vascular dementia. Acta Biochim. Biophys. Sin. 2017, 49, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Shi, L.; Gong, M.; Xu, H.; Li, F.; et al. Efficacy and safety of the compound Chinese medicine SaiLuoTong in vascular dementia: A randomized clinical trial. Alzheimers Dement. 2018, 4, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Paterson, K.E.; Myint, P.K.; Jennings, A.; Bain, L.K.M.; Lentjes, M.A.H.; Khaw, K.T.; Welch, A.A. Mediterranean Diet Reduces Risk of Incident Stroke in a Population With Varying Cardiovascular Disease Risk Profiles. Stroke 2018, 49, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martinez-Gonzalez, M.A.; Estruch, R.; Salas-Salvado, J.; Fito, M.; Martinez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Bonapace, S.; Lunardi, G.; Salgarello, M.; Dugo, C.; Canali, G.; Byrne, C.D.; Gori, S.; Barbieri, E.; Targher, G. Association between plasma ceramides and inducible myocardial ischemia in patients with established or suspected coronary artery disease undergoing myocardial perfusion scintigraphy. Metabolism 2018, 85, 305–312. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Nissensohn, M.; Roman-Vinas, B.; Sanchez-Villegas, A.; Piscopo, S.; Serra-Majem, L. The Effect of the Mediterranean Diet on Hypertension: A Systematic Review and Meta-Analysis. J. Nutr. Educ. Behav. 2016, 48, 42–53.e41. [Google Scholar] [CrossRef]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martinez-Gonzalez, M.A.; Martinez-Lapiscina, E.H.; Fito, M.; Perez-Heras, A.; Salas-Salvado, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The effect of a high-polyphenol Mediterranean diet (Green-MED) combined with physical activity on age-related brain atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Ranson, J.M.; Gregory, S.; Macpherson, H.; Milte, C.; Lentjes, M.; Mulligan, A.; McEvoy, C.; Griffiths, A.; Matu, J.; et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med. 2023, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef]
- Quetglas-Llabres, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gomez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 91. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wallin, A.; Wolk, A. Dietary Approaches to Stop Hypertension Diet and Incidence of Stroke: Results From 2 Prospective Cohorts. Stroke 2016, 47, 986–990. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- de Crom, T.O.E.; Mooldijk, S.S.; Ikram, M.K.; Ikram, M.A.; Voortman, T. MIND diet and the risk of dementia: A population-based study. Alzheimers Res. Ther. 2022, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, G.; Abbas-Zadeh, M.; Eftekhari, M.H. Effect of MIND diet intervention on cognitive performance and brain structure in healthy obese women: A randomized controlled trial. Sci. Rep. 2022, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Escher, C.E.; Asken, B.M.; VandeBunte, A.; Fonseca, C.; You, M.; Kramer, J.H.; Casaletto, K.B. Roles of physical activity and diet in cognitive aging: Is more better? Clin. Neuropsychol. 2023, 37, 286–303. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Geda, Y.E.; Graff-Radford, N.R.; Petersen, R.C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc. 2011, 86, 876–884. [Google Scholar] [CrossRef]
- Ohman, H.; Savikko, N.; Strandberg, T.E.; Pitkala, K.H. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement. Geriatr. Cogn. Disord. 2014, 38, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.; van Berckel, B.N.; Scheltens, P.; Scherder, E.J.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Best, J.R.; Davis, J.C.; Eng, J.J.; Lee, P.E.; Jacova, C.; Boyd, L.A.; Brasher, P.M.; Munkacsy, M.; Cheung, W.; et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016, 87, 2082–2090. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Kwon, C.Y.; Lee, B. Complementary and integrative medicines for behavioral and psychological symptoms of dementia: Overview of systematic reviews. Explore 2023, 19, 176–194. [Google Scholar] [CrossRef]
- Holmes, C.; Hopkins, V.; Hensford, C.; MacLaughlin, V.; Wilkinson, D.; Rosenvinge, H. Lavender oil as a treatment for agitated behaviour in severe dementia: A placebo controlled study. Int. J. Geriatr. Psychiatry 2002, 17, 305–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).