Abstract
The history of cannabis research spans centuries, with a focus on isolating and understanding its active compounds. Although plants like opium and coca yielded active alkaloids relatively early, cannabis posed unique challenges due to its active substances existing in oily mixtures that were difficult to isolate. Early studies in the 19th century, such as Ferdinand Tscheppe’s 1821 research, debunked claims of opioid-like substances in hemp, setting the stage for further exploration. In the late 19th and early 20th centuries, key figures like Sir William Brooke O’Shaughnessy and Robert Sidney Cahn made significant contributions to the understanding of cannabis’s chemical components, though major breakthroughs were delayed due to technical limitations. In the 1940s, Ghosh and Adams independently elucidated the structure of cannabinol, marking the first cannabinoid identified from cannabis. Subsequent work, including Šantavý’s 1964 and Mechoulam and Gaoni’s 1964 identification of ∆9-THC, confirmed the psychoactive compound’s structure and absolute configuration. This article traces these incremental advancements, highlighting the critical role of each researcher’s contributions in piecing together the puzzle of cannabinoid chemistry. It underscores that scientific progress thrives on collaboration and shared knowledge.
1. Introduction
People have been interested in plants since ancient times [1]. However, it was not until the early 19th century that the study of plants began to intensify, particularly the effort to clarify their active compounds. Although research started around the same time, progress was not the same for all these significant plants. While research into opium, the dried sap obtained from the unripe seed pods of the opium poppy (Papaver somniferum), and into coca leaves (Erythroxylum coca) progressed quickly, leading to the isolation of active natural substances, the isolation and identification of the active compound in hemp (Cannabis sativa) was unsuccessful. All three of these plants began to be studied roughly simultaneously.
Morphine was first isolated from opium in an impure form 220 years ago by a German pharmacist and pioneer of alkaloid chemistry, Friedrich Wilhelm Sertürner (1805). It was isolated in its pure form 208 years ago [2,3], and its structure was determined 203 years ago by John Masson Gulland and Robert Robinson [4,5]. It was synthesized by Marshall Gates and Gilg Tschudi [6,7]. Cocaine was isolated from coca leaves 170 years ago by Friedrich Gaedcke under the name erythroxyline [8]. Albert Niemann, 165 years ago, developed an improved purification process [9,10,11], and its structure was determined and it was synthesized 127 years ago by Richard Willstätter [12,13]. However, the chemistry of cannabis was still unfinalized, as the active compound was not isolated in pure form and its structure was unknown. I believe the explanation is straightforward. Opium and coca contain alkaloids that can easily be separated and crystallized as salts. This made it easy to isolate them, and the same was true for other plants where crystalline substances could be isolated. However, in many other plants—cannabis being one of the best examples—the active ingredients were found as part of mixtures. The methods available at the time were not sufficient for chemists to isolate these substances. The problem was that these substances did not crystallize; in their pure form, they were oily, and the same was true for their mixtures. At the time, isolating a pure oily substance from an oily mixture was nearly impossible. So, what was the case with cannabis? The following will shed light on this problem and its history.
Cannabis is an ancient, cultivated crop. It has been used as a valuable domestic and industrial plant, a recreational drug, and medicine since ancient times [14,15]. While morphine and cocaine were discovered relatively quickly and soon used in medicine, the case with cannabis was entirely different. The identification of its active substance took a long time, and its use in medicine is still progressing slowly and facing challenges today, even though in the past it was used for medical treatment without any issues.
2. Part I: First Steps to Chemical Research
The oldest scientific work dealing with chemical research on cannabis is probably a publication from 1821 (see Figure 1), i.e., 204 years old [16]. Medical student Ferdinand Tscheppe, in his dissertation, chemically examined the leaves of the local Cannabis sativa. As the subject of his dissertation, he chose to analyze hemp leaves to investigate whether any of the active ingredients of opium or similar substances could be found in them. The main and most significant result of Tscheppe’s work was the finding that European hemp (Cannabis sativa) does not contain morphine or other opioid-like significantly narcotic substances, as previously claimed. Among other things, he found out that there is no morphine in hemp leaves. Tscheppe also suggested that the differences in effects between European and Indian hemp (Cannabis indica) might be due to climatic and soil conditions.

Figure 1.
Dissertation of Ferdinand Tscheppe.
The first European to investigate the action of Indian hemp with any degree of scientific accuracy was Irish physician Sir William Brooke O’Shaughnessy [17].
Schlesinger [18] studied pollen qualitatively and quantitatively and was apparently the first investigator to obtain active extracts from the leaves and flowers of hemp.
Another attempt at cannabis analysis dates to the 1840s, when Bohlig [19] thoroughly chemically studied various parts of hemp and stinging nettle and prepared essential oil from them.
Smith and Smith [20] prepared from gunjah (cannabis) after purification an alcoholic extract that gave, after evaporation, powerful and completely intoxicating resin.
DeCourtive, a student of the French psychiatrist Jacques Joseph Moreau [21], wrote the first known thesis on hashish. Moreau is known for conducting the first psychopharmacological study of hashish. In his research, DeCourtive [22,23,24] conducted chemical research on hashish and its effects on animals and humans. He also prepared for Moreau, who conducted observations on himself, extracts, and following his example, also prepared these extracts himself and described his experiences and observations. Then, he tested these hashish extracts on animals and his friends. It was, for the time, excellent scientific work.
3. Part II: Starting to Understand the Chemistry
Bolas and Francis [25] heated the resinous extract of Indian hemp with nitric acid. They called the resulting substance of this drastic method oxy-cannabin (molecular formula C5H6O2) and stated that this compound was probably formed by the oxidation of some substance in the extract.
In 1896, Wood [26] isolated a compound with the formula C18H24O2 from charas, the exuded resin of Indian hemp. They named the compound cannabinol, as it was undoubtedly a hydroxyl derivative. This compound was extremely stable, but its formula was not yet correct.
C. R. Marshall [27,28]—assistant to the Downing professor of medicine, Cambridge—tested different concentrations and isolates from charas on laboratory animals (cats, dogs, and rabbits) and more than thirty times on himself. In the journal The Lancet, he described his personal experience with the substances isolated by Wood, Spivey, and Easterfield (Figure 2): “February 19th, 1898, I took from 0.1 to 0.15 gramme (from 1½ to 2 grains) of the pure substance … The substance very gradually dissolved in my mouth … I had the most irresistible desire to laugh … I sat upon the stool and laughed incessantly for several minutes. … When reclining in a chair I was happy beyond description, and afterward I was told that I constantly exclaimed, ‘This is lovely’! But I do not remember having any hallucinations: the happiness seemed rather to result from an absence of all external irritation. … The most peculiar effect was a complete loss of time relation: time seemed to have no existence: I appeared to be living in a present without a future or a past. … a complete loss of memory for recent events”.

Figure 2.
Thomas Barlow Wood (21 January 1869–6 November 1929); Thomas Hill Easterfield (4 March 1866–1 March 1949).
Dunstan and Henry [29] reported the isolation of a crystalline cannabinol acetate, but they gave no experimental details (Scheme 1).
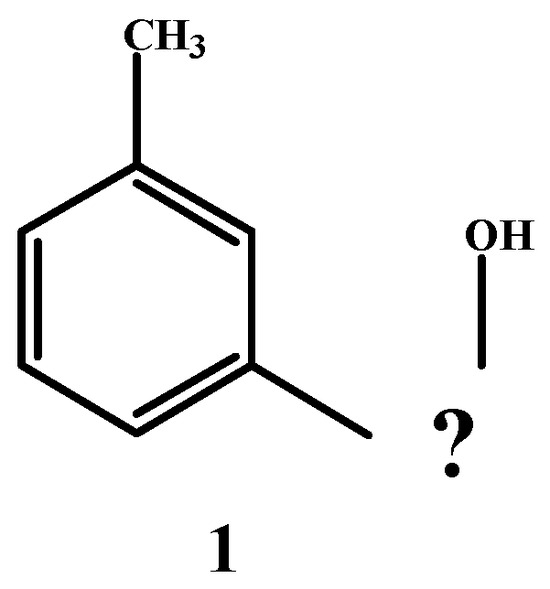
Scheme 1.
Identified parts of the cannabinol molecule.
4. Part III: Real Chemistry with the Structures of Chemical Compounds
In 1899, Wood et al. [30] determined that the compound known as cannabinol has the molecular formula C21H26O2. They also confirmed that this compound contains a hydroxyl group and a benzene nucleus, with two side chains in the meta-position relative to each other, one of which is a methyl group (1). Additionally, the compound is optically inactive.
This research ultimately led to a major disaster in the laboratory [31]: “On October 9th, 1901, a serious accident occurred in the University Chemical Laboratory, Cambridge, which a fortnight later led to the death of Mr. William Thomas Newton Spivey… It was while he was preparing material for the synthesis of cannabinolactone, a derivative of cannabinol, the narcotic principle of Indian hemp, that he met with the sad accident that ultimately caused his death. A flask containing a considerable quantity of carbon disulphide, which he had been using as a diluent in Etard’s reaction for preparing aromatic aldehydes, accidentally broke in his hand after the reaction had been completed. Some of the disulphide saturated his clothes, the rest vaporized, and the explosive mixture of vapor and air ignited. The explosion caused several wounds, but the most serious injuries were the burns due to the ignition of the disulphide with which his clothes were saturated. All appeared to be going well with him for a week after the accident, when pneumonia, which so frequently follows severe burns, supervened and caused his death after a second week’s painful illness”. Thomas Barlow Wood was seriously poisoned when he voluntarily tested the effects of “red oil” on himself [32]. It was also reported that T. H. Easterfield died during this research because of an explosion during the hydrogenation of “red oil”. As it later turned out, this report was not based on truth. In 1899, Easterfield was appointed professor at the Victoria University of Wellington, New Zealand. Sir Easterfield died in Nelson, New Zealand, on 1 March 1949.
The term “red oil” was historically used to refer to a viscous, reddish-brown extract obtained from Cannabis sativa. In the 19th and early 20th centuries, scientists were unable to isolate individual cannabinoids but recognized that this oil contained active compounds responsible for the effects of cannabis. It was not until the 1940s that cannabinol and cannabidiol were isolated, followed by tetrahydrocannabinol. Until then, “red oil” was an undefined mixture containing cannabinoids, terpenes, and other compounds, with its exact composition remaining unknown.
Robert Sidney Cahn [33,34,35,36] was a distinguished British chemist who made significant contributions to the study of cannabis compounds (Figure 3). In a series of four research papers, he explored the chemical structure of cannabinol. In the fourth paper, he tentatively proposed a structural formula for the compound, successfully determining the basic skeleton of cannabinoids. Cahn’s proposed structure (2) left the positions of the hydroxyl group and the n-pentyl group uncertain, yet his work represented a critical milestone in the identification of cannabinoid structures. As noted at the end of the publication, experiments conducted by Dr. H. R. Ing at University College, London, and Dr. H. Marx at the Medizinische Klinik, Heidelberg, revealed that cannabinol is not the pharmacologically active component of Cannabis resin (Scheme 2).

Figure 3.
Robert Sidney Cahn (9 June 1899–15 June 1981).
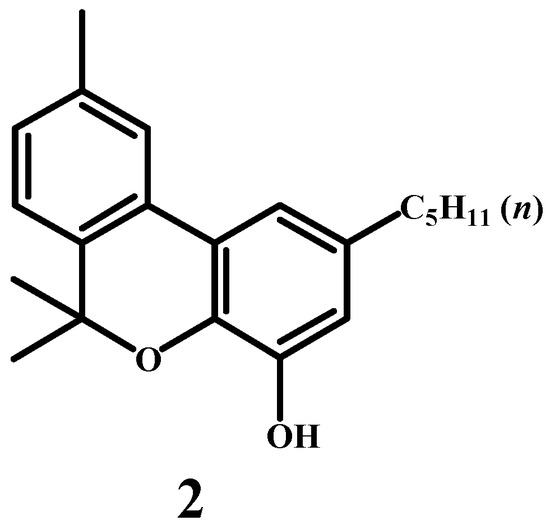
Scheme 2.
Tentative structure of the cannabinol molecule.
After solving the main problem—the structure of the basic skeleton and almost the entire molecule of cannabinol—Cahn surprisingly did not pursue this issue further. It then took a full seven lean years before progress gained full momentum, and the correct structure of cannabinol was finally resolved—the first correct structure of any cannabinoid ever, even though it was later revealed to be an artifact formed from tetrahydrocannabinol [37,38].
5. Part IV: Golden Age of Identifications
Jacob and Todd (Figure 4) successfully isolated cannabidiol and cannabinol from Egyptian hashish in approximately equal amounts [39]. Their study highlighted significant differences in the composition of cannabis resin depending on the plant’s geographical origin. Cannabidiol was identified as an unsaturated derivative with two double bonds, while the structure of cannabinol required further investigation. Spectral analysis and chemical reactions confirmed that the double bonds in cannabidiol are not conjugated with the aromatic nucleus. Four potential structures for cannabinol were proposed, labeled as (3), (4), (5), and (6) (Scheme 3), with structures (3) and (5) deemed the most probable. A later communication from Professor Adams suggested that cannabidiol is a resorcinol derivative. If this is accurate, structure (5) for cannabinol is more likely than (3), as it better aligns with its reducing properties.

Figure 4.
Alexander Robertus Todd (2 October 1907–10 January 1997); Roger Adams (2 January 1889–6 July 1971).
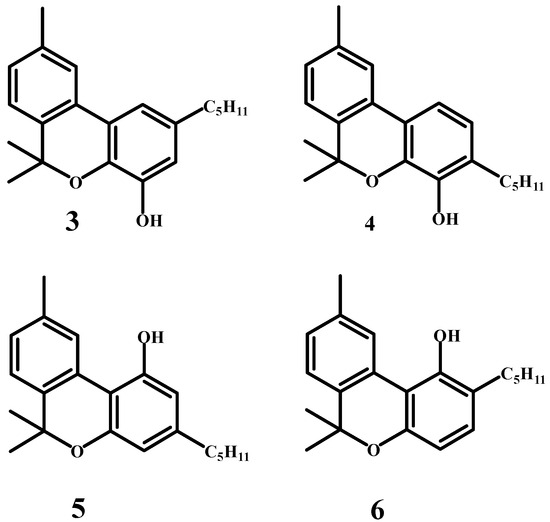
Scheme 3.
Four predicted structures of the cannabinol molecule.
In 1940, Ghosh [40] elucidated a structure of cannabinol (7) (Scheme 4), the first known cannabinoid from the hemp plant (Cannabis sativa L.). Simultaneously, in the same year, an American organic chemist, Adams (Figure 4), and his colleagues [41] also resolved this structure independently. There is no need to ponder who was first. History shows us that many discoveries occurred independently and simultaneously in different laboratories. We will see this later with other cannabinoids as well.
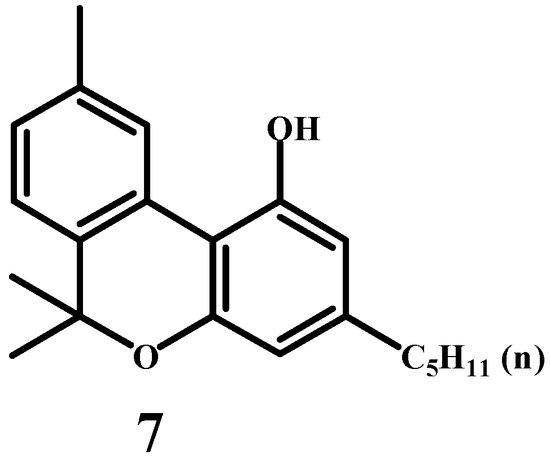
Scheme 4.
The correct structure of the cannabinol molecule.
Next, Adams’ publication [42] focuses on the structure of cannabidiol (CBD), isolated from the “red oil” extract of Minnesota wild hemp (Cannabis sativa). It represents an early and significant step in understanding cannabidiol’s chemistry. This compound does not exhibit the psychoactive properties typical of Cannabis sativa extracts, distinguishing it from tetrahydrocannabinol. The exact positions of double bonds in cannabidiol’s left-hand ring remain unresolved. The publication presents a tentative structure for cannabidiol, referred to as Structure (8) (Scheme 5).
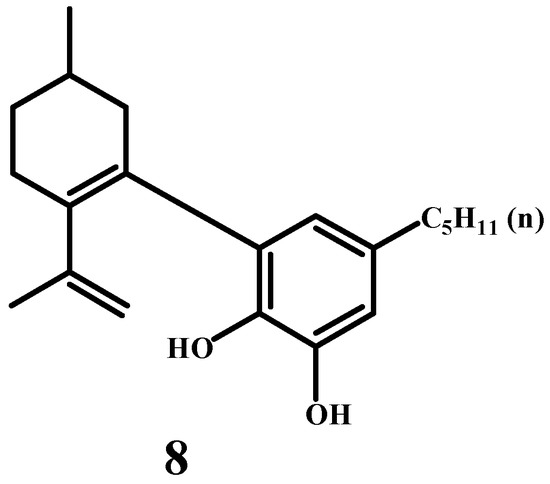
Scheme 5.
The predicted structure of the cannabidiol molecule.
In their later publication [43], the authors discuss the structure of cannabidiol and analyze the position of alicyclic double bonds in the molecule. The structures (9–12) mentioned in the publication represent possible configurations of the left-hand rings in the cannabidiol molecule (Scheme 6). These structures aim to delineate the arrangement of double bonds and other functional groups in the molecule’s alicyclic and aromatic regions. The publication concludes that structure (9) is the most probable configuration of cannabidiol (in fact, the correct structure is (12)). Unfortunately, Adams was unable to determine with certainty the correct structure of cannabidiol, nor was he ever able to do so later.
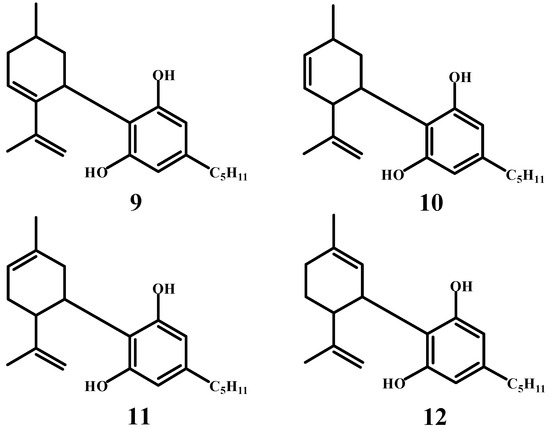
Scheme 6.
Other possible structures of the cannabidiol molecule.
The primary goal of Adams’ group was to convert cannabidiol, isolated from Cannabis sativa extracts, into tetrahydrocannabinol (THC) and study the resulting compound’s structure, properties, and physiological activity [44,45]. The dehydrogenation of THC yielded cannabinol, a compound that had already been confirmed through synthesis. This step solidified the identification of THC’s structure (13) as a precursor to cannabinol. The structure of tetrahydrocannabinol was essentially almost solved (Scheme 7). There was only one problem left—where exactly in the terpene cycle is the double bond located? The absolute configuration of tetrahydrocannabinol was not yet considered at that time.
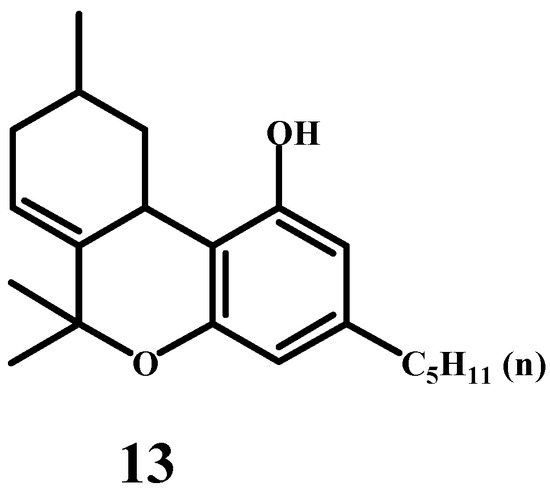
Scheme 7.
The predicted structure of the tetrahydrocannabidiol molecule.
Adams worked intensively in cannabis research. In 1940, he published 22 scientific papers, 15 of which were dedicated to cannabinoids. Interestingly, he never attempted to isolate tetrahydrocannabinol (THC) from cannabis. The reason for this remains unclear, but it is possible that he lacked access to marijuana or hashish with high THC content. Nevertheless, he worked extensively on the topic of tetrahydrocannabinols. Two of his works stand out. In one of them [46], he identifies compounds (14) and (15) as tetrahydrocannabinols, attributing the highest likelihood of natural THC to the latter. In another publication [47], he describes additional tetrahydrocannabinols, specifically compounds (16) and (17) (Scheme 8). It is particularly intriguing that he never considered tetrahydrocannabinol with the double bond in the correct position of the natural compound, specifically Δ9-THC.
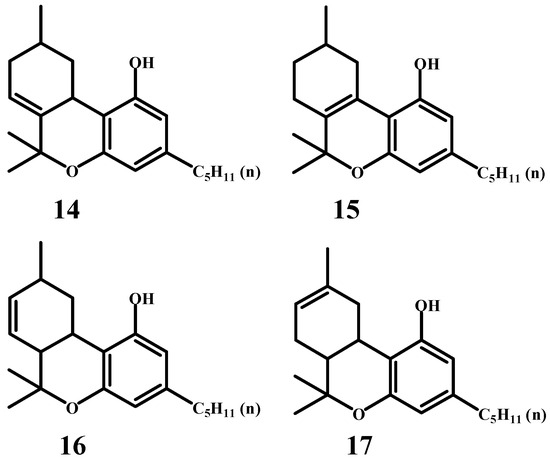
Scheme 8.
Other possible tetrahydrocannabidiol molecules.
Ghosh [48] obtained compound (15) as an intermediate in the cannabinol synthesis and mentioned that this compound is probably a tetrahydrocannabinol.
Adams [49] conducted a series of experiments with tetrahydrocannabinol (15), modifying its pentyl side chain to longer chains and subjecting these compounds to pharmacological tests on dogs to observe their ataxia (see Figure 5). He found that 1’,2’-dimethylheptyl-tetrahydrocannabinol was 512 times more potent in this test than tetrahydrocannabinol (15).
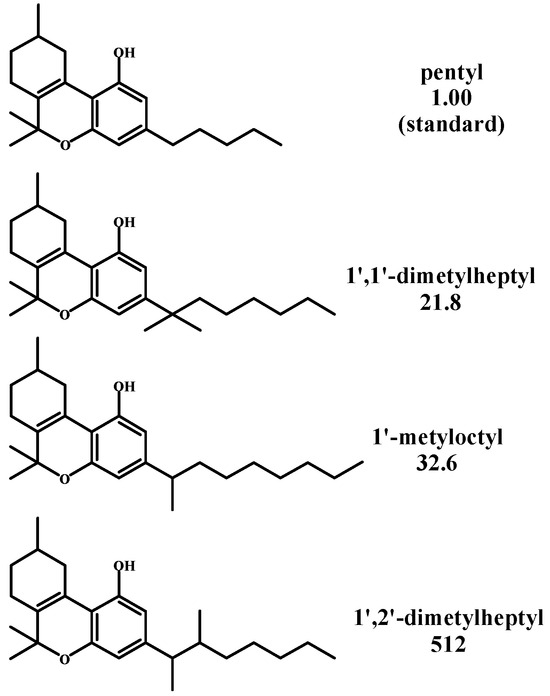
Figure 5.
Side chain derivatives of tetrahydrocannabinol.
Ten years after the correct structure of cannabinol discovery, Krejčí [50,51,52,53] was the first to demonstrate and describe the prominent antibacterial effect of cannabis upon Gram-positive microorganisms, including several pathogenic microbes. Krejčí and Šantavý [54] isolated the main substance responsible for the antibacterial effect and named it cannabidiolic acid (18). This was the first real cannabinoid compound ever discovered. From that year until 1990, the Faculty Hospital in Olomouc, Czechoslovakia, was the only medical facility in the world where patients were treated with cannabis. At that time, the structure of the compound C22H30O3 was only tentative (see Figure 6).
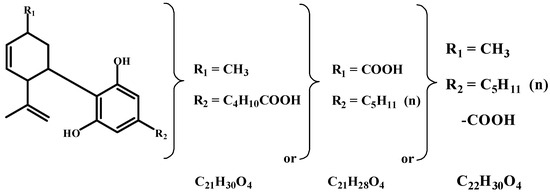
Figure 6.
The authors’ approach to cannabidiolic acid identification.
Later, it was determined exactly where the carboxyl group is in the molecule [53]. However, the position of the double bond remained incorrect within the molecule (see Figure 7 and Figure 8).
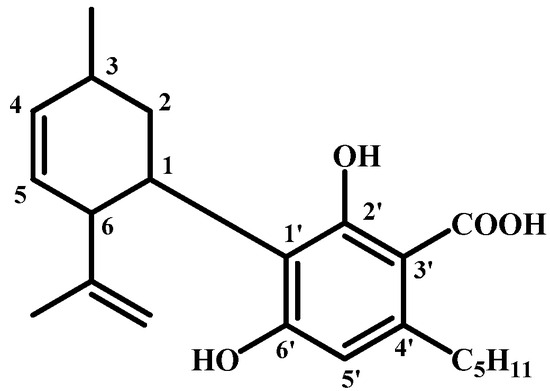
Figure 7.
The structure of CBDA with the correct position of the carboxyl group.

Figure 8.
Zdeněk Krejčí (25 March 1923–31 March 1992).
In 1963, the “ice finally broke”. Mechoulam and Shvo [55] published the correct structure of cannabidiol, but with the incorrect absolute configuration (see Figure 9). In the “Note added in proof”, Mechoulam writes: “Prof. F. Šantavý of Palacký University, Olomouc, Czechoslovakia has kindly informed us that, mainly based on optical rotation data, he has reached the same conclusion as reported in this paper regarding the structure of cannabidiol and that his manuscript is in preparation”.
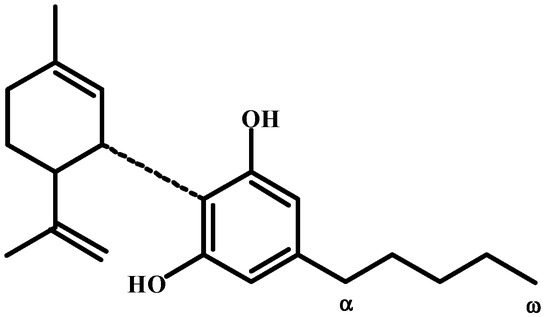
Figure 9.
The absolute configuration of cannabidiol as proposed by Mechoulam.
Šantavý published a manuscript in 1964 [56], which had been submitted in 1963 [57], presenting the correct structures and absolute configurations of cannabidiol (CBD) (19), cannabidiolic acid (CBDA) (18), ∆9-tetrahydrocannabinol (∆9-THC) (20), and ∆8-tetrahydrocannabinol (∆8-THC) (21) (Scheme 9). I have the original Šantavý manuscript, as provided by Professor Mechoulam (see Figure 10). Three years later, in 1967, Mechoulam and Gaoni [58] determined the absolute configuration of ∆9-tetrahydrocannabinol based on its correlation with known terpenoids. It was identical to the absolute configuration established by Professor Šantavý in 1964.
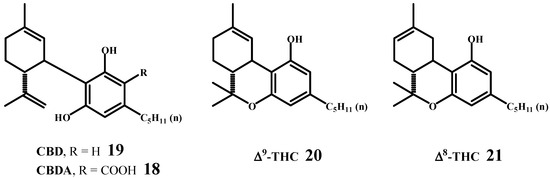
Scheme 9.
The first correct structures and absolute configurations of the molecules of cannabidiol, cannabidiolic acid, ∆9-tetrahydrocannabidiol and ∆8-tetrahydrocannabinol.

Figure 10.
The first page of Šantavý’s original manuscript.
In 1964, Gaoni and R. Mechoulam [59] isolated and identified a psychotomimetically active compound in hashish, (-)-trans-∆9-tetrahydrocannabinol (∆9-THC), with the correct structure but an incorrect absolute configuration (Figure 11). I have the original Mechoulam manuscript, as provided by Professor Šantavý (Figure 12). All three of these scientists are in Figure 13.
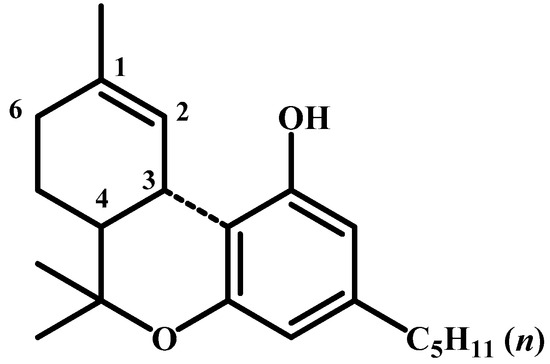
Figure 11.
The absolute configuration of Δ1-3,4-trans-tetrahydrocannabinol as proposed by Mechoulam.

Figure 12.
The first page of Mechoulam’s original manuscript.

Figure 13.
František Šantavý (23 April 1915–27 March 1983); Yehiel Gaoni (1 September 1928–14 May 2017); Raphael Mechoulam (5 November 1930–9 March 2023).
Mechoulam is appreciated worldwide for his basic discoveries in the field of cannabis. These discoveries led to the clarification of the biogenesis of these substances in the plant.
I cannot fail to mention, at the end of this historical article, my (L.H.) good friend Professor Yehiel Gaoni, who shared with me details of one of his experiments that he never published [60]. He was a colleague of Professor Raphael Mechoulam and performed isolations and syntheses in the laboratory. Professor Mechoulam was said to be a capable organizer. Gaoni also once synthesized 1′,1′-dimethylheptyl-Δ9-tetrahydrocannabinol (Δ9-THC-DMH, 22) (Scheme 10). To test this substance, he put a little on a spatula and put it under his tongue. He did not tell anyone and kept it as his secret. He drove home from the Weizmann Institute, and at home, it had a strong effect on him for three days. One of the experiences coincided with an experience that Albert Hofmann [61,62] had in 1943 after taking LSD (“Occasionally I felt as being outside my body … I thought I had died. My ‘ego’ was suspended somewhere in space and I saw my body lying dead on the sofa”). Gaoni felt like he was out of his body. He saw himself as an old and dead person. He told his wife that he felt that he was dying, but she laughed at him (she was a doctor, so she took care of him). The effect was said to be strong.
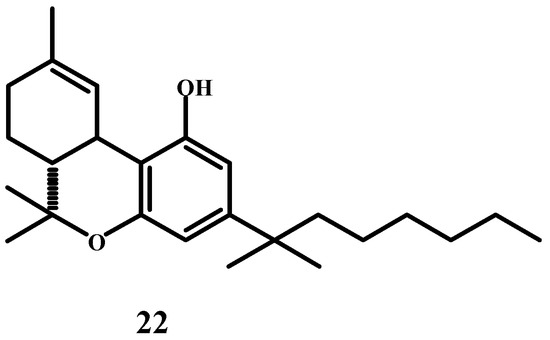
Scheme 10.
Structure of 1′,1′-dimethylheptyl-Δ9-tetrahydrocannabinol.
It is interesting to mention how people still worked in his time. Gaoni worked in the laboratory without a fume hood, distilled benzene, and pyridine, and perhaps from these or other chemicals he already had Parkinson’s disease by the time we met (his hands and lips were shaking slightly). Once upon a time, when they were training in fire rescue, he jumped into a firefighter’s rescue tarp. Those below, who were supposed to catch him safely, did not hold the tarp properly, so he fell to the ground with it and broke his spine. Of course, he fought his illness and went to the gym for an hour on the treadmill five times a week at the age of 78. He did his best, but unfortunately, we are not immortal.
6. Conclusions
As we have seen, it took a long time to uncover the structure of cannabinoids. The beginnings of scientific research are often challenging, but with the accumulation of knowledge, the path forward becomes clearer and the progress faster. It is important to recognize the contributions of every scientist in this process. The final result can be seen as a complete mosaic, with each piece playing an essential role. Some scientists contributed just one piece, others more, but even the smallest contribution was crucial to completing the picture. I want to acknowledge the efforts of all those involved, regardless of the scale of their contribution. The joy of discovery and sincere dedication were present in all. I firmly believe that collaboration, rather than competition or rivalry, should form the foundation of scientific progress.
Future cannabinoid research will focus on precision medicine, rare and novel cannabinoids, endocannabinoid system modulation, synthetic and semi-synthetic cannabinoids, nanotechnology and advanced drug delivery, interactions with the gut microbiome, and neuroprotection and aging.
Author Contributions
Conceptualization, L.O.H. and L.N.M.; writing—original draft preparation, L.O.H.; writing—review and editing, L.O.H. and L.N.M.; supervision, L.O.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abel, E.L. Marihuana: The First Twelve Thousand Years; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Sertürner, F.W. Über das Morphium, eine neue salzfähige Grundlage, und die Mekonsäure, als Hauptbestandteile des Opiums (On Morphine, a New Salt Base, and Meconic Acid, as Main Components of Opium). Ann. Phys. 1817, 25, 56–90. [Google Scholar] [CrossRef]
- Sertuerner, F.W. De la morphine et de l’acide meconique, consideres comme parties essentielles de l’opium (Morphine and meconic acid, considered essential parts of opium). Ann. Chim. Phys. 1817, 5, 21–42. [Google Scholar]
- Gulland, J.M.; Robinson, R. The morphine group. Part I. A discussion of the constitutional problem. J. Chem. Soc., Transact. 1923, 123, 980–998. [Google Scholar] [CrossRef]
- Gulland, J.M.; Robinson, R. Constitution of codeine and thebaine. Mem. Proc. Manch. Lit. Philos. Soc. 1925, 69, 79–86. [Google Scholar]
- Gates, M.; Tschudi, G. The synthesis of morphine. J. Am. Chem. Soc. 1952, 74, 1109–1110. [Google Scholar] [CrossRef]
- Gates, M.; Tschudi, G. The synthesis of morphine. J. Am. Chem. Soc. 1956, 78, 1380–1393. [Google Scholar] [CrossRef]
- Gaedcke, F. Ueber das Erythroxylin, dargestellt aus den Blättern des in Südamerika cultivirten Strauches Erythroxylon Coca Lam (On erythroxylin, prepared from the leaves of the shrub Erythroxylon Coca Lam, cultivated in South America). Arch. Pharm. 1855, 132, 141–150. [Google Scholar] [CrossRef]
- Niemann, A. Ueber eine neue organische Base in den Cocablättern. (On a New Organic Base in Coca Leaves). Arch. Pharm. 1860, 153, 129–155. [Google Scholar] [CrossRef]
- Niemann, A. Ueber eine neue organische Base in den Cocablättern. Arch. Pharm. 1860, 153, 291–308. [Google Scholar] [CrossRef]
- Niemann, A. Über Eine Neue Organische Base in Den Cocablättern. Inaugural Dissertation, Druck der Universitäts-Buchdruckerei von A. Huth, Göttingen, Germany, 1860. [Google Scholar]
- Willstätter, R.; Müller, W. Ueber Tropylamine (About Tropylamine). Ber. d. Dt. Chem. Ges. 1898, 31, 1202–1214. [Google Scholar] [CrossRef]
- Willstätter, R. Ueber die Constitution der Spaltungsproducte von Atropin und Cocaine (On the Constitution of the Cleavage Products of Atropine and Cocaine). Ber. d. Dt. Chem. Ges. 1898, 31, 1534–1553. [Google Scholar] [CrossRef]
- Hanuš, L.O. Pharmacological and Therapeutic Secrets of Plant and Brain (Endo) Cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef]
- Lee, M.A. Smoke Signals: A Social History of Marijuana—Medical, Recreational, and Scientific; Scribner: New York, NY, USA, 2012. [Google Scholar]
- Tscheppe, F. Chemische Untersuchung der Hanfblätter (Chemical analysis of hemp leaves). In Eine Inaugural-Dissertationwelche zur Erlangung der Doctorwürde in der Medizin; Tübingen, Germany; Volume 1821, 28p.
- O’Shaughnessy, W.B. On the preparations of the Indian hemp, or gunjah (Cannabis indica); their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Transact. Med. Phys. Soc. Bengal 1843, 5, 363. [Google Scholar]
- Schlesinger, S. Untersuchung der Cannabis sativa (Examination of Cannabis sativa). Repert. Pharm. 1840, 71, 190–208. [Google Scholar]
- Bohlig, J.F. Cannabis sativa und Urtica dioica chemisch analysiert (Cannabis sativa and Urtica dioica chemically analysed). Jahrb. Für Prakt. Pharm. Und Verwandte Fächer 1840, 3, 1–58. [Google Scholar]
- Smith, T.; Smith, H. Process for preparing cannabine (?), or hemp resin. Pharm. J. Transact. 1847, VI, 171–173. [Google Scholar]
- Moreau, J. Du Hachisch Et De L’aliénation Mentale: Études Psychologiques (Hashish and Mental Insanity: Psychological Studies); Fortin, Masson: Paris, France, 1845. [Google Scholar]
- DeCourtive, E. Haschish: Étude Historique, Chimique et Physiologique (Hashish: Historical, Chemical and Physiological Study); Thèse présentée a L’école de pharmacie de Paris, imprimerie d’Édouard Bautruche: Paris, France, 1848; 56p. [Google Scholar]
- DeCourtive, E. Note sur le haschisch (Note on Hashish). C. R. Acad. Sci. 1848, 26, 509–510. [Google Scholar]
- DeCourtive, E. Sur le haschisch (On Hashish). J. Pharm. Chim. 1848, 13, 427–441. [Google Scholar]
- Bolas, T.; Francis, E.E.H. On the products of the action of nitric acid on the resinous extract of Indian hemp. J. Chem. Soc. 1869, 22, 417–419. [Google Scholar] [CrossRef]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. Charas. The resin of Indian hemp. J. Chem. Soc. 1896, 69, 539–546. [Google Scholar] [CrossRef]
- Marshall, C.R. The active principle of Indian hemp: A preliminary communication. Lancet 1897, 149, 235–238. [Google Scholar] [CrossRef]
- Marshall, C.R. A contribution to the pharmacology of Cannabis indica. J. Am. Med. Assoc. 1898, 31, 882–891. [Google Scholar] [CrossRef][Green Version]
- Dunstan, W.R.; Henry, T.A. On oxycannabin from Indian hemp. Proc. Chem. Soc. 1898, 14, 44–45. [Google Scholar]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. Cannabinol. Part I. J. Chem. Soc. 1899, 75, 20–36. [Google Scholar] [CrossRef]
- Wood, T.B. Obituary notice: William Thomas Newton Spivey. J. Chem. Soc. Transact. 1902, 81, 635–636. [Google Scholar]
- Walton, R.P. Marihuana, America’s New Drug Problem; J.B. Lippincott Company: Philadelphia, PA, USA, 1938. [Google Scholar]
- Cahn, R.S. Cannabis indica resin, Part I. The Constitution of Nitrocannabinolactone (Oxycannabin). J. Chem. Soc. 1930, 986–992. [Google Scholar] [CrossRef]
- Cahn, R.S. Cannabis indica resin, Part II. J. Chem. Soc. 1931, 630–638. [Google Scholar] [CrossRef]
- Cahn, R.S. Cannabis indica resin, Part III. The constitution of Cannabinol. J. Chem. Soc. 1932, 1342–1353. [Google Scholar] [CrossRef]
- Cahn, R.S. Cannabis indica resin. Part IV. The synthesis of some 2:2-dimethyldibenzopyrans, and conformation of the structure of cannabinol. J. Chem. Soc. 1933, 1400–1405. [Google Scholar] [CrossRef]
- Hanuš, L.; Tesařík, K.; Krejčí, Z. Capillary gas chromatography of natural substances from Cannabis sativa L. I. Cannabinol and cannabinolic acid—artefacts. Acta Univ. Palacki. Olomuc. Fac. Med. 1985, 108, 29–38. [Google Scholar]
- Patil, A.S.; Mahajan, U.B.; Agrawal, Y.O.; Patil, K.R.; Patil, C.R.; Ojha, S.; Sharma, C.; Goyal, S.N. Plant-derived natural therapeutics targeting cannabinoid receptors in metabolic syndrome and its complications: A review. Biomed. Pharmacother. 2020, 132, 110889. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Todd, A.R. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J. Chem. Soc. 1940, 649–653. [Google Scholar] [CrossRef]
- Ghosh, R.; Todd, A.R.; Wilkinson, S. Cannabis indica, Part V. The synthesis of cannabinol. J. Chem. Soc. 1940, 1393–1396. [Google Scholar] [CrossRef]
- Adams, R.; Baker, B.R.; Wearn, R.B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-amyl-6,6,9-trimethyl-6-dibenzopyran. J. Am. Chem. Soc. 1940, 62, 2204–2207. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Adams, R.; Wolff, H.; Cain, C.K.; Clark, J.H. Structure of Cannabidiol. V. Position of the Alicyclic Double Bonds. J. Am. Chem. Soc. 1940, 62, 2215–2219. [Google Scholar] [CrossRef]
- Adams, R.; Pease, D.C.; Cain, C.K.; Baker, B.R.; Clark, J.H.; Wolff, H.; Wearn, R.B. Conversion of cannabidiol to a product with marihuana activity. A type reaction for synthesis of analogous substances. Conversion of cannabidiol to cannabinol. J. Am. Chem. Soc. 1940, 62, 2245–2246. [Google Scholar] [CrossRef]
- Adams, R.; Pease, D.C.; Cain, C.K.; Clark, J.H. Structure of Cannabidiol. VI. Isomerization of Cannabidiol to Tetrahydrocannabinol, a Physiologically Active Product. Conversion of Cannabidiol to Cannabinol. J. Am. Chem. Soc. 1940, 62, 2402–2405. [Google Scholar] [CrossRef]
- Adams, R.; Baker, B.R. Structure of Cannabidiol. VII. A Method of Synthesis of Tetrahydrocannabinol which Possesses Marihuana Activity. J. Am. Chem. Soc. 1940, 62, 2405–2408. [Google Scholar] [CrossRef]
- Adams, R.; Loewe, S.; Pease, D.C.; Cain, C.K.; Wearn, R.B.; Baker, B.R.; Wolff, H. Structure of cannabidiol. VIII. Position of the double bonds in cannabidiol. Marihuana activity of tetrahydrocannabinols. J. Am. Chem. Soc. 1940, 62, 2566–2567. [Google Scholar] [CrossRef]
- Ghosh, R.; Todd, A.R.; Wilkinson, S. Cannabis Indica. Part IV. The Synthesis of Some Tetrahydrodibenzopyran Derivatives. J. Chem. Soc. 1940, 1121–1125. [Google Scholar] [CrossRef]
- Adams, R.; MacKenzie, S., Jr.; Loewe, S. Tetrahydrocannabinol Homologs with Doubly Branched Alkyl Groups in the 3-Position. J. Am. Chem. Soc. 1949, 70, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Krejčí, Z. Antibiotický Účinek Cannabis Indica (The Antibiotic Effect of Cannabis indica). Dissertation, Masaryk University Brno, Brno, Czech Republic, 1950; 106p. [Google Scholar]
- Krejčí, Z. Antibakteriální účinek Cannabis indica (The antibacterial effect of Cannabis indica). Lék. Listy 1952, 7, 500. [Google Scholar]
- Krejčí, Z. Antibakteriální účin látek z Cannabis indica L. (The antibacterial effect of Cannabis indica L.). Acta Univ. Palacki. Olomuc. 1955, 6, 43–57. [Google Scholar]
- Kabelík, J.; Krejčí, Z.; Šantavý, F. Cannabis as a medicament. Bull.Narcot. 1960, 12, 5–23. [Google Scholar]
- Krejčí, Z.; Šantavý, F. Isolace dalších látek z listí indického konopí Cannabis sativa L. (Isolation of other substances from the leaves of the Indian hemp Cannabis sativa L., varietas indica). Acta Univ. Palacki. Olomuc. 1955, 6, 59–66. [Google Scholar]
- Mechoulam, R.; Shvo, Y. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Šantavý, F. Notes on the structure of cannabidiol compounds. Acta Univ. Palacki. Olomuc. Fac. Med. 1964, 35, 5–9. [Google Scholar]
- Šimánek, V. Formal editor of ACTA Universitatis Palackianae Olomucensis. Private communication, 2011. [Google Scholar]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of Δ1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Gaoni, Y. Private communication, 2006.
- Hofmann, A. LSD—Mein Sorgenkind, 1st ed.; Klett-Cotta: Stuttgart, Genmany, 1979. [Google Scholar]
- Hofmann, A. LSD: My Problem Child, 1st ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).