Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims and Scope
2.2. Search Strategy
2.3. Data Extraction
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. Current NPS Threats Volume VI, November 2021. Vienna. Available online: https://www.unodc.org/res/scientists/ewa/Current_NPS_Threats_VI.pdf (accessed on 16 September 2023).
- Winstock, A.R.; Lynskey, M.; Borschmann, R.; Waldron, J. Risk of emergency medical treatment following consumption of cannabis or synthetic cannabinoids in a large global sample. J. Psychopharmacol. 2015, 29, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Manning, J.J.; Finlay, D.B.; Javitch, J.A.; Banister, S.D.; Grimsey, N.L.; Glass, M. Signalling profiles of a structurally diverse panel of synthetic cannabinoid receptor agonists. Biochem. Pharmacol. 2020, 175, 113871. [Google Scholar] [CrossRef]
- Petitet, F.; Jeantaud, B.; Reibaud, M.; Imperato, A.; Dubroeucq, M.C. Complex pharmacology of natural cannabinoids: Evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998, 63, PL1–PL6. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, C.S.; Childers, S.R. Cannabinoid agonist signal transduction in rat brain: Comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J. Pharmacol. Exp. Ther. 2000, 295, 328–336. [Google Scholar] [PubMed]
- Govaerts, S.J.; Hermans, E.; Lambert, D.M. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur. J. Pharm. Sci. 2004, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Atwood, B.K.; Huffman, J.; Straiker, A.; Mackie, K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010, 160, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.; Takahashi, M.; Seto, T.; Kanai, C.; Suzuki, J.; Yoshida, M.; Hamano, T. Identification and quantitation of two benzoylindoles AM-694 and (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone, and three cannabimimetic naphthoylindoles JWH-210, JWH-122, and JWH-019 as adulterants in illegal products obtained via the Internet. Forensic Toxicol. 2011, 29, 95–110. [Google Scholar] [CrossRef]
- Gamage, T.F.; Farquhar, C.E.; Lefever, T.W.; Marusich, J.A.; Kevin, R.C.; McGregor, I.S.; Wiley, J.L.; Thomas, B.F. Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J. Pharmacol. Exp. Ther. 2018, 365, 437–446. [Google Scholar] [CrossRef]
- Finlay, D.B.; Manning, J.J.; Ibsen, M.S.; Macdonald, C.E.; Patel, M.; Javitch, J.A.; Banister, S.D.; Glass, M. Do toxic synthetic cannabinoid receptor agonists have signature in vitro activity profiles? A case study of AMB-FUBINACA. ACS Chem. Neurosci. 2019, 10, 4350–4360. [Google Scholar] [CrossRef]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [PubMed]
- Rhee, M.H.; Vogel, Z.; Barg, J.; Bayewitch, M.; Levy, R.; Hanus, L.; Breuer, A.; Mechoulam, R. Cannabinol derivatives: Binding to cannabinoid receptors and inhibition of adenylylcyclase. J. Med. Chem. 1997, 40, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Abbate, V.; Schwenk, M.; Presley, B.C.; Uchiyama, N. The ongoing challenge of novel psychoactive drugs of abuse. Part I. Synthetic cannabinoids (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1255–1282. [Google Scholar] [CrossRef]
- Angerer, V.; Mogler, L.; Steitz, J.P.; Bisel, P.; Hess, C.; Schoeder, C.T.; Müller, C.E.; Huppertz, L.M.; Westphal, F.; Schäper, J.; et al. Structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE. Drug Test. Anal. 2018, 10, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological evaluation of new constituents of “Spice”: Synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.L.A.; Logan, B.K.; Fogarty, M.F.; Krotulski, A.J.; Papsun, D.M.; Kacinko, S.L.; Huestis, M.A.; Ropero-Miller, J.D. Reports of Adverse Events Associated with Use of Novel Psychoactive Substances, 2017–2020: A Review. J. Anal. Toxicol. 2022, 46, 116–185. [Google Scholar] [CrossRef] [PubMed]
- Trecki, J.; Gerona, R.R.; Schwartz, M.D. Synthetic cannabinoid-related illnesses and deaths. N. Engl. J. Med. 2015, 373, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hermanns-Clausen, M.; Kneisel, S.; Szabo, B.; Auwarter, V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: Clinical and laboratory findings. Addiction 2013, 108, 534–544. [Google Scholar] [CrossRef]
- Courts, J.; Maskill, V.; Gray, A.; Glue, P. Signs and symptoms associated with synthetic cannabinoid toxicity: Systematic review. Australas. Psychiatry 2016, 24, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Busardo, F.P.; Tittarelli, R.; Auwarter, V.; Giorgetti, R. Post-mortem toxicology: A systematic review of death cases involving synthetic cannabinoid receptor agonists. Front. Psychiatry 2020, 11, 464. [Google Scholar] [CrossRef]
- Morrow, P.L.; Stables, S.; Kesha, K.; Tse, R.; Kappatos, D.C.; Pandey, R.; Russell, S.; Linsell, O.; McCarthy, M.J.; Spark, A.; et al. An outbreak of deaths associated with AMB-FUBINACA in Auckland NZ. eClinicalMedicine 2020, 25, 100460. [Google Scholar] [CrossRef] [PubMed]
- Bonar, E.E.; Ashrafioun, L.; Ilgen, M.A. Synthetic cannabinoid use among patients in residential substance use disorder treatment: Prevalence, motives, and correlates. Drug Alcohol Depend. 2014, 143, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Winstock, A.R.; Barratt, M.J. Synthetic cannabis: A comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. 2013, 131, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Abouchedid, R.; Ho, J.H.; Hudson, S.; Dines, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I. Acute toxicity associated with use of 5F-derivations of synthetic cannabinoid receptor agonists with analytical confirmation. J. Med. Toxicol. 2016, 12, 396–401. [Google Scholar] [CrossRef]
- Adamowicz, P.; Meissner, E.; Maslanka, M. Fatal intoxication with new synthetic cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin. Toxicol. 2019, 57, 1103–1108. [Google Scholar] [CrossRef]
- Allibe, N.; Richeval, C.; Willeman, T.; Humbert, L.; Allorge, D.; Maignan, M.; Eysseric-Guerin, H.; Stanke-Labesque, F.; Gaulier, J.M. Case reports: Four concomitant non-fatal intoxications with AB-FUBINACA and MDMA. Toxicol. Anal. Clin. 2017, 29, 101–110. [Google Scholar] [CrossRef]
- Angerer, V.; Jacobi, S.; Franz, F.; Auwarter, V.; Pietsch, J. Three fatalities associated with the synthetic cannabinoids 5F-ADB, 5F-PB-22, and AB-CHMINACA. Forensic Sci. Int. 2017, 281, 9–15. [Google Scholar] [CrossRef]
- Apirakkan, O.; Hudson, S.; Couchman, L.; Cowan, D.; Morley, S.; Abbate, V. The first reported case of a synthetic cannabinoid ethyl ester detected in a postmortem blood toxicological analysis. J. Anal. Toxicol. 2021, 44, 1052–1056. [Google Scholar] [CrossRef]
- Bäckberg, M.; Tworek, L.; Beck, O.; Helander, A. Analytically confirmed intoxications involving MDMB-CHMICA from the STRIDA project. J. Med. Toxicol. 2017, 13, 52–60. [Google Scholar] [CrossRef][Green Version]
- Barcelo, B.; Pichini, S.; Lopez-Corominas, V.; Gomila, I.; Yates, C.; Busardo, F.P.; Pellegrini, M. Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: A case series. Forensic Sci. Int. 2017, 273, 10–14. [Google Scholar] [CrossRef]
- Behonick, G.; Shanks, K.G.; Firchau, D.J.; Mathur, G.; Lynch, C.F.; Nashelsky, M.; Jskierny, D.J.; Meroueh, C. Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22. J. Anal. Toxicol. 2014, 38, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Vaiano, F.; Milia, M.G.D.; Mari, F. In vivo detection of the new psychoactive substance AM-694 and its metabolites. Forensic Sci. Int. 2015, 256, 21–27. [Google Scholar] [CrossRef]
- Brandehoff, N.; Adams, A.; McDaniel, K.; Banister, S.D.; Gerona, R.; Monte, A.A. Synthetic cannabinoid “Black Mamba” infidelity in patients presenting for emergency stabilization in Colorado: A P SCAN cohort. Clin. Toxicol. 2018, 56, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Wood, D.M.; Hudson, S.; Dargan, P.I. Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J. Med. Toxicol. 2013, 9, 278–281. [Google Scholar] [CrossRef][Green Version]
- Chan, S.; Wu, J.; Lee, B. Fatalities related to new psychoactive substances in Singapore—A case series. Forensic Sci. Int. 2019, 304, 109892. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Duflou, J.; Farrell, M.; Peacock, A.; Lappin, J. Characteristics and circumstances of synthetic cannabinoid-related death. Clin. Toxicol. 2020, 58, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Elena-González, A.; Cuadros-Tito, P.; Esteban-Gutiérrez, G. Spice intoxication and hyperglycemia. An. Sist. Sanit. Navar. 2020, 43, 87–91. [Google Scholar] [CrossRef]
- Engelgardt, P.; Krzyżanowski, M.; Piotrowski, P.; Borkowska-Sztachańska, M.; Wasilewska, A. Analytically confirmed presence of psychoactive substances, especially new psychoactive substances in a group of patients hospitalized with mental and behavioural disorders due to the use of psychoactive substances diagnosis. Int. J. Occup. Med. Environ. Health 2022, 35, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Gaunitz, F.; Andresen-Streichert, H. Analytical findings in a non-fatal intoxication with the synthetic cannabinoid 5F-ADB (5F-MDMB-PINACA): A case report. Int. J. Leg. Med. 2022, 136, 577–589. [Google Scholar] [CrossRef]
- Gaunitz, F.; Lehmann, S.; Thomas, A.; Thevis, M.; Rothschild, M.A.; Mercer-Chalmers-Bender, K. Post-mortem distribution of the synthetic cannabinoid MDMB-CHMICA and its metabolites in a case of combined drug intoxication. Int. J. Leg. Med. 2018, 132, 1645–1657. [Google Scholar] [CrossRef]
- Giorgetti, A.; Mogler, L.; Halter, S.; Haschimi, B.; Alt, A.; Rentsch, D.; Schmidt, B.; Thoma, V.; Vogt, S.; Auwarter, V. Four cases of death involving the novel synthetic cannabinoid 5F-Cumyl-PEGACLONE. Forensic Toxicol. 2020, 38, 314–326. [Google Scholar] [CrossRef]
- Giorgetti, A.; Zschiesche, A.; Groth, O.; Haschimi, B.; Scheu, M.; Pelletti, G.; Fais, P.; Musshoff, F.; Auwärter, V. ADB-HEXINACA—A Novel Synthetic Cannabinoid with a Hexyl Substituent: Phase I Metabolism in Authentic Urine Samples, a Case Report and Prevalence on the German Market. Drug Test. Anal. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Labadie, M.; Chouraqui, S.; Peyré, A.; Castaing, N.; Daveluy, A.; Molimard, M. Involuntary MDMB-4en-PINACA intoxications following cannabis consumption: Clinical and analytical findings. Clin. Toxicol. 2022, 60, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.J.; Keyfes, V.; Banka, S.S. Synthetic cannabinoid abuse resulting in ST-segment elevation myocardial infarction requiring percutaneous coronary intervention. J. Emerg. Med. 2017, 52, 496–498. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: Usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol. 2015, 33, 45–53. [Google Scholar] [CrossRef]
- Hasegawa, K.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Identification and quantification of predominant metabolites of synthetic cannabinoid MAB-CHMINACA in an authentic human urine specimen. Drug Test. Anal. 2018, 10, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hermanns-Clausen, M.; Kneisel, S.; Hutter, M.; Szabo, B.; Auwarter, V. Acute intoxication by synthetic cannabinoids—Four case reports. Drug Test. Anal. 2013, 5, 790–794. [Google Scholar] [CrossRef]
- Hill, S.L.; Najafi, J.; Dunn, M.; Acheampong, P.; Kamour, A.; Grundlingh, J.; Blain, P.G.; Thomas, S.H.L. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB-CHMICA. A report from the identification of novel psychoactive substances (IONA) study. Clin. Toxicol. 2016, 54, 638–643. [Google Scholar] [CrossRef]
- Institóris, L.; Kovács, K.; Sija, É.; Berkecz, R.; Körmöczi, T.; Németh, I.; Elek, I.; Bakos, Á.; Urbán, I.; Pap, C.; et al. Clinical symptoms and blood concentration of new psychoactive substances (NPS) in intoxicated and hospitalized patients in the Budapest region of Hungary (2018–2019). Clin. Toxicol. 2022, 60, 18–24. [Google Scholar] [CrossRef]
- Katz, K.D.; Leonetti, A.L.; Bailey, B.C.; Surmaitis, R.M.; Eustice, E.R.; Kacinko, S.; Wheatley, S.M. Case series of synthetic cannabinoid intoxication from one toxicology center. West. J. Emerg. Med. 2016, 17, 290–294. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Hill, S.L.; Pucci, M.; Bailey, G.; Keating, L.; Macfarlane, R.; Cantle, F.; Hudson, S.; Thomas, S.H.L. Clinical features associated with ADB-BUTINACA exposure in patients attending emergency departments in England. Clin. Toxicol. 2022, 60, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Klavz, J.; Gorenjak, M.; Marinsek, M. Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC. Forensic Sci. Int. 2016, 265, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Kleis, J.; Germerott, T.; Halter, S.; Heroux, V.; Roehrich, J.; Schwarz, C.S.; Hess, C. The synthetic cannabinoid 5F-MDMB-PICA: A case series. Forensic Sci. Int. 2020, 314, 110410. [Google Scholar] [CrossRef]
- Kovács, K.; Kereszty, E.; Berkecz, R.; Tiszlavicz, L.; Sija, E.; Kormoczi, T.; Jenei, N.; Revesz-Schmehl, H.; Institoris, L. Fatal intoxication of a regular drug user following N-ethyl-hexedrone and ADB-FUBINACA consumption. J. Forensic Leg. Med. 2019, 65, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.; Fels, H.; Dame, T.; Musshoff, F.; Halter, S.; Mogler, L.; Hess, C.; Madea, B.; Maas, A. Mono-/polyintoxication with 5F-ADB: A case series. Forensic Sci. Int. 2019, 301, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Zaitsu, K.; Taki, K.; Hisatsune, K.; Nakajima, J.; Moriyasu, T.; Asano, T.; Hayashi, Y.; Tsuchihashi, H.; Ishii, A. Fatal intoxication by 5F-ADB and diphenidine: Detection, quantification, and investigation of their main metabolic pathways in humans by LC/MS/MS and LC/Q-ToFMS. Drug Test. Anal. 2018, 10, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Labay, L.M.; Caruso, J.L.; Gilson, T.P.; Phipps, R.J.; Knight, L.D.; Lemos, N.P.; McIntyre, I.M.; Stoppacher, R.; Tormos, L.M.; Wiens, A.L.; et al. Synthetic cannabinoid drug use as a cause or contributory cause of death. Forensic Sci. Int. 2016, 260, 31–39. [Google Scholar] [CrossRef]
- Lam, R.P.K.; Tang, M.H.Y.; Leung, S.C.; Chong, Y.K.; Tsui, M.S.H.; Mak, T.W.L. Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: A case report with quantitative analysis of serum drug concentrations. Clin. Toxicol. 2017, 55, 662–667. [Google Scholar] [CrossRef]
- Langford, A.M.; Bolton, J.R. Synthetic cannabinoids: Variety is definitely not the spice of life. J. Forensic Leg. Med. 2018, 59, 36–38. [Google Scholar] [CrossRef]
- Lapoint, J.; James, L.P.; Moran, C.L.; Nelson, L.S.; Hoffman, R.S.; Moran, J.H. Severe toxicity following synthetic cannabinoid ingestion. Clin. Toxicol. 2011, 49, 760–764. [Google Scholar] [CrossRef]
- Larabi, I.A.; Riffi, M.; Fabresse, N.; Etting, I.; Alvarez, J.C. Validation of an UPLC-MS/MS method for the determination of sixteen synthetic cannabinoids in human hair. Application to document chronic use of JWH-122 following a non-fatal overdose. Toxicol. Anal. Clin. 2019, 31, 283–292. [Google Scholar] [CrossRef]
- Lonati, D.; Buscaglia, E.; Papa, P.; Valli, A.; Coccini, T.; Giampreti, A.; Petrolini, V.M.; Vecchio, S.; Serpelloni, G.; Locatelli, C.A. MAM-2201 (analytically confirmed) intoxication after “Synthacaine” consumption. Ann. Emerg. Med. 2014, 64, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B.; Kernbach-Wighton, G.; Bicker, W.; Kneisel, S.; Hutter, M.; Auwärter, V. Driving under the influence of synthetic cannabinoids (“Spice”): A case series. Int. J. Leg. Med. 2013, 128, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Nacca, N.; Schult, R.; Loflin, R.; Weltler, A.; Gordetsky, R.; Kacinko, S.; Moran, J.; Krotulski, A.; Wiegand, T. Coma, seizures, atriovetrivular block, and hypoglycemia in an ADB-FUBINACA body-packer. Emerg. Med. J. 2018, 55, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Neukamm, M.A.; Halter, S.; Auwärter, V.; Schmitt, G.; Giorgetti, A.; Bartel, M. Death after smoking of fentanyl, 5F-ADB, 5F-MDMB-P7AICA and other synthetic cannabinoids with a bucket bong. Forensic Toxicol. 2024, 42, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Deshmukh, A.; Dholaria, B.; Kaur, V.; Ramavaram, S.; Ukor, M.; Teran, G.A. Spicy seizure. Am. J. Med. Sci. 2012, 344, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Pieprzyca, E.; Skowronek, R.; Czekaj, P. Toxicological Analysis of Cases of Mixed Poisonings with Synthetic Cathinones and Other Drugs of Abuse. J. Anal. Toxicol. 2023, 46, 1008–1015. [Google Scholar] [CrossRef]
- Pucci, M.; Singh Jutley, G.; Looms, J.; Ford, L. N-desethyl isotonitazene detected in polydrug users admitted to hospital in Birmingham, United Kingdom. Clin. Toxicol. 2024, 62, 19–25. [Google Scholar] [CrossRef]
- Rice, K.; Hikin, L.; Lawson, A.; Smith, P.R.; Morley, S. Quantification of Flualprazolam in Blood by LC-MS-MS: A Case Series of Nine Deaths. J. Anal. Toxicol. 2021, 45, 410–416. [Google Scholar] [CrossRef]
- Rojek, S.; Korczynska-Albert, M.; Kulikowska, J.; Klys, M. New challenges in toxicology of new psychoactive substances exemplified by fatal cases after UR-144 and UR-144 with pentedrone administration determined by LC-ESI-MS-MS in blood samples. Arch. Med. Sadowej Kryminol. 2017, 67, 104–120. [Google Scholar] [CrossRef]
- Seywright, A.; Irvine, A.F.D.; McKeown, D.A.; Wylie, F.M.; Torrance, H.J. Synthetic cannabinoid receptor agonists in post-mortem casework in Scotland. J. Anal. Toxicol. 2022, 46, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Shanks, K.G.; Dahn, T.; Terrell, A.R. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in post-mortem whole blood casework. J. Anal. Toxicol. 2012, 36, 145–152. [Google Scholar] [CrossRef]
- Shanks, K.G.; Winston, D.; Heidingsfelder, J.; Behonick, G. Case reports of synthetic cannabinoid XLR-11 associated fatalities. Forensic Sci. Int. 2015, 252, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Shanks, K.G.; Clark, W.; Behonick, G. Death associated with the use of the synthetic cannabinoid ADB-FUBINACA. J. Anal. Toxicol. 2016, 40, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Tóth, D.; Heckmann, V.; Kuzma, M.; Mayer, M. Lethal case of myocardial ischemia following overdose of the synthetic cannabinoid ADB-FUBINACA. Leg. Med. 2022, 54, 1002004. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Tóth, D.; Heckmann, V.; Mayer, M.; Kuzma, M. Simultaneous fatal poisoning of two victims with 4F-MDMB-BINACA and ethanol. Forensic Toxicol. 2023, 41, 151–157. [Google Scholar] [CrossRef]
- Simon, G.; Kuzma, M.; Mayer, M.; Petrus, K.; Tóth, D. Fatal Overdose with the Cannabinoid Receptor Agonists MDMB-4en-PINACA and 4F-ABUTINACA: A Case Report and Review of the Literature. Toxics 2023, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.E.J.; Ng, M.; Chong, T.K.L.; Tan, B.K.K.; Ponampalam, R. A case of persistent refractory hypoglycemia from polysubstance recreational drug use. World J. Emerg. Med. 2023, 14, 75–77. [Google Scholar] [CrossRef]
- Steele, R.W.; Moran, J.H.; Patton, A.L.; Kokes, C.P.; James, L.P.; Storm, E.A.; Schexnayder, S.M. The spice of death: Sudden cardiac arrest after novel psychoactive substance exposure. Pediatr. Emerg. Care 2022, 38, 63–64. [Google Scholar] [CrossRef]
- Theofel, N.; Möller, P.; Vejmelka, E.; Kramer, C.; Tsokos, M.; Scholtis, S. A Fatal Case Report Resulting from the Abuse of the Designer Benzodiazepines Clonazolam and Flualprazolam in Conjunction with Dried Opium Poppy Pods. J. Anal. Toxicol. 2023, 46, 285–290. [Google Scholar] [CrossRef]
- Tiemensma, M.; Rutherford, J.D.; Scott, T.; Karch, S. Emergence of Cumyl-PEGACLONE-related fatalities in the Northern Territory of Australia. Forensic Sci. Med. Pathol. 2021, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tokarczyk, B.; Jurczyk, A.; Krupińska, J.; Adamowicz, P. Fatal intoxication with new synthetic cannabinoids 5F-MDMB-PICA and 4F-MDMB-BINACA-parent compounds and metabolite identification in blood, urine and cerebrospinal fluid. Forensic Sci. Med. Pathol. 2022, 18, 393–402. [Google Scholar] [CrossRef]
- Van Rafelghem, B.; Covaci, A.; Anseeuw, K.; van Nuijs, A.L.N.; Neels, H.; Mahieu, B.; Jacobs, W. Suicide by vaping the synthetic cannabinoid 4F-MDMB-BINACA: Cannabinoid receptors and fluoride at the crossroads of toxicity? Forensic Sci. Med. Pathol. 2021, 17, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Westin, A.A.; Frost, J.; Brede, W.R.; Gundersen, P.O.M.; Einvik, S.; Aarset, H.; Slordal, L. Sudden cardiac death following use of the synthetic cannabinoid MDMB-CHMICA. J. Anal. Toxicol. 2016, 40, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, I.; Minakata, K.; Nozawa, H.; Hasegawa, K.; Suzuki, M.; Kitamoto, T.; Suzuki, O.; Watanabe, K. A case of intoxication with a mixture of synthetic cannabinoids EAM-2201, AB-PINACA and AB-FUBINACA, and a synthetic cathinone α-PVP. Leg. Med. 2018, 35, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, C.O.; Jacob, J.; Monte, A.A.; Al-Jumaan, M.; Bronstein, A.C.; Heard, K.J. A characterization of synthetic cannabinoid exposures reported to the National Poison Data system in 2010. Ann. Emerg. Med. 2012, 60, 435–438. [Google Scholar] [CrossRef]
- Barratt, M.J.; Cakic, V.; Lenton, S. Patterns of synthetic cannabinoid use in Australia. Drug Alcohol Rev. 2012, 32, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Hudson, S.; Hikin, L.; Smith, P.R.; Morley, S.R. The changing pattern of synthetic cannabinoid use within England, April 2014 to March 2018. Med. Sci. Law 2019, 59, 180–186. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Current NPS Threats Volume I, March 2019. Vienna. Available online: https://www.unodc.org/documents/scientific/Current_NPS_Threats_Volume_I.pdf (accessed on 16 June 2023).
- Tettey, J.N.; Levissianos, S. The global emergence of NPS: An analysis of a new drug trend. In Novel Psychoactive Substances; Corazza, O., Roman-Urrestarazu, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar] [CrossRef]
- Growth, O.; Roider, G.; Angerer, V.; Schäper, J.; Graw, M.; Musshoff, F.; Auwärter, V. “pice”-related deaths in and around Munich, Germany: A retrospective look at the role of synthetic cannabinoid receptor agonists in our post-mortem cases over a seven-year period (2014–2020). Int. J. Leg. Med. 2023, 137, 1059–1069. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Vides, M.C.; Lassi, D.L.S.; Torales, J.; Ventriglio, A.; Bombana, H.S.; Leyton, V.; Périco, C.A.; Negrão, A.B.; Malbergier, A.; et al. Toxicity of synthetic cannabinoids in K2/Spice: A systematic review. Brain Sci. 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Charlson, F.; Ferrari, A.; Santomauro, D.; Erskine, H.; Mantilla-Herrara, A.; Whiteford, H.; Leung, J.; Naghavi, M.; Griswold, M.; et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Hjemsæter, A.J.; Bramness, J.G.; Drake, R.; Skeie, I.; Monsbakken, B.; Benth, J.Š.; Landheim, A.S. Mortality, cause of death and risk factors in patients with alcohol use disorder alone or poly-substance use disorders: A 19-year prospective cohort study. BMC Psychiatry 2019, 19, 101. [Google Scholar] [CrossRef]
- Mahtta, D.; Ramsey, D.; Krittanawong, C.; Al Rifai, M.; Khurram, N.; Samad, Z.; Jneid, H.; Ballantyne, C.; Petersen, L.A.; Virani, S.S. Recreational substance use among patients with premature atherosclerotic cardiovascular disease. Heart 2021, 107, 650–656. [Google Scholar] [CrossRef]
- Vandrey, R.; Dunn, K.E.; Fry, J.A.; Girling, E.R. A survey study to characterize use of spice products (synthetic cannabinoids). Drug Alcohol Depend. 2012, 120, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.B.; Hanly, M.G. Fatal caffeine overdose: A case report and review of literature. Am. J. Forensic Med. Pathol. 2013, 34, 321–324. [Google Scholar] [CrossRef]
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef]
- Pendleton, M.; Brown, S.; Thomas, C.M.; Odle, B. Potential toxicity of caffeine when used as a dietary supplement for weight loss. J. Diet. Suppl. 2013, 10, 1–5. [Google Scholar] [CrossRef]
- Ferrari Júnior, E.; Dos Santos, J.B.A.; Caldas, E.D. Drugs, pesticides and metabolites in forensic post-mortem blood samples. Med. Sci. Law 2021, 61, 97–104. [Google Scholar] [CrossRef]
- Hayaki, J.; Anderson, B.J.; Stein, M.D. Dual cannabis and alcohol use disorders in young adults: Problems magnified. Subst. Abus. 2016, 37, 579–583. [Google Scholar] [CrossRef]
- Yurasek, A.M.; Aston, E.R.; Metrik, J. Co-use of alcohol and cannabis: A review. Curr. Addict. Rep. 2017, 4, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Funada, M.; Takebayashi-Ohsawa, M.; Tomiyama, K.I. Synthetic cannabinoids enhanced ethanol-induced motor impairments through reduction of central glutamate neurotransmission. Toxicol. Appl. Pharmacol. 2020, 408, 115283. [Google Scholar] [CrossRef] [PubMed]
- Lukas, S.E.; Benedikt, R.; Mendelson, J.H.; Kouri, E.; Sholar, M.; Amass, L. Marijuana attenuates the rise in plasma ethanol levels in human subjects. Neuropsychopharmacology 1992, 7, 77–81. [Google Scholar] [PubMed]
- Lukas, S.E.; Orozco, S. Ethanol increases plasma delta(9)-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 2001, 64, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, M.S.; Kerr, W.C. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol Clin. Exp. Res. 2015, 39, 872–879. [Google Scholar] [CrossRef]
- Hartman, R.L.; Brown, T.L.; Milavetz, G.; Spurgin, A.; Gorelick, D.A.; Gaffney, G.; Huestis, M.A. Controlled cannabis vaporiser administration: Blood and plasma cannabinoids with and without alcohol. Clin. Chem. 2015, 61, 850–869. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Osei-Hyiaman, D.; Park, O.; Liu, J.; Batkai, S.; Mukhopadhyay, P.; Horiguchi, N.; Harvey-White, J.; Marsicano, G.; Lutz, B.; et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008, 7, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gautam, S.; Aseer, K.R.; Kim, J.; Chandrasekaran, P.; Mazucanti, C.H.; Ghosh, P.; O’Connell, J.F.; Doyle, M.E.; Appleton, A.; et al. Hepatocyte cannabinoid 1 receptor nullification alleviates toxin-induced liver damage via NF-κB signalling. Cell Death Dis. 2020, 11, 1044. [Google Scholar] [CrossRef]
- Alzu’bi, A.; Al Zoubi, M.S.; Al-Trad, B.; AbuAlArjah, M.I.; Shehab, M.; Alzoubi, H.; Albals, D.; Abdelhady, G.T.; El-Huneidi, W. Acute hepatic injury associated with acute administration of synthetic cannabinoid XLR-11 in mouse animal model. Toxics 2022, 10, 668. [Google Scholar] [CrossRef]
- Shahbaz, A.; Gaviria, R.E.; Shahid, M.F.; Yasin, M.A.; Ashraf, A.; Zaman, M.A. Acute liver injury induced by synthetic cannabinoid abuse. Cureus 2018, 10, e3257. [Google Scholar] [CrossRef]
- Burrowes, K.S.; Fuge, C.; Murray, T.; Amos, J.; Pitama, S.; Beckert, L. An evaluation of a New Zealand “vape to quit smoking” programme. N. Z. Med. J. 2022, 135, 45–55. [Google Scholar] [PubMed]
- Ball, J.; Zhang, J.; Stanley, J.; Boden, J.; Waa, A.; Hammond, D.; Edwards, R. Early-onset smoking and vaping of cannabis: Prevalence, correlates and trends in New Zealand 14–15-year-olds. Drug Alcohol Rev. 2023, 42, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.I.; Ind, P.W. Effect of cannabis smoking on lung function and respiratory symptoms: A structured literature review. npj Prim. Care Respir. Med. 2016, 26, 16071. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.; Sathiadoss, P.; Salameh, J.P.; Mcinnes, M.D.F.; Revah, G. Chest CT findings in marijuana smokers. Radiology 2023, 307, e212611. [Google Scholar] [CrossRef]
- Tan, W.C.; Lo, C.; Jong, A.; Xing, L.; Fitzgerald, M.J.; Vollmer, W.M.; Buist, S.A.; Sin, D.D.; Vancouver Burden of Obstructive Lung Disease (BOLD) Research Group. Marijuana and chronic obstructive lung disease: A population-based study. CMAJ 2009, 180, 814–820. [Google Scholar] [CrossRef]
- Kaplan, A.G. Cannabis and lung health: Does the bad outweigh the good? Pulm. Ther. 2021, 7, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Zawatsky, C.N.; Abdalla, J.; Cinar, R. Synthetic cannabinoids induce acute lung inflammation via cannabinoid receptor 1 activation. ERJ Open Res. 2020, 6, 00121–02020. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Young, R.; Kavanagh, D. Cannabis use and misuse prevalence among people with psychosis. Br. J. Psychiatry 2005, 187, 306–313. [Google Scholar] [CrossRef][Green Version]
- Barnett, J.H.; Werners, U.; Secher, S.M.; Hill, K.E.; Brazil, R.; Masson, K.; Pernet, D.E.; Kirkbride, J.B.; Murray, G.K.; Bullmore, E.T.; et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br. J. Psychiatry 2007, 190, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Welter, S.; Lücke, C.; Lam, A.P.; Custal, C.; Moeller, S.; Sörös, P.; Thiel, C.M.; Philipsen, A.; Müller, H.H.O. Synthetic cannabinoid use in a psychiatric patient population: A pilot study. Eur. Addict. Res. 2017, 23, 182–193. [Google Scholar] [CrossRef]
- Marel, C.; Sunderland, M.; Mills, K.L.; Slade, T.; Teesson, M.; Chapman, C. Conditional probabilities of substance use disorders and associated risk factors: Progression from first use to use disorder on alcohol, cannabis, stimulants, sedatives and opioids. Drug Alcohol Depend. 2019, 194, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.; Patel, R.; Morrison, P.D.; Kalk, N.; Stone, J.M. Synthetic cannabinoid use in psychiatric patients and relationship to hospitalisation: A retrospective electronic case register study. J. Psychopharmacol. 2020, 34, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Hurst, D.; Loeffler, G.; McLay, R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am. J. Psychiatry 2011, 168, 1119. [Google Scholar] [CrossRef] [PubMed]
- Roberto, A.J.; Lorenzo, A.; Li, K.J.; Young, J.; Mohan, A.; Pinnaka, S.; Lapidus, K.A. First-episode of synthetic cannabinoid-induced psychosis in a young adult, successfully managed with hospitalisation and risperidone. Case Rep. Psychiatry 2016, 2016, 7257389. [Google Scholar] [CrossRef]
- Teixeira, J.; Alexandre, S.; Cunha, C.; Raposo, F.; Costa, J.P. Impact of clozapine as the mainstay therapeutical approach to schizophrenia and substance use disorder: A retrospective inpatient analysis. Psychiatry Res. Commun. 2022, 2, 100056. [Google Scholar] [CrossRef]
- Swanson, J.; Van Dorn, R.A.; Swartz, M.S. Effectiveness of atypical antipsychotics for substance use in schizophrenia patients. Schizophr. Res. 2007, 94, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.M.; Daley, D.C.; Douaihy, A.B. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict. Behav. 2012, 37, 11–24. [Google Scholar] [CrossRef]
- Stapleton, J.; West, R.; Hajek, P.; Wheeler, J.; Vangeli, E.; Abdi, Z.; O’Gara, C.; McRobbie, H.; Humphrey, K.; Ali, R.; et al. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: Effectiveness in clinical practice. Addiction 2013, 108, 2193–2201. [Google Scholar] [CrossRef]
- Franck, J.; Jayaram-Lindström, N. Pharmacotherapy for alcohol dependence: Status of current treatments. Curr. Opin. Neurobiol. 2013, 23, 692–699. [Google Scholar] [CrossRef]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Bobashev, G.; Thomas, K.; Wines, R.; Kim, M.M.; Shanahan, E.; Gass, C.E.; Rowe, C.J.; et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 2014, 311, 1889–1900. [Google Scholar] [CrossRef]
- Beaglehole, B.; Foulds, J.; Mulder, R.T.; Boden, J.M. Dispensing of medication for alcohol use disorder; an examination of large databases in a New Zealand context. N. Z. Med. J. 2019, 132, 48–53. [Google Scholar] [PubMed]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009, 3, CD002209. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.V., Jr.; Scodes, J.M.; Pavlicova, M.; Lee, J.D.; Novo, P.; Campbell, A.N.C.; Rotrosen, J. Sublingual buprenorphine-naloxone compared with injection naltrexone for opioid use disorder: Potential utility of patient characteristics in guiding choice of treatment. Am. J. Psychiatry 2021, 178, 660–671. [Google Scholar] [CrossRef]
- Yoganathan, P.; Claridge, H.; Chester, L.; Englund, A.; Kalk, N.J.; Copeland, C.S. Synthetic cannabinoid-related deaths in England, 2012–2019. Cannabis Cannabinoid Res. 2022, 7, 516–525. [Google Scholar] [CrossRef]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Przybyla, J.A.; Watts, V.J. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J. Pharmacol. Exp. Ther. 2010, 332, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.M.; Young, A.P.; Laprairie, R.B.; Toguri, J.T.; Kelly, M.E.M.; Denovan-Wright, E.M. Heteromer formation between cannabinoid type 1 and dopamine type 2 receptors is altered by combination cannabinoid and antipsychotic treatments. J. Neurosci. Res. 2020, 98, 2496–2509. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Lo, Y.N.; Chen, J.C. Crosstalk between dopamine D₂ receptors and cannabinoid CB₁ receptors regulates CNR1 promoter activity via ERK1/2 signaling. J. Neurochem. 2013, 127, 163–176. [Google Scholar] [CrossRef]
- Nasrallah, H.A. Atypical antipsychotic-induced metabolic side effects: Insights from receptor-binding profiles. Mol. Psychiatry 2008, 13, 27–35. [Google Scholar] [CrossRef]
- Albizu, L.; Holloway, T.; González-Maeso, J.; Sealfon, S.C. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology 2011, 61, 770–777. [Google Scholar] [CrossRef]
- Viñals, X.; Moreno, E.; Lanfumey, L.; Cordomí, A.; Pastor, A.; de La Torre, R.; Gasperini, P.; Navarro, G.; Howell, L.A.; Pardo, L.; et al. Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLOS Biol. 2015, 13, 1002194. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Sun, J.C.; Tse, M.T.; Gorzalka, B.B. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int. J. Neuropsychopharmacol. 2006, 9, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lecue, I.; Mollinedo-Gajate, I.; Meana, J.J.; Callado, L.F.; Diez-Alarcia, R.; Urigüen, L. Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology 2018, 43, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lecue, I.; Unzueta-Larrinaga, P.; Barrena-Barbadillo, R.; Villate, A.; Horrillo, I.; Mendivil, B.; Landabaso, M.A.; Meana, J.J.; Etxebarria, N.; Callado, L.F.; et al. Cannabis use selectively modulates circulating biomarkers in the blood of schizophrenia patients. Addict. Biol. 2022, 27, 13233. [Google Scholar] [CrossRef] [PubMed]
- Louh, I.K.; Freeman, W.D. A ‘spicy’ encephalopathy: Synthetic cannabinoids as cause of encephalopathy and seizure. Crit. Care 2014, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; Bilel, S.; Tirri, M.; Arfè, R.; Corli, G.; Roda, E.; Locatelli, C.A.; Cavarretta, E.; De Giorgio, F.; Marti, M. The old and the new: Cardiovascular and respiratory alterations induced by acute JWH-018 administration compared to Δ9-THC-A preclinical study in mice. Int. J. Mol. Sci. 2023, 24, 1631. [Google Scholar] [CrossRef] [PubMed]
- Alon, M.H.; Saint-Fleur, M.O. Synthetic cannabinoid induced acute respiratory depression: Case series and literature review. Respir. Med. Case Rep. 2017, 22, 137–141. [Google Scholar] [CrossRef]
- Manini, A.F.; Krotulski, A.J.; Schimmel, J.; Allen, L.; Hurd, Y.L.; Richardson, L.D.; Vidal, K.; Logan, B.K. Respiratory failure in confirmed synthetic cannabinoid overdose. Clin. Toxicol. 2022, 60, 524–526. [Google Scholar] [CrossRef]
- Pirnay, S.; Borron, S.W.; Giudicelli, C.P.; Tourneau, J.; Baud, F.J.; Ricordel, I. A critical review of the causes of death among post-mortem toxicological investigations: Analysis of 34 buprenorphine-associated and 35 methadone-associated deaths. Addiction 2004, 99, 978–988. [Google Scholar] [CrossRef]
- Montandon, G.; Qin, W.; Liu, H.; Ren, J.; Greer, J.J.; Horner, R.L. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J. Neurosci. 2011, 31, 1292–1301. [Google Scholar] [CrossRef]
- Watkins, J.; Aradi, P.; Hahn, R.; Katona, I.; Mackie, K.; Makriyannis, A.; Hohmann, A.G. CB 1 Cannabinoid Receptor Agonists Induce Acute Respiratory Depression in Awake Mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wiese, B.M.; Liktor-Busa, E.; Couture, S.A.; Nikas, S.P.; Ji, L.; Liu, Y.; Makriyannis, A.; Spigelman, I.; Vanderah, T.W.; Largent-Milnes, T.M. Brain penetrant, but not peripherally restricted, synthetic cannabinoid 1 receptor agonists promote morphine-mediated respiratory depression. Cannabis Cannabinoid Res. 2022, 7, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, C.; Berthou, F.; Baird, S.; Dréano, Y.; Picart, D.; Bail, J.P.; Beaune, P.; Ménez, J.F. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem. Res. Toxicol. 1996, 9, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Chevillard, L.; Declèves, X.; Baud, F.J.; Risède, P.; Mégarbane, B. Respiratory effects of diazepam/methadone combination in rats: A study based on concentration/effect relationships. Drug Alcohol Depend. 2013, 131, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Coccia, C.P.; Bertolini, A.; Sternieri, E. Methadone–metabolism, pharmacokinetics and interactions. Pharmacol. Res. 2004, 50, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Eap, C.B.; Bertschy, G.; Powell, K.; Baumann, P. Fluvoxamine and fluoxetine do not interact in the same way with the metabolism of the enantiomers of methadone. J. Clin. Psychopharmacol. 1997, 17, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, C.; Picart, D.; Dréano, Y.; Berthou, F. In vitro interactions between fluoxetine or fluvoxamine and methadone or buprenorphine. Fundam. Clin. Pharmacol. 1998, 12, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Uehlinger, C.; Crettol, S.; Chassot, P.; Brocard, M.; Koeb, L.; Brawand-Amey, M.; Eap, C.B. Increased (R)-methadone plasma concentrations by quetiapine in cytochrome P450s and ABCB1 genotyped patients. J. Clin. Psychopharmacol. 2007, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Nadpara, P.A.; Joyce, A.R.; Murrelle, E.L.; Carroll, N.W.; Carroll, N.V.; Barnard, M.; Zedler, B.K. Risk factors for serious prescription opioid-induced respiratory depression or overdose: Comparison of commercially insured and veterans health affairs populations. Pain Medicat. 2018, 19, 79–96. [Google Scholar] [CrossRef]
- Calcaterra, S.; Glanz, J.; Binswanger, I.A. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013, 131, 263–270. [Google Scholar] [CrossRef]


| Author | Year | Country | n | Sex (M/F) | Mortalities | Non-Fatal Intoxications |
|---|---|---|---|---|---|---|
| Abouchedid et al. [26] | 2016 | UK | 1 | F | - | 1 |
| Adamowicz et al. [27] | 2019 | Poland | 1 | M | 1 | - |
| Allibe et al. [28] | 2017 | France | 4 | M | - | 4 |
| Angerer et al. [29] | 2017 | Germany | 3 | M | 3 | - |
| Apirakkan et al. [30] | 2021 | UK | 1 | M | 1 | - |
| Bäckberg et al. [31] | 2017 | Sweden | 8 | 7M/1F | - | 8 |
| Barcelo et al. [32] | 2017 | Spain | 2 | 1M/1F | - | 2 |
| Behonick et al. [33] | 2014 | USA | 2 | M | 2 | - |
| Bertol et al. [34] | 2015 | Italy | 1 | M | - | 1 |
| Brandehoff et al. [35] | 2018 | USA | 4 | 2M/2F | - | 4 |
| Chan et al. [36] | 2013 | UK | 1 | M | - | 1 |
| Chan et al. [37] | 2019 | Singapore | 2 | M | 2 | - |
| Darke et al. [38] | 2020 | Australia | 42 | - | 42 | - |
| Elena-González et al. [39] | 2020 | Spain | 1 | M | - | 1 |
| Engelgardt et al. [40] | 2022 | Poland | 10 | 9M/1F | - | 10 |
| Gaunitz and Andresen-Streichert [41] | 2022 | Germany | 1 | M | - | 1 |
| Gaunitz et al. [42] | 2018 | Germany | 1 | M | 1 | - |
| Giorgetti et al. [43] | 2020a | Germany | 4 | 3M/1F | 4 | - |
| Giorgetti et al. [44] | 2024 | Germany | 1 | M | 1 | - |
| Goncalves et al. [45] | 2022 | France | 8 | 7M/1F | - | 8 |
| Hamilton et al. [46] | 2017 | USA | 1 | M | - | 1 |
| Hasegawa et al. [47] | 2015 | Japan | 1 | M | 1 | - |
| Hasegawa et al. [48] | 2018 | Japan | 1 | M | 1 | - |
| Hermanns-Clausen et al. [18] | 2013a | Germany | 7 | 6M/1F | - | 7 |
| Hermanns-Clausen et al. [49] | 2013b | Germany | 1 | M | - | 1 |
| Hill et al. [50] | 2016 | UK | 4 | M | - | 4 |
| Institóris et al. [51] | 2022 | Hungary | 13 | 12M/1F | - | 13 |
| Katz et al. [52] | 2016 | USA | 10 | 6M/4F | 1 | 9 |
| King et al. [53] | 2022 | UK | 7 | 6M/1F | - | 7 |
| Klavz et al. [54] | 2016 | Slovenia | 1 | M | - | 1 |
| Kleis et al. [55] | 2020 | Germany | 9 | 8M/1F | 3 | 6 |
| Kovács et al. [56] | 2019 | Hungary | 1 | M | 1 | - |
| Kraemer et al. [57] | 2019 | Germany | 3 | 2M/1F | 3 | - |
| Kusano et al. [58] | 2018 | Japan | 1 | M | 1 | - |
| Labay et al. [59] | 2016 | USA | 19 | 15M/4F | 19 | - |
| Lam et al. [60] | 2017 | China | 1 | M | - | 1 |
| Langford and Bolton [61] | 2018 | UK | 1 | M | 1 | - |
| Lapoint et al. [62] | 2011 | USA | 1 | M | - | 1 |
| Larabi et al. [63] | 2019 | France | 1 | M | - | 1 |
| Lonati et al. [64] | 2014 | Italy | 1 | M | - | 1 |
| Morrow et al. [21] | 2020 | New Zealand | 51 | - | 51 | - |
| Musshoff et al. [65] | 2013 | Germany | 1 | M | - | 1 |
| Nacca et al. [66] | 2018 | USA | 1 | M | - | 1 |
| Neukamm et al. [67] | 2024 | Germany | 1 | M | 1 | - |
| Pant et al. [68] | 2012 | USA | 1 | M | - | 1 |
| Pieprzyca et al. [69] | 2023 | Poland | 3 | 3M | 3 | - |
| Pucci et al. [70] | 2024 | UK | 6 | 4M/2F | 1 | 5 |
| Rice et al. [71] | 2021 | UK | 2 | 1M/1F | 2 | - |
| Rojek et al. [72] | 2017 | Poland | 1 | M | 1 | - |
| Seywright et al. [73] | 2022 | UK | 11 | 10M/1F | 11 | - |
| Shanks et al. [74] | 2012 | USA | 1 | M | 1 | - |
| Shanks et al. [75] | 2015 | USA | 1 | F | 1 | - |
| Shanks et al., [76] | 2016 | USA | 1 | F | 1 | - |
| Simon et al. [77] | 2022 | Hungary | 1 | M | 1 | - |
| Simon et al. [78] | 2023a | Hungary | 2 | 2M | 2 | - |
| Simon et al. [79] | 2023b | Hungary | 1 | M | 1 | - |
| Soo et al. [80] | 2023 | Singapore | 1 | M | - | 1 |
| Steele et al. [81] | 2022 | USA | 1 | M | 1 | - |
| Theofel et al. [82] | 2023 | Germany | 1 | M | 1 | - |
| Tiemensma et al. [83] | 2021 | Australia | 4 | M | 4 | - |
| Tokarczyk et al. [84] | 2022 | Poland | 1 | M | 1 | - |
| Van Rafelghem et al. [85] | 2021 | Belgium | 1 | M | 1 | - |
| Westin et al. [86] | 2016 | Norway | 1 | M | 1 | - |
| Yamagishi et al. [87] | 2018 | Japan | 1 | M | 1 | - |
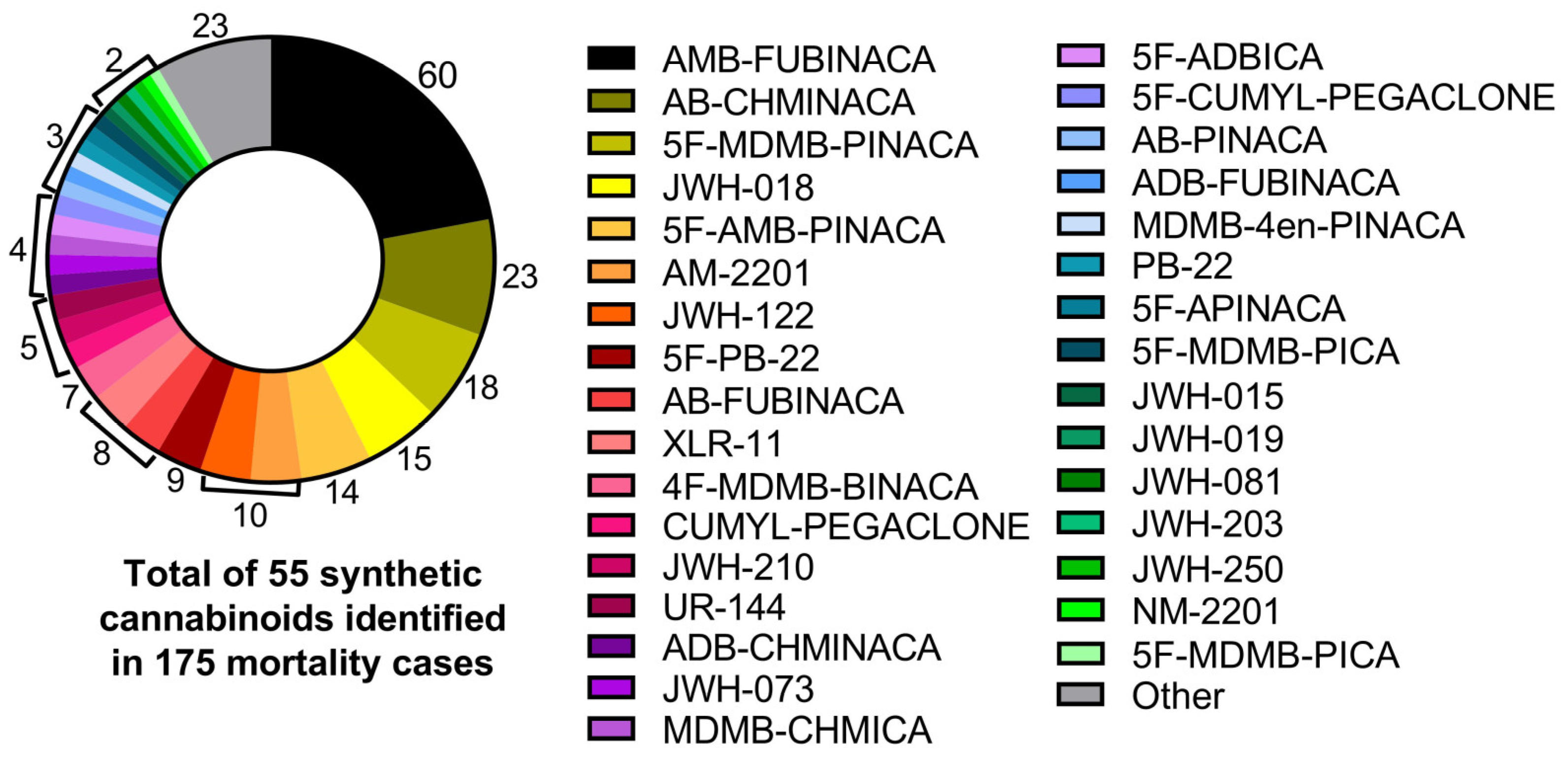
| Total | 278 | 185 | 175 | 103 |
| Case Features | Mean | Range |
|---|---|---|
| Age | 32 years | 13–64 years |
| No. of substances | 4 | 2–18 |
| Route of administration | n | % |
| Inhalation | 152 | 95.6% |
| Oral | 7 | 4.4% |
| Comorbidities | n | % |
| Drug-use history | 142 | 63.4% |
| Mental illness | 53 | 23.7% |
| HASCVD 1 | 45 | 20.1% |
| Cause of death | n | % |
| Mixed drug toxicity | 36 | 29.5% |
| Synthetic cannabinoid toxicity | 31 | 25.4% |
| Cardiovascular disease | 21 | 17.2% |
| Stroke, hypoxic brain injury, or encephalopathy | 8 | 6.6% |
| Asphyxia | 5 | 4.1% |
| Other | 21 | 17.2% |
| Synthetic cannabinoid quantification | n | Range (ng/mL) |
| Plasma | 6 | 0.20–44 |
| Serum | 29 | 0.11–230 |
| Urine | 7 | 0.08–24 |
| Whole blood | 69 | 0.01–204 |
| Co-Exposure | Specific Drug | n |
|---|---|---|
| Opioids | Methadone | 24 |
| Morphine | 22 | |
| Codeine | 14 | |
| Tramadol | 10 | |
| Fentanyl | 6 | |
| Antipsychotics/antidepressants | Risperidone | 13 |
| Mirtazapine | 9 | |
| Olanzapine | 8 | |
| Citalopram | 7 | |
| Fluoxetine | 7 | |
| Haloperidol | 7 | |
| Quetiapine | 7 | |
| Benzodiazepines | Diazepam | 9 |
| Alprazolam | 7 | |
| Lorazepam | 7 | |
| Midazolam | 6 | |
| Nordazepam | 6 | |
| Amphetamines | Amphetamine | 25 |
| Methamphetamine | 18 | |
| MDMA | 8 | |
| Miscellaneous | pFPP | 18 |
| Cocaine | 17 | |
| Pregabalin | 14 | |
| Lidocaine | 10 | |
| Zopiclone | 10 | |
| Diphenhydramine | 7 |
| Co-Exposure | Mortalities (n = 175) n (%) | Non-Fatal Intox. (n = 103) n (%) |
|---|---|---|
| Antipsychotics/antidepressants | 60 (34.3%) * | 11 (10.7%) |
| Alcohol | 69 (39.4%) * | 26 (25.2%) |
| Δ-9-THC | 54 (30.9%) | 37 (35.9%) |
| Tobacco | 57 (32.6%) * | 4 (3.9%) |
| Benzodiazepines | 35 (20.0%) | 30 (29.1%) |
| Opioids | 32 (18.3%) | 27 (26.2%) |
| Amphetamines | 19 (10.9%) | 20 (19.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, L.R.; Rosengren, R.J.; Glass, M. Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies. Psychoactives 2024, 3, 365-383. https://doi.org/10.3390/psychoactives3030023
Thomsen LR, Rosengren RJ, Glass M. Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies. Psychoactives. 2024; 3(3):365-383. https://doi.org/10.3390/psychoactives3030023
Chicago/Turabian StyleThomsen, Lucy R., Rhonda J. Rosengren, and Michelle Glass. 2024. "Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies" Psychoactives 3, no. 3: 365-383. https://doi.org/10.3390/psychoactives3030023
APA StyleThomsen, L. R., Rosengren, R. J., & Glass, M. (2024). Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies. Psychoactives, 3(3), 365-383. https://doi.org/10.3390/psychoactives3030023






