Diabetes Psychiatry: The Missing Piece of the Puzzle to Prevent Complications of the Diabetes Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. The Bidirectional Relationship Between Diabetes and Depression
2.2. Hypothalamic–Pituitary–Adrenal Axis Dysregulation and Brain Metabolism
2.3. Inflammation, Insulin Resistance, and Neurotransmitter Dysfunction
2.4. Obesity, High BMI, and Depression Risk
2.5. Depression and T2DM Progression: Impact on Complications and Severity
2.6. Dyslipidemia and Serotonin Dysregulation in T2DM-Related Depression
3. Antidepressants and Diabetes Mellitus
3.1. Positive Impacts: Enhancing Metabolic Health with Antidepressants
3.1.1. Glycemic Control: General Improvements Across Studies
3.1.2. Glycemic Control: Targeted Benefits from Specific Antidepressants
3.1.3. Insulin Regulation: Fine-Tuning Sensitivity and Secretion
3.1.4. Cardiometabolic Health: Broad Protective Effects
3.1.5. Cardiometabolic Health: Drug-Specific Actions for Cardiovascular Wellness
3.2. Negative Impacts: Potential Risks Associated with Antidepressant Use in Diabetic Patients
3.2.1. Increased Risk of Type 2 Diabetes
3.2.2. Adverse Effects on Glucose Regulation and Insulin Sensitivity
3.2.3. Metabolic Side Effects and Cardiovascular Risks
3.2.4. Other Adverse Effects
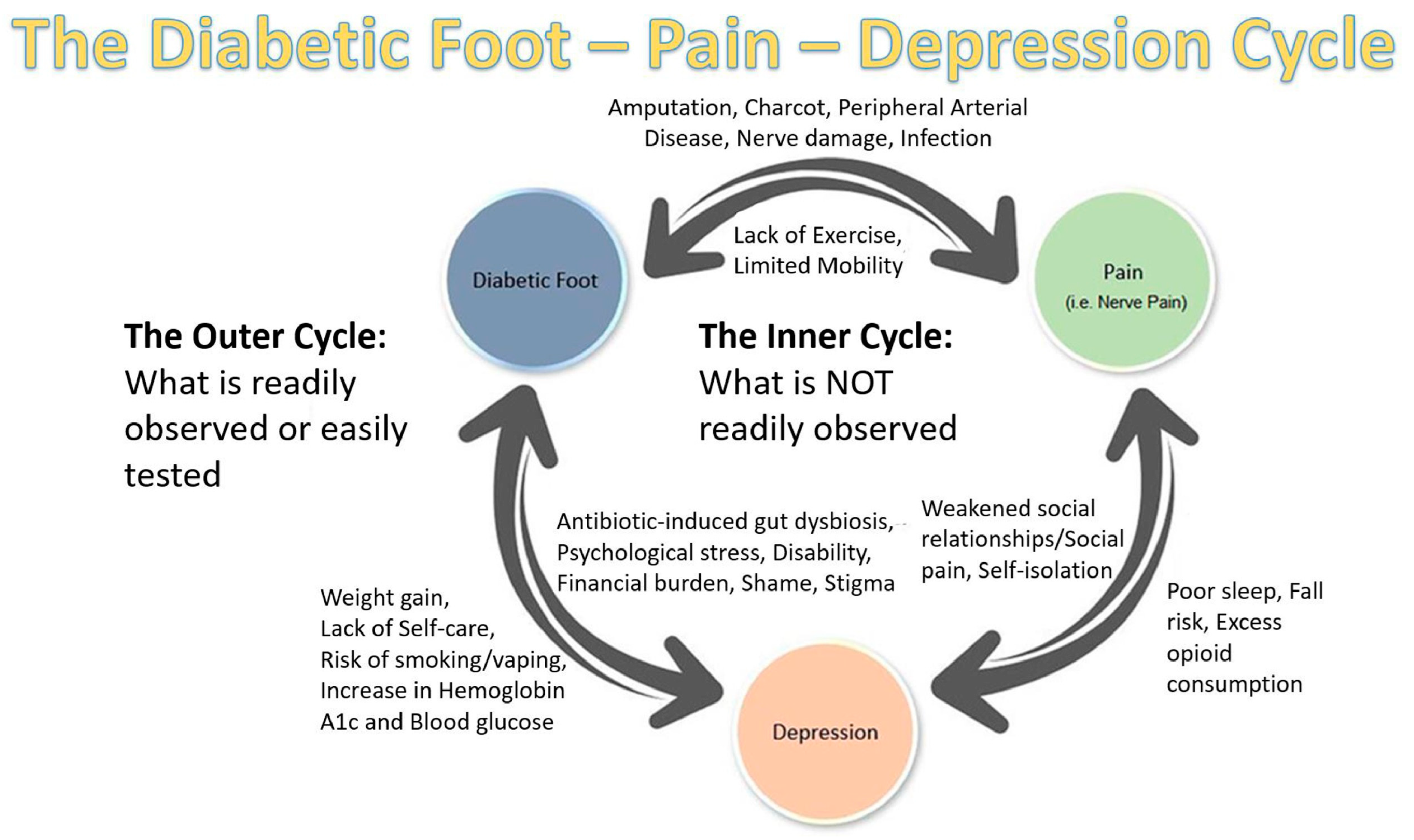
3.3. Antidepressants and Diabetic Foot Complications
4. Discussion: The Role of Psychiatrists in Antidepressant Management of the Diabetic Patient
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| SSRIs | Selective serotonin reuptake inhibitors |
| TCAs | Tricyclic antidepressants |
| SNRIs | Serotonin-norepinephrine reuptake inhibitors |
| MAOIs | Monoamine oxidase inhibitors |
References
- Brooks, L.M.; Brooks, B.M.; Arp, A.S.; Dove, C.R.; Rogers, L.C.; Michel, R.; Clinton, V.; Labovitz, J.; Brooks, B.M.; Armstrong, D.G. Diabetes-Related Extremity Amputation Depression and Distress (DREADD): A Multimethod Study. Semin. Vasc. Surg. 2025, 38, 94–100. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.D.; Ong, S.C.; Rafiq, A.; Kalam, M.N.; Sajjad, A.; Abdullah, M.; Malik, T.; Yaseen, F.; Babar, Z.-U.-D. A systematic review of the economic burden of diabetes mellitus: Contrasting perspectives from high and low middle-income countries. J. Pharm. Policy Pract. 2024, 17, 2322107. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 14 March 2025).
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Mullins, R.J.; Diehl, T.C.; Chia, C.W.; Kapogiannis, D. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front. Aging Neurosci. 2017, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Wimberley, T.; Horsdal, H.T.; Brikell, I.; Laursen, T.M.; Astrup, A.; Fanelli, G.; Bralten, J.; Poelmans, G.; Gils, V.V.; Jansen, W.J. Temporally ordered associations between type 2 diabetes and brain disorders–a Danish register-based cohort study. BMC Psychiatry 2022, 22, 573. [Google Scholar] [CrossRef]
- Possidente, C.; Fanelli, G.; Serretti, A.; Fabbri, C. Clinical insights into the cross-link between mood disorders and type 2 diabetes: A review of longitudinal studies and Mendelian randomisation analyses. Neurosci. Biobehav. Rev. 2023, 152, 105298. [Google Scholar] [CrossRef]
- Ai, Y.; Xu, R.; Liu, L. The Prevalence and Risk Factors of Sarcopenia in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2021, 13, 93. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Zong, Q.Q.; Zhang, Q.; Ng, C.; Ungvari, G.; Xiang, Y.T. Prevalence of comorbid major depressive disorder in Type 2 diabetes: A meta-analysis of comparative and epidemiological studies. Diabet. Med. 2019, 36, 961–969. [Google Scholar] [CrossRef]
- Khaledi, M.; Haghighatdoost, F.; Feizi, A.; Aminorroaya, A. The prevalence of comorbid depression in patients with type 2 diabetes: An updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019, 56, 631–650. [Google Scholar] [CrossRef]
- WHO. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 15 March 2025).
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, E.S.; Chen, P.R.; Kim, E. Dysregulated hypothalamic–pituitary–adrenal axis is associated with increased inflammation and worse outcomes after ischemic stroke in diabetic mice. Front. Immunol. 2022, 13, 864858. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, A.; Maconick, L.; Francis, E.; Walters, K.; Wong, I.C.; Osborn, D.; Hayes, J.F. Prevalence and characteristics of antidepressant prescribing in adults with comorbid depression and type 2 diabetes mellitus: A systematic review and meta-analysis. Health Sci. Rev. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Akhaury, K.; Chaware, S. Relation between diabetes and psychiatric disorders. Cureus 2022, 14, e30733. [Google Scholar] [CrossRef]
- Goldney, R.D.; Phillips, P.J.; Fisher, L.J.; Wilson, D.H. Diabetes, depression, and quality of life: A population study. Diabetes Care 2004, 27, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.; Makubate, B.; Hernandez-Santiago, V.; Dreischulte, T. The rising tide of polypharmacy and drug-drug interactions: Population database analysis 1995–2010. BMC Med. 2015, 13, 74. [Google Scholar] [CrossRef]
- Fanelli, G.; Serretti, A. Depression, antidepressants, and insulin resistance: Which link? Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2022, 60, 4–6. [Google Scholar] [CrossRef]
- Gonzalez Heredia, T.; González-Ramírez, L.P.; Hernández-Corona, D.M.; Maciel-Hernández, E.A. Anxious depression in patients with Type 2 Diabetes Mellitus and its relationship with medication adherence and glycemic control. Glob. Public Health 2021, 16, 460–468. [Google Scholar] [CrossRef]
- Detka, J.; Kurek, A.; Kucharczyk, M.; Głombik, K.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Brain glucose metabolism in an animal model of depression. Neuroscience 2015, 295, 198–208. [Google Scholar] [CrossRef]
- Hoogendoorn, C.J.; Roy, J.F.; Gonzalez, J.S. Shared dysregulation of homeostatic brain-body pathways in depression and type 2 diabetes. Curr. Diabetes Rep. 2017, 17, 90. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Evans, K.R.; Krüger, S.; Mayberg, H.S.; Meyer, J.H.; McCann, S.; Arifuzzman, A.I.; Houle, S.; Vaccarino, F.J. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am. J. Psychiatry 2001, 158, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Hamer, M.; Batty, G.D.; Geddes, J.R.; Tabak, A.G.; Pentti, J.; Virtanen, M.; Vahtera, J. Antidepressant medication use, weight gain, and risk of type 2 diabetes: A population-based study. Diabetes Care 2010, 33, 2611–2616. [Google Scholar] [CrossRef]
- Naumovs, V.; Groma, V.; Mednieks, J. From low-grade inflammation in osteoarthritis to neuropsychiatric sequelae: A narrative review. Int. J. Mol. Sci. 2022, 23, 16031. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Shams, H.A.; Al-Mamorri, F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. J. Lab. Physicians 2019, 11, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Hamada, M.T.; Al-Samerraie, A.Y. Effects of metformin on omentin-1 serum levels in a newly diagnosed type 2 diabetes mellitus: Randomized, placebo controlled study. Mustansiriya Med. J. 2016, 15, 49–56. [Google Scholar] [CrossRef]
- Lyra e Silva, N.d.M.; Lam, M.P.; Soares, C.N.; Munoz, D.P.; Milev, R.; De Felice, F.G. Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front. Psychiatry 2019, 10, 420372. [Google Scholar] [CrossRef]
- Mansur, R.B.; Fries, G.R.; Subramaniapillai, M.; Frangou, S.; De Felice, F.G.; Rasgon, N.; McEwen, B.; Brietzke, E.; McIntyre, R.S. Expression of dopamine signaling genes in the post-mortem brain of individuals with mental illnesses is moderated by body mass index and mediated by insulin signaling genes. J. Psychiatr. Res. 2018, 107, 128–135. [Google Scholar] [CrossRef]
- Martin, H.; Bullich, S.; Guiard, B.P.; Fioramonti, X. The impact of insulin on the serotonergic system and consequences on diabetes-associated mood disorders. J. Neuroendocrinol. 2021, 33, e12928. [Google Scholar] [CrossRef]
- Kraus, C.; Kautzky, A.; Watzal, V.; Gramser, A.; Kadriu, B.; Deng, Z.-D.; Bartova, L.; Zarate, C.A., Jr.; Lanzenberger, R.; Souery, D. Body mass index and clinical outcomes in individuals with major depressive disorder: Findings from the GSRD European Multicenter Database. J. Affect. Disord. 2023, 335, 349–357. [Google Scholar] [CrossRef]
- Kloiber, S.; Ising, M.; Reppermund, S.; Horstmann, S.; Dose, T.; Majer, M.; Zihl, J.; Pfister, H.; Unschuld, P.G.; Holsboer, F. Overweight and obesity affect treatment response in major depression. Biol. Psychiatry 2007, 62, 321–326. [Google Scholar] [CrossRef]
- Khan, P.; Qayyum, N.; Malik, F.; Khan, T.; Khan, M.; Tahir, A.; Siddiqui, A. Incidence of anxiety and depression among patients with type 2 diabetes and the predicting factors. Cureus 2019, 11, e4254. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Gillies, C.; Sathanapally, H.; Abner, S.; Seidu, S.; Davies, M.J.; Polonsky, W.H.; Khunti, K. A systematic review and meta-analysis to compare the prevalence of depression between people with and without Type 1 and Type 2 diabetes. Prim. Care Diabetes 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Alruwaili, N.S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Ragab, A.E.; Alenazi, A.A.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Antidepressants and type 2 diabetes: Highways to knowns and unknowns. Diabetol. Metab. Syndr. 2023, 15, 179. [Google Scholar] [CrossRef]
- Nouwen, A.; Adriaanse, M.; van Dam, K.; Iversen, M.M.; Viechtbauer, W.; Peyrot, M.; Caramlau, I.; Kokoszka, A.; Kanc, K.; de Groot, M. Longitudinal associations between depression and diabetes complications: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1562–1572. [Google Scholar] [CrossRef]
- Wu, C.-S.; Hsu, L.-Y.; Wang, S.-H. Association of depression and diabetes complications and mortality: A population-based cohort study. Epidemiol. Psychiatr. Sci. 2020, 29, e96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.; Luo, B.; Cui, J.; Liu, Y.; Liu, Y. Comorbidity of type 2 diabetes mellitus and depression: Clinical evidence and rationale for the exacerbation of cardiovascular disease. Front. Cardiovasc. Med. 2022, 9, 861110. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.J.; Gebregziabher, M.; Martin-Harris, B.; Egede, L.E. Understanding the influence of psychological and socioeconomic factors on diabetes self-care using structured equation modeling. Patient Educ. Couns. 2015, 98, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Strine, T.W.; Mokdad, A.H. Prevalence of depression among US adults with diabetes: Findings from the 2006 behavioral risk factor surveillance system. Diabetes Care 2008, 31, 105–107. [Google Scholar] [CrossRef]
- D’Amato, C.; Morganti, R.; Greco, C.; Di Gennaro, F.; Cacciotti, L.; Longo, S.; Mataluni, G.; Lauro, D.; Marfia, G.A.; Spallone, V. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Vasc. Dis. Res. 2016, 13, 418–428. [Google Scholar] [CrossRef]
- Dziemidok, P.; Dąbrowski, M.; Makara-Studzińska, M. Relationship between diabetic neuropathy and occurrence of depression among diabetic patients. Psychiatr. Pol. 2016, 50, 407–415. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.; Giltay, E.J.; van Veen, T.; Zitman, F.G.; Penninx, B.W. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom. Med. 2013, 75, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-G.; Cai, D.-B.; Liu, J.; Liu, R.-X.; Wang, S.-B.; Tang, Y.-Q.; Zheng, W.; Wang, F. Cholesterol and triglyceride levels in first-episode patients with major depressive disorder: A meta-analysis of case-control studies. J. Affect. Disord. 2020, 266, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Roohafza, H.; Sadeghi, M.; Afshar, H.; Mousavi, G.; Shirani, S. Evaluation of lipid profile in patient with major depressive disorder and generalized anxiety disorder. ARYA Atheroscler. 2010, 1. [Google Scholar]
- Bampi, S.R.; Casaril, A.M.; Domingues, M.; de Andrade Lourenço, D.; Pesarico, A.P.; Vieira, B.; Begnini, K.R.; Seixas, F.K.; Collares, T.V.; Lenardão, E.J. Depression-like behavior, hyperglycemia, oxidative stress, and neuroinflammation presented in diabetic mice are reversed by the administration of 1-methyl-3-(phenylselanyl)-1H-indole. J. Psychiatr. Res. 2020, 120, 91–102. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, B.; Ju, C.; Jin, H.; Ye, X.; Yao, L.; Jia, M.; Sun, Z.; Yuan, Y. The association of decreased serum gdnf level with hyperglycemia and depression in type 2 diabetes mellitus. Endocr. Pract. 2019, 25, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Manjarrez, G.; Hernandez, J. Inhibition and kinetic changes of brain tryptophan-5-hydroxylase during insulin-dependent diabetes mellitus in the rat. Nutr. Neurosci. 2005, 8, 57–62. [Google Scholar] [CrossRef]
- Wilhelm, K.; Gillis, I.; Reddy, J.; Mitchell, P.B.; Campbell, L.; Dobson-Stone, C.; Pierce, K.D.; Schofield, P.R. Association between serotonin transporter promoter polymorphisms and psychological distress in a diabetic population. Psychiatry Res. 2012, 200, 343–348. [Google Scholar] [CrossRef]
- Oury, F.; Karsenty, G. Towards a serotonin-dependent leptin roadmap in the brain. Trends Endocrinol. Metab. 2011, 22, 382–387. [Google Scholar] [CrossRef]
- Khawagi, W.Y.; Al-kuraishy, H.M.; Hussein, N.R.; Al-Gareeb, A.I.; Atef, E.; Elhussieny, O.; Alexiou, A.; Papadakis, M.; Jabir, M.S.; Alshehri, A.A. Depression and type 2 diabetes: A causal relationship and mechanistic pathway. Diabetes Obes. Metab. 2024, 26, 3031–3044. [Google Scholar] [CrossRef]
- Lam, R.W.; Kennedy, S.H.; Adams, C.; Bahji, A.; Beaulieu, S.; Bhat, V.; Blier, P.; Blumberger, D.M.; Brietzke, E.; Chakrabarty, T. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023: Mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can. J. Psychiatry 2024, 69, 641–687. [Google Scholar]
- Dai Cao, T.X.; Filliter, C.; Montastruc, F.; Yu, O.H.Y.; Fergusson, E.; Rej, S.; Azoulay, L.; Renoux, C. Selective serotonin reuptake inhibitors and the risk of type 2 diabetes mellitus in youths. J. Affect. Disord. 2022, 318, 231–237. [Google Scholar]
- Bansal, N.; Hudda, M.; Payne, R.A.; Smith, D.J.; Kessler, D.; Wiles, N. Antidepressant use and risk of adverse outcomes: Population-based cohort study. BJPsych Open 2022, 8, e164. [Google Scholar] [CrossRef]
- Allen, S.N.; Fisher, M.; Phipps, N. The correlation between depression and diabetes. US Pharm. 2014, 39, 12–15. [Google Scholar]
- Rohde, C.; Thomsen, R.W.; Østergaard, S.D. A Within-Subject Before-After Study of the Impact of Antidepressants on Hemoglobin A1c and Low-Density Lipoprotein Levels in Type 2 Diabetes. J. Clin. Psychopharmacol. 2022, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Brieler, J.A.; Salas, J.; Keegan-Garrett, E.; Scherrer, J.F. Achievement of glycemic control and antidepressant medication use in comorbid depression and type 2 diabetes. J. Affect. Disord. 2023, 324, 1–7. [Google Scholar] [CrossRef]
- Khapre, M.; Kant, R.; Sharma, D.; Sharma, A. Antidepressant use and glycemic control in diabetic population: A meta-analysis. Indian J. Endocrinol. Metab. 2020, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- van der Feltz-Cornelis, C.; Allen, S.F.; Holt, R.I.; Roberts, R.; Nouwen, A.; Sartorius, N. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: Systematic review and meta-analysis. Brain Behav. 2021, 11, e01981. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Shahriarirad, R.; Kwon, K.; Bejarano-Pineda, L.; Waryasz, G.; Ashkani-Esfahani, S. Comparative analysis of the therapeutic effects of pregabalin, gabapentin, and duloxetine in diabetic peripheral neuropathy: A retrospective study. J. Diabetes Its Complicat. 2025, 39, 109001. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Shiha, N.A.; Attia, A.S. Escitalopram ameliorates cardiomyopathy in type 2 diabetic rats via modulation of receptor for advanced glycation end products and its downstream signaling cascades. Front. Pharmacol. 2020, 11, 579206. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, L.; Yang, Z.; Li, Q.; Huang, Y.; He, M.; Zhang, S.; Zhang, Z.; Wang, X.; Zhao, W. Metabolic effects of fluoxetine in adults with type 2 diabetes mellitus: A meta-analysis of randomized placebo-controlled trials. PLoS ONE 2011, 6, e21551. [Google Scholar] [CrossRef]
- Abrahamian, H.; Hofmann, P.; Kinzl, J.; Toplak, H. Diabetes mellitus and comorbid depression: Improvement of both diseases with milnacipran. A replication study (results of the Austrian Major Depression Diabetes Mellitus study group). Neuropsychiatr. Dis. Treat. 2012, 8, 355–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abrahamian, H.; Hofmann, P.; Prager, R.; Toplak, H. Diabetes mellitus and co-morbid depression: Treatment with milnacipran results in significant improvement of both diseases (results from the Austrian MDDM study group). Neuropsychiatr. Dis. Treat. 2009, 5, 261–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laimer, M.; Kramer-Reinstadler, K.; Rauchenzauner, M.; Lechner-Schoner, T.; Strauss, R.; Engl, J.; Deisenhammer, E.A.; Hinterhuber, H.; Patsch, J.R.; Ebenbichler, C.F. Effect of mirtazapine treatment on body composition and metabolism. J. Clin. Psychiatry 2006, 67, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Hennings, J.; Ising, M.; Grautoff, S.; Himmerich, H.; Pollmächer, T.; Schaaf, L. Glucose tolerance in depressed inpatients, under treatment with mirtazapine and in healthy controls. Exp. Clin. Endocrinol. Diabetes 2010, 118, 98–100. [Google Scholar] [CrossRef]
- Zhao, Z.; Low, Y.S.; Armstrong, N.A.; Ryu, J.H.; Sun, S.A.; Arvanites, A.C.; Hollister-Lock, J.; Shah, N.H.; Weir, G.C.; Annes, J.P. Repurposing cAMP-modulating medications to promote β-cell replication. Mol. Endocrinol. 2014, 28, 1682–1697. [Google Scholar] [CrossRef]
- Guaiana, G.; Gupta, S.; Chiodo, D.; Davies, S.J.; Haederle, K.; Koesters, M. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst. Rev. 2013, 12. [Google Scholar] [CrossRef]
- Hollander, P.; Gupta, A.K.; Plodkowski, R.; Greenway, F.; Bays, H.; Burns, C.; Klassen, P.; Fujioka, K.; COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013, 36, 4022–4029. [Google Scholar] [CrossRef]
- Raskin, J.; Smith, T.R.; Wong, K.; Pritchett, Y.L.; D’souza, D.N.; Iyengar, S.; Wernicke, J. Duloxetine versus routine care in the long-term management of diabetic peripheral neuropathic pain. J. Palliat. Med. 2006, 9, 29–40. [Google Scholar] [CrossRef]
- Crucitti, A.; Zhang, Q.; Nilsson, M.; Brecht, S.; Yang, C.R.; Wernicke, J. Duloxetine treatment and glycemic controls in patients with diagnoses other than diabetic peripheral neuropathic pain: A meta-analysis. Curr. Med. Res. Opin. 2010, 26, 2579–2588. [Google Scholar] [CrossRef]
- Cipriani, A.; La Ferla, T.; Furukawa, T.A.; Signoretti, A.; Nakagawa, A.; Churchill, R.; McGuire, H.; Barbui, C. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst. Rev. 2009, 2. [Google Scholar] [CrossRef]
- Echeverry, D.; Duran, P.; Bonds, C.; Lee, M.; Davidson, M.B. Effect of pharmacological treatment of depression on A1C and quality of life in low-income Hispanics and African Americans with diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Care 2009, 32, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kosaka, Y.; Kishimoto, N.; Wang, J.; Smith, S.B.; Honig, G.; Kim, H.; Gasa, R.M.; Neubauer, N.; Liou, A. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 2011, 60, 3208–3216. [Google Scholar] [CrossRef]
- Paulmann, N.; Grohmann, M.; Voigt, J.-P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevšek, M.; Fink, H.; Rupnik, M. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Imaizumi, M.; Kim, H.; Yoshida, M.; Fujiwara, T.; Aoyagi, K.; Toyofuku, Y.; Nakamichi, Y.; Nishiwaki, C.; Okamura, T.; Uchida, T. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc. Natl. Acad. Sci. USA 2013, 110, 19420–19425. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, H.; Kim, H.; Park, J.; Choi, W.; Choi, W.; Hong, H.J.; Ro, H.-J.; Jun, S.; Choi, S.H. Lactation improves pancreatic β cell mass and function through serotonin production. Sci. Transl. Med. 2020, 12, eaay0455. [Google Scholar] [CrossRef]
- Ferrero, E.; Masini, M.; Carli, M.; Moscato, S.; Beffy, P.; Vaglini, F.; Mattii, L.; Corti, A.; Scarselli, M.; Novelli, M. Dopamine-mediated autocrine inhibition of insulin secretion. Mol. Cell. Endocrinol. 2024, 592, 112294. [Google Scholar] [CrossRef]
- Kadioglu, S.; Muci, E.; Kesim, M.; Ulku, C.; Duman, M.; Kalyoncu, N.; Yariş, E. The effect of paroxetine, a selective serotonin reuptake inhibitor, on blood glucose levels in mice. Int. J. Pharmacol. 2011, 7, 283–290. [Google Scholar] [CrossRef]
- Chang, H.H.; Chi, M.H.; Lee, I.H.; Tsai, H.C.; Gean, P.W.; Yang, Y.K.; Lu, R.-B.; Chen, P.S. The change of insulin levels after six weeks antidepressant use in drug-naive major depressive patients. J. Affect. Disord. 2013, 150, 295–299. [Google Scholar] [CrossRef]
- Briscoe, V.J.; Ertl, A.C.; Tate, D.B.; Dawling, S.; Davis, S.N. Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes 2008, 57, 2453–2460. [Google Scholar] [CrossRef]
- Briscoe, V.J.; Ertl, A.C.; Tate, D.B.; Davis, S.N. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes 2008, 57, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.; Knudsen, J.S.; Schmitz, N.; Østergaard, S.D.; Thomsen, R.W. The impact of hospital-diagnosed depression or use of antidepressants on treatment initiation, adherence and HbA 1c/LDL target achievement in newly diagnosed type 2 diabetes. Diabetologia 2021, 64, 361–374. [Google Scholar] [CrossRef]
- Wu, C.-S.; Hsu, L.-Y.; Pan, Y.-J.; Wang, S.-H. Associations between antidepressant use and advanced diabetes outcomes in patients with depression and diabetes mellitus. J. Clin. Endocrinol. Metab. 2021, 106, e5136–e5146. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.-C.; Huang, K.-L.; Chen, H.-M.; Chen, P.-C.; Chen, V.C.-H.; Chiu, W.-C. Antidepressants and the risk of myocardial infarction among patients with diabetes: A population-based cohort study. J. Affect. Disord. 2021, 294, 109–114. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, T.-Y.; Lee, C.-P.; Yang, Y.-H.; McIntyre, R.S.; Subramaniapillai, M.; Lee, Y.; Chen, V.C.-H. Association between selective serotonin reuptake inhibitor and risk of peripheral artery disease in diabetes mellitus: Propensity score matching and landmark analysis. Medicine 2022, 101, e29202. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Yang, Y.-H.; Chen, K.-J.; Lee, Y.; McIntyre, R.S.; Lu, M.-L.; Lee, Y.-C.; Hsieh, M.-C.; Chen, V.C.-H. Antidepressants reduced risk of mortality in patients with diabetes mellitus: A population-based cohort study in Taiwan. J. Clin. Endocrinol. Metab. 2019, 104, 4619–4625. [Google Scholar] [CrossRef]
- Kauffman, R.P.; Castracane, V.D.; White, D.L.; Baldock, S.D.; Owens, R. Impact of the selective serotonin reuptake inhibitor citalopram on insulin sensitivity, leptin and basal cortisol secretion in depressed and non-depressed euglycemic women of reproductive age. Gynecol. Endocrinol. 2005, 21, 129–137. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Shults, J.; Rutherford, N.; Schwartz, S. Safety and efficacy of s-citalopram in patients with co-morbid major depression and diabetes mellitus. Neuropsychobiology 2007, 54, 208–214. [Google Scholar] [CrossRef]
- Dhavale, H.; Panikkar, V.; Jadhav, B.S.; Ghulghule, M.; Agari, A.D. Depression and diabetes: Impact of antidepressant medications on glycaemic control. J. Assoc. Physicians India 2013, 61, 896–899. [Google Scholar]
- Buhl, E.S.; Jensen, T.K.; Jessen, N.; Elfving, B.; Buhl, C.S.; Kristiansen, S.B.; Pold, R.; Solskov, L.; Schmitz, O.; Wegener, G. Treatment with an SSRI antidepressant restores hippocampo-hypothalamic corticosteroid feedback and reverses insulin resistance in low-birth-weight rats. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E920–E929. [Google Scholar] [CrossRef]
- Meng, H.; Lu, J.; Zhang, X. Metabolic Influences of Commonly used Antidepressants on Blood Glucose Homeostasis. Indian J. Pharm. Sci. 2019, 81, 188. [Google Scholar] [CrossRef]
- Knol, M.J.; Geerlings, M.I.; Egberts, A.C.G.; Gorter, K.J.; Grobbee, D.E.; Heerdink, E.R. No increased incidence of diabetes in antidepressant users. Int. Clin. Psychopharmacol. 2007, 22, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Farino, Z.J.; Morgenstern, T.J.; Maffei, A.; Quick, M.; De Solis, A.J.; Wiriyasermkul, P.; Freyberg, R.J.; Aslanoglou, D.; Sorisio, D.; Inbar, B.P. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol. Psychiatry 2020, 25, 2070–2085. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, C.; Wu, C.; Wu, T.; Pang, J.; Zhou, P.; Cao, Y. Antidepressant effect of carvedilol on streptozotocin-induced diabetic peripheral neuropathy mice by altering gut microbiota. Biochem. Biophys. Res. Commun. 2024, 730, 150374. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, V.; Sarzi-Puttini, P.; Pellegrino, G.; Sirotti, S.; Atzeni, F.; Alciati, A.; Torta, R.; Varrassi, G.; Fornasari, D.; Coaccioli, S. Pharmacological treatment of fibromyalgia syndrome: A practice-based review. Curr. Pain Headache Rep. 2024, 28, 1349–1363. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Y.; Chen, L.; Liu, Y.; Du, B. Effects of the selective serotonin reuptake inhibitor fluoxetine on glucose metabolism: A systematic review. Asian J. Psychiatry 2022, 73, 103092. [Google Scholar] [CrossRef]
- Sun, J.W.; Hernández-Díaz, S.; Haneuse, S.; Bourgeois, F.T.; Vine, S.M.; Olfson, M.; Bateman, B.T.; Huybrechts, K.F. Association of selective serotonin reuptake inhibitors with the risk of type 2 diabetes in children and adolescents. JAMA Psychiatry 2021, 78, 91–100. [Google Scholar] [CrossRef]
- Andersohn, F.; Schade, R.; Suissa, S.; Garbe, E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am. J. Psychiatry 2009, 166, 591–598. [Google Scholar] [CrossRef]
- Yoon, J.M.; Cho, E.-G.; Lee, H.-K.; Park, S.M. Antidepressant use and diabetes mellitus risk: A meta-analysis. Korean J. Fam. Med. 2013, 34, 228. [Google Scholar] [CrossRef]
- Azevedo Da Silva, M.; Fournier, A.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Nabi, H.; Fagherazzi, G. Increased risk of type 2 diabetes in antidepressant users: Evidence from a 6-year longitudinal study in the E3N cohort. Diabet. Med. 2020, 37, 1866–1873. [Google Scholar] [CrossRef]
- Raeder, M.B.; Bjelland, I.; Vollset, S.E.; Steen, V.M. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: The Hordaland Health Study. J. Clin. Psychiatry 2006, 67, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Metabolic disorders induced by psychotropic drugs. Ann. Endocrinol. 2023, 84, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Liu, D.; Sun, W.; Xu, C.; Chen, M.; Cao, H.; Zhang, F. Antidepressants account for the causal effect of major depressive disorder on type 2 diabetes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 136, 111164. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Ben-Shushan, G.; Hershkovitz, A.; Isaac, R.; Gil-Ad, I.; Shvartsman, D.; Ronen, D.; Weizman, A.; Zick, Y. Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol. Cell. Neurosci. 2007, 36, 305–312. [Google Scholar] [CrossRef]
- Brown, L.C.; Majumdar, S.R.; Johnson, J.A. Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res. Clin. Pract. 2008, 79, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Shen, Y.-C.; Hung, Y.-J.; Chao-Ha, C.; Yeh, C.-B.; Perng, C.-H. Comparisons of glucose–insulin homeostasis following maprotiline and fluoxetine treatment in depressed males. J. Affect. Disord. 2007, 103, 257–261. [Google Scholar] [CrossRef]
- Zilliox, L.; Russell, J.W. Treatment of diabetic sensory polyneuropathy. Curr. Treat. Options Neurol. 2011, 13, 143–159. [Google Scholar] [CrossRef] [PubMed]
- De Long, N.E.; Gutgesell, M.K.; Petrik, J.J.; Holloway, A.C. Fetal exposure to sertraline hydrochloride impairs pancreatic β-cell development. Endocrinology 2015, 156, 1952–1957. [Google Scholar] [CrossRef]
- Ruiz-Santiago, C.; Rodríguez-Pinacho, C.V.; Pérez-Sánchez, G.; Acosta-Cruz, E. Effects of selective serotonin reuptake inhibitors on endocrine system. Biomed. Rep. 2024, 21, 128. [Google Scholar] [CrossRef]
- Biagetti, B.; Corcoy, R. Hypoglycemia associated with fluoxetine treatment in a patient with type 1 diabetes. World J. Clin. Cases WJCC 2013, 1, 169. [Google Scholar] [CrossRef]
- Liu, B.; Ruz-Maldonado, I.; Toczyska, K.; Olaniru, O.E.; Zariwala, M.G.; Hopkins, D.; Zhao, M.; Persaud, S.J. The selective serotonin reuptake inhibitor fluoxetine has direct effects on beta cells, promoting insulin secretion and increasing beta-cell mass. Diabetes Obes. Metab. 2022, 24, 2038–2050. [Google Scholar] [CrossRef]
- Eckel, R.H.; Bornfeldt, K.E.; Goldberg, I.J. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021, 33, 1519–1545. [Google Scholar] [CrossRef]
- Li, X.-Q.; Tang, X.-R.; Li, L.-L. Antipsychotics cardiotoxicity: What’s known and what’s next. World J. Psychiatry 2021, 11, 736. [Google Scholar] [CrossRef]
- AL-Jahwari, L.; Qutishat, M.; Almaqbali, M.; Al Risi, K.; AlBreiki, M.; Albalushi, M.; Al-Huseini, S. Effects of antidepressants on body weight in patients treated in a naturalistic setting in Oman. Middle East Curr. Psychiatry 2025, 32, 3. [Google Scholar] [CrossRef]
- Boyce, P.; Ma, C. Choosing an antidepressant. Aust. Prescr. 2021, 44, 12. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Meehan, S.R.; Chen, D.; Brubaker, M.; Weiss, C. Changes in metabolic parameters and body weight in patients with prediabetes treated with adjunctive brexpiprazole for major depressive disorder: Pooled analysis of short-and long-term clinical studies. J. Clin. Psychiatry 2023, 84, 48721. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Yasuda, H.; Hotta, N.; Kasuga, M.; Kashiwagi, A.; Kawamori, R.; Yamada, T.; Baba, Y.; Alev, L.; Nakajo, K. Efficacy and safety of 40 mg or 60 mg duloxetine in J apanese adults with diabetic neuropathic pain: Results from a randomized, 52-week, open-label study. J. Diabetes Investig. 2016, 7, 100–108. [Google Scholar] [CrossRef]
- Greene, C.R.; Blackbourn, L.A.; McGurnaghan, S.J.; Mercer, S.W.; Smith, D.J.; Wild, S.H.; Wu, H.; Jackson, C.A.; On behalf of the Scottish Diabetes Research Network epidemiology group. Antidepressant and antipsychotic prescribing in patients with type 2 diabetes in Scotland: A time-trend analysis from 2004 to 2021. Br. J. Clin. Pharmacol. 2024, 90, 2802–2810. [Google Scholar] [CrossRef]
- Kim, J.; Hurh, K.; Han, S.; Kim, H.; Park, E.-C.; Jang, S.-Y. Association between antidepressants and the risk of diabetic foot ulcers and amputation in antidepressant-naïve type 2 diabetes mellitus patients: A nested case-control study. Diabetes Res. Clin. Pract. 2024, 209, 111591. [Google Scholar] [CrossRef]
- Wu, C.-S.; Huang, Y.-J.; Ko, Y.-C.; Lee, C.-H. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: A systematic review and meta-analysis of randomized controlled trials. Syst. Rev. 2023, 12, 53. [Google Scholar] [CrossRef]
- Brooks, B.M.; Shih, C.-D.; Brooks, B.M.; Tower, D.E.; Tran, T.T.; Simon, J.E.; Armstrong, D.G. The Diabetic Foot–Pain–Depression Cycle: A Multidisciplinary Cohort Study. J. Am. Podiatr. Med. Assoc. 2023, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.; Simó, R.; Sanchís, P.; Ayala, L.; Fortuny, R.; Zubillaga, I.; Masmiquel, L. Eating disorders are frequent among type 2 diabetic patients and are associated with worse metabolic and psychological outcomes: Results from a cross-sectional study in primary and secondary care settings. Acta Diabetol. 2015, 52, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.M.; Bratches, R.W.R.; Wolff, K.B.; Stapp, M.D.; Bruce, K.W.; Tower, D.E. Opioid-Prescribing Approaches-One-Size-Fits-All versus Patient-Centric and Procedure-Focused-Among Podiatric Physicians: A Cross-Sectional Study. J. Am. Podiatr. Med. Assoc. 2023, 113, 21–246. [Google Scholar] [CrossRef]
- Ryans, C.P.; Brooks, B.M.; Tower, D.E.; Robbins, J.M.; Butterworth, M.L.; Stapp, M.D.; Nettles, A.M.; Brooks, B.M. Evidence-Based Opioid Education That Reduces Prescribing: The 10 Principles of Opioid Prescribing in Foot and Ankle Surgery. J. Foot Ankle Surg. 2024, 63, 214–219. [Google Scholar] [CrossRef] [PubMed]
| Antidepressant Drug Class | Effect(s)/Potential Impact on Diabetes Mellitus and its Complications |
|---|---|
| SSRIs: Selective serotonin reuptake inhibitors | Inhibition of insulin release secondary to pancreatic cell dysfunction; dysregulation of the HPA axis and development of insulin resistance; weight changes (gain or loss); weak anticoagulants (may increase the risk of bleeding with the potential to reduce vascular inflammation and improve endothelial function). |
| TCAs: Tricyclic antidepressants | Weight gain and insulin resistance. |
| SNRIs: Serotonin-norepinephrine reuptake inhibitors | Weight changes (gain or loss). Potential for neuropathic pain relief in the treatment of diabetic peripheral neuropathy (DPN). |
| MAOIs: Monoamine oxidase inhibitors | Weight gain; hydrazine-type MAOIs have been observed to decrease the fasting blood glucose and improve glucose tolerance in diabetic patients. |
| NDRIs: Norepinephrine-dopamine reuptake inhibitors | Associated with a potential risk of type 2 diabetes, especially with long-term use and in higher doses. |
| NMDA-RAs: N-methyl-D-aspartate receptor antagonists | Enhanced glucose tolerance; some research suggests that NMDA-RAs can help protect or even regenerate pancreatic islet cells. |
| NRIs: Norepinephrine reuptake inhibitors | Potentially worsening glycemic control in some cases and improving it in others. |
| 5-HT2 antagonists | Weight gain is rare. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, B.M.; Nettles, A.M.; Brooks, B.M. Diabetes Psychiatry: The Missing Piece of the Puzzle to Prevent Complications of the Diabetes Pandemic. Psychoactives 2025, 4, 13. https://doi.org/10.3390/psychoactives4020013
Brooks BM, Nettles AM, Brooks BM. Diabetes Psychiatry: The Missing Piece of the Puzzle to Prevent Complications of the Diabetes Pandemic. Psychoactives. 2025; 4(2):13. https://doi.org/10.3390/psychoactives4020013
Chicago/Turabian StyleBrooks, Bradley M., Ashley M. Nettles, and Brandon M. Brooks. 2025. "Diabetes Psychiatry: The Missing Piece of the Puzzle to Prevent Complications of the Diabetes Pandemic" Psychoactives 4, no. 2: 13. https://doi.org/10.3390/psychoactives4020013
APA StyleBrooks, B. M., Nettles, A. M., & Brooks, B. M. (2025). Diabetes Psychiatry: The Missing Piece of the Puzzle to Prevent Complications of the Diabetes Pandemic. Psychoactives, 4(2), 13. https://doi.org/10.3390/psychoactives4020013






