1. Introduction
Inflammation represents a physiological reaction orchestrated by the immune system and can be activated by a diverse array of stimuli, involving pathogens, compromised cells, and harmful substances. These triggers have the capacity to elicit acute or chronic inflammatory reactions within vital organs, potentially culminating in tissue injury or the onset of various diseases. Both infectious and non-infectious agents, as well as cellular damage, have the ability to activate inflammatory cells and initiate intricate signaling pathways associated with inflammation, with the most frequently involved pathways being nuclear factor-κB (NF-κB), inflammasomes, and JAK-STAT pathways [
1,
2,
3,
4,
5].
LPS has the capacity to function as both Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs). It can engage Toll-like receptors, specifically TLR4, initiating a cascade of signaling pathways, including NF-κB, interferon regulatory factor (IRF), and MAPK pathways. These signaling events subsequently trigger the synthesis and release of proinflammatory cytokines and chemokines, facilitating an immune response characterized by inflammation [
6,
7,
8].
Likewise, transcription factors STAT3 and STAT1 play a substantial role in the expression of multiple genes encoding proinflammatory cytokines. The IL-6/JAK/STAT3 pathway has gained significant attention as a potential target for innovative therapeutic strategies in the treatment of diverse inflammatory conditions [
9,
10,
11].
Additionally, immune cells possess NOD-like receptors (NLRs) that detect pathogenic patterns in the cell’s cytoplasm, leading to the activation of inflammasomes. The NLRP3 inflammasome is extensively studied due to its association with inflammatory disorders, obesity, diabetes, and neurodegenerative diseases [
12,
13]. The activation of the NLRP3 inflammasome occurs in two steps. Initially, immune cells recognize insults, activating NF-κB and increasing the expression of IL-1β, IL-18, and NLRP3. In the second step, various processes like potassium ion efflux, reactive oxygen species generation, and decreased cAMP levels collectively activate the NLRP3 inflammasome. This activation induces the cleavage of Pro-Caspase-1 and thereby the conversion of Pro-IL-1β and Pro-IL-18 into their active forms, IL-1β and IL-18, respectively [
12,
14,
15,
16]. The activity of certain membrane channels, such as Pannexin-1 (PANX-1)/P2X7, can influence the assembly of the NLRP3 inflammasome. PANX-1 activation is linked to ATP efflux, subsequently triggering the activation of P2X7. This, in turn, causes alterations in the intracellular ion content and thereby the activation of the NLRP3 inflammasome [
17,
18].
Dysregulated NLRP3 inflammasome activation and subsequent increased IL-1β production by macrophages resident in the pancreas have been linked to β-cell apoptosis, loss of β-cells, and the progression of T2DM [
19].
Serotonin, also referred to as 5-hydroxytryptamine (5-HT), plays a vital role as a peripheral hormone in various organs. Its activity is mediated through various components such as different classes of mammalian serotonin receptor subtypes, the serotonin transporter (SERT), and its binding to effector proteins. Serotonin signaling is present in almost all immune cells, and recent research has shed light on its immunoregulatory functions. For instance, in monocytes/macrophages, serotonin has been observed to influence the release of proinflammatory cytokines by activating serotonin receptors [
20]. In this line, it has been found that the selective activation of the 5-HT2A receptor can suppress inflammatory responses in in primary aortic smooth muscle cells induced by TNFα [
21]. In vivo studies have substantiated the efficacy of activation of the 5-HT2A receptor in mitigating proinflammatory responses. In this context, it has been observed that the systemic and selective activation of the 5-HT2A receptor is associated with the downregulation of proinflammatory genes, contributing to the reduction of systemic inflammation [
22].
Psychedelic compounds interact with serotonin receptors and their different subtypes located throughout the brain. These receptors play a role in regulating various processes such as emotions, moods (including anxiety and aggression), cognition, sexual behavior, learning, memory, and appetite [
23,
24]. It is important to remember that these receptors are present throughout the entire nervous system in the body [
25].
Psilocybin, among all psychedelic drugs, is known for its notably favorable safety profile [
26]. Psilocybin, a natural compound which is produced by the
Psilocybe genus of mushrooms, has agonistic effects on many serotonin receptors, in particular on the 5-HT2A receptor. In fact, it has been demonstrated that psilocybin is able to modulate 5-HT2A receptor activation and modify downstream gene transcription and translation, and thereby it is considered as a safe serotonin receptor agonist [
27]. Previously, we demonstrated that psilocybin could regulate proinflammatory responses in the brains of LPS-induced mice. Building on this, we observed that psilocybin reduces inflammation in the brains of animals with LPS-induced neuroinflammation [
28]. The findings from this study prompted further investigation into the anti-inflammatory effects of psilocybin.
In the present study, our objective was to elucidate the effects of psilocybin on lipopolysaccharide (LPS)-induced inflammation in THP-1 macrophages. Our findings indicate that psilocybin has the potential to modify the inflammatory responses in LPS-induced THP-1 macrophages, and this effect is, at least in part, attributed to alterations in NF-κB, STAT3, and STAT1 phosphorylation.
2. Materials and Methods
2.1. Cell Cultures and Experimental Conditions
In this study, THP-1 monocytes (catalog number: ATCC TIB-202, sourced from the American Type Culture Collection, Rockville, MD, USA) were employed. The THP-1 cell line, a human leukemia monocytic cell line, is widely utilized for exploring responses and mechanisms related to inflammation. Cell cultures were established in cell culture plates utilizing RPMI 1640 medium (catalog number: 350-000-CL, procured from Wisent Inc., Saint-Jean-Baptiste, QC, Canada). Further, 10% fetal bovine serum (FBS) was added to the medium (catalog number: 10082147, obtained from Fisher Scientific Company, Ottawa, ON, Canada). The incubation of cultures was conducted at +37 °C with a CO2 concentration of 5%. Subculture was executed every 48 h, and cells at passages 8 to 13 were selected for all experimental procedures.
For the differentiation of monocytes into macrophages, non-adherent monocytes underwent exposure to 50 ng/mL phorbol-12-myristate-13-acetate (PMA) (product number: BML-PE160-0005, procured from Enzo Farmingdale, NY, USA) for 48 h. To nullify the effects of PMA on macrophage responses to LPS/ATP and psilocybin (product number: 520-52-50, Applied Pharmaceutical Innovation, Edmonton, AB, Canada), the medium was discarded, cells were rinsed twice with PBS 1X (without calcium and magnesium, catalog number: 311-010-CL, Wisent Inc., Saint-Jean-Baptiste, QC, Canada), followed by a 24 h recovery period in a PMA-free medium. Note that the psilocybin employed in this study was synthesized and purified by HPLC, and its purity, as per HPLC analysis, was found to be 99.12%. After purchase, psilocybin was stored at 20 °C, in a dark place.
Subsequently, cells were pre-treated with psilocybin for 1 h, followed by incubation with a medium containing 500 ng/mL lipopolysaccharide (LPS) from Escherichia coli O111:B4 (catalog number: L4391, sourced from EMD Millipore Corporation, Temecula, CA, USA) for 4 h to induce proinflammatory responses. The LPS stock solution was prepared by dissolving 1 mg of sterile powder in 1 mL of medium, and the resulting stock was stored at −20 °C.
Cells treated with LPS underwent incubation with 5 mM adenosine 5′-triphosphate (ATP) disodium salt hydrate (product ID: A6419, sourced from EMD Millipore Corporation, Temecula, CA, USA) for 30 min to initiate the assembly of the NLRP3 inflammasome. It is essential to mention that, for the preparation of the ATP stock, the powder was dissolved in the medium, and the pH was adjusted to 7 using NaOH, followed by filtration of the stock, and storage at −20 °C. In this study, we examined the effects of three different doses of psilocybin on both LPS-stimulated and unstimulated THP-1 macrophages. The objective was to understand the impact of psilocybin on general inflammatory responses and also on LPS-mediated inflammatory responses in THP-1 macrophages. To ensure that psilocybin’s activity does not interfere with LPS induction, we pre-treated the cells with psilocybin before the induction with LPS. It is important to highlight that we conducted optimization studies to determine the optimal dose of LPS and the duration of LPS and ATP incubation. Moreover, as extracellular ATP levels primarily contribute to the formation of the NLRP3 inflammasome, we studied the response of NLRP3, Pro-IL-1β, and IL-1β to psilocybin in LPS + ATP (LA)-induced THP-1 macrophages, whereas for other proteins and gene expression studies, we used LPS-induced THP-1 macrophages.
2.2. Cytotocity Assay
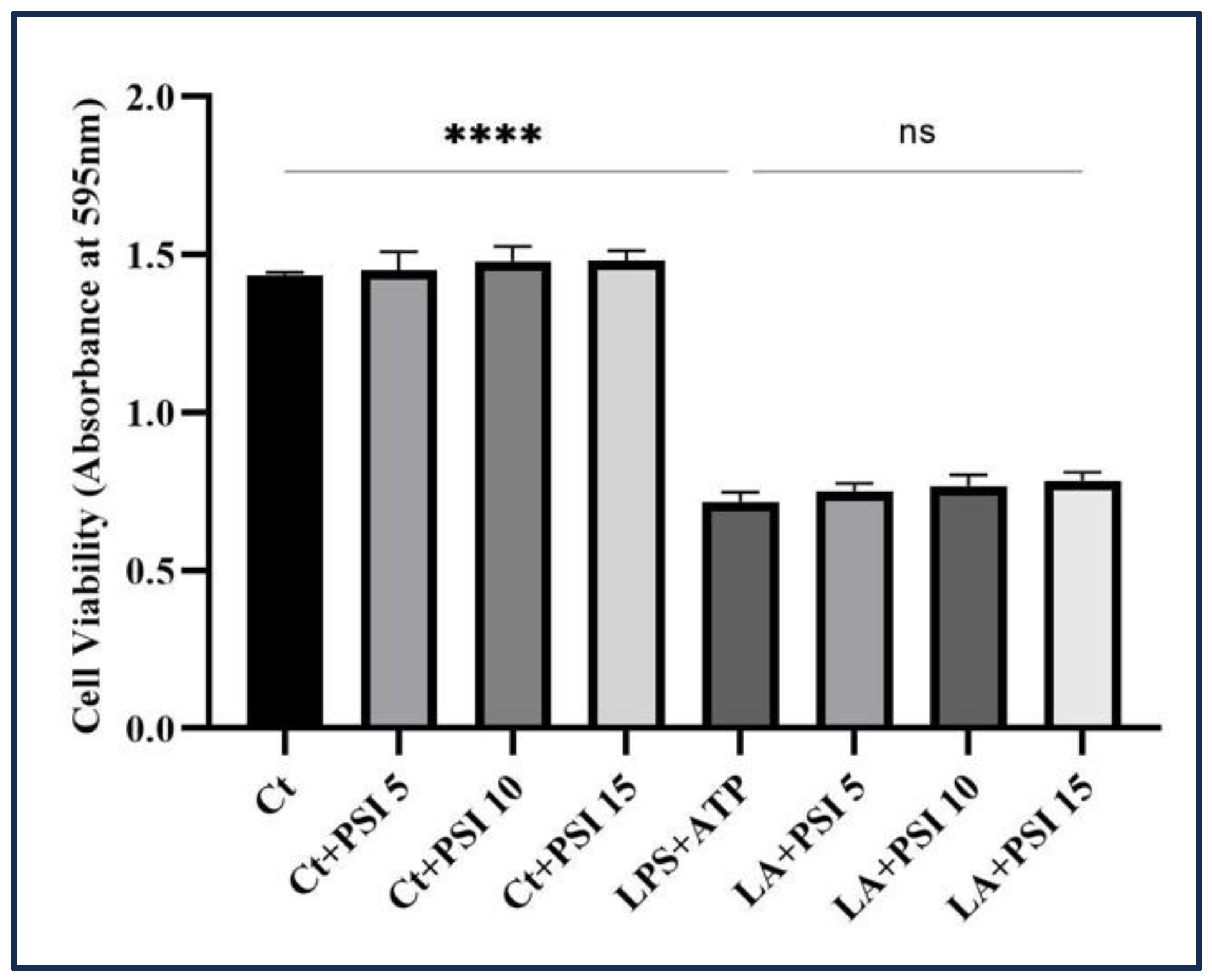
To evaluate the effect of psilocybin on THP-1 macrophages’ survival induced by LA, we employed an MTT assay. Initially, THP-1 monocyte cells were treated with PMA and subsequently dispensed into separate wells with 1 × 10
4 cells/mL in a 96-well plate. It is worth mentioning that we seeded a high number of THP-1 monocyte cells in each well. This decision is based on the fact that THP-1 monocytes, upon differentiation into macrophages, acquire macrophage-like properties, including the loss of their ability to undergo cell division [
29]. After one-hour pretreatment with various concentrations of psilocybin, the cells were then incubated with LPS (500 ng/mL) and ATP (5 mM), followed by incubation at +37 °C with a 5% carbon dioxide concentration for the following 24 h.
Following the incubation period, a metabolic activity assay was performed. The content of each well was incubated with a solution of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) labeling reagent (product ID: 11465007001, Millipore Sigma Canada Ltd., Oakville, ON, Canada). Subsequently, the microplate was placed in a suitable environment for 4 h. Subsequently, a solubilizing solution was added, and the plate was left to incubate overnight. The solubilizing solution consists of SDS 10% in diluted hydrochloric acid (HCl). The absorbance of the resultant solution was assessed at a particular wavelength using a plate reader (FLUOstar Omega, BMG LABTECH, Offenburg, Germany).
2.3. Immunoblotting
Post cellular treatments, cell lysis was carried out with RIPA lysis buffer (comprising 150 mM NaCl, 50 mM Tris pH 8, 0.5% Deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mM Na
3Vo
4, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM EDTA, sourced from Abcam). Whole cell lysates were subsequently stored under suitable conditions. After quantifying total protein, a specific quantity of proteins was loaded for gel electrophoresis. Depending on the abundance of the studied proteins, varying amounts of total proteins (50 µg for low abundance and 100 µg for high abundance proteins) were utilized for gel electrophoresis. The resolved proteins underwent electrotransfer onto polyvinylidene difluoride (PVDF) membranes (Amersham Hybond
® P membranes, product number: RPN2020F, obtained from GE Healthcare, Oakville, ON, Canada); membranes were then blocked with 5% nonfat milk in PBS + 0.1% Tween
® 20 Detergent (PBST) before being incubated with primary antibodies. This was succeeded by an overnight incubation with primary antibodies at 4 °C. Information regarding the primary antibodies is provided in
Table S1. The membranes were subjected to three washes with PBST, followed by incubation with relevant secondary antibodies for 2 h at room temperature, and then a second round of wash with PBST. Immunoreactivity was detected using peroxidase-conjugated antibodies, and the reaction was visualized using the ECL Plus Western Blotting Detection System (GE Healthcare, Oakville, ON, Canada). Densitometric analyses were conducted using ImageJ 1.53K. Band intensities were quantified and normalized against the intensity of housekeeping proteins. Two housekeeping proteins, GAPDH and β-actin, were employed to normalize western blot results. The use of two housekeeping proteins as controls aimed to ensure that the quantification of bands was not affected by incomplete blotting due to air bubbles. To ensure consistent results, the responses of all examined proteins on the same mem-brane were normalized against the same controls; thus the same control image is pre-sented on all figures. For membrane stripping, a mild stripping buffer was employed, consisting of 10 mL Tween 20, 1 g SDS, 15 g glycine, per 1 L of distilled water, with a pH of 2.2 as per the instructions provided by Abcam.
2.4. Gene Expression Profiling by Real-Time PCR
In the examination of gene transcription, RNA extraction from the cells was carried out employing a commercially available TRIzol
® Reagent (CAT#15596018, purchased from Invitrogen, Life Technologies Inc., Burlington, ON, Canada), adhering to the guidelines provided by the manufacturer. Following the measurement of RNA quantity using Nanodrop 2000c (ThermoFisher Scientific, Waltham, MA, USA), the complementary DNA was synthesized with the iScript™ Reverse Transcription Supermix (CAT: 1708841, obtained from BioRad Laboratories, Saint-Laurent, QC, Canada); total RNA in the amount of 1 μg was used. To set up qRT-PCR reactions, 1 μL of cDNA was combined with SsAdvancedTM Universal Inhibitor-Tolerant SYBR Green Supermix (product ID: 1725017, Bio-Rad Laboratories, Saint-Laurent, QC, Canada), along with 2.5 mM of each primer according to the manufacturer’s instructions. The qRT-PCR primers were designed using the PrimerQuest™ Tool, an online software, and procured from Eurofins (Ottawa, ON, Canada) (refer to
Table S2).
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
The quantification of IL-β release and its response to the treatments in LPS + ATP groups were assessed using the Human IL-1β/IL-1F2 Quantikine ELISA Kit (Cat number DLB50 from R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. After the addition of the Stop Solution, optical density measurements were recorded at 450 nm and 570 nm employing SpectraMax (SpectraMax i3x from Molecular Devices, San Jose, CA, USA) microplate reader. To compensate for potential optical distortions in the plate, the values at 570 nm were subtracted from those at 450 nm. Due to the notably high quantity of secreted IL-1β, the supernatants underwent a 50-fold dilution.
2.6. Statistical Analysis
The data analysis was conducted utilizing GraphPad Prism 8.0 software. All results are expressed as mean values ± standard deviation (SD). Statistical significance was evaluated employing one-way analysis of variance (ANOVA), followed by the application of Dunnett’s test for comparing mean values between the LPS and LA groups with other treatment groups.
4. Discussion
Psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine), classified as a substituted indole alkylamine, is categorized as a hallucinogenic tryptamine. Psilocybin is the primary mind-altering substance found in so-called magic mushrooms [
34]. Alongside its recognized recreational, spiritual, and religious applications, there is substantial medical potential supported by both personal accounts and scientific research [
35].
In this study, our objective was to examine the impact of psilocybin on proinflammatory responses using an in vitro model.
There is limited research on the impact of psilocybin on inflammation. One such study by Nkadimeng et al. (2020) explored its potential anti-inflammatory effects. They focused on a specific species of magic mushrooms called
Psilocybe natalensis. The study demonstrated that the analgesic, antioxidant, and anti-inflammatory properties of these mushrooms varied depending on the dosage administered. To evaluate these effects, the researchers treated LPS-induced macrophages with three different extracts derived from
Psilocybe natalensis mushrooms, namely ethanol and cold and hot water extracts. The results of the study confirmed the antioxidant capabilities of
Psilocybe natalensis mushrooms, as all three extracts effectively inhibited the nitric oxide induced by LPS. Additionally, the study showed that these mushrooms possessed anti-inflammatory characteristics through the inhibition of prostaglandin E2 and IL-1β cytokine synthesis [
36].
The present study initially aimed to examine the ability of psilocybin to decrease COX-2, Pro-TNFα, IL-1β, IL-6 and Pro-IL-1β levels in LA/LPS-induced THP-1 macrophages. We found that the administration of certain doses of psilocybin significantly reduced IL-6 and COX-2 protein levels in THP-1 macrophages challenged by LPS (see
Figure 2). These results align with the study by Nkadimeng (2020) et al., which demonstrated that extract treatment leads to a decrease in prostaglandin E2 production mediated by COX-2 and IL-6. Psilocybin also decreased Pro-IL-1β, mature IL-1β (see
Figure 3a), and secreted IL-1β in a dose-response manner (see
Figure 8); Nkadimeng et al. (2020) also found psilocybin to decrease the level of secreted IL-1β.
To understand the potential mechanisms of psilocybin’s effects on
IL-6,
COX-2, and
IL-1β mRNA levels, we examined its impact on key inflammation-related transcription factors: NF-κB, STAT1, and STAT3. Our results indicate that psilocybin at doses of 10 and 15 μM downregulated the elevated levels of phosphorylated NF-κB in LPS-induced macrophages (
Figure 4a), thereby suppressing its activity. Psilocybin also attenuated the activation of TYK2, STAT3, and STAT1 transcription factors, further supporting its inhibitory influence on the transcription of proinflammatory proteins.
Additionally, the qRT-PCR results revealed that certain doses of psilocybin downregulated IL-1β and TNFα at the transcriptional level. This effect may be linked to psilocybin’s inhibitory impact on NF-kB, STAT3, and STAT1 activation in LPS-induced macrophages. The inhibitory effect of psilocybin on the activation of NF-kB and subsequent transcription of proinflammatory genes is likely mediated through the downregulation of IL-6/TYK2 proteins, observed in our experiments. This may be further supported by the fact that only the 10 μM and 15 μM doses of psilocybin mitigated the phosphorylation of both NF-kB and TYK2, while 5 μM psilocybin did not show any significant impact on the phosphorylation of TYK2, and it even increased the phosphorylation of NF-kB. Accordingly, Yang et al. (2005) established that interferon α induces the activation of NF-κB in cells lacking JAK1 by utilizing a pathway that relies on TYK2. This observation implies that TYK2 phosphorylation is involved in the phosphorylation of NF-κB and the consequent proinflammatory responses [
37]. STAT3 functions as a transcription factor that, once activated, promotes the expression of various proinflammatory cytokines, including IL-17, IL-23, and IL-8 [
11]. Notably, IL-6 plays a crucial role in activating STAT3 during inflammation, and the IL-6/STAT3 axis has emerged as a promising target for the development of novel treatments for inflammatory disorders. Excessive activation of NF-κB results in elevated levels of IL-6, which in turn can initiate the phosphorylation of JAKs such as TYK2. This phosphorylation event subsequently activates STAT3 and even NF-kB itself, facilitating their movement into the cell nucleus and ultimately leading to an increased transcription of cytokine genes [
38]. The activation of STAT3 leads to a cooperative interplay with NF-κB, resulting in an increase in the phosphorylation and subsequent translocation of NF-κB into the nucleus [
39,
40], which ultimately results in cytokine storm. According to our findings, 10 μM and 15 μM of psilocybin significantly inhibit the TYK2/STAT3 pathway (
Figure 4), suggesting a potential mechanism through which psilocybin may modulate the proinflammatory responses leading to a cytokine storm.
Apart from the suppressive effects of psilocybin on the TYK2/STAT3 axis, it appears to have a mitigating effect on the TYK2/STAT-1 axis as well (
Figure 4). This suggests an additional mechanism by which psilocybin can downregulate proinflammatory responses in macrophages stimulated with LPS. Accordingly, it has been established that the activation of STAT1 induced by LPS is responsible for the production of IL-6, involving both NF-κB-dependent and independent pathways [
9]. It is worth mentioning that while 5 μM does not show any significant impact on the mitigation of TYK2 phosphorylation, this dose mitigates the phosphorylation of both STAT3 and STAT1, suggesting the possible involvement of other JAKs in mediating the inhibitory impact of 5 μM psilocybin on the phosphorylation of these transcription factors.
It is worth mentioning that, despite psilocybin’s inhibitory effects on IL-6 and COX-2 protein levels, no effect on the mRNA levels of IL-6 and COX-2 was observed (
Figure 7). This suggests that specific dose(s) of psilocybin may exert their inhibitory effects on the post-transcriptional regulation of these proteins. The pattern of expression of
COX-2 and
IL-6 and
COX-2 mRNA to psilocybin in macrophages treated with LPS was consistent with that of the
TNFα transcript and was associated with changes in P-NF-κB upon psilocybin administration. This may suggest a potential role of NF-κB as a target through which psilocybin exerts its effects on the transcription of these proinflammatory cytokines.
LPS induction of macrophages results in an increase in the transcription of
Pro-IL-1β and
NLRP3 [
41], which in turn results in the formation of NLRP3 inflammasome and subsequent production of active IL-1β. Exposure to 5 and 10 μM psilocybin lowered the concentration of NLRP3 in THP-1 cells challenged by LA. This reduction is likely due to post-translational processes, as no inhibitory effect was observed in the mRNA level (
Figure 7). In addition, the alleviating effect of 10 and 15 μM of psilocybin on the increased levels of Pro-IL-1β, which is likely mediated through the downregulation of its transcription (
Figure 3a and
Figure 7), provides substantial evidence of psilocybin’s inhibitory influence on the initial stage of activation of the NLRP3 inflammasome. Furthermore, the administration of psilocybin resulted in the downregulation of the IL-1β/Pro-IL-1β ratio in LA-induced THP-1 macrophages (see
Figure 3a). This suggests that all three doses of psilocybin may also inhibit the second phase of NLRP3 inflammasome activation in addition to suppressing the initial phase.
During apoptosis, PANX-1, a transmembrane protein found in various cells including macrophages, undergoes cleavage by Caspase-3 and 7 at its C-terminus. This cleavage leads to the opening of PANX-1 channels and an increase in membrane permeability [
42,
43]. As a result, extracellular ATP levels rise, triggering the activation of the P2X7 receptor and leading to an influx of potassium ions into the cell. Intracellular potassium levels are known to be associated with the initiation of the second phase of NLRP3 inflammasome activation [
17]. Exposure to psilocybin diminished the levels of total PANX-1 (see
Figure 5). This reduction is likely due to its influence on post-transcriptional processes, as indicated by the qRT-PCR results (see
Figure 7). This suggests that psilocybin may target an additional pathway through which it exerts inhibitory effects on the second phase of NLRP3 inflammasome activation. Furthermore, we observed that 5 μM psilocybin increased the cleavage and subsequent opening of PANX-1 channel, while neither 10 μM nor 15 μM had a significant impact on this process (see
Figure 5). This provides evidence of a potential link between apoptosis, PANX-1 cleavage, and the stimulatory effect of 5 μM psilocybin on the response of P-NF-κB, Pro-IL-1β, IL-6, and COX-2.
In general, our results show that psilocybin impacts the production of inflammation-related cytokines in LPS/LA-induced macrophages in a complex manner. The findings suggest that the effect of psilocybin on the production of proinflammatory cytokines is dose dependent and sometimes bi-phasic or has a bell-curve response. For instance, the 10 and 15 μM concentrations of psilocybin decreased the protein levels of IL-1β and COX-2, while 5 μM increased the levels of these proteins. Additionally, 10 μM psilocybin reduced the level of IL-6, but 15 μM increased the level of this protein. Moreover, while 10 μM psilocybin did not impact the level of Pro-TNFα, the 5 and 15 μM concentrations increased its level. The dose-dependent effects of psilocybin on the production of proinflammatory cytokines could be attributed to its complex interactions with the immune system. Additional research is required for a comprehensive understanding of the underlying mechanisms involved.
Monocytes/macrophages possess various serotonin receptors such as 5-HT1E, 5-HT2B, 5-HT1A, 5-HT2A, 5-HT3, 5-HT4, and 5-HT7 [
20]. Psilocybin exhibits agonistic effects on several serotonin receptors, particularly 5-HT2A and 5-HT2B receptors. Previous studies have demonstrated that psilocybin can modulate the activation of these receptors, leading to alterations in downstream gene transcription and translation [
27]. The relationship between the activation of 5-HT2 receptor(s) and anti-inflammatory properties has been extensively established [
20,
44,
45]. In our current report, we demonstrated that psilocybin was capable of restoring the diminished levels of serotonin receptors 5-HT2A and 5-HT2B in THP-1 macrophages induced by LPS (
Figure 6). This discovery may introduce a novel pathway through which psilocybin exerts its inhibitory effects on the dysregulated proinflammatory cytokines and proteins.
In summary, the key findings of this study are as follows (
Figure 9):
- 1.
Psilocybin demonstrates a dose-dependent mitigation of elevated levels of Pro-IL-1β, IL-1β, IL-6, and COX-2 proteins in LA/LPS-induced THP-1 macrophages.
- 2.
Specific doses of psilocybin mitigate the activities of NF-κB, STAT1, and STAT3 transcription factors, potentially serving as a target through which psilocybin downregulates TNFα and IL-1β expression.
- 3.
Psilocybin, at certain doses, appears to exert mitigatory effects on STAT3 activation by influencing TYK-2 activation.
- 4.
The reduction in PANX-1 levels likely contributes to the mitigatory impact of specific psilocybin doses on the NLRP3 assembly in LPS-induced THP-1 macrophages.
- 5.
Psilocybin demonstrates a stimulatory effect on the levels of SR-2A and SR-2B in LPS-induced THP-1 macrophages (
Figure 9).
6. Future Direction
In this work, we have provided evidence of the inhibitory impact of specific doses of psilocybin on the production of proinflammatory proteins and cytokines in LPS/LA-induced THP-1 macrophages. To further validate our results, it is recommended to conduct experiments with other cell lines and in vivo systems.
Additionally, considering that psilocybin inhibits the IL-1β/Pro-IL-1β ratio, future research could explore the impact of psilocybin on the second phase of NLRP3 inflammasome activation, focusing on post-translational modifications of NLRP3 in response to psilocybin.
We have also observed the inhibitory impact of psilocybin on STAT3 and STAT1 activation. Further investigations could explore the response of proinflammatory cytokines controlled by these transcription factors, including IL-8, IL-17, and IL-23.
While our study found that psilocybin administration increased the levels of two serotonin receptors in LPS-induced macrophages, further research is needed to establish connections between these findings and the responses of the proinflammatory proteins and cytokines studied here.