Abstract
Psychotropic medications, commonly prescribed for psychiatric disorders, can have underappreciated dermatological side effects. This in-depth review explores the intricate relationship between psychotropic drugs and the skin, emphasizing the significance of recognizing and managing these side effects in clinical practice. It categorizes the dermatological side effects associated with different classes of psychotropic medications. These include antidepressants, antipsychotics, mood stabilizers, and anxiolytics. We delve into the spectrum of dermatological conditions, from mild issues like dry skin and acne to severe complications such as Stevens–Johnson syndrome and drug-induced lupus erythematosus. In conclusion, a comprehensive understanding of the dermatological side effects of psychotropic medications is essential for healthcare providers, enabling a holistic approach to patient care. This review is a valuable resource for clinicians, researchers, and educators, facilitating better-informed decision-making in the treatment of mental health disorders while prioritizing skin health and overall well-being.
1. Introduction
1.1. Background
Psychotropic medications, used to treat primary psychiatric disorders as well as psychodermatologic conditions, constitute one of the most frequently prescribed drugs in outpatients of a hospital setting given that psychiatric disorders represent a significant comorbidity in the United States (ranging from approximately 1% to 22%) [1,2,3]. Adverse reactions (ADRs) to these medications are a leading cause of discontinuations and therefore can lead to poor compliance [4]. ADRs have been broadly classified in the literature into type A—representing predictable reactions such as anticholinergic and antipyramidal signs—and type B—representing often idiosyncratic reactions such as adverse cutaneous drug reactions (ACDRs)—the latter of which has been under-reported due to the fact that most of these are benign and easily manageable [5]. Nevertheless, dermatological reactions account for the majority of side effects from drugs including psychotropic medication, with some prospective studies estimating a prevalence of approximately 4 to 7 in 1000 hospitalisations [6,7]. Similarly, ACDRs account for the majority (2 to 5%) of ADRs to psychotropic medication [8]. As per one report, the prevalence of dermatological symptoms in psychiatric patients was as high as 8.4%, with 50% of them having received their first treatment with psychotropic medications [9]. ACDRs have been reported in all classes of psychotropic drugs—mood stabilizers (39%), antidepressants (29%), and antipsychotics (19%) [10]. Additionally, cross-sensitivity is a concern due to the common utilization of multiple drugs to achieve remission [11]. However, most of the existing data focus on hospital settings, where multiple drugs are commonly prescribed, which can make the establishment of causal drugs difficult to interpret for an appropriate withdrawal strategy. We have comprehensively reviewed the risks, mechanisms, and current management practices of the ACDRs associated with psychotropics and also discussed the potential of a multidisciplinary approach to patient care [12].
1.2. Pathogenesis: Mechanism and Risk Factors
Both immunogenic as well as non-immunogenic mechanisms have been described in the literature; the former accounts for 5–10% of all ACDRs and can be mediated by immunoglobulin E (IgE), circulating immune complexes, or by lymphocytes [13,14]. Predominantly, the expansion of T cells represents the most common immune response to a drug, recognizing it as foreign and orchestrating a delayed immune response, often manifesting as pruritic rashes affecting the skin. In the context of psychiatric medications, the immunogenic role is underscored by factors such as Human Leukocyte Antigen (HLA) subtype and viral infections, and emerging evidence suggests a strong interrelation between thoughts, emotional patterns, psychological dynamics, and the immune response in psychiatric conditions [15]. The immunogenic mechanism has been highlighted by the role of factors such as Human Leukocyte Antigen (HLA) subtype or viral infections [16]. Substance abuse, being a woman (particularly in the reproductive age group), and the use of antiepileptic drugs (AEDs) and selective serotonin reuptake inhibitors (SSRIs) were identified as independent risk factors from regression analyses [10,17]. Hormonal influences and a predisposition to autoimmune disease likely cause women to be at higher risk, although the exact reason remains unclear [18,19]. The HLA system, located on the short arm of chromosome 6p21.31, plays a pivotal role in immune recognition, and abnormalities in HLA expression can impact antigen processing and presentation [20,21]. The distribution of HLA genes across chromosomes is categorized into three distinct regions—class-I, class-II, and class-III [22]. Additionally, it is important to note that HLA serves as a genetic marker and remains unchanged throughout an individual’s lifespan [23]. Moreover, the classical grouping of HLA-I genes comprises three subtypes: HLA-A, HLA-B, and HLA-C [24]. The role of HLA polymorphism has been extensively studied over the years to provide an understanding of the pathogenic mechanisms of ACDRs and atopic conditions [25]. Of note is the particular HLA subtype that has been observed to impact different ethnicities based on carbamazepine (CBZ) studies (Table 1). In particular, HLA-A has been observed to be most frequently associated with SJS/TEN reactions induced by the AEDs carbamazepine, lamotrigene, phenytoin, and zonisamide, followed by HLA-B or C subtypes [26]. Furthermore, ethnicity has a role to play in the type of reaction observed [27]. Pharmacogenomic considerations such as HLA subtype can be used to guide dose adjustments or drug selections to avoid any life-threatening ACDRs [28]. A higher risk has also been observed in the elderly population, particularly those with multiple chronic medical conditions and polypharmacy [29].

Table 1.
Risk factors like ethnicity, genetic predisposition, and miscellaneous factors associated with dermatological side effects of psychotropic medications [10,17,29,30,31,32,33].
1.3. Clinical Picture
The diagnosis of drug-induced dermatological reactions is a significant challenge due to factors such as multifactorial aetiology and complex presentation. A comprehensive patient history is crucial, including details about the rash’s onset, its pattern, and the patient’s medication history [34]. In some cases, confirmation of the diagnosis may necessitate skin testing or in vitro testing [35]. Exanthematous eruptions, the most common dermatological reaction, typically manifest initially on the trunk or in areas subjected to pressure or trauma [36]. Upon discontinuation of the offending drug, these eruptions tend to spread to the extremities and eventually subside. The rash commonly emerges within a few hours after the initial drug administration, although the timeline can vary for previously sensitized individuals [37]. Other dermatological presentations encompass drug-induced urticaria, characterized by erythematous wheals, and erythroderma, a generalized skin erythema [38]. Drug-induced photosensitivity is a rare phenomenon which occurs due to cellular damage caused by cross-reaction in sun-exposed areas and results in oedema and erythema [39]. Although it is considered a rare ACDR, it may have potential significance due to its association with SSRIs, which are widely prescribed globally for a variety of psychiatric conditions apart from depression. Thus, patients on these photosensitizing drugs must be aware of the potential risk and must practice sun protection. Sunlight avoidance is the ideal measure, but individuals who have to stay outdoors must use broad-spectrum sunscreens, which offer protection against both UVB and UVA; these also must contain a high sun protection factor (SPF). In addition to this, smartphone apps can alert the user about UV levels and offer personalized protection, and other measures like the use of UPF-rated clothing have also proved to be beneficial [39]. In severe cases, drug-induced dermatological reactions can escalate to conditions like Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and drug reactions with eosinophilia and systemic symptoms (DRESS) [40,41,42]. Also, certain mood stabilizers like lithium have caused notable instances of drug induced alopecia (12–18%). Apart from this, other drugs like TCAs, benzodiazepines, and newer antidepressants are also responsible for hair loss, although the incidence remains rare [43]. Discontinuation or dose reduction usually result in complete hair regrowth, but the therapeutic value of mineral supplements remains uncertain. Such severe reactions necessitate immediate hospitalization and the prompt discontinuation of the causative drugs to mitigate further complications.
2. Methodology
We conducted a comprehensive search of the Pubmed and Scopus databases to identify all the relevant articles for this study, reviewing all relevant articles like case series, case reports, cohort studies, editorials, and brief reports up until November 2023. The search terms included “psychotropics”, “antipsychotics”, “mood stabilizers”, “antidepressants”, “cutaneous reactions”, and “dermatological reactions”, with appropriate use of the Boolean operators AND, OR, NOT. Individual drug names and groups were also searched to avoid missing any relevant articles. We manually searched the reference lists of the included studies as well as previous reviews to ensure that our search strategy did not overlook any potentially relevant studies. No language restriction was used, and commentaries or studies conducted solely in animal models were excluded. Additionally, articles that did not mention the dermatological side effects of psychotropic medications were also excluded. The articles were reviewed according to the inclusion and exclusion criteria (IC and EC). The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart enumerating the step-by-step inclusion of studies is reported in Figure 1. The articles considered in this review were selected with a particular emphasis on those that were deemed to hold significant clinical and medical relevance.
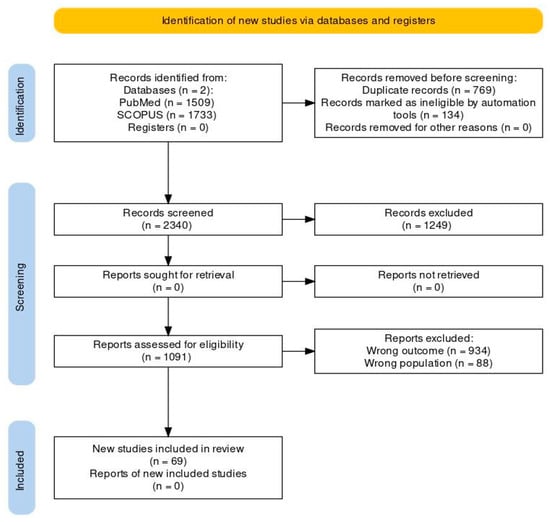
Figure 1.
PRISMA (2020) flowchart to depict the inclusion of studies.
3. Morphology and Diagnosis
3.1. Morphology and Basics of Skin Lesions
Drug-induced cutaneous reactions pose a significant challenge in diagnosis and management. Among the enhanced spectrum of skin lesions seen, 51–95% of them are exanthematous eruptions [44]. Exanthematous eruptions typically manifest as diffuse erythematous macules and papules, predominantly on the upper half of the body. They often emerge within ten days of initiating the offending drug but can also appear after drug discontinuation. The notorious agent attributed to this kind of skin eruption is phenothiazine neuroleptics. Notably, CBZ can induce a similar eruption that tends to initiate on the face and spread throughout the body [45]. Barbiturates have also been implicated in causing exanthematous eruptions, typically manifesting as macular lesions [45].
Urticarial reactions are another type of drug-induced cutaneous response which can appear anywhere on the body, often within minutes to hours of drug intake, and usually resolve within 24 h [35]. They potentially impact the airway and necessitate urgent medical intervention because of their association with anaphylaxis and angioedema. Fixed drug eruptions present as well-defined, solitary, red to purple lesions, sometimes with bullae or erosions. These eruptions typically occur within 30 min to 8 h after drug ingestion [46]. Barbiturates are commonly associated with fixed drug eruptions, and they have also been reported with certain antidepressants [45]. Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS) is characterized by a macular exanthematous eruption, fever, lymphadenopathy, and multiorgan involvement, with the liver being most affected. About 25% of DIHS sufferers have significant facial oedema, and about 30% have eosinophilia [47]. Usually occurring two to six weeks after starting medication, CBZ and aromatic anticonvulsants such as phenytoin and phenobarbital are most commonly linked to this condition’s symptoms. DIHS can result in a 10% death rate, mostly from hepatic necrosis and fulminant hepatitis [47]. Erythema multiforme is characterized by well-defined erythematous macules or papules with central bullae or crusts, often forming “target” lesions. Typically, this condition affects the extremities and palmoplantar surfaces, with the potential involvement of mucous membranes. Erythema multiforme is commonly linked to infections, with drug-induced cases primarily associated with AEDs [48]. Cutaneous pseudolymphoma is a benign condition that mimics cutaneous lymphoma. It typically presents as red to violaceous smooth papules, nodules, or plaques, often accompanied by an exanthem. A dermatology consult and biopsy may be necessary to differentiate this condition from malignancy.
3.2. Diagnosis
Diagnosing cutaneous side effects can be a challenging endeavour due to the involved complexities of the interplay of etiologic factors and complex cross-reactions due to multiple therapeutic agents prescribed to the patient. The burden of antipsychotic side effects is estimated using validated tools such as the Glasgow Antipsychotic Side Effect Scale (GASS) or the Udvalg for Kliniske Undersøgelser (UKU) SE scale, which could provide clues about any dermatological side effects from antipsychotics. Physical examination is pivotal in understanding the pattern, distribution, and characteristics of the cutaneous eruption, offering essential diagnostic information. A high level of suspicion is warranted given the fact that ACDRs are non-specific and mimic any skin lesions. Numerous algorithms have been devised to assess the likelihood that a drug is responsible for a cutaneous manifestation. One such example is the algorithm of drug causality for EN (ALDEN) scale, which can be helpful in determining drug causality and has been found to be more sensitive than general methods [49]. Constructing a timeline that includes the drug’s initiation, duration of use, the last time it was taken, and when the cutaneous symptoms appeared can aid in diagnosis. Drugs initiated within the previous three months, particularly within the last six weeks, are often the primary suspects, as are drugs used intermittently [50]. Therefore, a comprehensive drug history with proper information regarding dosage and duration must be obtained before starting a new agent. Prior ACDRs in a patient’s history should be considered as a potential diagnostic clue. Moreover, the response of cutaneous symptoms to reduced drug dosages or discontinuation is a valuable indicator of a drug-induced reaction. Exploring the patient’s family history for instances of hypersensitivity reactions to drugs may provide additional context for diagnosis [51].
In cases where drug-induced skin reactions raise concerns, certain clinical findings should be closely monitored [50]. Identifying these symptoms may necessitate immediate discontinuation of the offending drug and a consultation with a dermatologist. Moreover, it is crucial to differentiate between ACDRs caused by psychotropic drugs and self-inflicted skin lesions, often resulting from psychiatric conditions like trichotillomania [52]. Therefore, comprehensive documentation of cutaneous findings during psychotropic medication treatment, including detailed descriptions, the timing of onset, and the progression of the skin eruption, plays a significant role in achieving an accurate diagnosis and ensuring appropriate management.
4. Cutaneous Adverse Drug Reactions
The side effects in the case of psychotropic medications can range from mild exanthematous reactions or urticaria to potentially life-endangering ones such as Stevens–Johnson syndrome, TEN, erythema multiforme, and anticonvulsant hypersensitivity syndrome [53]. In addition to this, some rare events including angioedema, anaphylaxis, hypersensitivity vasculitis, drug-induced lupus erythematosus, alopecia, pigmentary disorders, photosensitivity, lichenoid lesions, fixed drug eruptions, psoriasiform eruptions, acne, and seborrheic eruptions encompass the spectrum. A classification of adverse effects according to specific drug classes is summarized in Table 2. The adverse effects are majorly attributed to an immunological basis; hence, cross-reactions are probable due to the response of antibodies and lymphocytes to shared molecular components among drugs belonging to the same class [54]. The characteristics of mild and severe side effects are summarized in Table 3 and Table 4.

Table 2.
Common drugs and their side effects [10,51,54,55].

Table 3.
Common side effects and their details [4,5,6,7,10].

Table 4.
Severe side effects and their details [10,51,52,53,54].
A Polish study carried out on hospitalized psychiatric patients reported an 8.4% prevalence of cutaneous side effects with the usage of psychotropic medications; out of the symptomatic patients, more than half of the patients were treated with psychotropic agents as the first treatment. Consistent with these results, it was more common to observe dermatologic symptoms in patients receiving treatment with more than two psychotropic medications, especially during the early stages of their medication regimen. Notably, approximately one-third of these individuals displayed symptoms associated with allergic disorders [9]. A study by Greil et al. analysed 594 severe drug reaction cases and 8085 other adverse drug reaction cases from a psychiatry pharmacovigilance program in the German population, identifying risk factors through logistic regression. Women, especially those under 50 years old, were more vulnerable (67%). Substance abuse and clomethiazole were newly identified risk factors [17].
In a separate study, around 5% of individuals using antipsychotic medications experienced adverse skin reactions [35]. Tricyclic antidepressants, like amitriptyline, could lead to adverse cutaneous drug reactions (ACDRs), including skin rashes and hypersensitivity responses like urticaria, photosensitivity, and hyperpigmentation [7]. Reports from different countries, such as Malaysia, Thailand, India, Italy, and China, pointed to anticonvulsants as common culprits for severe cutaneous drug reactions, notably Stevens–Johnson syndrome and toxic epidermal necrolysis (TEN). Other implicated agents encompassed antibiotics, analgesics, Non-Steroidal Anti-inflammatory Drugs (NSAIDs), and antitubercular and antirheumatic drugs. It is crucial to highlight that these reports covered anticonvulsants in general, emphasizing that not all anticonvulsant medications serve as mood stabilizers [56].
A Chinese case report describes a patient who developed SJS due to CBZ, with a positive HLA-A * 3101 gene; no prior exposure was recorded. SJS symptoms included skin eruptions, mucosal erosions, fever, and pain, affecting approximately 3% of her body surface. Genetic testing revealed a positive HLA-A * 3101 gene but negative HLA-B * 1502 and HLA-B * 5801 genes. Treatment involved intravenous infliximab and care for the affected mucosa. Within six days, the skin rash improved, and the patient was discharged [57]. Another Turkish case report of a 51-year-old man reported pruritic eczema after topical doxepin use; the symptoms were monitored at a regular follow-up of 2 months and were successfully treated with systemic corticosteroids. After six months, she underwent a patch test, which was strongly positive for doxepin. She then presented again with positive papulovesicular reactions at the site of positive patch tests and was further treated with intravenous triamcinolone [58].
5. Management of ACDRs
Psychotropic medications are invaluable tools in the treatment of psychiatric disorders, substantially improving the quality of life for countless individuals. Yet, the therapeutic benefits of these medications can be accompanied by potentially distressing, and, in some instances, severe dermatological side effects. Severe ACDRs such as SJS/TEN must be treated immediately because these are medical emergencies associated with significant mortality. The first step towards managing ACDRs is the identification and prompt withdrawal of the offending drug [59]. Discontinuing the causative drug often resolves cutaneous adverse effects, accounting for 93.7% of these reactions [10]. This decision should be made carefully, weighing the skin reaction’s severity against the underlying illness the medication was intended to treat [60]. A notable difficulty encountered in the context of ACDRs arising from psychotropic medications is where patients receive multiple drugs. Most ACDRs are not diagnosed as being caused by drugs. In such scenarios, the discontinuation of non-essential medications can be considered. For lithium and CBZ, reducing the dose can improve the skin condition. With CBZ, starting with lower doses and gradually increasing can prevent cutaneous eruptions. In cases of mild alopecia, continuation of the drug is generally preferred unless severe adverse effects occur [61]. Other treatments primarily focus on symptom management. Pruritus can be relieved with antihistamines, either topical or systemic. Lichenification responds to topical steroids, while severe exanthematous reactions may require systemic steroids. Hyperhidrosis can be managed by adjusting the dose, switching drugs, or discontinuing the medication [62]. Topical aluminium chloride hexahydrate can help in mild cases. If these options are unsuitable, medications like glycopyrrolate, benztropine, terazosin, mirtazapine, cyproheptadine, or clonidine may be considered, considering comorbidities like glaucoma and dementia [62].
In severe cases, immediate drug discontinuation and a dermatology consultation are crucial [63]. Supportive care for exfoliative syndromes such as SJS/TEN includes aggressive fluid management, nutritional support, and antibiotics [64]. Owing to the rarity of the condition, there are still no well-defined randomised control trials to support the use of any particular strategy in the management of SJS/TEN. Methyprednisolone administered at a dose of 500 mg/day for 3 days was reported to be successful in managing SJS/TEN [65]. Apart from steroids, IV immunoglobulin is therefore the preferred treatment for SJS and TEN [66]. Likewise, the management of DRESS constitutes supportive care such as hydration and systemic corticosteroids. Due to the lack of sufficient clinical evidence regarding the use of any specific agent in its management, practices are mostly based on expert opinions [67]. It is crucial to observe the patient carefully for potential recurrence of the adverse reaction upon reintroduction of the medication. This necessitates vigilant monitoring and close examination of the skin for any indications of a reaction.
6. Multidisciplinary Approach
6.1. Collaboration across Specialties
Psychiatrists occupy a central role in prescribing and monitoring psychotropic medications. They are responsible for evaluating a patient’s mental health needs, selecting the most suitable drug, and determining the appropriate dosage. However, the consideration of potential dermatological side effects is an added layer that necessitates collaboration with dermatologists. Working together, these specialists can accurately identify at-risk patients, promptly recognize and address emerging skin issues, and make informed decisions regarding medication adjustments.
Dermatologists bring invaluable expertise to this multidisciplinary team. Their specialized knowledge allows for precise diagnosis and management of dermatological side effects. Furthermore, dermatologists serve as educators, imparting essential knowledge about side effect recognition and management to patients and their psychiatric counterparts. A holistic figure involving the key aspects of multimodal management is highlighted in Figure 2.
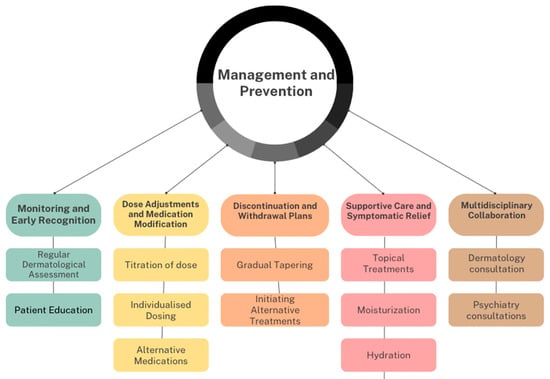
Figure 2.
Aspects of multimodal management.
6.2. Integral Role of Nursing
Nursing staff play a crucial role in the healthcare system. Their direct patient interaction positions them to observe and report early changes in the skin, which may serve as indicators of ADRs. Additionally, they are pivotal in patient education. Nursing professionals ensure that individuals are well informed about potential side effects, understand the importance of monitoring their skin, and know how to report any concerning changes. Their contributions to effective collaboration within the healthcare team enhance patient safety and treatment adherence.
6.3. Pharmacovigilance
Pharmacovigilance is central to monitoring and managing the dermatological side effects of psychotropic medications. It involves the systematic reporting, tracking, and analysis of ADRs. Pharmacists are key contributors to this process. They help ensure that reports of skin reactions are communicated effectively between healthcare providers, facilitating timely intervention and minimizing the risk of severe complications. The intricate relationship between psychiatric care and the emergence of dermatological side effects during psychotropic medication use underscores the imperative need for a multidisciplinary approach. In addition to this, certain initiatives like The AMSP (Arzneimittelsicherheit in der Psychiatrie) is a prospective multicentre initiative across European countries like Germany, Switzerland, and Austria. It takes into account the continuous assessment of severe ACDRs to psychotropic agents in psychiatric inpatients, it uses active screening of CDR cases, and documents them in their database, which has existed since 1993 [68]. This database serves as a treasure chest for pharmacovigilance and assessing cause–effect relationships. Apart from this, this database is instrumental in enhancing awareness among healthcare workers on drug safety issues. Collaboration between psychiatrists, dermatologists, nursing staff, and pharmacists maximizes patient safety and treatment efficacy. By fostering clear communication channels and embracing a holistic perspective, healthcare providers can significantly enhance patient care and well-being. This approach exemplifies effective and patient-centred care in the pursuit of improved patient outcomes.
Prevention may soon focus on genetic testing to identify individuals with specific HLA subtypes associated with drug-induced cutaneous hypersensitivities. The Food and Drug Administration (FDA) recommends genetic testing before starting CBZ therapy, particularly for Asians or those with HLA-B 1502 [69]. However, the cost-effectiveness and general applicability of this approach are yet to be determined.
7. Conclusions
This in-depth review has shed light on the intricate relationship between psychotropic medications and dermatological side effects. While psychotropic drugs have revolutionized the treatment of psychiatric disorders and greatly improved the quality of life for countless individuals, they can also introduce the challenge of adverse skin reactions. It was also noted that the incidence of ACDRs was more commonly associated with carbamazepine, lamotrigine, and barbiturates; a significantly low ACDR incidence was reported with the use of modern antipsychotics like SSRIs and dual-mechanism antipsychotics. However, the lowest incidence of ACDRs were seen with conventional and atypical antipsychotics. Recognizing and managing these side effects is vital in ensuring holistic patient care. Through a multidisciplinary approach involving collaboration between psychiatrists, dermatologists, nursing staff, and pharmacists, we can enhance patient safety and treatment efficacy. The clear communication channels and holistic perspective fostered in this collaborative model exemplify effective and patient-centred care. This comprehensive approach is essential in addressing the dermatological concerns that may arise during psychotropic medication use and in achieving improved patient outcomes. As we move forward in the field of psychiatric pharmacotherapy, it is imperative to continue research into prevention strategies, such as genetic testing, to identify individuals at risk of cutaneous hypersensitivities. By staying committed to the well-being of psychiatric patients and addressing the dermatological aspects of their treatment, we can ensure that the benefits of psychotropic medications are maximized while minimizing potential side effects.
Author Contributions
Conceptualization: N.D.; data curation: N.D.; formal analysis: N.D.; methodology: D.D.; project administration: D.D.; resources: D.D.; supervision: P.R.; validation: P.R.; visualization: P.R.; writing—original draft: N.D., D.D. and P.R.; writing—review and editing: N.D. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACDRs | Adverse cutaneous drug reactions |
| ADRs | Adverse reactions |
| AGEP | Acute generalized exanthematous pustulosis |
| CBZ | Carbamazepine |
| DRESS | Drug reactions with eosinophilia and systemic symptoms |
| HLA | Human Leukocyte Antigen |
| IV | Intravenous |
| SJS | Stevens–Johnson syndrome |
| SSRIs | Selective serotonin reuptake inhibitors |
| TEN | Toxic epidermal necrolysis |
| IgE | Immunoglobulin E |
| AED | Antiepileptic drugs |
References
- Jafferany, M.; Stamu-O’Brien, C.; Mkhoyan, R.; Patel, A. Psychotropic drugs in dermatology: A dermatologist’s approach and choice of medications. Dermatol. Ther. 2020, 33, e13385. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Madriz, J.A.; Serrano-Arias, B.; Zavaleta-Monestel, E.; Chaverri-Fernández, J.M.; Covarrubias-Gómez, A.; Villalobos-Madriz, J.A.; Villalobos, J.A.; Arguedas-Chacon, S.; Zavaleta, E.; Rodriguez-Miranda, R. Prescribing Trends in Psychotropic Medications Among Outpatients of a Latin American Healthcare Setting: A Five-Year Retrospective Study. Cureus 2023, 15, e37832. [Google Scholar] [CrossRef]
- Barr, P.B.; Bigdeli, T.B.; Meyers, J.L. Prevalence, Comorbidity, and Sociodemographic Correlates of Psychiatric Disorders Reported in the All of Us Research Program. JAMA Psychiatry 2022, 79, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Mago, R. Adverse Effects of Psychotropic Medications: A Call to Action. Psychiatr. Clin. N. Am. 2016, 39, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Iasella, C.J.; Johnson, H.J.; Dunn, M.A. Adverse Drug Reactions: Type A (Intrinsic) or Type B (Idiosyncratic). Clin. Liver Dis. 2017, 21, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Fiszenson-Albala, F.; Auzerie, V.; Mahe, E.; Farinotti, R.; Durand-Stocco, C.; Crickx, B.; Descamps, V. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br. J. Dermatol. 2003, 149, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Salazar, A.; de Leon-Rosales, S.P.; Rangel-Frausto, S.; Criollo, E.; Archer-Dubon, C.; Orozco-Topete, R. Epidemiology of adverse cutaneous drug reactions. A prospective study in hospitalized patients. Arch. Med. Res. 2006, 37, 899–902. [Google Scholar] [CrossRef]
- Bliss, S.A.; Warnock, J.K. Psychiatric medications: Adverse cutaneous drug reactions. Clin. Dermatol. 2013, 31, 101–109. [Google Scholar] [CrossRef]
- Murak-Kozanecka, E.; Rabe-Jabłońska, J. Prevalence and type of dermatologic disorders in psychiatric patients treated with psychotropic drugs. Psychiatr. Pol. 2004, 38, 491–505. [Google Scholar]
- Lange-Asschenfeldt, C.; Grohmann, R.; Lange-Asschenfeldt, B.; Engel, R.R.; Rüther, E.; Cordes, J. Cutaneous adverse reactions to psychotropic drugs: Data from a multicenter surveillance program. J. Clin. Psychiatry 2009, 70, 1258–1265. [Google Scholar] [CrossRef]
- Khairkar, P.; Bang, G.; Singh, A.; Tiple, P. Possible cross-sensitivity between sertraline and paroxetine in a panic disorder patient. Indian J. Pharmacol. 2010, 42, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Lucca, J.M.; Vamsi, A.; Kurian, S.J.; Ebi, S. A prospective observational study on psychotropic drug use in non psychiatric wards. Indian J. Psychiatry 2019, 61, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Deshazo, R.D.; Kemp, S.F. Allergic reactions to drugs and biologic agents. JAMA 1997, 278, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, L. Mechanisms of cutaneous drug reactions. Rev. Prat. 2000, 50, 1294–1299. [Google Scholar] [PubMed]
- Vasile, C. Mental health and immunity (Review). Exp. Ther. Med. 2020, 20, 211. [Google Scholar] [CrossRef] [PubMed]
- Seishima, M.; Yamanaka, S.; Fujisawa, T.; Tohyama, M.; Hashimoto, K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2006, 155, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Greil, W.; Zhang, X.; Stassen, H.; Grohmann, R.; Bridler, R.; Hasler, G.; Toto, S.; Bleich, S.; Kasper, S. Cutaneous adverse drug reactions to psychotropic drugs and their risk factors—A case-control study. Eur. Neuropsychopharmacol. 2019, 29, 111–121. [Google Scholar] [CrossRef]
- Alvestad, S.; Lydersen, S.; Brodtkorb, E. Rash from antiepileptic drugs: Influence by gender, age, and learning disability. Epilepsia 2007, 48, 1360–1365. [Google Scholar] [CrossRef]
- Invernizzi, P.; Pasini, S.; Selmi, C.; Gershwin, M.E.; Podda, M. Female predominance and X chromosome defects in autoimmune diseases. J. Autoimmun. 2009, 33, 12–16. [Google Scholar] [CrossRef]
- Mungall, A.J.; Palmer, S.A.; Sims, S.K.; Edwards, C.A.; Ashurst, J.L.; Wilming, L.; Jones, M.C.; Horton, R.; Hunt, S.E.; Scott, C.E.; et al. The DNA sequence and analysis of human chromosome 6. Nature 2003, 425, 805–811. [Google Scholar] [CrossRef]
- Hazini, A.; Fisher, K.; Seymour, L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J. Immunother. Cancer 2021, 9, e002899. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Barker, D.J.; Georgiou, X.; Cooper, M.A.; Flicek, P.; Marsh, S.G.E. IPD-IMGT/HLA Database. Nucleic Acids Res. 2020, 48, D948–D955. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.Q.; Zheng, X.F. HLA gene polymorphism and forensic medicine. Fa Yi Xue Za Zhi 2003, 19, 51–53. [Google Scholar] [PubMed]
- Djajadiningrat, R.S.; Horenblas, S.; Heideman, D.A.; Sanders, J.; De Jong, J.; Jordanova, E.S. Classic and nonclassic HLA class I expression in penile cancer and relation to HPV status and clinical outcome. J. Urol. 2015, 193, 1245–1251. [Google Scholar] [CrossRef]
- Kloypan, C.; Koomdee, N.; Satapornpong, P.; Tempark, T.; Biswas, M.; Sukasem, C. A Comprehensive Review of HLA and Severe Cutaneous Adverse Drug Reactions: Implication for Clinical Pharmacogenomics and Precision Medicine. Pharmaceuticals 2021, 14, 1077. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Rajan, A.K.; Chhabra, M.; Kashyap, A.; Chandran, V.P.; Venkataraman, R.; Nair, S.; Thunga, G. Role of human leukocyte antigen in anti-epileptic drugs-induced Stevens–Johnson Syndrome/toxic epidermal necrolysis: A meta-analysis. Seizure 2022, 102, 36–50. [Google Scholar] [CrossRef]
- Jordan, I.K.; Sharma, S.; Nagar, S.D.; Mariño-Ramírez, L. The Apportionment of Pharmacogenomic Variation: Race, Ethnicity, and Adverse Drug Reactions. Med. Res. Arch. 2022, 10, 10.18103/mra.v10i9.2986. [Google Scholar] [CrossRef]
- Mushiroda, T. Avoidance of cutaneous adverse drug reactions induced by antiepileptic drugs based on pharmacogenomics. J. Hum. Genet. 2023, 68, 227–230. [Google Scholar] [CrossRef]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef]
- Mehta, T.; Prajapati, L.; Mittal, B.; Joshi, C.; Sheth, J.; Patel, D.; Dave, D.; Goyal, R. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 579–582. [Google Scholar] [CrossRef]
- Tassaneeyakul, W.; Tiamkao, S.; Jantararoungtong, T.; Chen, P.; Lin, S.; Chen, W.; Konyoung, P.; Khunarkornsiri, U.; Auvichayapat, N.; Pavakul, K.; et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 2010, 51, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Kaniwa, N.; Saito, Y.; Aihara, M.; Matsunaga, K.; Tohkin, M.; Kurose, K.; Furuya, H.; Takahashi, Y.; Muramatsu, M.; Kinoshita, S.; et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 2010, 51, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; De Bakker, P.I.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Nigen, S.; Knowles, S.R.; Shear, N.H. Drug eruptions: Approaching the diagnosis of drug-induced skin diseases. J. Drugs Dermatol. JDD 2003, 2, 278–299. [Google Scholar] [PubMed]
- Warnock, J.K.; Morris, D.W. Adverse cutaneous reactions to antipsychotics. Am. J. Clin. Dermatol. 2002, 3, 629–636. [Google Scholar] [CrossRef]
- Marzano, A.V.; Borghi, A.; Cugno, M. Adverse drug reactions and organ damage: The skin. Eur. J. Intern. Med. 2016, 28, 17–24. [Google Scholar] [CrossRef]
- Khandpur, S.; Ahuja, R. Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma. Dermatopathology 2022, 9, 164–171. [Google Scholar] [CrossRef]
- Bigby, M.; Jick, S.; Jick, H.; Arndt, K. Drug-Induced Cutaneous Reactions: A Report From the Boston Collaborative Drug Surveillance Program on 15 438 Consecutive Inpatients, 1975 to 1982. JAMA 1986, 256, 3358–3363. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Irrera, N.; Campo, G.M.; Borgia, F.; Motolese, A.; Vaccaro, F.; Squadrito, F.; Altavilla, D.; Condorelli, A.G.; Motolese, A.; et al. Drug-Induced Photosensitivity: Clinical Types of Phototoxicity and Photoallergy and Pathogenetic Mechanisms. Front. Allergy 2022, 3, 876695. [Google Scholar] [CrossRef]
- Coulson, C.J.; Yrastorza-Daghman, M.B. Aripiprazole-Induced Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Case Study. Clin. Neuropharmacol. 2023, 46, 153–156. [Google Scholar] [CrossRef]
- Ghozlane, L.; Asma, J.; Ahmed, Z.; Ons, C.; Sarrah, K.; Riadh, D.; Sihem, E.A. Antipsychotics Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: Literature Review and a Report of a Suspected Case Related to Chlorpromazine. Curr. Drug Saf. 2023, 18, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, J.; Badyal, R.; Kumar, S.; Prasad, S. Olanzapine-induced acute generalized exanthematous pustulosis: A case report. Indian J. Psychiatry 2021, 63, 411–413. [Google Scholar] [CrossRef]
- Mercke, Y.; Sheng, H.; Khan, T.; Lippmann, S. Hair loss in psychopharmacology. Ann. Clin. Psychiatry 2000, 12, 35–42. [Google Scholar] [CrossRef]
- Allain, H.; Chevrant-Breton, J.; Beneton, C.; Bousser, A.M.; Bentue-Ferrer, D.; Mazeas, D.; Van den Driessche, J. Conséquences dermatologiques indésirables des médicaments. Résultat d’une enquête de pharmacovigilance [Undesirable dermatologic results of drugs. Result of a drug monitoring survey]. Ann. Med. Interne 1983, 134, 530–536. [Google Scholar]
- Garnis-Jones, S. Dermatologic side effects of psychopharmacologic agents. Dermatol. Clin. 1996, 14, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Drug, S.D. Reactions. American Academy of Dermatology. 2011. Available online: www.aad.org/education-and-quality-care (accessed on 6 January 2024).
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome: Part I. Clinical perspectives. J. Am. Acad. Dermatol. 2013, 68, 693.e1–693.e14. [Google Scholar] [CrossRef]
- Sokumbi, O.; Wetter, D.A. Clinical features, diagnosis, and treatment of erythema multiforme: A review for the practising dermatologist. Int. J. Dermatol. 2012, 51, 889–902. [Google Scholar] [CrossRef]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Le Louet, H. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef]
- A Drake, L.; Dinehart, S.M.; Farmer, E.R.; Goltz, R.W.; Graham, G.F.; Hordinsky, M.K.; Lewis, C.W.; Pariser, D.M.; Skouge, J.M.; Webster, S.B.; et al. Guidelines of care for cutaneous adverse drug reactions. Am. Acad. Dermatol. J. Am. Acad. Dermatol. 1996, 35 Pt 1, 458–461. [Google Scholar]
- Schnyder, B.; Pichler, W.J. Mechanisms of drug-induced allergy. Mayo Clin. Proc. 2009, 84, 268–272. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K.; Haberman, H.F. The self-inflicted dermatoses: A critical review. Gen. Hosp. Psychiatry 1987, 9, 45–52. [Google Scholar] [CrossRef]
- Mufaddel, A.; Osman, O.T.; Almugaddam, F. Adverse cutaneous effects of psychotropic medications. Expert Rev. Dermatol. 2013, 8, 681–692. [Google Scholar] [CrossRef]
- Mitkov, M.V.; Trowbridge, R.M.; Lockshin, B.N.; Caplan, J.P. Dermatologic Side Effects of Psychotropic Medications. Psychosomatics 2013, 55, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.A.; Jick, H. Rates of cutaneous reactions to drugs. A report from the Boston Collaborative Drug Surveillance Program. J. Am. Med. Assoc. 1976, 235, 918–923. [Google Scholar] [CrossRef]
- Martin, T.; Li, H. Severe cutaneous adverse drug reactions: A review on epidemiology, etiology, clinical manifestation and pathogenesis. Chin. Med. J. (Engl.) 2008, 121, 756–761. [Google Scholar] [PubMed]
- Xu, Y.Y.; Sun, Z.L.; Zhang, X.L.; Liu, Z.L.; Liu, W.; Guan, X. Carbamazepine induced Stevens-Johnson syndrome in Han Chinese with positive HLA-A * 3101 gene: A case report. Beijing Da Xue Xue Bao Yi Xue Ban 2023, 55, 755–757. [Google Scholar] [CrossRef]
- Özkaya, E.; Sirkeci, E.G.; Mangir, Ö. Long-lasting allergic contact dermatitis and positive patch test reactions to doxepin. Contact Dermat. 2023, 89, 209–212. [Google Scholar] [CrossRef]
- Watanabe, T.; Go, H.; Saigusa, Y.; Takamura, N.; Watanabe, Y.; Yamane, Y.; Totsuka, M.; Ishikawa, H.; Nakamura, K.; Matsukura, S.; et al. Mortality and risk factors on admission in toxic epidermal necrolysis: A cohort study of 59 patients. Allergol. Int. 2021, 70, 229–234. [Google Scholar] [CrossRef]
- Deandrea, D.; Walker, N.; Mehlmauer, M.; White, K. Dermatological reactions to lithium: A critical review of the literature. J. Clin. Psychopharmacol. 1982, 2, 199–204. [Google Scholar] [CrossRef]
- Gautam, M. Alopecia due to psychotropic medications. Ann. Pharmacother. 1999, 33, 631–637. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Fealey, R.D. Drug-induced hyperhidrosis and hypohidrosis: Incidence, prevention and management. Drug Saf. 2008, 31, 109–126. [Google Scholar] [CrossRef] [PubMed]
- MacMorran, W.S.; Krahn, L.E. Adverse cutaneous reactions to psychotropic drugs. Psychosomatics 1997, 38, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Konstantinow, A.; Mühlbauer, W.; Balda, B.-R.; Ring, J. Toxische epidermale Nekrolysen (Arzneimittel-induziertes Lyell-Syndrom). Teil 2: Therapie [Toxic epidermal necrolysis (drug-induced Lyell’s syndrome). 2. Treatment]. DMW—Dtsch. Med. Wochenschr. 2001, 126, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-T.; Chu, C.-Y. Treatments for Severe Cutaneous Adverse Reactions. J. Immunol. Res. 2017, 2017, 1503709. [Google Scholar] [CrossRef]
- Bang, D.; Shah, T.; Thakker, D.; Shah, Y.; Raval, A.D. Drug-induced Stevens–Johnson syndrome: Case series from tertiary care centre in Gujarat. Pharmacoepidemiol. Drug Saf. 2012, 21, 384–395. [Google Scholar] [CrossRef]
- Cho, Y.-T.; Yang, C.-W.; Chu, C.-Y. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System. Int. J. Mol. Sci. 2017, 18, 1243. [Google Scholar] [CrossRef]
- Grohmann, R.; Rüther, E.; Engel, R.; Hippius, H. Assessment of adverse drug reactions in psychiatric inpatients with the AMSP drug safety program: Methods and first results for tricyclic antidepressants and SSRI. Pharmacopsychiatry 1999, 32, 21–29. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Information on Carbamazepine (Marketed as Carbatrol, Equetro, Tegretol, and Generics) with FDA Alerts. 2007. Available online: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107834.html (accessed on 6 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).