Rapid and Prolonged Antidepressant and Antianxiety Effects of Psychedelics and 3,4-Methylenedioxy-methamphetamine—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- Pharmacological differences exist among psychedelics overall, and each one of them may have a different clinical response when used to treat MDD or anxiety disorders. Thus, we investigated the benefits of each psychedelic individually.
- We assessed whether psychedelics produce a long-lasting effect beyond a quick relief of depressive or anxiety symptoms.
- We based our analysis on core instruments: clinician-reported or self-reported outcome measures to quantify hallucinogens’ clinical responses to mitigate discrepancies between outcome measures.
- We investigated the potential benefits of MDMA in reducing symptoms of depression and anxiety.
- We investigated psychedelics and MDMA safety profiles.
2. Materials and Methods
2.1. Criteria for Considering Studies
2.1.1. Types of Studies
2.1.2. Types of Participants
2.2. Types of Interventions
2.2.1. Experimental Intervention
2.2.2. Control Intervention
2.3. Types of Outcome Measures
2.3.1. Clinical Response
2.3.2. Adverse Events
2.4. Types of Settings
2.5. Search Methods for Identification of Studies
Electronic Searches
2.6. Data Collection and Analysis
- Publication status, title, authors’ names, source, country, and year of publication.
- Trial characteristics: design and setting.
- Interventions: type of pharmacological and control intervention, dose, and duration.
- Number of participants, age, gender, loss to follow-up, and race.
- Outcomes.
2.7. Evaluation of the Methodological Quality of Randomized Controlled Trials, Bias Risk, and Heterogeneity
- I2 = 0% to 40%: might not be important.
- I2 = 30% to 60%: may represent moderate heterogeneity.
- I2 = 50% to 90%: may represent substantial heterogeneity.
- I2 = 75% to 100%: considerable heterogeneity.
2.8. Data Synthesis
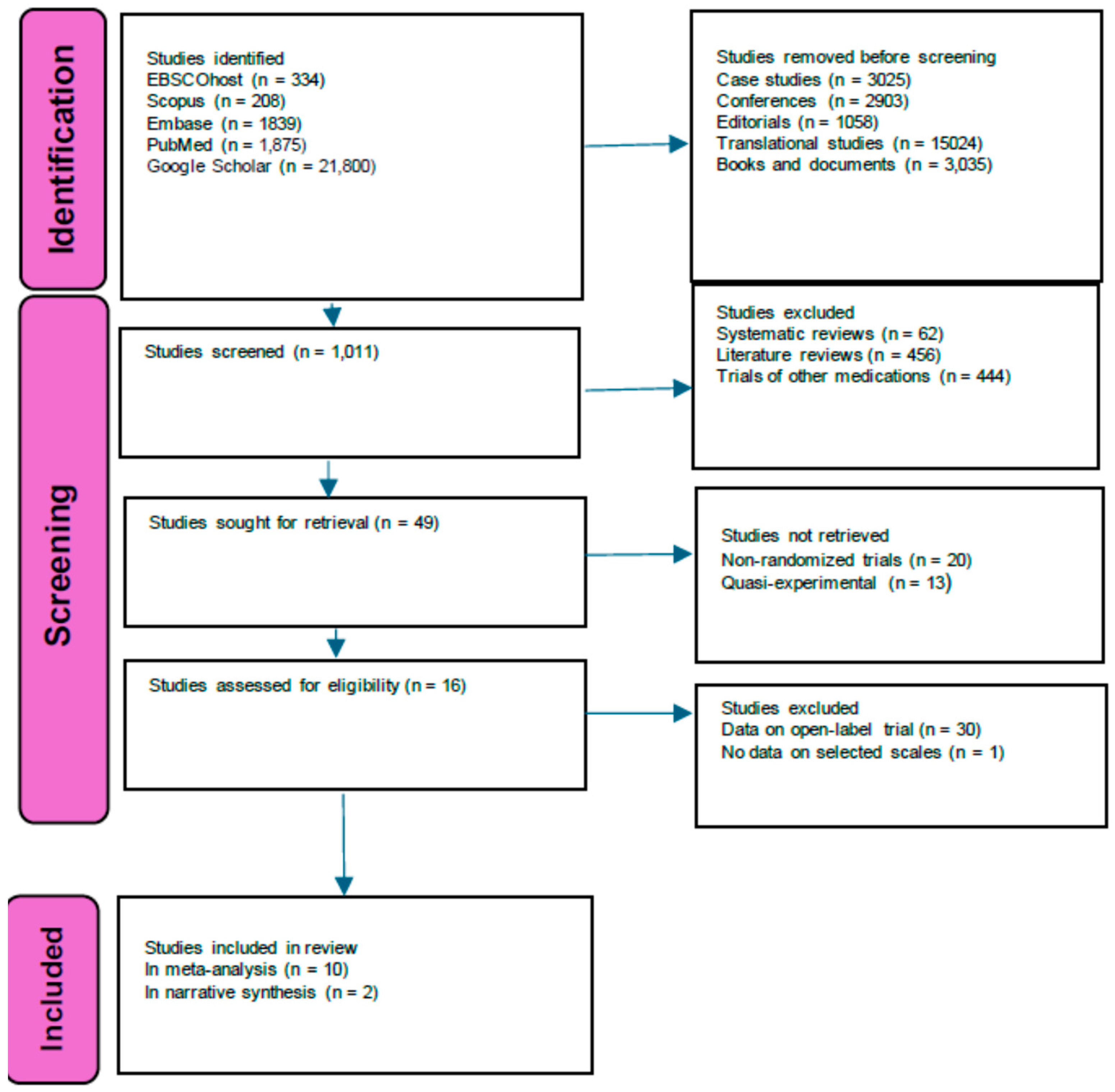
3. Results
3.1. Description of Studies
3.2. Risk of Bias in Included Studies
3.3. Synthesis of Results
3.3.1. Depressive Symptoms—Clinician-Rated Measures
3.3.2. Depressive Symptoms—Self-Rated Measures
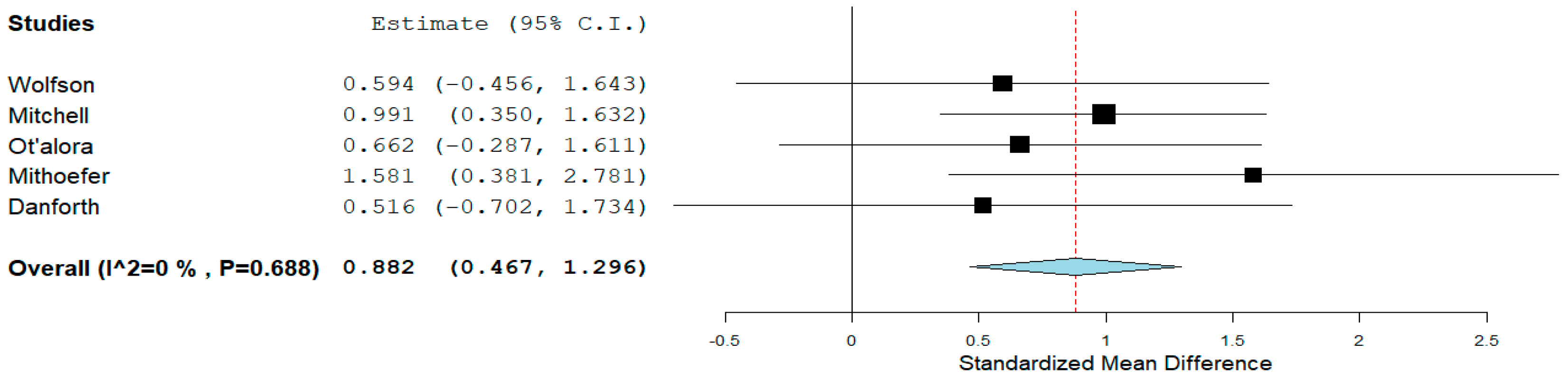
3.3.3. Anxiety Symptoms—Clinician-Rated Measures
3.3.4. Anxiety Symptoms—Self-Rated Measures
3.3.5. Meta-Regression
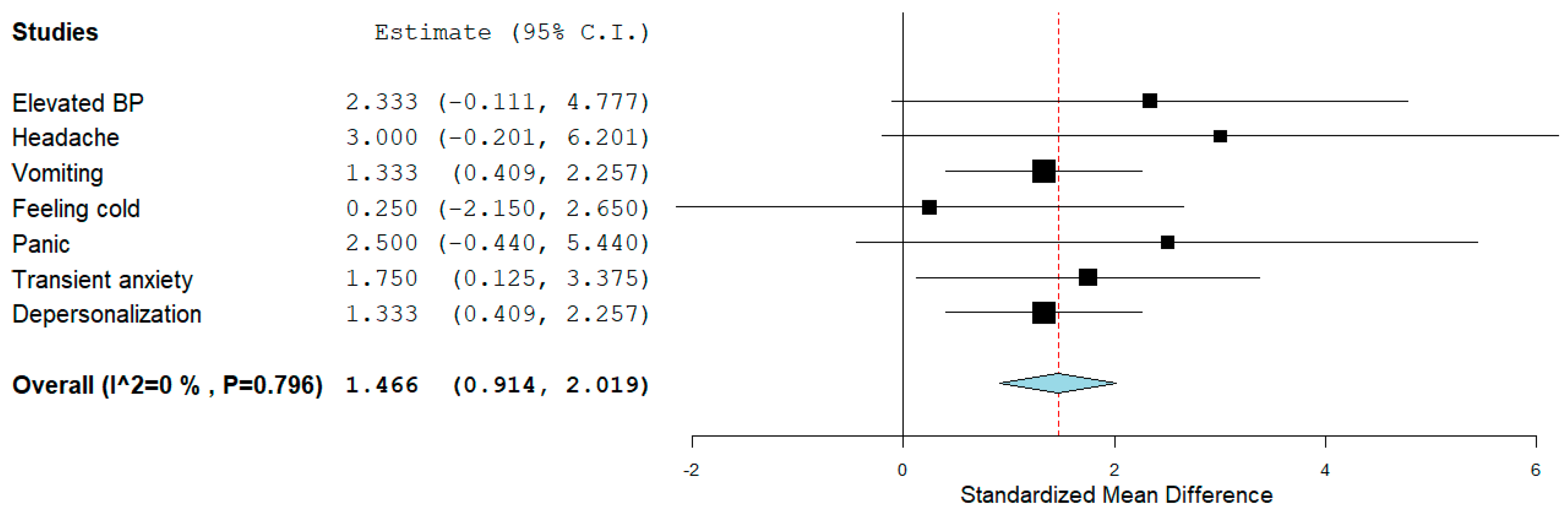
3.3.6. Adverse Effects
4. Discussion
4.1. Summary of Main Results
4.2. Overall Completeness and Applicability of Evidence
4.3. Abuse and Misuse Concerns
4.4. Legal Aspects
4.5. Limitations
5. Conclusions
5.1. Implications for Practice and Policy
5.2. Implications for Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salas-Wright, C.P.; Hodges, J.C.; Hai, A.H.; Alsolami, A.; Vaughn, M.G. Toward a typology of hallucinogen users in the United States. Drug Alcohol Depend. 2021, 229, 109139. [Google Scholar] [CrossRef] [PubMed]
- Volgin, A.D.; Yakovlev, O.A.; Demin, K.A.; Alekseeva, P.A.; Kyzar, E.J.; Collins, C.; Nichols, D.E.; Kalueff, A.V. Understanding Central Nervous System Effects of Deliriant Hallucinogenic Drugs through Experimental Animal Models. ACS Chem. Neurosci. 2019, 10, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X. Brain Mechanisms of Hallucinogens and Entactogens. Dialogues Clin. Neurosci. 2001, 3, 265–279. [Google Scholar] [CrossRef]
- Baumeister, D.; Barnes, G.; Giaroli, G.; Tracy, D. Classical Hallucinogens as Antidepressants? A Review of Pharmacodynamics and Putative Clinical Roles. Ther. Adv. Psychopharmacol. 2014, 4, 156–169. [Google Scholar] [CrossRef]
- Weston, N.M.; Gibbs, D.; Bird, C.I.V.; Daniel, A.; Jelen, L.A.; Knight, G.; Goldsmith, D.; Young, A.H.; Rucker, J.J. Historic Psychedelic Drug Trials and the Treatment of Anxiety Disorders. Depress. Anxiety 2020, 37, 1261–1279. [Google Scholar] [CrossRef]
- Sessa, B.; Higbed, L.; Nutt, D. A Review of 3,4-Methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy. Front. Psychiatry 2019, 10, 138. [Google Scholar] [CrossRef]
- Wolfson, P.E.; Andries, J.; Feduccia, A.A.; Jerome, L.; Wang, J.B.; Williams, E.; Carlin, S.C.; Sola, E.; Hamilton, S.; Yazar-Klosinski, B.; et al. MDMA-Assisted Psychotherapy for Treatment of Anxiety and Other Psychological Distress Related to Life-Threatening Illnesses: A Randomized Pilot Study. Sci. Rep. 2020, 10, 20442. [Google Scholar] [CrossRef] [PubMed]
- Smausz, R.; Neill, J.; Gigg, J. Neural Mechanisms Underlying Psilocybin’s Therapeutic Potential—The Need for Preclinical In Vivo Electrophysiology. J. Psychopharmacol. 2022, 36, 781–793. [Google Scholar] [CrossRef]
- Aronson, J.K. Psilocybin. In Meyler’s Side Effects of Drugs; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1048–1051. [Google Scholar]
- Halberstadt, A.L.; Geyer, M.A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef]
- Andrade, R. Serotonergic Regulation of Neuronal Excitability in the Prefrontal Cortex. Neuropharmacology 2011, 61, 382–386. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Gates, M.J. Hallucinogens and Psychedelics. In Principles of Forensic Toxicology; Springer: Cham, Switzerland, 2020; pp. 467–489. [Google Scholar]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin—Summary of Knowledge and New Perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The Abuse Potential of Medical Psilocybin According to the 8 Factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Aguilar-Valles, A.; Preller, K.H.; Heifets, B.D.; Hibicke, M.; Mitchell, J.; Gobbi, G. Hallucinogens in Mental Health: Preclinical and Clinical Studies on LSD, Psilocybin, MDMA, and Ketamine. J. Neurosci. 2021, 41, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Rose, A.J. LSD: Its Use, Abuse, and Suggested Treatment (Observations by the Haight-Ashbury Medical Clinic). J. Psychedelic Drugs 1968, 1, 117–123. [Google Scholar] [CrossRef]
- Horowitz, M.J. Flashbacks: Recurrent Intrusive Images after the Use of LSD. Am. J. Psychiatry 1969, 126, 565–569. [Google Scholar] [CrossRef]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The Pharmacology of Lysergic Acid Diethylamide: A Review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef]
- Killion, B.; Hai, A.H.; Alsolami, A.; Vaughn, M.G.; Sehun Oh, P.; Salas-Wright, C.P. LSD Use in the United States: Trends, Correlates, and a Typology of Us. Drug Alcohol Depend. 2021, 223, 108715. [Google Scholar] [CrossRef]
- Frecska, E.; Bokor, P.; Winkelman, M. The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization. Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef]
- Jacob, M.S.; Presti, D.E. Endogenous Psychoactive Tryptamines Reconsidered: An Anxiolytic Role for Dimethyltryptamine. Med. Hypotheses 2005, 64, 930–937. [Google Scholar] [CrossRef]
- Liester, M.B.; Prickett, J.I. Hypotheses Regarding the Mechanisms of Ayahuasca in the Treatment of Addictions. J. Psychoact. Drugs 2012, 44, 200–208. [Google Scholar] [CrossRef]
- Ruffell, S.; Netzband, N.; Bird, C.; Young, A.H.; Juruena, M.F. The Pharmacological Interaction of Compounds in Ayahuasca: A Systematic Review. Rev. Bras. Psiquiatr. 2020, 42, 646–656. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Valle, M.; Bouso, J.C.; Nomdedéu, J.F.; Rodríguez-Espinosa, J.; McIlhenny, E.H.; Barker, S.A.; Barbanoj, M.J.; Riba, J. Autonomic, Neuroendocrine, and Immunological Effects of Ayahuasca: A Comparative Study with d-Amphetamine. J. Clin. Psychopharmacol. 2011, 31, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Estrella-Parra, E.A.; Almanza-Pérez, J.C.; Alarcón-Aguilar, F.J. Ayahuasca: Uses, Phytochemical and Biological Activities. Nat. Prod. Bioprospect. 2019, 9, 251–265. [Google Scholar] [CrossRef]
- Wiltshire, P.E.J.; Hawksworth, D.L.; Edwards, K.J. Light Microscopy Can Reveal the Consumption of a Mixture of Psychotropic Plant and Fungal Material in Suspicious Death. J. Forensic Leg. Med. 2015, 34, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.-M.; Ferreira, S.M.; Ávila, A.-A.L.; Perazzo, F.F.; Schneedorf, J.M.; Hinsberger, A.; Carvalho, J.C.T. Les effets de l’ayahuasca sur le système nerveux central: Étude comportementale. Phytothérapie 2007, 5, 254–257. [Google Scholar] [CrossRef]
- Pitol, D.L.; Siéssere, S.; Dos Santos, R.G.; Rosa, M.L.N.M.; Hallak, J.E.C.; Scalize, P.H.; Pereira, B.F.; Iyomasa, M.M.; Semprini, M.; Riba, J.; et al. Ayahuasca Alters Structural Parameters of the Rat Aorta. J. Cardiovasc. Pharmacol. 2015, 66, 58–62. [Google Scholar] [CrossRef]
- Fábregas, J.M.; González, D.; Fondevila, S.; Cutchet, M.; Fernández, X.; Barbosa, P.C.R.; Alcázar-Córcoles, M.Á.; Barbanoj, M.J.; Riba, J.; Bouso, J.C. Assessment of Addiction Severity among Ritual Users of Ayahuasca. Drug Alcohol Depend. 2010, 111, 257–261. [Google Scholar] [CrossRef]
- Kalant, H. The Pharmacology and Toxicology of “Ecstasy” (MDMA) and Related Drugs. CMAJ 2001, 165, 917–928. [Google Scholar]
- Green, A.R.; Mechan, A.O.; Elliott, J.M.; O’Shea, E.; Colado, M.I. The Pharmacology and Clinical Pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”). Pharmacol. Rev. 2003, 55, 463–508. [Google Scholar] [CrossRef]
- Kamilar-Britt, P.; Bedi, G. The Prosocial Effects of 3,4-Methylenedioxymethamphetamine (MDMA): Controlled Studies in Humans and Laboratory Animals. Neurosci. Biobehav. Rev. 2015, 57, 433–446. [Google Scholar] [CrossRef]
- Bosch, O.G.; Halm, S.; Seifritz, E. Psychedelics in the Treatment of Unipolar and Bipolar Depression. Int. J. Bipolar Disord. 2022, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Liechti, M.E. Pharmacology of MDMA- and Amphetamine-like New Psychoactive Substances. Handb. Exp. Pharmacol. 2018, 252, 143–164. [Google Scholar]
- Holze, F.; Vizeli, P.; Müller, F.; Ley, L.; Duerig, R.; Varghese, N.; Eckert, A.; Borgwardt, S.; Liechti, M.E. Distinct Acute Effects of LSD, MDMA, and D-Amphetamine in Healthy Subjects. Neuropsychopharmacology 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Meyer, J. 3,4-Methylenedioxymethamphetamine (MDMA): Current Perspectives. Subst. Abus. Rehabil. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Bruno, R.; Topp, L. Is Ecstasy a Drug of Dependence? Drug Alcohol Depend. 2010, 107, 1–10. [Google Scholar] [CrossRef]
- Li, N.-X.; Hu, Y.-R.; Chen, W.-N.; Zhang, B. Dose Effect of Psilocybin on Primary and Secondary Depression: A Preliminary Systematic Review and Meta-Analysis. J. Affect. Disord. 2022, 296, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Romeo, B.; Karila, L.; Martelli, C.; Benyamina, A. Efficacy of Psychedelic Treatments on Depressive Symptoms: A Meta-Analysis. J. Psychopharmacol. 2020, 34, 1079–1085. [Google Scholar] [CrossRef]
- Leger, R.F.; Unterwald, E.M. Assessing the Effects of Methodological Differences on Outcomes in the Use of Psychedelics in the Treatment of Anxiety and Depressive Disorders: A Systematic Review and Meta-Analysis. J. Psychopharmacol. 2022, 36, 20–30. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Pace, B.T.; Nicholas, C.R.; Raison, C.L.; Hutson, P.R. The Experimental Effects of Psilocybin on Symptoms of Anxiety and Depression: A Meta-Analysis. Psychiatry Res. 2020, 284, 112749. [Google Scholar] [CrossRef]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases-A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; De Manincor, M.; Perkins, D.; Sarris, J. Classic Serotonergic Psychedelics for Mood and Depressive Symptoms: A Metaanalysis of Mood Disorder Patients and Healthy Participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; Mcquay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Metaanalyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased Global Integration in the Brain after Psilocybin Therapy for Depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Martin, S.F.; Yazar-Klosinski, B.; Michel, Y.; Brewerton, T.D.; Doblin, R. Durability of Improvement in Post-Traumatic Stress Disorder Symptoms and Absence of Harmful Effects or Drug Dependency after 3,4-Methylenedioxymethamphetamine-Assisted Psychotherapy: A Prospective Long-Term Follow-up Study. J. Psychopharmacol. 2013, 27, 28–39. [Google Scholar] [CrossRef]
- Jerome, L.; Feduccia, A.A.; Wang, J.B.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. Long-Term Follow-up Outcomes of MDMA-Assisted Psychotherapy for Treatment of PTSD: A Longitudinal Pooled Analysis of Six Phase 2 Trials. Psychopharmacology 2020, 237, 2485–2497. [Google Scholar] [CrossRef]
- Uthaug, M.V.; Mason, N.L.; Toennes, S.W.; Reckweg, J.T.; de Sousa Fernandes Perna, E.B.; Kuypers, K.P.C.; van Oorsouw, K.; Riba, J.; Ramaekers, J.G. A Placebo-Controlled Study of the Effects of Ayahuasca, Set and Setting on Mental Health of Participants in Ayahuasca Group Retreats. Psychopharmacology 2021, 238, 1899–1910. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot Study of Psilocybin Treatment for Anxiety in Patients with Advanced-Stage Cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Ot’alora, G.M.; Grigsby, J.; Poulter, B.; Van Derveer, J.W., 3rd; Giron, S.G.; Jerome, L.; Feduccia, A.A.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine-Assisted Psychotherapy for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Phase 2 Controlled Trial. J. Psychopharmacol. 2018, 32, 1295–1307. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy for Post-Traumatic Stress Disorder in Military Veterans, Firefighters, and Police Officers: A Randomised, Double-Blind, Dose-Response, Phase 2 Clinical Trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Danforth, A.L.; Grob, C.S.; Struble, C.; Feduccia, A.A.; Walker, N.; Jerome, L.; Yazar-Klosinski, B.; Emerson, A. Reduction in Social Anxiety after MDMA-Assisted Psychotherapy with Autistic Adults: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Psychopharmacology 2018, 235, 3137–3148. [Google Scholar] [CrossRef]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated with Life-Threatening Diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid Antidepressant Effects of the Psychedelic Ayahuasca in Treatment-Resistant Depression: A Randomized Placebo-Controlled Trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and Safety of Psilocybin-Assisted Treatment for Major Depressive Disorder: Prospective 12-Month Follow-Up. J. Psychopharmacol. 2022, 36, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Agin-Liebes, G.I.; Malone, T.; Yalch, M.M.; Mennenga, S.E.; Ponté, K.L.; Guss, J.; Bossis, A.P.; Grigsby, J.; Fischer, S.; Ross, S. Long-Term Follow-up of Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Patients with Life-Threatening Cancer. J. Psychopharmacol. 2020, 34, 155–166. [Google Scholar] [CrossRef]
- Cowen, P.J. Serotonin Receptor Subtypes: Implications for Psychopharmacology. Br. J. Psychiatry 1991, 159, 7–14. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef]
- Gregorio, D.; Enns, D.; Nuñez, J.P.; Posa, N.A.; Gobbi, L. D-Lysergic Acid Diethylamide, Psilocybin, and Other Classic Hallucinogens: Mechanism of Action and Potential Therapeutic Applications in Mood Disorders. Prog. Brain Res. 2018, 242, 69–96. [Google Scholar]
- Mak, L.E.; Minuzzi, L.; MacQueen, G.; Hall, G.; Kennedy, S.H.; Milev, R. The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Connect. 2017, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Grieder, M.; Wang, D.J.J.; Dierks, T.; Wahlund, L.-O.; Jann, K. Default Mode Network Complexity and Cognitive Decline in Mild Alzheimer’s Disease. Front. Neurosci. 2018, 12, 770. [Google Scholar] [CrossRef]
- Posner, J.; Cha, J.; Wang, Z.; Talati, A.; Warner, V.; Gerber, A.; Peterson, B.S.; Weissman, M. Increased Default Mode Network Connectivity in Individuals at High Familial Risk for Depression. Neuropsychopharmacology 2016, 41, 1759–1767. [Google Scholar] [CrossRef]
- Schultz, D.H.; Ito, T.; Solomyak, L.I.; Chen, R.H.; Mill, R.D.; Anticevic, A.; Cole, M.W. Global Connectivity of the Fronto-Parietal Cognitive Control Network Is Related to Depression Symptoms in the General Population. Netw. Neurosci. 2019, 3, 107–123. [Google Scholar] [CrossRef]
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin Acutely Alters the Functional Connectivity of the Claustrum with Brain Networks That Support Perception, Memory, and Attention. Neuroimage 2020, 218, 116980. [Google Scholar] [CrossRef]
- Müller, F.; Liechti, M.E.; Lang, U.E.; Borgwardt, S. Advances and Challenges in Neuroimaging Studies on the Effects of Serotonergic Hallucinogens: Contributions of the Resting Brain. Prog. Brain Res. 2018, 242, 159–177. [Google Scholar] [PubMed]
- Young, M.B.; Norrholm, S.D.; Khoury, L.M.; Jovanovic, T.; Rauch, S.A.; Reiff, C.M.; Dunlop, B.W.; Rothbaum, B.O.; Howell, L.L. Inhibition of Serotonin Transporters Disrupts the Enhancement of Fear Memory Extinction by 3,4-Methylenedioxymethamphetamine (MDMA). Psychopharmacology 2017, 234, 2883–2895. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Richards, W.; Griffiths, R. Human Hallucinogen Research: Guidelines for Safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Kenward, M.G.; Fairclough, D.L.; Horton, N.J. Differential Dropout and Bias in Randomised Controlled Trials: When It Matters and When It May Not. BMJ 2013, 346, e8668. [Google Scholar] [CrossRef]
- Rocha, J.M.; Rossi, G.N.; Osório, F.L.; Hallak, J.E.C.; Dos Santos, R.G. Adverse Effects after Ayahuasca Administration in the Clinical Setting. J. Clin. Psychopharmacol. 2022, 42, 321–324. [Google Scholar] [CrossRef]
- Goldman, S.; Galarneau, D.; Friedman, R. New Onset LSD Flashback Syndrome Triggered by the Initiation of SSRIs. Ochsner J. 2007, 7, 37–39. [Google Scholar]
- Das, S.; Barnwal, P.; Ramasamy, A.; Sen, S.; Mondal, S. Lysergic Acid Diethylamide: A Drug of “Use”? Ther. Adv. Psychopharmacol. 2016, 6, 214–228. [Google Scholar] [CrossRef]
- Rucker, J.J.H.; Iliff, J.; Nutt, D.J. Psychiatry & the Psychedelic Drugs. Past, Present & Future. Neuropharmacology 2018, 142, 200–218. [Google Scholar]
- United States Drug Enforcement Administration. Ecstasy or MDMA (Also Known as Molly). Available online: https://www.dea.gov/factsheets/ecstasy-or-mdma-also-known-molly (accessed on 14 June 2024).


| Study | N | Age | Setting/Country | Hallucinogens | Design | Dose | Measures | Diagnosis |
|---|---|---|---|---|---|---|---|---|
| Davis et al. (2021) [53] | 27 | 21–75 | Medical Center/USA | Psilocybin | R, waiting list | 20–30 mg/70 kg | BDI, HAM-D | Moderate to severe major depressive disorder |
| Ross et al. (2016) [54] | 29 | 22–75 | Academic medical/USA | Psilocybin | R, P, cross-over | 21 mg/70 kg | BDI, STAI T, state, HADS | Life-threatening cancer |
| Griffiths et al. (2016) [55] | 51 | 56.3 (average) | USA | Psilocybin | R, P, double-blind, cross-over | 22–30 mg/70 kg | BDI, HAM-D, STAI T, state, HAM-A, HADS | Life-threatening cancer |
| Grob et al. (2011) [56] | 12 | 36–58 | Hospital research/USA | Psilocybin | R, P, double-blind | 30 mg/70 kg | BDI, STAI T, state | Life-threatening cancer |
| Carhart-Harris et al. (2021) [57] | 59 | 18–80 | University/UK | Psilocybin | R, escitalopram, double-blind | 25 mg | BDI, HAM-D | Moderate to severe major depressive disorder |
| Wolfson et al. (2020) [7] | 18 | >18 | Outpatient/USA | 3,4-methylenedioxy-methamphetamine | R, P, double-blind, cross-over | 125 mg | BDI, HAM-D, STAI T, state | Life-threatening cancer |
| Mitchell et al. (2021) [58] | 90 | 41 (average) | Multisite/USA, Canada, Israel | 3,4-methylenedioxy-methamphetamine | R, P, double-blind | 80–180 mg | BDI | Post-traumatic stress disorder |
| Ot’alora et al. (2018) [59] | 28 | >18 | Outpatient/USA | 3,4-methylenedioxy-methamphetamine | R, blinded, cross-over | 100–125 mg | BDI | Post-traumatic stress disorder |
| Mithoefer et al. (2018) [60] | 26 | >18 | Outpatient/USA | 3,4-methylenedioxy-methamphetamine | R, double-blind, cross-over | 100–125 mg | BDI | Post-traumatic stress disorder |
| Danforth (2018) [61] | 12 | 31.3 (average) | Multisite/USA | 3,4-methylenedioxy-methamphetamine | R, P, double-blind | 75–125 mg | BDI, LSAS | Autism spectrum disorder |
| Gasser et al. (2014) [62] | 12 | 39–64 | Switzerland | Lysergic acid diethylamide | R, P, double-blind, cross-over | 200 μg | STAI T, state | Life-threatening cancer |
| Palhano-Fontes et al. (2019) [63] | 29 | 18–60 | University Hospital/Brazil | Ayahuasca | R, P, double-blind, parallel arm | 25 mg/70 kg | HAM-D, MADRS | Treatment-resistant depression |
| 95% CI for Cohen’s d | |||||
|---|---|---|---|---|---|
| z | p | Cohen’s d | Lower | Upper | |
| Psilocybin | 15.275 | 1.118 × 10−52 | 3.333 | 2.906 | 3.761 |
| LSD | 5.692 | 1.255 × 10−8 | 1.800 | 1.180 | 2.420 |
| MDMA | 13.671 | 1.519 × 10−42 | 3.222 | 2.760 | 3.684 |
| Ayahuasca | 3.578 | 3.466 × 10−4 | 1.600 | 0.723 | 2.477 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fluyau, D.; Kailasam, V.K.; Revadigar, N. Rapid and Prolonged Antidepressant and Antianxiety Effects of Psychedelics and 3,4-Methylenedioxy-methamphetamine—A Systematic Review and Meta-Analysis. Psychoactives 2024, 3, 476-490. https://doi.org/10.3390/psychoactives3040029
Fluyau D, Kailasam VK, Revadigar N. Rapid and Prolonged Antidepressant and Antianxiety Effects of Psychedelics and 3,4-Methylenedioxy-methamphetamine—A Systematic Review and Meta-Analysis. Psychoactives. 2024; 3(4):476-490. https://doi.org/10.3390/psychoactives3040029
Chicago/Turabian StyleFluyau, Dimy, Vasanth Kattalai Kailasam, and Neelambika Revadigar. 2024. "Rapid and Prolonged Antidepressant and Antianxiety Effects of Psychedelics and 3,4-Methylenedioxy-methamphetamine—A Systematic Review and Meta-Analysis" Psychoactives 3, no. 4: 476-490. https://doi.org/10.3390/psychoactives3040029
APA StyleFluyau, D., Kailasam, V. K., & Revadigar, N. (2024). Rapid and Prolonged Antidepressant and Antianxiety Effects of Psychedelics and 3,4-Methylenedioxy-methamphetamine—A Systematic Review and Meta-Analysis. Psychoactives, 3(4), 476-490. https://doi.org/10.3390/psychoactives3040029





