Abstract
Alcohol Use Disorder (AUD) profoundly impacts individuals and society, driven by neurobiological adaptations that sustain chronicity and relapse. Emerging research highlights neuroinflammation, particularly microglial activation, as a central mechanism in AUD pathology. Ethanol engages microglia—the brain’s immune cells—through key signaling pathways such as Toll-like receptor 4 (TLR4) and the NLRP3 inflammasome, triggering the release of proinflammatory cytokines (IL-1β, TNF-α, IL-6). These mediators alter synaptic plasticity in addiction-related brain regions, including the ventral tegmental area, nucleus accumbens, amygdala, and lateral habenula, thereby exacerbating cravings, withdrawal symptoms, and relapse risk. Rodent models reveal that microglial priming disrupts dopamine signaling, heightening impulsivity and anxiety-like behaviors. Human studies corroborate these findings, demonstrating increased microglial activation markers in postmortem AUD brains and neuroimaging analyses. Notably, sex differences influence microglial reactivity, complicating AUD’s neuroimmune landscape and necessitating sex-specific research approaches. Microglia-targeted therapies—including minocycline, ibudilast, GLP-1 receptor agonists, and P2X7 receptor antagonists—promise to mitigate neuroinflammation and reduce alcohol intake, yet clinical validation remains limited. Addressing gaps such as biomarker identification, longitudinal human studies, and developmental mechanisms is critical. Leveraging multi-omics tools and advanced neuroimaging can refine microglia-based therapeutic strategies, offering innovative avenues to break the self-sustaining cycle of AUD.
1. Introduction
Alcohol Use Disorder (AUD) is a chronic, relapsing neuropsychiatric condition marked by compulsive alcohol consumption, loss of control over drinking, and persistent negative emotional states. It affects millions globally, imposing significant health and societal burdens due to its compulsive nature and high relapse rates. While traditional research has focused on neurotransmitter and circuit dysfunction, recent advances underscore the importance of neuroimmune mechanisms in AUD pathophysiology.
Emerging evidence highlights the central role of neuroimmune processes, particularly microglial activation and neuroinflammation, in driving AUD-related neuropathology [1]. The brain’s primary immune cells, microglia, regulate inflammation and neuronal activity. Chronic alcohol exposure can dysregulate microglial function, leading to a proinflammatory state that contributes to neuronal damage and behavioral impairments. This review synthesizes current knowledge on ethanol-induced microglial activation, its impact on addiction-related neural circuits, sex-specific neuroimmune responses, and emerging therapies targeting microglial modulation.
2. Methods
2.1. Literature Search and Selection
This review employed a systematic search strategy following PRISMA 2020 guidelines [2]. Four databases—PubMed (Medline), Google Scholar, Cochrane Library, and ClinicalTrials.gov—were searched for studies examining microglial involvement in AUD, particularly concerning sex-specific outcomes. The search focused on 2005 publications targeting neuroimmune signaling, microglial function, and AUD pathology. Manual screening supplemented automated retrieval.
Studies were included if they were English-language randomized controlled trials, observational studies, or animal models directly investigating microglial activation in AUD. Articles lacking methodological rigor or primary data were excluded.
An initial yield of 1056 studies were retrieved: 1030 from Google Scholar, 24 from PubMed Central, and 2 combined from Cochrane Library and ClinicalTrials.gov. After removing duplicates, screening titles, abstracts, and keywords, 359 studies were excluded. The remaining 697 underwent full-text review, resulting in 236 articles that met the predefined eligibility criteria (Figure 1).
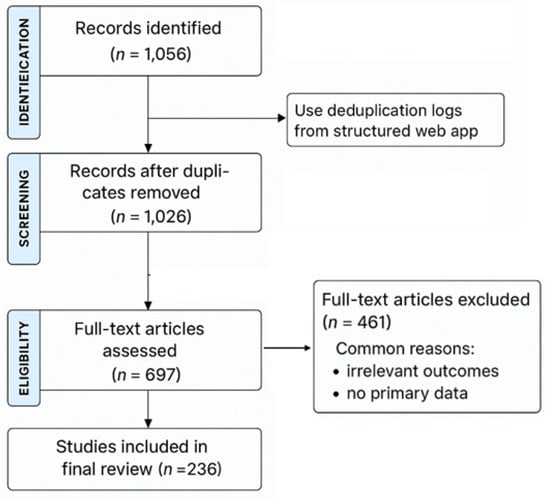
Figure 1.
A PRISMA flow diagram with the respective stages of selecting studies for inclusion/exclusion in the systematic review.
2.2. Data Extraction and Bias Assessment
A structured web application enabled standardized extraction of study characteristics, interventions, microglial biomarkers, neuroimmune pathways, and AUD-related behavioral outcomes. Two reviewers independently evaluated the risk of bias using validated tools, with discrepancies resolved through consensus.
This review utilized a systematic search strategy to synthesize evidence on microglial activation in AUD. The study is not registered on PROSPERO.
3. Results
3.1. Overview of Microglia and Neuroimmune Signaling
Microglia serve as CNS immune sentinels, performing surveillance synaptic pruning and responding to injury. They detect pathogens and damage-associated signals via pattern recognition receptors (PRRs) like Toll-like receptors (TLRs), triggering intracellular signaling cascades leading to inflammatory mediator release [1]. Activated microglia exhibits phenotypes beyond the conventional M1/M2 classification, dynamically modulating CNS immunity and neural activity. Transcriptome and single-cell studies have shown that microglial activation is highly heterogeneous, with M1 and M2 representing a continuum of activation states rather than discrete subtypes [3]. There is extensive overlap in gene expression, and microglia can shift phenotypes in response to environmental cues, ensuring functional plasticity [3,4]. This plasticity is critical for maintaining CNS homeostasis and mediating pathological processes such as neuroinflammation. Fine modulation of microglial activation is essential to prevent neurodegenerative diseases and maintain normal CNS function [3,4]. Their functional plasticity is critical in maintaining CNS homeostasis and mediating pathological processes such as neuroinflammation in AUD.
3.2. Ethanol-Induced Microglial Activation: Molecular Pathways
Ethanol induces neuroimmune activation predominantly via pattern recognition receptors (PRRs) expressed on microglia, with Toll-like receptor 4 (TLR4) playing a pivotal role [5,6]. Ethanol activates microglia via TLR4, NLRP3, and P2X7 pathways, releasing cytokines that disrupt AUD circuits (Figure 2). TLR4 detects damage-associated molecular patterns (DAMPs) triggered by ethanol-induced neuronal and glial stress, including endogenous molecules such as high mobility group box 1 protein (HMGB1) [7,8]. Activation of TLR4 initiates downstream signaling through MyD88-dependent and independent pathways, culminating in nuclear factor kappa B (NF-κB) translocation and transcription of proinflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) [6,9,10].
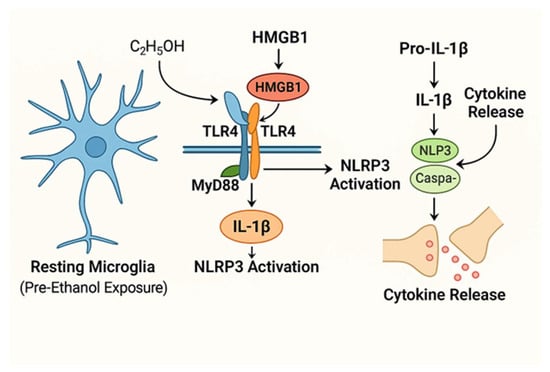
Figure 2.
Schematic representation of ethanol activates microglia via TLR4/NLRP3, driving cytokine release and synaptic disruption in AUD. Ethanol (C2H5OH) and HMGB1 activate TLR4 on microglia, initiating NF-κB signaling and releasing proinflammatory cytokines IL-1β, TNF-α, and IL-6. Concurrent activation of the NLRP3 inflammasome facilitates IL-1β maturation. Cytokines modulate synaptic transmission and plasticity, contributing to AUD-related neural circuit dysfunction.
Simultaneously, ethanol activates the nucleotide-binding domain and leucine-rich repeat pyrin domain-containing 3 (NLRP3) inflammasome complex in microglia, which cleaves pro-IL-1β into its active form, IL-1β, thereby amplifying neuroinflammation [11,12]. The released cytokines disrupt neuronal functions by altering neurotransmission, synaptic receptor trafficking, and neuroplasticity [13,14].
Peripheral immune activation is driven by increased gut permeability to bacterial endotoxins (e.g., lipopolysaccharide, LPS), further fueling central neuroinflammation through cytokine signaling across the blood–brain barrier, highlighting the gut–brain axis’s involvement in AUD [15,16,17]. Rodent models show that systemic LPS injection increases brain cytokines and promotes escalated alcohol intake, establishing a direct link between peripheral immune challenge and central microglial activation [18,19].
3.2.1. Toll-like Receptor 4 (TLR4)
TLR4 is a pattern recognition receptor expressed on microglia, playing a key role in ethanol-induced neuroimmune activation. Ethanol and its metabolites trigger the release of endogenous danger signals (e.g., HMGB1), which engage TLR4 and initiate NF-κB signaling. This results in transcriptional upregulation of proinflammatory cytokines (IL-1β, TNF-α, IL-6) that propagate neuroinflammation and disrupt synaptic function in addiction-related brain regions (hippocampus, ventral tegmental area, nucleus accumbens) [20].
Chronic ethanol exposure increases microglial TLR4 expression, amplifying NF-κB activity and inflammatory cytokine release. Genetic or pharmacological inhibition of TLR4 attenuates ethanol-induced neuroinflammation, demonstrating its role in AUD pathophysiology. However, regional and model-specific variations exist in TLR4’s effects [5,6,21].
By fostering a proinflammatory microglial phenotype, TLR4 signaling contributes to craving, withdrawal, and relapse vulnerability in AUD. Targeting TLR4-mediated neuroimmune pathways represents a promising therapeutic approach to mitigate alcohol-induced neuroinflammation and neural dysfunction [6,22,23].
3.2.2. NLRP3 Inflammasome
The NLRP3 inflammasome is a cytosolic protein complex crucial for the maturation of IL-1β and IL-18 via caspase-1 activation [24]. Chronic ethanol exposure potentiates TLR4-driven NLRP3 activation in microglia, further amplifying neuroinflammatory cascades [25].
IL-1β release through NLRP3 signaling modulates synaptic function and contributes to neurodegeneration in AUD, reinforcing ethanol-driven neuroimmune dysregulation [26].
3.2.3. Purinergic Signaling Via P2RY12
Beyond TLR4 and NLRP3, purinergic signaling is critical in microglial activation and heterogeneity in AUD. P2RY12, a G-protein-coupled purinergic receptor, is predominantly expressed on homeostatic microglia, regulating their surveillance and motility. Unlike TLR4-driven inflammatory activation, P2RY12 signaling is associated with microglial responses to neuronal distress rather than overt neuroinflammation [27,28].
Chronic ethanol exposure downregulates P2RY12 expression, shifting microglia toward a proinflammatory phenotype. This loss of P2RY12 function impairs microglial–neuronal interactions, reducing their ability to maintain synaptic integrity and exacerbating neuroimmune dysregulation.
Sex-Specific Differences in P2RY12 Expression and Microglial Responses to Ethanol
P2RY12 is a microglial signature gene that mediates microglial-neuronal interactions and is critical in maintaining microglial homeostasis [28,29].
Research demonstrates significant sex-specific differences in P2RY12 expression, with adult female mice exhibiting higher P2RY12 protein levels in microglia than males. Hormonal fluctuations influence these differences and are particularly pronounced during specific phases of the estrus cycle in females [28].
P2RY12 deficiency leads to sex-specific cellular and behavioral perturbations: Female mice lacking P2RY12 show more pronounced microglial morphological changes and behavioral anomalies than males, suggesting that microglial responses to insults such as ethanol may vary by sex [28].
Influence on AUD Susceptibility
Microglial responses to ethanol are implicated in alcohol use disorder (AUD) pathophysiology. Ethanol exposure triggers microglial activation, leading to morphological changes and heightened immune responses, with evidence that genetic background (including sex differences) modulates these responses and influences AUD susceptibility [30].
Transcriptomic analyses reveal that ethanol exposure alters the expression of microglial genes, including those associated with homeostatic states such as P2RY12, and these changes can be sex-dependent [30,31].
P2RY12 as a Neuroprotective Target
Targeting P2RY12-mediated pathways may offer neuroprotection: P2RY12 is essential for microglial process motility and their ability to respond to injury or inflammation. Its activity is associated with anti-inflammatory and neuroprotective effects in the central nervous system [27].
Studies show that ethanol exposure induces proinflammatory gene expression in microglia, but maintaining or enhancing P2RY12 signaling helps preserve microglial homeostasis and may mitigate ethanol-induced neuroinflammation [32].
Therapeutic strategies that modulate P2RY12 activity could potentially reduce neuroinflammatory responses in AUD and other neuroimmune conditions, highlighting its value as a pharmacological target [27,32].
3.2.4. Microglial Activation: Cause or Consequence of AUD?
A key unresolved question in AUD research is whether microglial activation is a driver or a secondary consequence of disease progression. Longitudinal studies using adolescent exposure models provide insights into this relationship.
Adolescent binge drinking induces persistent microglial activation, characterized by increased HMGB1-TLR4 signaling and neuroimmune priming [33,34,35]. This early neuroimmune dysregulation predisposes individuals to heightened neuroinflammatory responses in adulthood, suggesting that microglial activation may be an initiating factor rather than a mere consequence of chronic alcohol exposure [33,36].
Additionally, studies on repetitive mild traumatic brain injury (RmTBI) in adolescents highlight how microglial activation during critical developmental windows modifies long-term neuroimmune trajectories [37,38,39]. These findings parallel AUD-related neuroimmune alterations, reinforcing the hypothesis that early microglial priming contributes to AUD pathophysiology rather than simply responding to ethanol-induced damage.
Future research should focus on longitudinal imaging and biomarker studies to further delineate microglial activation dynamics across AUD stages.
3.3. Neuroimmune Modulation of Synaptic Plasticity and Neural Circuits
Microglial cytokines affect synaptic transmission by regulating receptor trafficking and neurotransmitter release. TNF-α promotes AMPA receptor insertion and GABAA receptor internalization, altering the excitation-inhibition balance. IL-1β modulates long-term potentiation mechanisms critical for learning and addiction-related plasticity. These neuroimmune effects impact key brain regions involved in reward (ventral tegmental area [VTA], nucleus accumbens [NAc]), stress (amygdala), and negative affect (lateral habenula [LHb]), contributing to craving, withdrawal, and relapse. Microglial cytokines disrupt VTA-NAc, amygdala-LHb-BNST, PFC, and hippocampal circuits, driving AUD pathology (Figure 3).
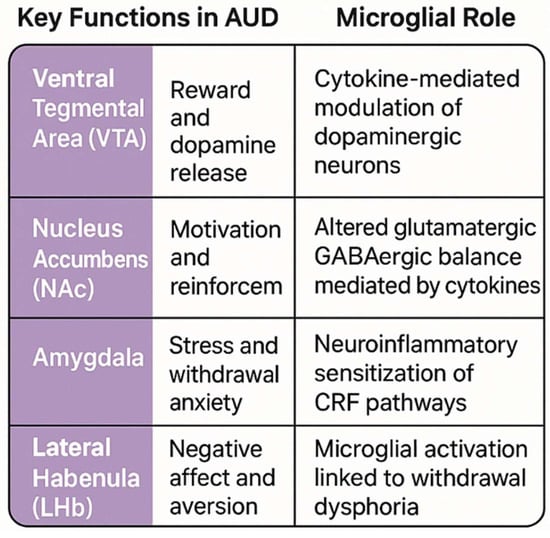
Figure 3.
Microglial activation disrupts key AUD circuits. In the VTA-NAc, IL-1β and TNF-α enhance dopamine signaling, driving craving. In the amygdala-LHb, IL-6 and HMGB1 amplify stress responses, promoting withdrawal anxiety. In the PFC, TNF-α and complement-mediated pruning impair executive control, increasing impulsivity. These circuits interact, sustaining AUD pathology.
Microglia influence addiction-related behaviors by modulating synaptic function through soluble factors and neuron-glia interactions [28,40,41,42,43,44,45]. Cytokines released by activated microglia regulate glutamatergic and GABAergic transmission in reward-related circuits [46,47]. For instance, TNF-α promotes endocytosis of GABAA receptors while enhancing surface expression of AMPA-type glutamate receptors, tipping the excitatory/inhibitory balance towards hyperexcitability.
In the VTA, microglial activation indirectly alters dopaminergic neuron firing patterns via cytokine-mediated receptor modulation, contributing to dysfunction of the mesolimbic reward system that underlies craving and compulsive alcohol seeking [16,48]. The LHb, critical in processing aversive stimuli and negative affect, exhibits microglial-driven neuroinflammation that heightens withdrawal-induced anxiety and dysphoria, thereby promoting relapses.
Neuroimmune factors also impact neurogenesis and neuronal survival in regions such as the hippocampus, affecting cognitive function and stress response regulation crucial in AUD pathology [49,50]. By releasing chemokines like CXCL12, microglia influence neuronal migration and circuit remodeling, which are altered in chronic alcohol exposure [16]. As microglial responses are highly plastic, priming by repeated ethanol exposure sensitizes these cells, exacerbating inflammatory responses and behavioral impairments during the withdrawal and relapse phases.
3.4. Microglial Priming and Behavioral Consequences in Rodent Models
Repeated ethanol exposure “primes” microglia, making them hyper-responsive to subsequent immune stimuli [51]. Rodent models reveal that priming leads to exaggerated neuroimmune responses, dopamine system dysregulation, impulsiveness, and anxiety-like behaviors that model addiction vulnerability [52]. Behavioral studies also demonstrate that microglial inhibition reduces ethanol consumption and withdrawal-related anxiety, underscoring microglial contributions to AUD progression.
3.5. Evidence from Human Studies: Postmortem and Neuroimaging Findings
Human postmortem brains from individuals with AUD show elevated microglial markers such as IBA1, CD11b, and MCP-1, alongside morphological activation indicative of chronic neuroinflammation [7,53,54].
Current neuroimmune imaging tools, including TSPO PET ligands, have key limitations. TSPO is not exclusive to microglia and is expressed in other brain cells, leading to non-specific signals. Genetic variability in TSPO expression also affects ligand binding and complicates individual comparisons. Additionally, many ligands show non-specific binding, reducing accuracy. While new imaging targets are being explored, challenges with specificity and validation remain, highlighting the need for more precise tools to assess neuroinflammation in AUD [55,56]
3.6. Sex Differences in Microglial Responses and AUD
Sex differences significantly influence microglial responses to ethanol, potentially shaping vulnerability to AUD (Table 1). Females exhibit higher microglial density and morphological activation, especially in limbic regions such as the hippocampus and amygdala, compared to males [17,26,57,58,59,60,61,62]. Moreover, females demonstrate heightened proinflammatory cytokine expression [63] and enhanced microglial reactivity to stressors, factors associated with increased susceptibility to stress-induced drinking and anxiety-like behaviors [60,62,64]. Transcript studies reveal distinct microglial gene-expression patterns between sexes, suggesting potential differences in microglial regulation and neural remodeling during alcohol exposure [65,66]. Female microglia exhibit increased excitatory synapse pruning, leading to altered neural circuit remodeling in addiction-relevant brain regions [59,64,67]. These neuroimmune sex differences correlate with clinical observations where females show increased stress-induced alcohol consumption, heightened anxiety-like behaviors, and impulsivity [68].

Table 1.
Sex Differences in Microglial Activation Correlate with AUD Vulnerability.
Hormonal Modulation of Microglial Priming
Sex hormones such as estrogen and progesterone are pivotal in modulating microglial activation and inflammatory signaling [69], contributing to sex-specific neuroimmune responses in AUD. Estrogen exhibits both anti-inflammatory and proinflammatory effects depending on its concentration and the specific receptor subtypes engaged [69]. At physiological levels, estrogen tends to suppress microglial activation by inhibiting NF-κB signaling and downregulating the production of proinflammatory cytokines [70]. However, during periods of hormonal fluctuation—such as menopause or stress—estrogen may paradoxically enhance microglial reactivity, thereby exacerbating neuroinflammatory responses to ethanol exposure [71].
Progesterone, in contrast, generally exerts immunosuppressive effects on microglia [72]. It reduces microglial priming by dampening the expression of inflammatory cytokines, including TNF-α, IL-1β, and IL-6, while promoting a homeostatic microglial phenotype [73]. Chronic alcohol exposure may interfere with progesterone signaling, particularly in females, leading to sustained microglial sensitization and heightened vulnerability to neuroimmune dysregulation [59].
Beyond their direct effects on microglia, estrogen and progesterone also influence microglial-neuronal crosstalk [66]. These hormones modulate synaptic plasticity, neuroprotective mechanisms, and neurotransmitter homeostasis, all of which are critical to maintaining neural integrity in the context of alcohol exposure [59,74]. Dysregulation of these hormonal pathways may underlie sex-specific differences in AUD susceptibility and the progression of alcohol-induced neurodegeneration.
These findings underscore the importance of incorporating sex-specific strategies into AUD treatment paradigms. Targeting estrogen receptor pathways may help mitigate microglial-driven neuroinflammation in females, while progesterone-based interventions offer neuroimmune protection against ethanol-induced sensitization. Furthermore, integrating hormonal status into biomarker development may enhance precision medicine approaches for AUD, enabling more tailored and effective interventions.
3.7. Therapeutic Interventions Targeting Microglial Activation in AUD
3.7.1. Preclinical and Emerging Clinical Evidence
Given microglia’s integral role in AUD pathology, pharmacological agents targeting microglial activation represent promising therapeutic candidates (Table 2).
- Minocycline: An antibiotic with anti-inflammatory properties that inhibits microglial activation and reduces proinflammatory cytokine release in rodent AUD models, reducing ethanol intake and anxiety-like behaviors [43,75].
- Ibudilast: A phosphodiesterase inhibitor and glial modulator, suppresses microglial proinflammatory signaling and attenuates relapse-like drinking behavior in multiple animal models. Clinical trials report decreases in heavy drinking days and alcohol cravings in AUD patients receiving ibudilast treatment, though larger randomized controlled trials are warranted [25]. The results found that the medication reduced craving but did not reduce alcohol use on the primary drinking outcome. Follow-up analyses identified possible sex-dependent effects, whereby females with alcohol use disorder showed a more beneficial response to ibudilast versus placebo and compared to male participants. Additional analyses are underway to test biomarkers and sex effects.
- Glucagon-like peptide-1 (GLP-1) receptor agonists: Initially developed to treat metabolic disorders, they demonstrate therapeutic potential for reducing alcohol-seeking behavior through dual modulation of neuroimmune pathways and reward circuitry. By suppressing microglial activation and curbing proinflammatory cytokine release, these agents target mechanisms implicated in AUD. Growing clinical interest in their application stems from their established safety profile and unique capacity to address metabolic dysfunction and neuroimmune dysregulation—key factors often intertwined in AUD pathophysiology [17].
- P2X7 Receptor Antagonists: By blocking ATP-gated ion channel-mediated microglial activation, these agents prevent the activation of the NLRP3 inflammasome and subsequent cytokine release, thereby mitigating neuroinflammation-related behavioral pathology in AUD [17].

Table 2.
Pharmacological Agents Targeting Microglial Activation in AUD.
Table 2.
Pharmacological Agents Targeting Microglial Activation in AUD.
| Agent | Mechanism | Preclinical Evidence | Clinical Evidence |
|---|---|---|---|
| Minocycline | Microglial activation inhibitor | Reduces ethanol intake withdrawal anxiety [76] | Limited trials; promising |
| Ibudilast | Phosphodiesterase inhibitor and glial modulator | Decreases alcohol consumption and craving [25] | Early clinical trials showed reduced heavy drinking [29,77] |
| GLP-1 Agonists | Modulates reward and inflammation | Reduces alcohol seeking | Clinical trials are ongoing; early positive |
| P2X7 Antagonists | Blocks ATP-induced inflammasome activation | Attenuates IL-1β release | Preclinical; human studies pending |
Therapeutics targeting microglial activation in AUD. Established agents (minocycline, ibudilast) and emerging drugs (semaglutide, P2X7 antagonists) show preclinical and clinical promise, while novel approaches (nanoparticles, probiotics) remain exploratory. Stages reflect current development as of 2025, with challenges in specificity and scalability.
3.7.2. Mechanistic Insights: GLP-1 Receptor Agonists
GLP-1 receptor agonists (GLP-1RAs) modulate microglial activity to reduce alcohol-seeking primarily by shifting microglia from a proinflammatory, neurotoxic state (often referred to as the M1 phenotype) toward an anti-inflammatory, neuroprotective state (the M2 phenotype), thereby attenuating neuroinflammation that contributes to AUD pathology [78,79].
Mechanisms of GLP-1R Agonists in Modulating Microglial Activity
a. Activation of GLP-1 Receptors on Microglia
GLP-1 receptors (GLP-1R) are expressed on microglia, neurons, and astrocytes within the central nervous system. Activation of these receptors by GLP-1RAs such as exendin-4 (Ex-4) leads to signaling cascades that influence microglial polarization and inflammatory responses [3,80].
b. Shift from Proinflammatory M1 to Anti-inflammatory M2 Microglial Phenotype
GLP-1R activation decreases markers of proinflammatory M1 microglia (e.g., inducible nitric oxide synthase [iNOS], cyclooxygenase-2 [COX-2], TNF-α, and IL-1β while increasing markers associated with M2 microglia, such as CD206 [3,81]. This phenotypic switch reduces the release of proinflammatory cytokines and reactive astrocyte activation, known contributors to neuroinflammation and neural damage that reinforce alcohol craving and seeking [3].
c. Involvement of PI3K/ARAP3/RhoA Signaling Pathway
Molecular studies using RNA sequencing and genetic knockdown have identified that GLP-1RA-induced microglial anti-inflammatory effects are mediated through upregulation of ARAP3 (ArfGAP with RhoGAP domain, Ankyrin repeat, and PH domain 3), a GTPase-activating protein that negatively regulates RhoA (Ras homolog family member A) activation. Activation of GLP-1R signaling increases ARAP3 expression, which inhibits RhoA, a proinflammatory pathway regulator, thus suppressing microglial inflammation [3]. Knockdown of ARAP3 reverses the anti-inflammatory and microglial polarization effects of GLP-1RAs, highlighting its significant role [3].
d. Inhibition of Microglia-Astrocyte Crosstalk
Proinflammatory microglia triggers reactive astrogliosis, exacerbating neuroinflammation and neuronal damage. GLP-1R agonists reduce microglial secretion of astrocyte-activating cytokines such as TNF-α and IL-1α, diminishing astrocyte activation and migration in co-culture models [3]. This interrupts the feed-forward neuroinflammatory cascade supporting alcohol-related neurocircuit dysfunction.
e. Reduction in Proinflammatory Cytokines in Brain Tissue
In vivo administration of GLP-1RAs in models such as spinal cord injury shows reductions in brain and spinal cord levels of TNF-α, IL-1β, and IL-6, consistent with attenuation of neuroinflammation via microglial modulation [3,82,83]. Although spinal cord injury models differ from AUD, similar neuroimmune pathways underpin addiction-related neuroinflammation.
Relation to Alcohol-Seeking Behavior
Alcohol use leads to microglial activation and neuroinflammation in reward- and stress-related brain regions, which enhances alcohol craving and relapse vulnerability. By attenuating microglial proinflammatory activation and promoting a neuroprotective phenotype, GLP-1RAs mitigate neuroinflammation-associated neural circuit dysfunction, thereby reducing alcohol-seeking behavior [84]. Preclinical studies in rodents demonstrate that GLP-1RAs reduce alcohol intake and alcohol-induced activation of dopaminergic reward pathways, likely mediated by these anti-inflammatory and neuromodulatory effects on microglia and neuronal circuits [84,85]. Clinical trials are beginning to confirm that GLP-1RAs, such as semaglutide, decrease alcohol craving and some drinking behaviors in adults with AUD, supporting the translational potential of microglial modulation via GLP-1RAs in alcohol addiction treatment [5].
Challenges in Clinical Translation
Despite promising preclinical findings, several clinical translation barriers hinder the development of neuroimmune-targeted therapies for AUD.
Blood–Brain Barrier (BBB) Penetration of P2X7 Antagonists
P2X7 receptor antagonists, such as JNJ-47965567, have demonstrated central nervous system (CNS) penetration, effectively reducing IL-1β release and neuroinflammation. However, BBB permeability remains challenging for many P2X7-targeting compounds, as high molecular weight and poor lipophilicity can limit CNS bioavailability. Additionally, species differences in P2X7 receptor pharmacodynamics complicate direct translation from animal models to human applications [86,87,88].
Off-Target Effects of Minocycline
Minocycline, a tetracycline antibiotic with anti-inflammatory properties, inhibits microglial activation and reduces ethanol-induced neuroinflammation. However, its broad immunosuppressive effects raise concerns about off-target consequences, including gut microbiome disruption, mitochondrial toxicity, and unintended neuronal suppression [10,76,89]. These effects may limit its long-term therapeutic viability, necessitating targeted delivery strategies or combination approaches to mitigate adverse outcomes.
Combination Therapies: GLP-1 Agonists and Naltrexone
The co-administration of GLP-1 receptor agonists—such as semaglutide or liraglutide—with naltrexone, an opioid receptor antagonist, offers a promising avenue for synergistic intervention in AUD. GLP-1 agonists have been shown to modulate dopaminergic reward pathways, thereby reducing alcohol-seeking behaviors and diminishing the reinforcing properties of ethanol [59,78,90]. Naltrexone complements this mechanism by blocking opioid-mediated reinforcement, which further decreases craving and lowers the risk of relapse [91].
Together, these agents may exert additive or synergistic effects on neuroimmune modulation. This combination could address both the neurobiological and behavioral dimensions of AUD by attenuating microglial-driven neuroinflammation and stabilizing reward circuit dysregulation. Such dual-action therapy holds promise for individuals with heightened neuroimmune sensitivity or those who exhibit resistance to monotherapies [92].
However, the therapeutic potential of this combination must be weighed against several risks [93]. Gastrointestinal side effects, including nausea and diarrhea—are more pronounced when these agents are used together. Additionally, metabolic interactions may impact glucose homeostasis, necessitating vigilant monitoring in patients with diabetes or other metabolic disorders [94]. The long-term efficacy and safety profile of this approach remains to be fully elucidated, underscoring the need for rigorous clinical trials to determine optimal dosing strategies and treatment durability.
Future Directions
Optimizing BBB-permeable P2X7 antagonists, refining targeted microglial modulation, and evaluating combination therapies in longitudinal human studies will be critical for advancing neuroimmune-based AUD interventions.
3.8. Emerging Technologies in Neuroimmune Research of AUD
3.8.1. Multi-Omics
Integration of transcriptomic, proteomic, and epigenomic data has identified key gene networks and microglial phenotypes associated with AUD, enhancing biomarker discovery and therapeutic targeting [8,28,95].
3.8.2. Molecular Imaging
Advances in PET imaging with novel microglial-specific ligands, including CX3CR1 and next-generation TSPO, allow in vivo assessment of microglial activation states, aiding patient stratification and treatment monitoring [55,56].
3.9. Comorbidities Share Underlying Neuroimmune Mechanisms
Comorbidities such as depression and PTSD frequently occur alongside AUD, and research suggests they may share underlying neuroimmune mechanisms. In both depression and PTSD, as with AUD, there is evidence of increased neuroinflammation and altered immune signaling, such as elevated proinflammatory cytokines and reduced neurotrophic factors like BDNF. These shared neuroimmune changes may contribute to the development and persistence of all three conditions, helping to explain their frequent co-occurrence. Understanding these overlapping pathways could inform new treatment strategies targeting neuroimmune dysfunction in individuals with AUD and comorbid psychiatric disorders [29,57,96].
3.10. Challenges, Knowledge Gaps, and Future Directions
Despite promising findings, several challenges remain:
- Multi-Omics and Biomarker Discovery
Integrating transcriptomic, proteomic, and epigenomic data from human postmortem and animal model microglia provides enriched molecular signatures of AUD-related neuroimmune alterations, facilitating the identification of actionable therapeutic targets and biomarkers [29,97]. For example, multi-omics analyses have revealed dysregulation in the microglial expression of TREM2 and PU.1, transcription factors linked to innate immunity and neurodegeneration that may contribute to AUD neuropathology [53]. Single-cell multi-omics methods coupled with spatial transcriptomics enable detailed depictions of microglial heterogeneity in AUD, highlighting discrete subpopulations with distinct functional profiles amenable to specific interventions [28].
- Advanced Molecular Imaging
Developing and applying novel PET ligands selective for microglial surface markers (e.g., CX3CR1) promise enhanced specificity in visualizing microglial activation and functional states in vivo [95,98]. Such tools would afford longitudinal monitoring of neuroinflammation, stratification of patients, and assessment of therapeutic response. Combining TSPO PET with complementary imaging modalities and fluid biomarkers (e.g., cytokines, microRNAs in CSF, and blood) may establish multi-dimensional profiling approaches essential for clinical translation [98].
- Inclusive Clinical Trials and Sex-Dependent Treatment Approaches
Future clinical studies must incorporate sex and age stratification alongside the characterization of comorbid psychiatric and medical conditions that modulate neuroimmune responses [59]. Trials testing microglial modulators should evaluate not just consumption metrics but also neuroimmune biomarker dynamics and neuroimaging endpoints to elucidate mechanisms. Moreover, elucidating the impact of adolescent alcohol exposure on microglial development and priming remains critical, as early-life microglial activation may predispose to later AUD vulnerability [95].
- Integration with Established Pharmacotherapies
Microglial-targeted treatments should be seen as complementary to FDA-approved medications like naltrexone and acamprosate, which target neurotransmitter systems implicated in reward and withdrawal pathways. Combining neuroimmune modulation with these agents could enhance efficacy and reduce relapses by simultaneously addressing neurochemical and inflammatory pathologies. Systems biology approaches integrating genomic, immunologic, behavioral, and environmental data will be indispensable to unraveling AUD’s complex interfacing mechanisms and designing multi-target therapies [17]. Important gaps include limited longitudinal human studies, insufficient biomarkers to discriminate microglial activation states, and a need for more sex- and age-inclusive clinical trials. Refining imaging modalities and expanding multi-omics will accelerate personalized medicine development for AUD [26,99].
- Challenges and Future Directions in Therapeutic Development
Despite encouraging preclinical data, clinical translation faces hurdles, including limited longitudinal human studies, lack of robust biomarkers for microglial activation states, and insufficient understanding of individual differences due to sex, developmental stage, and comorbidities [57]. Additionally, the heterogeneity of AUD pathophysiology necessitates precision targeting and patient stratification.
Future studies should integrate multi-omics approaches—transcriptomics, proteomics, and metabolomics—to delineate microglial phenotypes and signaling pathways specific to AUD stages and subtypes [57]. Advanced molecular imaging methods such as TSPO PET ligands and emerging CX3CR1-based probes will enhance the in vivo characterization of microglial dynamics [57]. Inclusive clinical trials incorporating sex and age stratification alongside biomarker development are imperative to optimize therapeutic efficacy.
- Integration with Existing Pharmacotherapies and Systems: A Biomedical Approach
Microglial modulation should be viewed as complementary to established AUD pharmacotherapies like naltrexone and acamprosate, which primarily target neurotransmitter systems. Given the entangled nature of neuroimmune and neurochemical alterations in AUD, an integrated systems biomedicine framework encompassing genetic, epigenetic, immunological, and environmental contributors will enhance mechanistic understanding and therapeutic development [57].
4. Discussion
The review highlights the crucial role of microglial activation and neuroimmune signaling in the pathophysiology of alcohol use disorder (AUD), integrating molecular, cellular, and behavioral evidence to deepen understanding of how ethanol-induced microglial changes contribute to craving, withdrawal, and relapse. By elucidating key mechanisms such as TLR4 and NLRP3 inflammasome activation and detailing how GLP-1 receptor agonists modulate microglial phenotype to reduce neuroinflammation and alcohol seeking, the review reinforces the therapeutic potential of targeting neuroimmune pathways. Furthermore, recognizing sex differences in microglial responses and the emerging multi-omics and imaging technologies accentuates the complexity and clinical relevance of personalized approaches to AUD treatment. These insights pave the way for developing more effective, mechanism-based interventions that complement existing pharmacotherapies and may significantly improve outcomes for individuals battling AUD.
While AUD research focuses primarily on microglia due to their central role in neuroinflammation, other immune-related cells, such as astrocytes and peripheral monocytes, also contribute to alcohol-induced brain pathology. Astrocytes, for example, show structural and molecular alterations in response to chronic alcohol exposure, affecting neurotransmission, neuroinflammation, and blood–brain barrier function [100,101]. Peripheral monocytes can infiltrate the brain and interact with resident microglia, further influencing neuroimmune responses. However, most current imaging and postmortem studies emphasize microglial markers, with less focus on these alternative cell types.
5. Limitations
This review’s findings must be interpreted in light of several key constraints. First, reliance on English-language studies from select databases risks publication and language bias, as non-English publications and unpublished negative results—such as failed TLR4 or P2X7-targeted interventions—may be underrepresented. This omission could skew perceptions of therapeutic potential.
Second, translational relevance is limited by species-specific differences in neuroimmune responses. For example, rodent models—the primary source of preclinical data—exhibit distinct microglial activation patterns, ethanol metabolism, and TLR4/cytokine signaling compared to primates. Such disparities may compromise the applicability of rodent-derived insights to human AUD pathophysiology.
Third, methodological heterogeneity across studies—including variations in experimental models, dosing protocols, and outcome measures—hinders direct comparisons and clinical generalizability. While preclinical findings clarify mechanistic pathways, they often oversimplify the complexity of microglial dynamics in human AUD.
Finally, technological gaps impede cohesive analysis. Current neuroimmune biomarkers lack specificity, and emerging tools like CX3CR1 PET ligands remain underdeveloped for clinical use, limiting real-time assessment of microglial activation in AUD-related neuroinflammation.
6. Future Research Priorities
Advancing neuroimmune-targeted interventions for alcohol use disorder (AUD) requires addressing critical translational gaps in biomarker development, sex-specific treatment responses, and human validation. Emerging PET ligands, such as CX3CR1-targeted tracers, offer improved specificity over conventional TSPO-based imaging, enabling more nuanced assessment of microglial phenotypes across the AUD continuum. Longitudinal PET studies are essential to map neuroimmune shifts from early alcohol exposure to chronic dependence, track therapeutic modulation of microglial dynamics, and validate these next-generation biomarkers in diverse populations. Concurrently, sex-stratified clinical trials are critical for optimizing neuroimmune therapies, given well-established differences in microglial density, cytokine signaling, and hormonal regulation. Trials investigating agents such as minocycline, ibudilast, and GLP-1 receptor agonists have shown promise in modulating neuroinflammation and reducing alcohol intake, with emerging evidence of sex-dependent efficacy [78,79]. For instance, GLP-1 receptor agonists like semaglutide and liraglutide have demonstrated reductions in alcohol consumption and relapse risk in both preclinical and clinical settings [78]. At the same time, sex-specific microglial responses to CSF1R inhibition suggest differential therapeutic windows. Integrating neuroimaging advances with personalized, sex-informed therapeutic strategies will accelerate clinical translation and enhance precision medicine approaches for AUD.
7. Conclusions
Microglia-mediated neuroimmune signaling is a mechanistic cornerstone in AUD pathophysiology, exerting profound effects on neural circuits governing reward, stress, and executive function. Ethanol-induced activation of TLR4 and NLRP3 inflammasome pathways leads to a sustained inflammatory state in the brain, driving behavioral impairments central to addiction chronicity and relapse. Sex differences in microglial responses further shape AUD vulnerability and treatment outcomes.
Pharmacological strategies targeting microglial activation present promising avenues, though clinical translation requires the resolution of critical gaps through longitudinal, multi-omics, and advanced imaging studies embedded within inclusive trial designs. Integrating neuroimmune modulation with existing AUD therapies aligns with evolving precision medicine paradigms and offers the transformative potential to disrupt AUD’s vicious neuroinflammatory cycle.
By advancing the understanding of microglial biology in AUD and bridging translational gaps, the field moves closer to novel, targeted therapeutics, thereby supporting Psychoactives’ mission to enhance addiction neuroscience and improve outcomes for millions afflicted by AUD worldwide.
Author Contributions
Conceptualization, J.-H.Y.; Visualization, J.-H.Y. and W.Z.; Writing and editing, J.-H.Y., F.C. and L.C.; writing—original draft, J.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ray, L.A.; Bujarski, S.; Shoptaw, S.; Roche, D.J.; Heinzerling, K.; Miotto, K. Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled, Human Laboratory Trial. Neuropsychopharmacology 2017, 42, 1776–1788. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, H.; Xia, M.; Chang, J.; Li, X.; Ye, S.; Wu, S.; Jiang, S.; Bao, J.; Wang, B.; et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int. J. Biol. Sci. 2022, 18, 1328–1346. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, H.; Yan, A.; Yin, F.; Qiao, X. DNA Methylation in Alcohol Use Disorder. Int. J. Mol. Sci. 2023, 24, 10130. [Google Scholar] [CrossRef]
- Fernández-Lizarbe, S.; Pascual, M.; Guerri, C. Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol1. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef] [PubMed]
- Holloway, K.; Douglas, J.; Rafferty, T.; Kane, C.; Drew, P. Ethanol Induces Neuroinflammation in a Chronic Plus Binge Mouse Model of Alcohol Use Disorder via TLR4 and MyD88-Dependent Signaling. Cells 2023, 12, 2109. [Google Scholar] [CrossRef]
- Walter, T.J.; Crews, F.T. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflamm. 2017, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Crews, F. Release of Neuronal HMGB1 by Ethanol through Decreased HDAC Activity Activates Brain Neuroimmune Signaling. PLoS ONE 2014, 9, e87915. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, X.; Xu, M.; Frank, J.A.; Luo, J. Minocycline attenuates ethanol-induced cell death and microglial activation in the developing spinal cord. Alcohol 2019, 79, 25–35. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Ureña-Peralta, J.; Morillo-Bargues, M.; Gómez-Pinedo, U.; Guerri, C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem. Res. 2016, 41, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef] [PubMed]
- Zipp, F.; Bittner, S.; Schafer, D. Cytokines as emerging regulators of central nervous system synapses. Immunity 2023, 56, 914–925. [Google Scholar] [CrossRef]
- Stück, E.; Christensen, R.; Huie, J.; Tovar, C.; Miller, B.; Nout, Y.; Bresnahan, J.; Beattie, M.; Ferguson, A. Tumor Necrosis Factor Alpha Mediates GABAA Receptor Trafficking to the Plasma Membrane of Spinal Cord Neurons In Vivo. Neural Plast. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Kearns, R. Gut–Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders. Cell. Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef]
- Namba, M.D.; Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F.; Neisewander, J.L. Neuroimmune Mechanisms as Novel Treatment Targets for Substance Use Disorders and Associated Comorbidities. Front. Neurosci. 2021, 15, 650785. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Arnold, M.; McConnell, K.; Solomon, M.; Amico, K.; Schank, J. The effects of lipopolysaccharide exposure on social interaction, cytokine expression, and alcohol consumption in male and female mice. Physiol. Behav. 2023, 265, 114159. [Google Scholar] [CrossRef]
- Singh, A.K.; Jiang, Y.; Gupta, S.; Benlhabib, E. Effects of chronic ethanol drinking on the blood brain barrier and ensuing neuronal toxicity in alcohol-preferring rats subjected to intraperitoneal LPS injection. Alcohol Alcohol. 2007, 42, 385–399. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Montagud-Romero, S.; Forteza, J.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J. Neuroinflamm. 2017, 14, 145. [Google Scholar] [CrossRef]
- Lawrimore, C.J.; Crews, F.T. Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol Clin. Exp. Res. 2017, 41, 939–954. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Wang, C.; Liu, Y.; You, J.; Wang, P.; Xu, G.; Shen, H.; Yao, H.; Lan, X.; et al. Chronic ethanol exposure induces neuroinflammation in H4 cells through TLR3/NF-κB pathway and anxiety-like behavior in male C57BL/6 mice. Toxicology 2020, 446, 152625. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.; Nabavi, S. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Meredith, L.R.; Grodin, E.N.; Montoya, A.K.; Miranda, R., Jr.; Squeglia, L.M.; Towns, B.; Evans, C.; Ray, L.A. The effect of neuroimmune modulation on subjective response to alcohol in the natural environment. Alcohol Clin. Exp. Res. 2022, 46, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Grodin, E.N.; Bujarski, S.; Towns, B.; Burnette, E.; Nieto, S.; Lim, A.; Lin, J.; Miotto, K.; Gillis, A.; Irwin, M.R.; et al. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: A randomized trial. Transl. Psychiatry 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Moura, H.F.; Hansen, F.; Galland, F.; Silvelo, D.; Rebelatto, F.P.; Ornell, F.; Massuda, R.; Scherer, J.N.; Schuch, F.; Kessler, F.H.; et al. Inflammatory cytokines and alcohol use disorder: Systematic review and meta-analysis. Braz. J. Psychiatry 2022, 44, 548–556. [Google Scholar] [CrossRef]
- Lou, N.; Takano, T.; Pei, Y.; Xavier, A.L.; Goldman, S.A.; Nedergaard, M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood–brain barrier. Proc. Natl. Acad. Sci. USA 2016, 113, 1074–1079. [Google Scholar] [CrossRef]
- Uweru, O.J.; Okojie, A.K.; Trivedi, A.; Benderoth, J.; Thomas, L.S.; Davidson, G.; Cox, K.; Eyo, U.B. A P2RY12 deficiency results in sex-specific cellular perturbations and sexually dimorphic behavioral anomalies. J. Neuroinflamm. 2024, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.K.; Grantham, E.K.; Warden, A.S.; Harris, R.A. Neuroimmune signaling in alcohol use disorder. Pharmacol. Biochem. Behav. 2019, 177, 34–60. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Boreland, A.J.; Kapadia, S.; Zhang, S.; Stillitano, A.C.; Abbo, Y.; Clark, L.; Lai, D.; Liu, Y.; et al. Polygenic risk for alcohol use disorder affects cellular responses to ethanol exposure in a human microglial cell model. Sci. Adv. 2024, 10, eado5820. [Google Scholar] [CrossRef]
- Liu, W.; Vetreno, R.P.; Crews, F.T. Hippocampal TNF-death receptors, caspase cell death cascades, and IL-8 in alcohol use disorder. Mol. Psychiatry 2021, 26, 2254–2262. [Google Scholar] [CrossRef]
- Iring, A.; Tóth, A.; Baranyi, M.; Otrokocsi, L.; Módis, L.V.; Gölöncsér, F.; Varga, B.; Hortobágyi, T.; Bereczki, D.; Dénes, Á.; et al. The dualistic role of the purinergic P2Y12-receptor in an in vivo model of Parkinson’s disease: Signalling pathway and novel therapeutic targets. Pharmacol. Res. 2022, 176, 106045. [Google Scholar] [CrossRef]
- Crews, F.T.; Fisher, R.P.; Qin, L.; Vetreno, R.P. HMGB1 neuroimmune signaling and REST-G9a gene repression contribute to ethanol-induced reversible suppression of the cholinergic neuron phenotype. Mol. Psychiatry 2023, 28, 5159–5172. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P.; Matheny, L.; Ankeny, S.; Qin, L.; Coleman, L.G.; Vetreno, R.P. Adolescent binge alcohol exposure accelerates Alzheimer’s disease-associated basal forebrain neuropathology through proinflammatory HMGB1 signaling. Front. Aging Neurosci. 2025, 17, 1531628. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Crews, F.T. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience 2012, 226, 475–488. [Google Scholar] [CrossRef]
- Nwachukwu, K.N.; Mohammed, H.E.; Mebane, D.R.; Barber, A.W.; Swartzwelder, H.S.; Marshall, S.A. Acute and Chronic Ethanol Effects during Adolescence on Neuroimmune Responses: Consequences and Potential Pharmacologic Interventions. Cells 2023, 12, 1423. [Google Scholar] [CrossRef]
- Fidan, E.; Lewis, J.; Kline, A.E.; Garman, R.H.; Alexander, H.; Cheng, J.P.; Bondi, C.O.; Clark, R.S.; Dezfulian, C.; Kochanek, P.M.; et al. Repetitive Mild Traumatic Brain Injury in the Developing Brain: Effects on Long-Term Functional Outcome and Neuropathology. J. Neurotrauma 2016, 33, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kaukas, L.; Krieg, J.; Collins-Praino, L.; Corrigan, F. Effects of Remote Immune Activation on Performance in the 5-Choice Serial Reaction Time Task Following Mild Traumatic Brain Injury in Adolescence. Front. Behav. Neurosci. 2021, 15, 659679. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Schneiders, A.G.; Vella, R.K.; Fenning, A.S. Repetitive mild traumatic brain injury affects inflammation and excitotoxic mRNA expression at acute and chronic time-points. PLoS ONE 2021, 16, e0251315. [Google Scholar] [CrossRef]
- Green, J.; Sundman, M.; Chou, Y. Opioid-induced microglia reactivity modulates opioid reward, analgesia, and behavior. Neurosci. Biobehav. Rev. 2022, 135, 104544. [Google Scholar] [CrossRef]
- Da Silva, M.C.M.; Iglesias, L.P.; Candelario-Jalil, E.; Khoshbouei, H.; Moreira, F.; De Oliveira, A.C.P. Role of Microglia in Psychostimulant Addiction. Curr. Neuropharmacol. 2022, 21, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.C.M.; Gomes, G.; De Barros Fernandes, H.; Da Silva, A.M.; Teixeira, A.; Moreira, F.; De Miranda, A.; De Oliveira, A. Inhibition of CSF1R, a receptor involved in microglia viability, alters behavioral and molecular changes induced by cocaine. Sci. Rep. 2021, 11, 15989. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.S.; Triplett, T.A.; Lyu, A.; Grantham, E.K.; Azzam, M.M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Ehrlich, L.I.R.; Mayfield, R.D.; et al. Microglia depletion and alcohol: Transcriptome and behavioral profiles. Addict. Biol. 2021, 26, e12889. [Google Scholar] [CrossRef]
- Soares, A.; Picciotto, M. Nicotinic regulation of microglia: Potential contributions to addiction. J. Neural Transm. 2023, 131, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.; Schafer, D. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef]
- Galic, M.; Riazi, K.; Pittman, Q. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Root, D.; Zhang, S.; Barker, D.; Miranda-Barrientos, J.; Liu, B.; Wang, H.; Morales, M. Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep. 2018, 23, 3465–3479. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Willis, C.M.; Krzak, G.; Hamel, R.; Pirvan, L.; Ionescu, R.B.; Reisz, J.A.; Prag, H.A.; Garcia-Segura, M.E.; Wu, V.; et al. Mitochondrial complex I activity in microglia sustains neuroinflammation. Nature 2024, 628, 195–203. [Google Scholar] [CrossRef]
- Macht, V.A.; Vetreno, R.P.; Crews, F.T. Cholinergic and Neuroimmune Signaling Interact to Impact Adult Hippocampal Neurogenesis and Alcohol Pathology Across Development. Front. Pharmacol. 2022, 13, 849997. [Google Scholar] [CrossRef]
- Wolf, S.A.; Steiner, B.; Akpinarli, A.; Kammertoens, T.; Nassenstein, C.; Braun, A.; Blankenstein, T.; Kempermann, G. CD4-Positive T Lymphocytes Provide a Neuroimmunological Link in the Control of Adult Hippocampal Neurogenesis1. J. Immunol. 2009, 182, 3979–3984. [Google Scholar] [CrossRef]
- Kazmi, N.; Wallen, G.R.; Yang, L.; Alkhatib, J.; Schwandt, M.L.; Feng, D.; Gao, B.; Diazgranados, N.; Ramchandani, V.A.; Barb, J.J. An exploratory study of proinflammatory cytokines in individuals with alcohol use disorder: MCP-1 and IL-8 associated with alcohol consumption, sleep quality, anxiety, depression, and liver biomarkers. Front. Psychiatry 2022, 13, 931280. [Google Scholar] [CrossRef]
- Xie, L.; Rungratanawanich, W.; Yang, Q.; Tong, G.; Fu, E.; Lu, S.; Liu, Y.; Akbar, M.; Song, B.J.; Wang, X. Therapeutic strategies of small molecules in the microbiota-gut-brain axis for alcohol use disorder. Drug. Discov. Today 2023, 28, 103552. [Google Scholar] [CrossRef]
- Ramos, A.; Joshi, R.S.; Szabo, G. Innate immune activation: Parallels in alcohol use disorder and Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 910298. [Google Scholar] [CrossRef]
- Kapoor, M.; Chao, M.J.; Johnson, E.C.; Novikova, G.; Lai, D.; Meyers, J.L.; Schulman, J.; Nurnberger, J.I., Jr.; Porjesz, B.; Liu, Y.; et al. Multi-omics integration analysis identifies novel genes for alcoholism with potential overlap with neurodegenerative diseases. Nat. Commun. 2021, 12, 5071. [Google Scholar] [CrossRef]
- Lähteenvuo, M.; Tiihonen, J.; Solismaa, A.; Tanskanen, A.; Mittendorfer-Rutz, E.; Taipale, H. Repurposing Semaglutide and Liraglutide for Alcohol Use Disorder. JAMA Psychiatry 2025, 82, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Garcia-Rivas, V.; Fai, C.; Thomas, M.A.; Zheng, X.; Picciotto, M.R.; Mineur, Y.S. Role of microglia in stress-induced alcohol intake in female and male mice. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Neupane, S.P. Neuroimmune Interface in the Comorbidity between Alcohol Use Disorder and Major Depression. Front. Immunol. 2016, 7, 655. [Google Scholar] [CrossRef]
- Barko, K.; Shelton, M.; Xue, X.; Afriyie-Agyemang, Y.; Puig, S.; Freyberg, Z.; Tseng, G.C.; Logan, R.W.; Seney, M.L. Brain region- and sex-specific transcriptional profiles of microglia. Front. Psychiatry 2022, 13, 945548. [Google Scholar] [CrossRef]
- Soares, A.R.; Garcia-Rivas, V.; Fai, C.; Thomas, M.; Zheng, X.; Picciotto, M.R.; Mineur, Y.S. Sex differences in the microglial response to stress and chronic alcohol exposure in mice. Biol. Sex. Differ. 2025, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Borgonetti, V.; Bajo, M.; Roberto, M. Sex-dependent factors of alcohol and neuroimmune mechanisms. Neurobiol. Stress 2023, 26, 100562. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.; Ferguson, L.; Harris, R.A. Neuroimmune signaling: A key component of alcohol abuse. Curr. Opin. Neurobiol. 2013, 23, 513–520. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Garcia-Rivas, V.; Thomas, M.A.; Soares, A.R.; McKee, S.A.; Picciotto, M.R. Sex differences in stress-induced alcohol intake: A review of preclinical studies focused on amygdala and inflammatory pathways. Psychopharmacology 2022, 239, 2041–2061. [Google Scholar] [CrossRef]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e2776. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Hammer, J.; Ugursu, B.; Schalbetter, S.; Richetto, J.; Weber-Stadlbauer, U.; Mueller, F.; Scarborough, J.; Wolf, S.A.; et al. Microglia undergo molecular and functional adaptations to dark and light phases in male laboratory mice. Brain Behav. Immun. 2024, 120, 571–583. [Google Scholar] [CrossRef]
- Sullivan, O.; Ciernia, A.V. Work hard, play hard: How sexually differentiated microglia work to shape social play and reproductive behavior. Front. Behav. Neurosci. 2022, 16, 989011. [Google Scholar] [CrossRef]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen Prevents the Lipopolysaccharide-Induced Inflammatory Response in Microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Garcia, C.; Atif, F.; Yousuf, S.; Sayeed, I.; Neigh, G.N.; Stein, D.G. Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy following Ischemic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3740. [Google Scholar] [CrossRef]
- Gupta, A.; Perkins, R.B.; Ortega, G.; Feldman, S.; Villa, A. Barrier use during oro-genital sex and oral Human Papillomavirus prevalence: Analysis of NHANES 2009–2014. Oral. Dis. 2019, 25, 609–616. [Google Scholar] [CrossRef]
- Portis, S.M.; Haass-Koffler, C.L. New Microglial Mechanisms Revealed in Alcohol Use Disorder: How Does That Translate? Biol. Psychiatry 2020, 88, 893–895. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Ralevski, E.; Gueorguieva, R.; Sloan, M.E.; Devine, L.; Yoon, G.; Arias, A.J.; Sofuoglu, M. Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology 2019, 236, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, C.S.; Bremmer, M.P.; Paladino, M.B.; Kostantinis, G.; Gilmore, T.A.; Sullivan, N.R.; Tow, A.C.; Dermody, S.S.; Prince, M.A.; Jordan, R.; et al. Once-Weekly Semaglutide in Adults with Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2025, 82, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E. GLP-1 Receptor Agonists: Promising Therapeutic Targets for Alcohol Use Disorder. Endocrinology 2025, 166, bqaf028. [Google Scholar] [CrossRef]
- Chuong, V.; Farokhnia, M.; Khom, S.; Pince, C.L.; Elvig, S.K.; Vlkolinsky, R.; Marchette, R.C.; Koob, G.F.; Roberto, M.; Vendruscolo, L.F.; et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 2023, 8, e170671. [Google Scholar] [CrossRef]
- Reiner, D.J.; Mietlicki-Baase, E.G.; McGrath, L.E.; Zimmer, D.J.; Bence, K.K.; Sousa, G.L.; Konanur, V.R.; Krawczyk, J.; Burk, D.H.; Kanoski, S.E.; et al. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J. Neurosci. 2016, 36, 3531–3540. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Noguchi, T.; Katoh, H.; Nomura, S.; Okada, K.; Watanabe, M. The GLP-1 receptor agonist exenatide improves recovery from spinal cord injury by inducing macrophage polarization toward the M2 phenotype. Front. Neurosci. 2024, 18, 1342944. [Google Scholar] [CrossRef]
- Kuthati, Y.; Davuluri, V.N.G.; Wong, C.-S. Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications. Biomolecules 2025, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Conigrave, J.H.; Lewohl, J.; Haber, P.; Morley, K.C. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 89, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef]
- Johns, A.E.; Taga, A.; Charalampopoulou, A.; Gross, S.K.; Rust, K.; McCray, B.A.; Sullivan, J.M.; Maragakis, N.J. Exploring P2X7 receptor antagonism as a therapeutic target for neuroprotection in an hiPSC motor neuron model. Stem Cells Transl. Med. 2024, 13, 1198–1212. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wang, Q.; Ao, H.; Shoblock, J.R.; Lord, B.; Aluisio, L.; Fraser, I.; Nepomuceno, D.; Neff, R.A.; Welty, N.; et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br. J. Pharmacol. 2013, 170, 624–640. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, C.; García-Magro, N.; Negredo, P.; Avendaño, C.; Bhattacharya, A.; Ceusters, M.; García, A.G. Chronic administration of P2X7 receptor antagonist JNJ-47965567 delays disease onset and progression, and improves motor performance in ALS SOD1G93A female mice. Dis. Models Mech. 2020, 13, dmm045732. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; de Diego-Garcia, L.; Vegliante, G.; Moreno, O.; Gil, B.; Ramos-Cabrer, P.; Mitra, M.; Martin, A.F.; Menendez-Mendez, A.; Wang, Y.; et al. P2X7R antagonism suppresses long-lasting brain hyperexcitability following traumatic brain injury in mice. Theranostics 2025, 15, 1399–1419. [Google Scholar] [CrossRef]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2021, 14, 614884. [Google Scholar] [CrossRef]
- Withey, S.L.; Deshpande, H.U.; Cao, L.; Bergman, J.; Kohut, S.J. Effects of chronic naltrexone treatment on relapse-related behavior and neural responses to fentanyl in awake nonhuman primates. Psychopharmacology 2024, 241, 2289–2302. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: Population based cohort study. BMJ 2024, 385, e078242. [Google Scholar] [CrossRef]
- Mousavi, A.; Shojaei, S.; Soleimani, H.; Semirani-Nezhad, D.; Ebrahimi, P.; Zafari, A.; Ebrahimi, R.; Roozbehi, K.; Harrison, A.; Syed, M.A.; et al. Safety, efficacy, and cardiovascular benefits of combination therapy with SGLT-2 inhibitors and GLP-1 receptor agonists in patients with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2025, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E. Combining Glucagon-Like Peptide 1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors to Target Multiple Organ Defects in Type 2 Diabetes. Diabetes Spectr. 2020, 33, 165–174. [Google Scholar] [CrossRef]
- Melbourne, J.K.; Chandler, C.M.; Van Doorn, C.E.; Bardo, M.T.; Pauly, J.R.; Peng, H.; Nixon, K. Primed for addiction: A critical review of the role of microglia in the neurodevelopmental consequences of adolescent alcohol drinking. Alcohol Clin. Exp. Res. 2021, 45, 1908–1926. [Google Scholar] [CrossRef]
- Neupane, S.P.; Bramness, J.G.; Lien, L. Comorbid post-traumatic stress disorder in alcohol use disorder: Relationships to demography, drinking and neuroimmune profile. BMC Psychiatry 2017, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Zillich, L.; Poisel, E.; Frank, J.; Foo, J.C.; Friske, M.M.; Streit, F.; Sirignano, L.; Heilmann-Heimbach, S.; Heimbach, A.; Hoffmann, P.; et al. Multi-omics signatures of alcohol use disorder in the dorsal and ventral striatum. Transl. Psychiatry 2022, 12, 190. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Sandiego, C.M.; Hannestad, J.; Angarita, G.A.; Kumar, A.; McGovern, E.M.; Huang, Y.; O’Connor, K.C.; Carson, R.E.; O’Malley, S.S.; et al. In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol. Psychiatry 2017, 22, 1759–1766. [Google Scholar] [CrossRef]
- Carlson, E.R.; Melbourne, J.K.; Nixon, K. Pharmacological depletion of microglia protects against alcohol-induced corticolimbic neurodegeneration during intoxication in male rats. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J. Molecular Neuropathology of Astrocytes and Oligodendrocytes in Alcohol Use Disorders. Front. Mol. Neurosci. 2018, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Adermark, L.; Bowers, M.S. Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcohol Clin. Exp. Res. 2016, 40, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).