The Significant Decrease of Serum Sodium and Blood Pressure following Thoracoscopic Left Atrial Appendage Clipping
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. Data Collection
2.3. Stand-Alone Thoracoscopic Left Atrial Appendage Clipping
2.4. Postoperative Care
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes of Electrolytes and Hemodynamics
3.3. Change of Brain Natriuretic Peptide
4. Discussion
4.1. Major Findings of Change in Blood Pressure and Serum Electrolytes
4.2. Proposed Hypothesis for the Current Observations
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, P.G.; Healey, J.S.; Raina, P.; Connolly, S.J.; Ibrahim, Q.; Gupta, R.; Avezum, A.; Dans, A.L.; Lopez-Jaramillo, P.; Yeates, K.; et al. Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153,152 middle-aged individuals. Cardiovasc. Res. 2021, 117, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- van Laar, C.; Verberkmoes, N.J.; van Es, H.W.; Lewalter, T.; Dunnington, G.; Stark, S.; Longoria, J.; Hofman, F.H.; Pierce, C.M.; Kotecha, D.; et al. Thoracoscopic Left Atrial Appendage Clipping: A Multicenter Cohort Analysis. JACC Clin. Electrophysiol. 2018, 4, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Roles of atrial natriuretic peptide and its therapeutic use. J. Cardiol. 2010, 56, 262–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Bold, A.J.; Bruneau, B.G.; Kuroski de Bold, M.L. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc. Res. 1996, 31, 7–18. [Google Scholar] [CrossRef]
- Garcia, R.; Cantin, M.; Thibault, G. Role of right and left atria in natriuresis and atrial natriuretic factor release during blood volume changes in the conscious rat. Circ. Res. 1987, 61, 99–106. [Google Scholar] [CrossRef]
- Maybrook, R.; Pillarisetti, J.; Yarlagadda, V.; Gunda, S.; Sridhar, A.R.; Deibert, B.; Afzal, M.R.; Reddy, M.; Atkins, D.; Earnest, M.; et al. Electrolyte and hemodynamic changes following percutaneous left atrial appendage ligation with the LARIAT device. J. Interv. Card. Electrophysiol. 2015, 43, 245–251. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Mahankali, A.S.; Kanmanthareddy, A.; Lee, R.; Badhwar, N.; Bartus, K.; Atkins, D.; Bommana, S.; Cheng, J.; Rasekh, A.; et al. Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2015, 1, 153–160. [Google Scholar] [CrossRef]
- Holmes, B.B.; Patel, N.; Lugo, R.; Richardson, T.; Metawee, M.; Rinke, L.L.; Meisch, C.; Shoemaker, M.B.; Ellis, C.R. Clinical predictors of acute hyponatremia following LARIAT ligation of the left atrial appendage. J. Cardiovasc. Electrophysiol. 2019, 30, 2501–2507. [Google Scholar] [CrossRef]
- Turagam, M.K.; Vuddanda, V.; Verberkmoes, N.; Ohtsuka, T.; Akca, F.; Atkins, D.; Bommana, S.; Emmert, M.Y.; Gopinathannair, R.; Dunnington, G.; et al. Epicardial Left Atrial Appendage Exclusion Reduces Blood Pressure in Patients With Atrial Fibrillation and Hypertension. J. Am. Coll. Cardiol. 2018, 72, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Hyponatremia. N. Engl. J. Med. 2000, 342, 1581–1589. [Google Scholar] [CrossRef]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Beigel, R.; Wunderlich, N.C.; Ho, S.Y.; Arsanjani, R.; Siegel, R.J. The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC Cardiovasc. Imaging 2014, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Oki, T.; Yamada, H.; Abe, M.; Onose, Y.; Thomas, J.D. Relationship between left atrial appendage function and plasma concentration of atrial natriuretic peptide. Eur. J. Echocardiogr. 2000, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sakellaridis, T.; Argiriou, M.; Charitos, C.; Tsakiridis, K.; Zarogoulidis, P.; Katsikogiannis, N.; Kougioumtzi, I.; Machairiotis, N.; Tsiouda, T.; Arikas, S.; et al. Left atrial appendage exclusion-Where do we stand? J. Thorac. Dis. 2014, 6 (Suppl. S1), S70–S77. [Google Scholar]
- Tang, M.; Wang, G.; Lu, P.; Karas, R.H.; Aronovitz, M.; Heximer, S.P.; Kaltenbronn, K.M.; Blumer, K.J.; Siderovski, D.P.; Zhu, Y.; et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat. Med. 2003, 9, 1506–1512. [Google Scholar] [CrossRef]
- Sabrane, K.; Kruse, M.N.; Fabritz, L.; Zetsche, B.; Mitko, D.; Skryabin, B.V.; Zwiener, M.; Baba, H.A.; Yanagisawa, M.; Kuhn, M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J. Clin. Investig. 2005, 115, 1666–1674. [Google Scholar] [CrossRef]
- Ramnarain, D.; Mehra, N. Natriuretic peptide-induced hyponatremia in a patient with left atrial myxoma. Crit. Care 2011, 15, P368. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Li, Y.; Han, J.; Li, Y.; Meng, X.; Zhang, H. The Effect of Minimally Invasive Thoracoscopic Left Atrial Appendage Excision on Cardiac Dynamic and Endocrine Function. Ann. Thorac. Cardiovasc. Surg. 2021, 27, 49–55. [Google Scholar] [CrossRef]
- Luani, B.; Rauwolf, T.; Groscheck, T.; Tanev, I.; Herold, J.; Isermann, B.; Schmeisser, A.; Braun-Dullaeus, R.C. Serial Assessment of Natriuretic Peptides in Patients Undergoing Interventional Closure of the Left Atrial Appendage. Heart Lung Circ. 2018, 27, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Majunke, N.; Sandri, M.; Adams, V.; Daehnert, I.; Mangner, N.; Schuler, G.; Moebius-Winkler, S. Atrial and Brain Natriuretic Peptide Secretion After Percutaneous Closure of the Left Atrial Appendage With the Watchman Device. J. Invasive Cardiol. 2015, 27, 448–452. [Google Scholar] [PubMed]
| Age | 68.50 (6.00) |
| Male | 42 (80.77%) |
| Type of Atrial fibrillation | |
| Paroxysmal | 12 (20.08%) |
| Non-Paroxysmal | 40 (76.92%) |
| Stroke History | 52 (100.00%) |
| Hypertension | 43 (82.69%) |
| DM | 17 (32.69%) |
| CVA/TIA | 52 (100.00%) |
| Heart failure | 4 (7.69%) |
| CHA2DS2-VASc Score | 5.00 (1.75) |
| Serum Level of Electrolytes | |
| Sodium (mmol/L) | 142.22 ± 2.59 |
| Potassium (mmol/L) | 3.89 ± 0.37 |
| BNP (mmol/L) | 243.11 ± 233.47 |
| Blood Pressure | |
| SBP (mmHg) | 122.93 ± 13.82 |
| DBP (mmHg) | 77.22 ± 7.72 |
| Baseline | Immediately | 1st Day Post | 2nd Day Post | Discharge | |
|---|---|---|---|---|---|
| Electrolytes | |||||
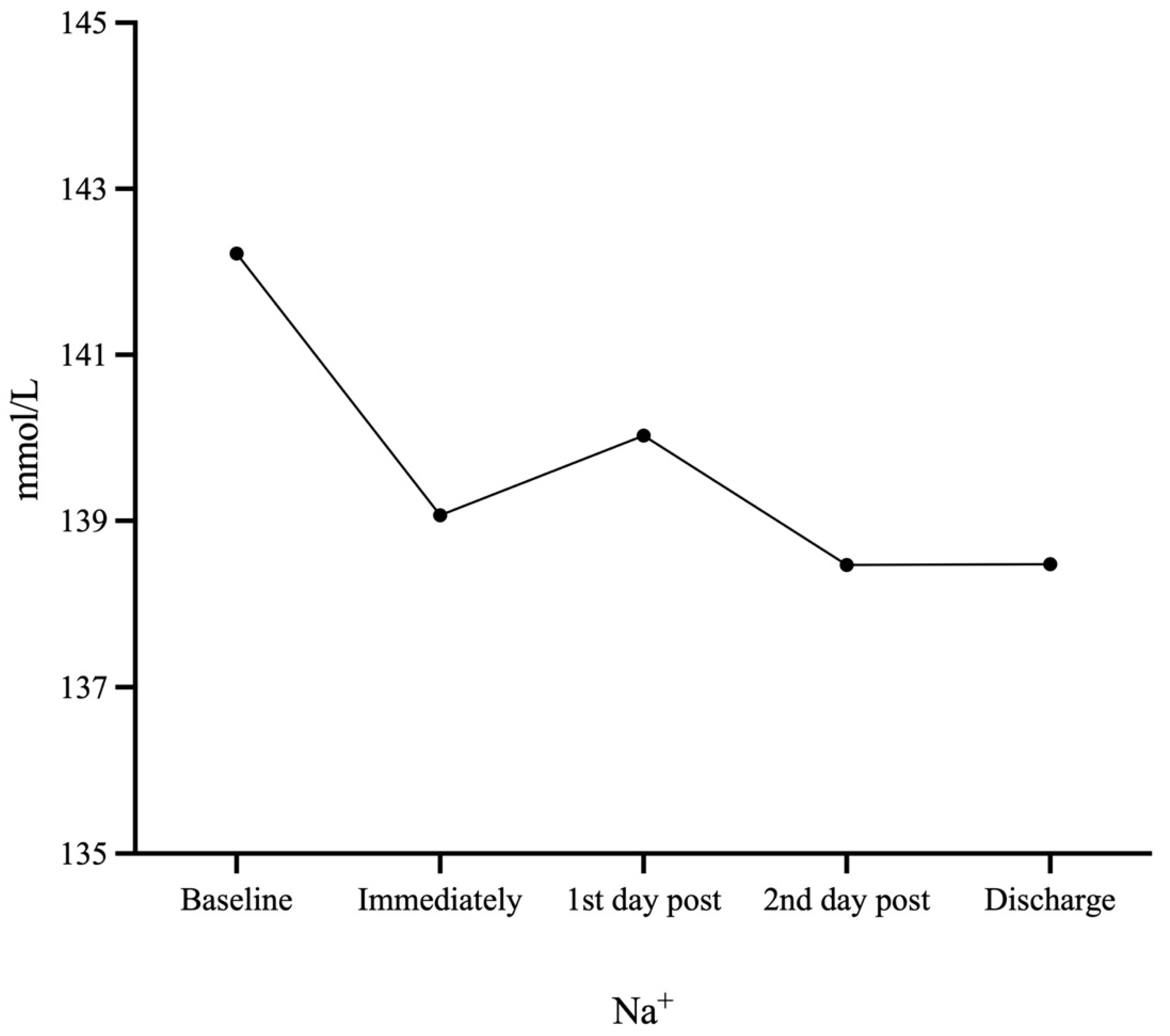
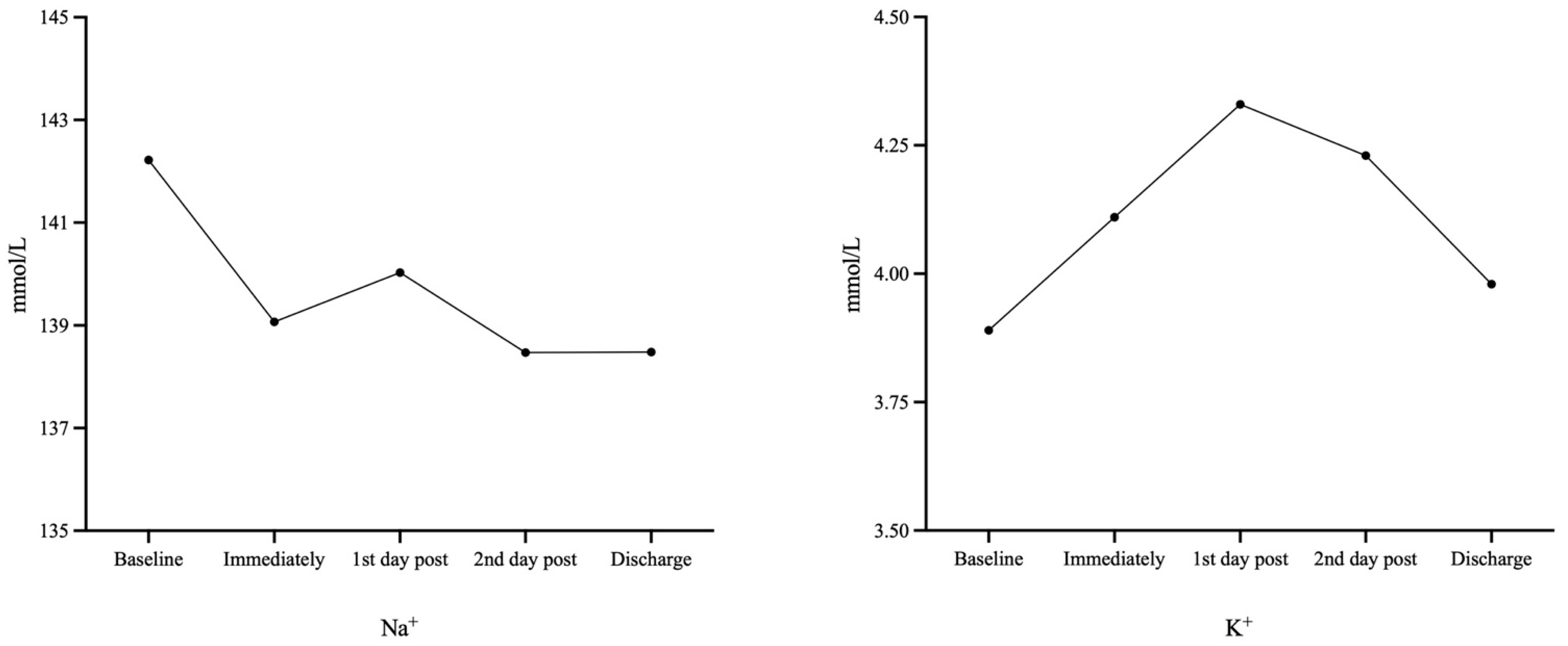
| Na+ (mmol/L) | 142.22 ± 2.59 | 139.07 ± 2.36 (p < 0.001) | 140.03 ± 2.84 (p < 0.001) | 138.47 ± 3.10 (p < 0.001) | 138.48 ± 2.63 (p < 0.001) |
| K+ (mmol/L) | 3.89 ± 0.37 | 4.11 ± 0.49 (p = 0.005) | 4.33 ± 1.01 (p = 0.005) | 4.23 ± 0.49 (p < 0.001) | 3.98 ± 0.39 (p = 0.149) |
| Blood Pressure | |||||
| SBP (mmHg) | 122.93 ± 13.82 | 131.94 ± 12.93 (p < 0.001) | 120.14 ± 10.68 (p = 0.193) | 119.12 ± 11.81 (p = 0.117) | 118.99 ± 12.29 (p = 0.034) |
| DBP (mmHg) | 77.22 ± 7.72 | 75.32 ± 7.59 (p = 0.218) | 66.35 ± 8.44 (p < 0.001) | 71.26 ± 10.31 (p = 0.001) | 78.00 ± 7.39 (p = 0.502) |
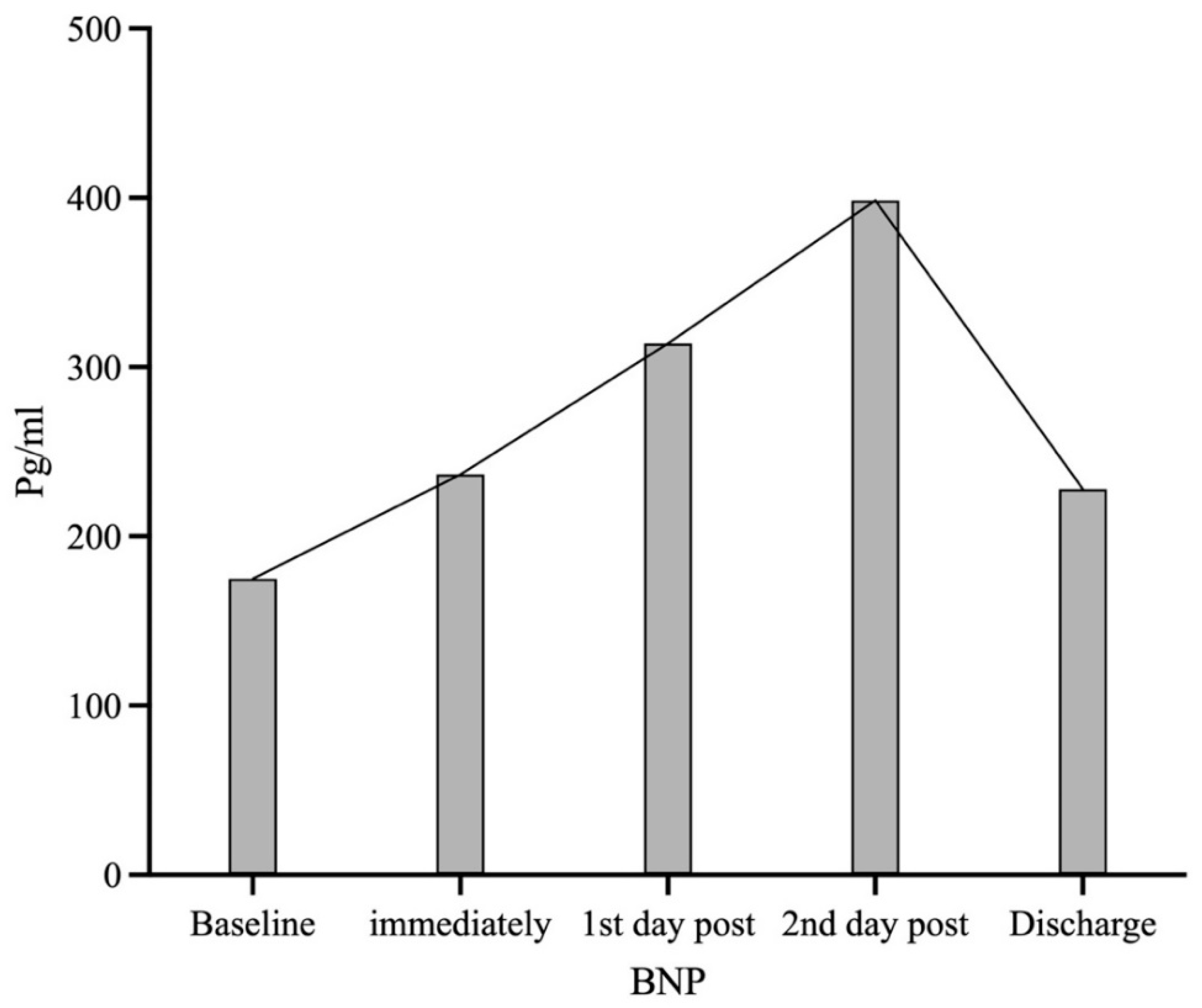
| BNP (pg/mL) | 174.90 (239.70) | 236.70 (207.30) (p = 0.030) | 314.00 (260.90) (p < 0.001) | 398.50 (337.70) (p < 0.001) | 227.90 (305.40) (p = 0.035) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Han, X.; Ye, C.; Xu, D. The Significant Decrease of Serum Sodium and Blood Pressure following Thoracoscopic Left Atrial Appendage Clipping. J. Vasc. Dis. 2022, 1, 97-104. https://doi.org/10.3390/jvd1020011
Chen Y, Han X, Ye C, Xu D. The Significant Decrease of Serum Sodium and Blood Pressure following Thoracoscopic Left Atrial Appendage Clipping. Journal of Vascular Diseases. 2022; 1(2):97-104. https://doi.org/10.3390/jvd1020011
Chicago/Turabian StyleChen, Yiming, Xuesong Han, Cong Ye, and Dong Xu. 2022. "The Significant Decrease of Serum Sodium and Blood Pressure following Thoracoscopic Left Atrial Appendage Clipping" Journal of Vascular Diseases 1, no. 2: 97-104. https://doi.org/10.3390/jvd1020011
APA StyleChen, Y., Han, X., Ye, C., & Xu, D. (2022). The Significant Decrease of Serum Sodium and Blood Pressure following Thoracoscopic Left Atrial Appendage Clipping. Journal of Vascular Diseases, 1(2), 97-104. https://doi.org/10.3390/jvd1020011





