Psychedelics for Moral Bioenhancement in Healthy Individuals—A Violation of the Non-Maleficence Principle?
Abstract
1. Introduction
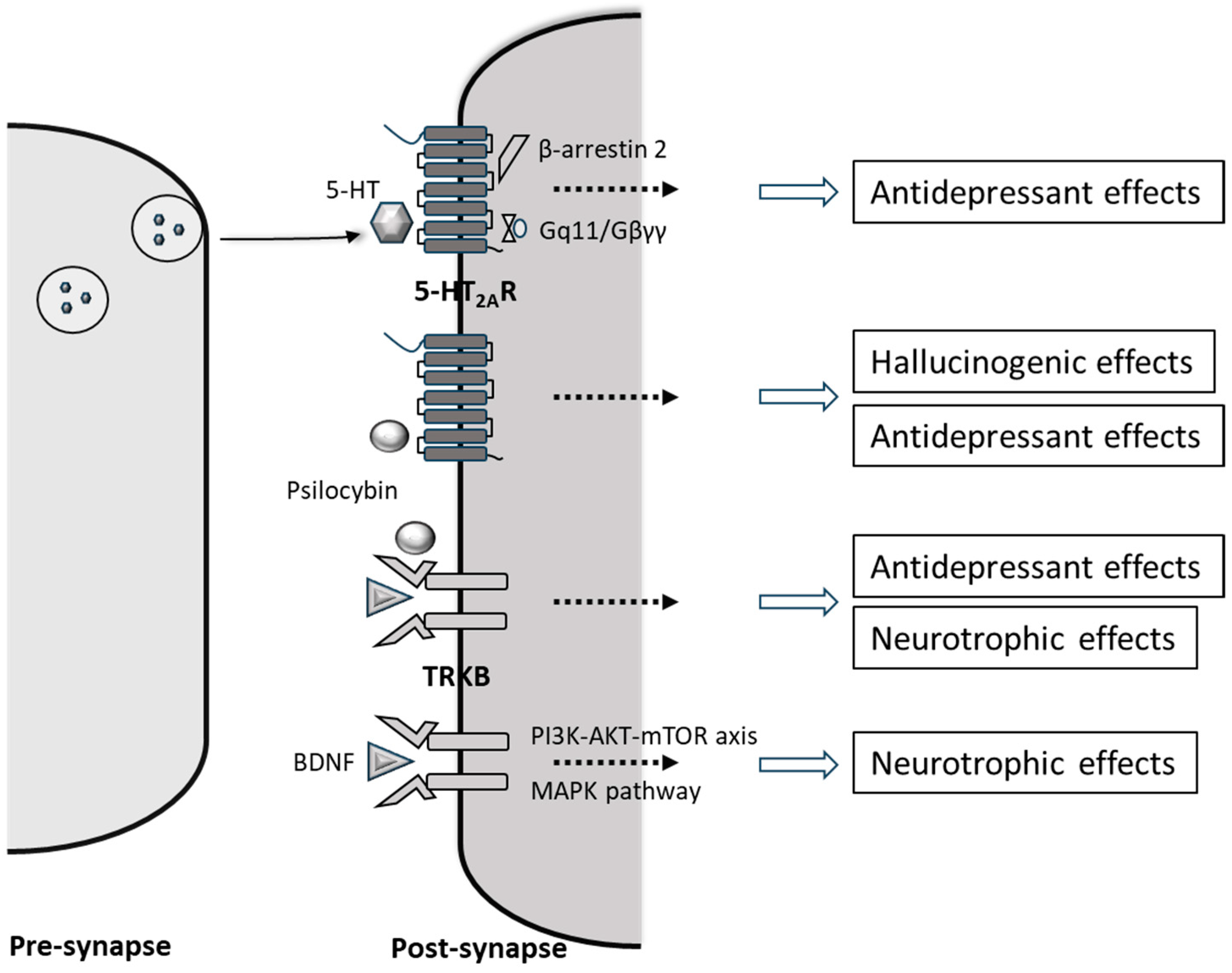
2. Psilocybin’s Mechanism of Action and Its Neuropharmacological Effects
3. Issues with Psilocybin’s Adverse Effects
4. Does Psilocybin Really Promote Morality or Alter Moral Mindsets?
5. The Use of Psychedelics Such as Psilocybin Specifically Only for MBE Is Unjustified
6. Attempts to Use Psilocybin Solely to Achieve MBE in Healthy Individuals Would Violate the Ethical Principle of Non-Maleficence
7. Concluding Remarks
Funding
Data Availability Statement
Conflicts of Interest
References
- Harris, J. Moral enhancement and freedom. Bioethics 2011, 25, 102–111. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Martin, I.; Salles, A. Moral bioenhancement: Much ado about nothing? Bioethics 2015, 29, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dubljević, V.; Racine, E. Moral Enhancement Meets Normative and Empirical Reality: Assessing the Practical Feasibility of Moral Enhancement Neurotechnologies. Bioethics 2017, 31, 338–348. [Google Scholar] [CrossRef]
- Paulo, N.; Bublitz, J.C. How (not) to argue for moral enhancement: Reflections on a decade of debate. Topoi 2019, 38, 95–109. [Google Scholar] [CrossRef]
- Douglas, T. Moral Enhancement. J. Appl. Philos. 2008, 25, 228–245. [Google Scholar] [CrossRef]
- Persson, I.; Savulescu, J. Unfit for the Future; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Earp, B.; Douglas, T.; Savulescu, J. Moral Neuroenhancement. In The Routledge Handbook of Neuroethics; Johnson, L., Rommelfanger, K., Eds.; Routledge: London, UK, 2017. [Google Scholar]
- Beck, B. Conceptual and practical problems of moral enhancement. Bioethics 2015, 29, 233–240. [Google Scholar] [CrossRef]
- Macpherson, I.; Roqué, M.V.; Segarra, I. Moral enhancement, at the peak of pharmacology and at the limit of ethics. Bioethics 2019, 33, 992–1001. [Google Scholar] [CrossRef]
- Neitzke-Spruill, L.; Beit, C.; Robinson, J.; Blevins, K.; Reynolds, J.; Evans, N.G.; McGuire, A.L. A Transformative Trip? Experiences of Psychedelic Use. Neuroethics 2024, 17, 33. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Preller, K.H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Vejmola, Č.; Tylš, F.; Piorecká, V.; Koudelka, V.; Kadeřábek, L.; Novák, T.; Páleníček, T. Psilocin, LSD, mescaline, and DOB all induce broadband desynchronization of EEG and disconnection in rats with robust translational validity. Transl. Psychiatry 2021, 11, 506. [Google Scholar] [CrossRef]
- Petridis, P.D. A psychedelic state arises from desynchronized brain activity. Nature 2024, 632, 32–33. [Google Scholar] [CrossRef]
- Siegel, J.S.; Subramanian, S.; Perry, D.; Kay, B.P.; Gordon, E.M.; Laumann, T.O.; Reneau, T.R.; Metcalf, N.V.; Chacko, R.V.; Gratton, C.; et al. Psilocybin desynchronizes the human brain. Nature 2024, 632, 131–138. [Google Scholar] [CrossRef]
- Ross, S.; Agin-Liebes, G.; Lo, S.; Zeifman, R.J.; Ghazal, L.; Benville, J.; Corso, S.F.; Real, C.B.; Guss, J.; Bossis, A.; et al. Acute and Sustained Reductions in Loss of Meaning and Suicidal Ideation Following Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Life-Threatening Cancer. ACS Pharmacol. Transl. Sci. 2021, 4, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.S.; Aaronson, S.T. The Emerging Field of Psychedelic Psychotherapy. Curr. Psychiatry Rep. 2022, 24, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Borissova, A.; Rucker, J.J. The development of psilocybin therapy for treatment-resistant depression: An update. Bjpsych Bull. 2023, 48, 38–44. [Google Scholar] [CrossRef]
- Whinkin, E.; Opalka, M.; Watters, C.; Jaffe, A.; Aggarwal, S. Psilocybin in Palliative Care: An Update. Curr. Geriatr. Rep. 2023, 12, 50–59. [Google Scholar] [CrossRef]
- Lee, H.J.; Tsang, V.W.; Chai, B.S.; Lin, M.C.; Howard, A.; Uy, C.; Elefante, J.O. Psilocybin’s Potential Mechanisms in the Treatment of Depression: A Systematic Review. J. Psychoact. Drugs 2023, 56, 301–315. [Google Scholar] [CrossRef]
- Nogrady, B. Australia’s approval of MDMA and psilocybin for PTSD and depression is premature, say critics. Br. Med. J. 2023, 382, 1599. [Google Scholar] [CrossRef]
- Haridy, R. Australia to prescribe MDMA and psilocybin for PTSD and depression in world first. Nature 2023, 619, 227–228. [Google Scholar] [CrossRef]
- Tennison, M.N. Moral transhumanism: The next step. J. Med. Philos. 2012, 37, 405–416. [Google Scholar] [CrossRef]
- Earp, B.D. Psychedelic Moral Enhancement. R. Inst. Philos. Suppl. 2018, 83, 415–439. [Google Scholar] [CrossRef]
- Gordon, E.C. Trust and Psychedelic Moral Enhancement. Neuroethics 2022, 15, 19. [Google Scholar] [CrossRef]
- Rakić, V. Psilocybin: The most effective moral bio-enhancer? Bioethics 2023, 37, 683–689. [Google Scholar] [CrossRef]
- de Vos, C.M.H.; Mason, N.L.; Kuypers, K.P.C. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef]
- Jaster, A.M.; González-Maeso, J. Mechanisms and molecular targets surrounding the potential therapeutic effects of psychedelics. Mol. Psychiatry 2023, 28, 3595–3612. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and Serotonin 5-HT(2A) Receptor-Mediated Signaling Pathways. Curr. Top. Behav. Neurosci. 2018, 36, 45–73. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Kometer, M.; Schmidt, A.; Jäncke, L.; Vollenweider, F.X. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Whalley, K. Unpicking the antidepressant actions of psychedelics. Nat. Rev. Neurosci. 2023, 24, 455. [Google Scholar] [CrossRef]
- O’Grady, C. No Trip Needed for Psychedelics to Lift Mood? 2023. Available online: https://www.science.org/content/article/psychedelic-inspired-drugs-could-relieve-depression-without-causing-hallucinations (accessed on 1 January 2024).
- Muir, J.; Lin, S.; Aarrestad, I.K.; Daniels, H.R.; Ma, J.; Tian, L.; Olson, D.E.; Kim, C.K. Isolation of psychedelic-responsive neurons underlying anxiolytic behavioral states. Science 2024, 386, 802–810. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Erritzoe, D.; Williams, T.M.; Stone, J.M.; Evans, J.; Sharp, D.J.; Feilding, A.; Wise, R.G.; Nutt, D.J. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr. Bull. 2013, 39, 1343–1351. [Google Scholar] [CrossRef]
- Barber, G.; Nemeroff, C.B.; Siegel, S. A Case of Prolonged Mania, Psychosis, and Severe Depression After Psilocybin Use: Implications of Increased Psychedelic Drug Availability. Am. J. Psychiatry 2022, 179, 892–896. [Google Scholar] [CrossRef]
- Hyde, C.; Glancy, G.; Omerod, P.; Hall, D.; Taylor, G.S. Abuse of indigenous psilocybin mushrooms: A new fashion and some psychiatric complications. Br. J. Psychiatry 1978, 132, 602–604. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.I.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Serotonergic hallucinogens as translational models relevant to schizophrenia. Int. J. Neuropsychopharmacol. 2013, 16, 2165–2180. [Google Scholar] [CrossRef]
- Van Kampen, J.; Katz, M. Persistent psychosis after a single ingestion of ‘ecstasy’. Psychosomatics 2001, 42, 525–527. [Google Scholar] [CrossRef]
- Patel, A.; Moreland, T.; Haq, F.; Siddiqui, F.; Mikul, M.; Qadir, H.; Raza, S. Persistent Psychosis After a Single Ingestion of “Ecstasy” (MDMA). Prim. Care Companion CNS Disord. 2011, 13, PCC.11l01200. [Google Scholar] [CrossRef] [PubMed]
- Espiard, M.-L.; Lecardeur, L.; Abadie, P.; Halbecq, I.; Dollfus, S. Hallucinogen persisting perception disorder after psilocybin consumption: A case study. Eur. Psychiatry 2005, 20, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H.; Lerner, A.G.; Passie, T. A Review of Hallucinogen Persisting Perception Disorder (HPPD) and an Exploratory Study of Subjects Claiming Symptoms of HPPD. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 333–360. [Google Scholar]
- Skryabin, V.Y.; Vinnikova, M.; Nenastieva, A.; Alekseyuk, V. Hallucinogen persisting perception disorder: A literature review and three case reports. J. Addict. Dis. 2018, 37, 268–278. [Google Scholar] [CrossRef]
- Müller, F.; Kraus, E.; Holze, F.; Becker, A.; Ley, L.; Schmid, Y.; Vizeli, P.; Liechti, M.E.; Borgwardt, S. Flashback phenomena after administration of LSD and psilocybin in controlled studies with healthy participants. Psychopharmacology 2022, 239, 1933–1943. [Google Scholar] [CrossRef]
- Pokorny, T.; Preller, K.H.; Kometer, M.; Dziobek, I.; Vollenweider, F.X. Effect of Psilocybin on Empathy and Moral Decision-Making. Int. J. Neuropsychopharmacol. 2017, 20, 747–757. [Google Scholar] [CrossRef]
- Schmid, Y.; Hysek, C.M.; Simmler, L.D.; Crockett, M.J.; Quednow, B.B.; E Liechti, M. Differential effects of MDMA and methylphenidate on social cognition. J. Psychopharmacol. 2014, 28, 847–856. [Google Scholar] [CrossRef]
- Gabay, A.S.; Carhart-Harris, R.L.; Mazibuko, N.; Kempton, M.J.; Morrison, P.D.; Nutt, D.J.; Mehta, M.A. Psilocybin and MDMA reduce costly punishment in the Ultimatum Game. Sci. Rep. 2018, 8, 8236. [Google Scholar] [CrossRef]
- Workman, C.I.; Yoder, K.J.; Decety, J. The Dark Side of Morality—Neural Mechanisms Underpinning Moral Convictions and Support for Violence. AJOB Neurosci. 2020, 11, 269–284. [Google Scholar] [CrossRef]
- Sai, L.; Bellucci, G.; Wang, C.; Fu, G.; Camilleri, J.A.; Eickhoff, S.B.; Krueger, F. Neural mechanisms of deliberate dishonesty: Dissociating deliberation from other control processes during dishonest behaviors. Proc. Natl. Acad. Sci. USA 2021, 118, e2109208118. [Google Scholar] [CrossRef]
- Koenigs, M.; Young, L.; Adolphs, R.; Tranel, D.; Cushman, F.; Hauser, M.; Damasio, A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 2007, 446, 908–911. [Google Scholar] [CrossRef]
- Young, L.; Bechara, A.; Tranel, D.; Damasio, H.; Hauser, M.; Damasio, A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron 2010, 65, 845–851. [Google Scholar] [CrossRef] [PubMed]
- van Honk, J.; Terburg, D.; Montoya, E.R.; Grafman, J.; Stein, D.J.; Morgan, B. Breakdown of utilitarian moral judgement after basolateral amygdala damage. Proc. Natl. Acad. Sci. USA 2022, 119, e2119072119. [Google Scholar] [CrossRef]
- Garrett, N.; Lazzaro, S.C.; Ariely, D.; Sharot, T. The brain adapts to dishonesty. Nat. Neurosci. 2016, 19, 1727–1732. [Google Scholar] [CrossRef]
- Persson, I.; Savulescu, J. Getting moral enhancement right: The desirability of moral bioenhancement. Bioethics 2013, 27, 124–131. [Google Scholar] [CrossRef]
- Specker, J.; Focquaert, F.; Raus, K.; Sterckx, S.; Schermer, M. The ethical desirability of moral bioenhancement: A review of reasons. BMC Med. Ethics 2014, 15, 67. [Google Scholar] [CrossRef]
- Simkulet, W. Intention and Moral Enhancement. Bioethics 2016, 30, 714–720. [Google Scholar] [CrossRef]
- Kudlek, K. Towards a systematic evaluation of moral bioenhancement. Theor. Med. Bioeth. 2022, 43, 95–110. [Google Scholar] [CrossRef]
- Greene, J.D.; Sommerville, R.B.; Nystrom, L.E.; Darley, J.M.; Cohen, J.D. An fMRI investigation of emotional engagement in moral judgment. Science 2001, 293, 2105–2108. [Google Scholar] [CrossRef]
- Greene, J.D.; Morelli, S.A.; Lowenberg, K.; Nystrom, L.E.; Cohen, J.D. Cognitive load selectively interferes with utilitarian moral judgment. Cognition 2008, 107, 1144–1154. [Google Scholar] [CrossRef]
- Parkinson, C.; Sinnott-Armstrong, W.; Koralus, P.E.; Mendelovici, A.; McGeer, V.; Wheatley, T. Is morality unified? Evidence that distinct neural systems underlie moral judgments of harm, dishonesty, and disgust. J. Cogn. Neurosci. 2011, 23, 3162–3180. [Google Scholar] [CrossRef]
- de Sio, F.S.; Maslen, H.; Faulmüller, N. The Necessity of Objective Standards for Moral Enhancement. AJOB Neurosci. 2012, 3, 15–16. [Google Scholar] [CrossRef]
- Jebari, K. What to Enhance: Behaviour, Emotion or Disposition? Neuroethics 2014, 7, 253–261. [Google Scholar] [CrossRef]
- Shook, J.R. Neuroethics and the Possible Types of Moral Enhancement. AJOB Neurosci. 2012, 3, 3–14. [Google Scholar] [CrossRef]
- Beauchamp, T.; Childress, J. Principles of Biomedical Ethics; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Kant, I. Groundwork for the Metaphysics of Morals; Yale University Press: New Haven, CT, USA, 2018; (originally published as Grundlegung zur Metaphysik der Sitten, 1785). [Google Scholar]
- Douglas, T. Criminal Rehabilitation Through Medical Intervention: Moral Liability and the Right to Bodily Integrity. J. Ethics 2014, 18, 101–122. [Google Scholar] [CrossRef]
- Birks, D.; Douglas, T. Treatment for Crime; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.L. Psychedelics for Moral Bioenhancement in Healthy Individuals—A Violation of the Non-Maleficence Principle? Psychoactives 2025, 4, 5. https://doi.org/10.3390/psychoactives4010005
Tang BL. Psychedelics for Moral Bioenhancement in Healthy Individuals—A Violation of the Non-Maleficence Principle? Psychoactives. 2025; 4(1):5. https://doi.org/10.3390/psychoactives4010005
Chicago/Turabian StyleTang, Bor Luen. 2025. "Psychedelics for Moral Bioenhancement in Healthy Individuals—A Violation of the Non-Maleficence Principle?" Psychoactives 4, no. 1: 5. https://doi.org/10.3390/psychoactives4010005
APA StyleTang, B. L. (2025). Psychedelics for Moral Bioenhancement in Healthy Individuals—A Violation of the Non-Maleficence Principle? Psychoactives, 4(1), 5. https://doi.org/10.3390/psychoactives4010005





