Stent-Graft Repair of Concomitant Occlusion and Anastomotic Pseudoaneurysm in a Prosthetic Femoropopliteal Bypass
Abstract
1. Introduction
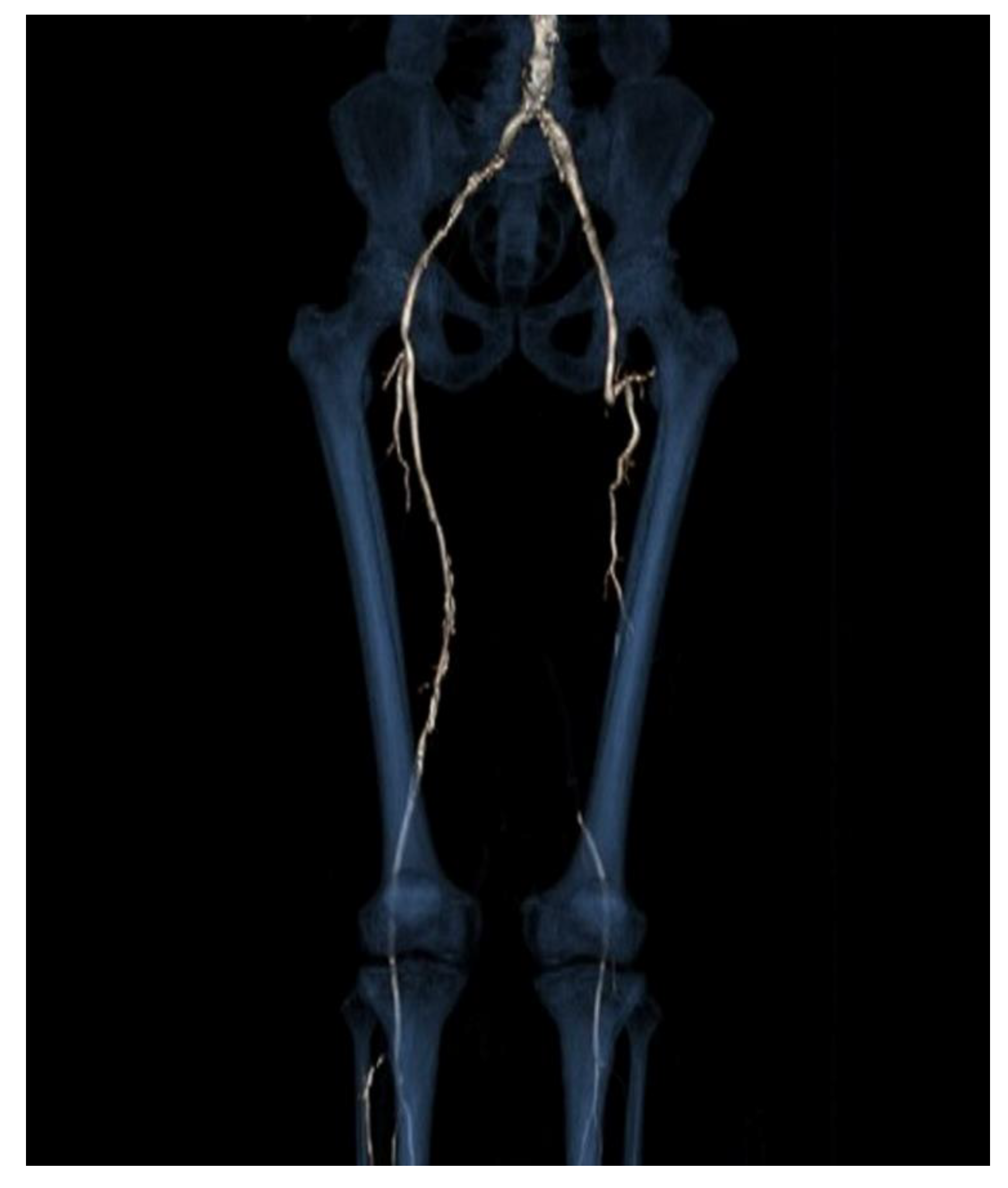
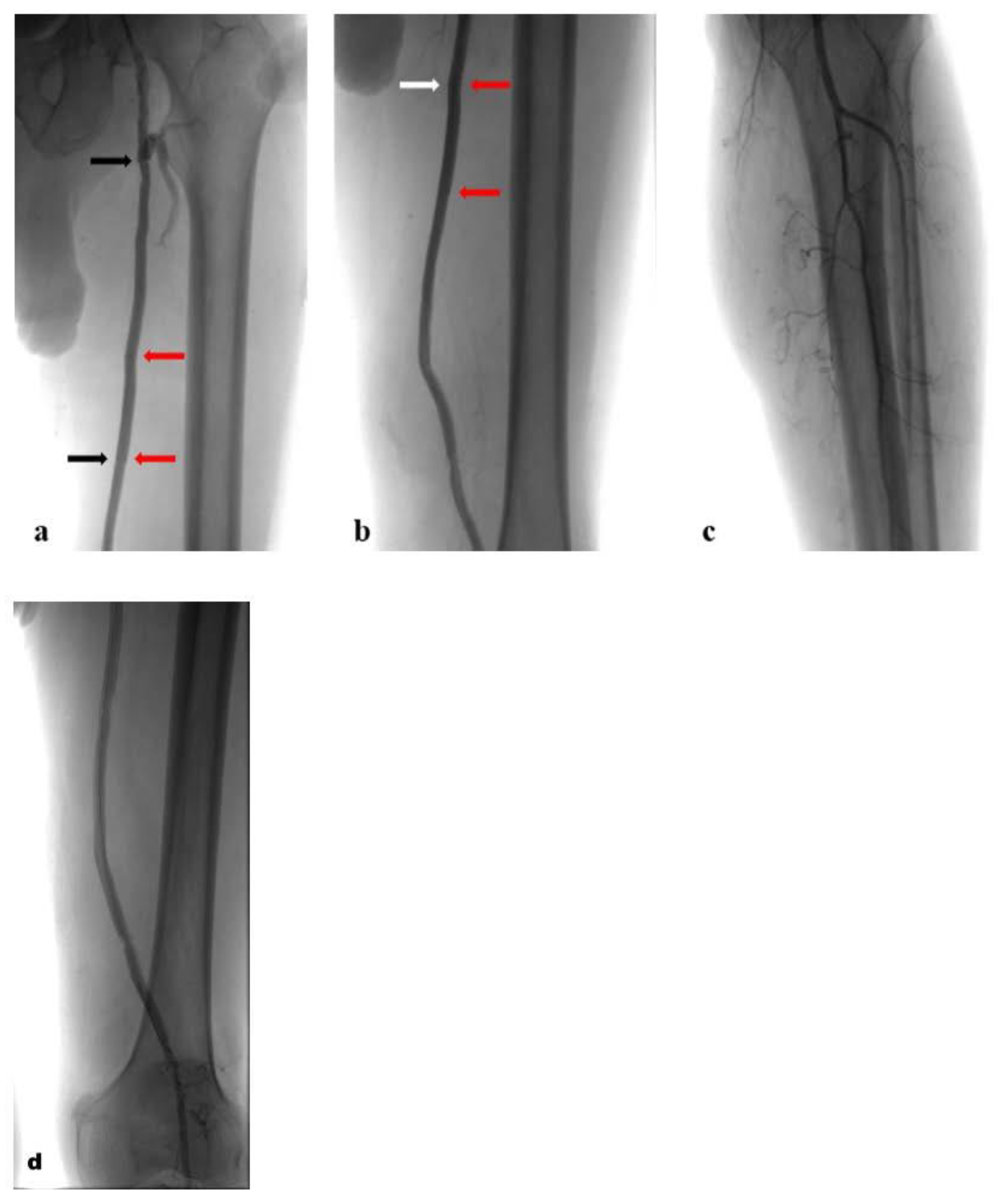
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taha, A.G.; Byrne, R.M.; Avgerinos, E.D.; Marone, L.K.; Makaroun, M.S.; Chaer, R.A. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J. Vasc. Surg. 2015, 61, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Magnetti, F.; Thalhammer, C.; Hechelhammer, L.; Husmann, M.; Pfammatter, T.; Amann-Vesti, B. Spontaneous pseudoaneurysm of a femoro-popliteal Omniflow II graft treated with a stentgraft. Vasa 2010, 39, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Derom, A.; Nout, E. Treatment of femoral pseudoaneurysms with endograft in high-risk patients. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 644–647.9. [Google Scholar] [CrossRef] [PubMed]
- Carollo, A.; Gagliardo, G.; DeVito, P.M.; Cicchillo, M. Stent graft repair of anastomotic pseudoaneurysm of femoral-popliteal bypass graft following patch angioplasty. J. Surg. Case Rep. 2016, 2016, rjw198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rundback, J.; Haug, J.; Herman, K.; Manno, J.; Cerda, M. Percutaneous stent-graft repair of anastomotic pseudoaneurysms following vascular bypass procedures: A report of two cases. Case Rep. Vasc. Med. 2013, 2013, 124832. [Google Scholar] [CrossRef] [PubMed]
- Begovac, P.C.; Thomson, R.C.; Fisher, J.L.; Hughson, A.; Gällhagen, A. Improvements in GORE-TEX® Vascular Graft performance by Carmeda® BioActive Surface heparin immobilization. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Bush, R.L.; Yao, Q.; Lumsden, A.B.; Chen, C. Evaluation of platelet deposition and neointimal hyperplasia of heparin-coated small-caliber ePTFE grafts in a canine femoral artery bypass model. J. Surg. Res. 2004, 118, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, N.; Obara, H.; Iwasa, K.; Hattori, T.; Yamamoto, H.; Watada, S.; Kobayashi, T.; Suematsu, N.; Mitsuoka, H.; Soga, Y.; et al. Preliminary Experience of Viabahn Stent Graft Inside the Occluded Prosthetic Bypass Graft for the Treatment of Above Knee Femoropopliteal Bypass Occlusion. Cardiovasc. Interv. Radiol. 2020, 43, 223–230. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzaretti, A.; Trevisan, D.; Beneggi, I.M.; Lomoro, P.; Fachinetti, C.; Mombelloni, S.; Togni, G.; Simonetti, I.; Vannelli, A. Stent-Graft Repair of Concomitant Occlusion and Anastomotic Pseudoaneurysm in a Prosthetic Femoropopliteal Bypass. J. Vasc. Dis. 2022, 1, 80-87. https://doi.org/10.3390/jvd1020009
Azzaretti A, Trevisan D, Beneggi IM, Lomoro P, Fachinetti C, Mombelloni S, Togni G, Simonetti I, Vannelli A. Stent-Graft Repair of Concomitant Occlusion and Anastomotic Pseudoaneurysm in a Prosthetic Femoropopliteal Bypass. Journal of Vascular Diseases. 2022; 1(2):80-87. https://doi.org/10.3390/jvd1020009
Chicago/Turabian StyleAzzaretti, Andrea, Daniele Trevisan, Irene Maria Beneggi, Pascal Lomoro, Camilla Fachinetti, Sara Mombelloni, Giorgio Togni, Igino Simonetti, and Alberto Vannelli. 2022. "Stent-Graft Repair of Concomitant Occlusion and Anastomotic Pseudoaneurysm in a Prosthetic Femoropopliteal Bypass" Journal of Vascular Diseases 1, no. 2: 80-87. https://doi.org/10.3390/jvd1020009
APA StyleAzzaretti, A., Trevisan, D., Beneggi, I. M., Lomoro, P., Fachinetti, C., Mombelloni, S., Togni, G., Simonetti, I., & Vannelli, A. (2022). Stent-Graft Repair of Concomitant Occlusion and Anastomotic Pseudoaneurysm in a Prosthetic Femoropopliteal Bypass. Journal of Vascular Diseases, 1(2), 80-87. https://doi.org/10.3390/jvd1020009





