Pharmacokinetic and Toxicological Aspects of 1,3-Dimethylamylamine with Clinical and Forensic Relevance
Abstract
1. Introduction
2. Methodology
3. Chemical Structure of 1,3-DMAA
4. Natural or Synthetic Origin of 1,3-DMAA
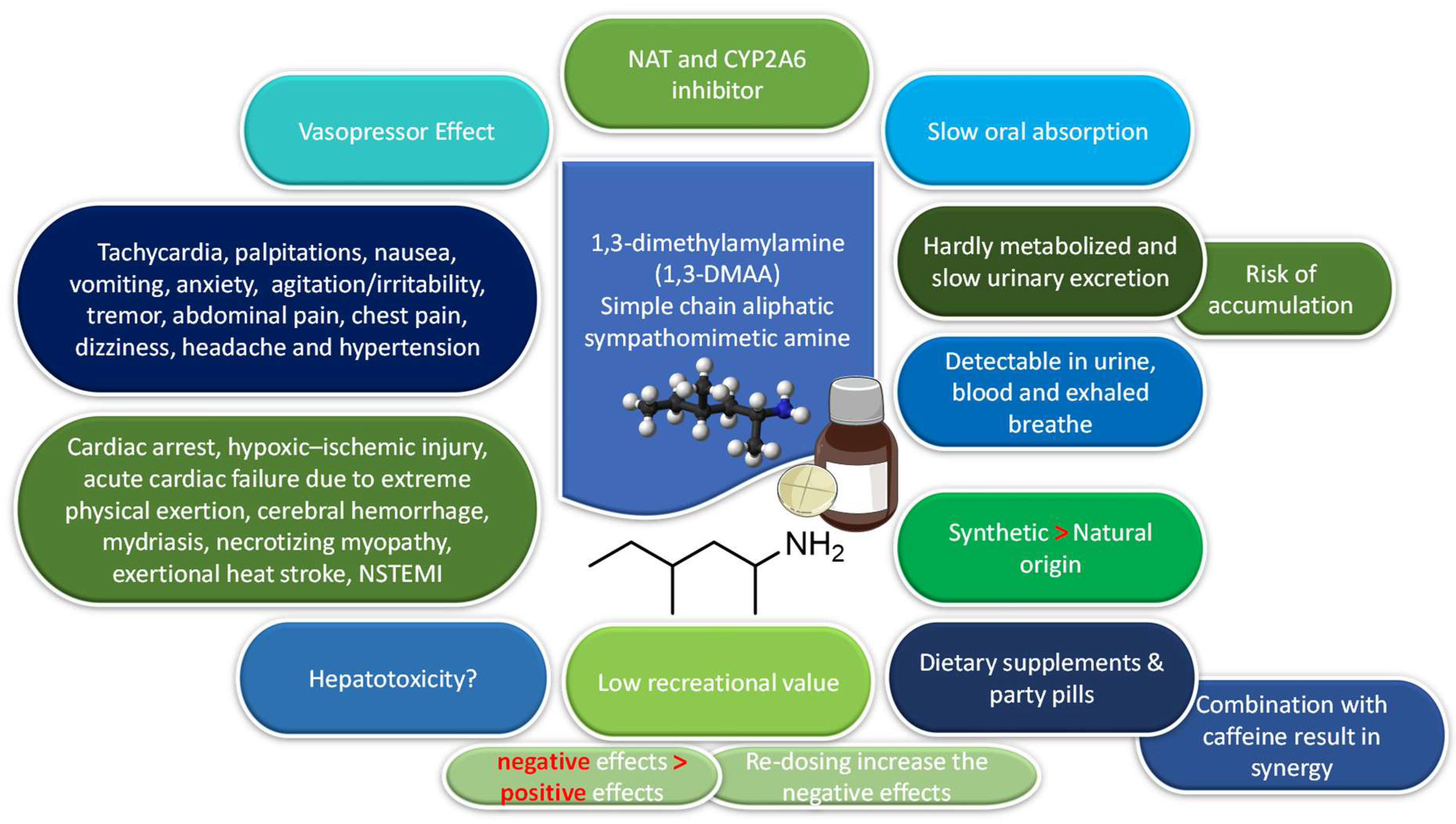
5. Pharmacokinetics
5.1. Absorption and Distribution
5.2. Metabolism and Interactions
5.3. Excretion
6. Pharmacodynamics
7. Effects of 1,3-DMAA
7.1. Consumption with Caffeine
7.1.1. Human Testing with 1,3-DMAA and Caffeine Alone or in Combination
7.1.2. Human Testing with Dietary Supplements Containing 1,3-DMAA and Caffeine
8. Critical Look at Human Studies Funded by the Industry
8.1. In Vitro Testing
8.2. Animal Studies
9. Adverse Effects
9.1. Consumption of 1,3-DMAA Party Pills
9.2. Consumption of Dietary Supplements Containing 1,3-DMAA
9.2.1. Cardiac Arrest
9.2.2. Cerebral Hemorrhage
9.2.3. Non-ST-Elevation Myocardial Infarction (NSTEMI)
9.2.4. Other Adverse Effects
9.2.5. Potential for Causality
9.3. Can 1,3-DMAA Cause Hepatotoxicity?
10. Acute Intoxication and Treatment
11. Potential for Abuse
12. Forensic Aspects and Doping
13. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pawar, R.S.; Tamta, H.; Ma, J.; Krynitsky, A.J.; Grundel, E.; Wamer, W.G.; Rader, J.I. Updates on chemical and biological research on botanical ingredients in dietary supplements. Anal. Bioanal. Chem. 2013, 405, 4373–4384. [Google Scholar] [CrossRef]
- Rasmussen, N.; Keizers, P.H.J. History full circle: ‘Novel’ sympathomimetics in supplements. Drug Test. Anal. 2016, 8, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Eliason, M.J.; Eichner, A.; Cancio, A.; Bestervelt, L.; Adams, B.D.; Deuster, P.A. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil. Med. 2012, 177, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Eichner, S.; Maguire, M.; Shea, L.A.; Fete, M.G. Banned and discouraged-use ingredients found in weight loss supplements. J. Am. Pharm. Assoc. 2016, 56, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Klontz, K.C.; DeBeck, H.J.; LeBlanc, P.; Mogen, K.M.; Wolpert, B.J.; Sabo, J.L.; Salter, M.; Seelman, S.L.; Lance, S.E.; Monahan, C.; et al. The role of adverse event reporting in the FDA response to a multistate outbreak of liver disease associated with a dietary supplement. Public Health Rep. 2015, 130, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Jackson, S.; Easton, J. Another bitter pill: A case of toxicity from DMAA party pills. N. Z. Med. J. 2010, 123, 124–127. [Google Scholar] [PubMed]
- Gee, P.; Tallon, C.; Long, N.; Moore, G.; Boet, R.; Jackson, S. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann. Emerg. Med. 2012, 60, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, A.D.; Adams, K.J.; Domin, M.A.; Henck, C.; Shepard, J.R.E. DART-MS for rapid, preliminary screening of urine for DMAA. Drug Test. Anal. 2014, 6, 788–796. [Google Scholar] [CrossRef]
- Benjamin, S.; Au, T.Y.; Assavarittirong, C. Lack of supplement regulation: A potential for ethical and physiological repercussions. Nutr. Health 2022, 28, 495–499. [Google Scholar] [CrossRef]
- Young, C.; Oladipo, O.; Frasier, S.; Putko, R.; Chronister, S.; Marovich, M. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil. Med. 2012, 177, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.; Butlin, E.; Shields, W.; Lacey, B. Experience with OxyELITE Pro and Acute Liver Injury in Active Duty Service Members. Dig. Dis. Sci. 2014, 59, 3117–3121. [Google Scholar] [CrossRef]
- Eudy, A.E.; Gordon, L.L.; Hockaday, B.C.; Lee, D.A.; Lee, V.; Luu, D.; Martinez, C.A.; Ambrose, P.J. Efficacy and safety of ingredients found in preworkout supplements. Am. J. Health Syst. Pharm. 2013, 70, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Ehlert, T.; Simon, P.; Neuberger, E.W.I. Boost Me: Prevalence and Reasons for the Use of Stimulant Containing Pre Workout Supplements Among Fitness Studio Visitors in Mainz (Germany). Front. Psychol. 2018, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Denham, B.E. When contaminated dietary supplements cause positive drug tests: Methylhexaneamine as a doping agent in sport. Int. J. Sport Policy 2017, 9, 677–689. [Google Scholar] [CrossRef]
- Dunn, M. Have prohibition policies made the wrong decision? A critical review of studies investigating the effects of DMAA. Int. J. Drug Policy 2017, 40, 26–34. [Google Scholar] [CrossRef]
- Lauritzen, F. Dietary Supplements as a Major Cause of Anti-doping Rule Violations. Front. Sport. Act. Living 2022, 4, 868228. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Deuster, P.; Venhuis, B.J. Four experimental stimulants found in sports and weight loss supplements: 2-amino-6-methylheptane (octodrine), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylamylamine (1,3-DMAA) and 1,3-dimethylbutylamine (1,3-DMBA). Clin. Toxicol. 2018, 56, 421–426. [Google Scholar] [CrossRef]
- van der Bijl, P. Dietary supplements containing prohibited substances: A review (Part 1). S. Afr. J. Sport. Med. 2014, 26, 59. [Google Scholar] [CrossRef]
- White, C.M. Continued Risk of Dietary Supplements Adulterated With Approved and Unapproved Drugs: Assessment of the US Food and Drug Administration’s Tainted Supplements Database 2007 Through 2021. J. Clin. Pharmacol. 2022, 62, 928–934. [Google Scholar] [CrossRef]
- Kerpel dos Santos, M.; Gleco, E.; Davidson, J.T.; Jackson, G.P.; Pereira Limberger, R.; Arroyo, L.E. DART-MS/MS screening for the determination of 1,3-dimethylamylamine and undeclared stimulants in seized dietary supplements from Brazil. Forensic Chem. 2018, 8, 134–145. [Google Scholar] [CrossRef]
- Pellegrini, M.; Rotolo, M.C.; Busardo, F.P.; Pacifici, R.; Pichini, S. Non-allowed Pharmacologically Active Substances in Physical and Sexual Performance Enhancing Products. Curr. Neuropharmacol. 2017, 15, 724–730. [Google Scholar] [CrossRef]
- Kimergård, A.; Walker, C.; Cowan, D. Potent and untested drugs sold as “dietary supplements”. BMJ 2015, 351, h4181. [Google Scholar] [CrossRef]
- Duiven, E.; van Loon, L.J.C.; Spruijt, L.; Koert, W.; de Hon, O.M. Undeclared doping substances are highly prevalent in commercial sports nutrition supplements. J. Sport. Sci. Med. 2021, 20, 328–338. [Google Scholar] [CrossRef]
- Diamanti, K.; Aalizadeh, R.; Alygizakis, N.; Galani, A.; Mardal, M.; Thomaidis, N.S. Wide-scope target and suspect screening methodologies to investigate the occurrence of new psychoactive substances in influent wastewater from Athens. Sci. Total Environ. 2019, 685, 1058–1065. [Google Scholar] [CrossRef]
- Archer, J.R.H.; Hudson, S.; Jackson, O.; Yamamoto, T.; Lovett, C.; Lee, H.M.; Rao, S.; Hunter, L.; Dargan, P.I.; Wood, D.M. Analysis of anonymized pooled urine in nine UK cities: Variation in classical recreational drug, novel psychoactive substance and anabolic steroid use. QJM Int. J. Med. 2015, 108, 929–933. [Google Scholar] [CrossRef]
- Sobolevsky, T.; Krotov, G.; Dikunets, M.; Nikitina, M.; Mochalova, E.; Rodchenkov, G. Anti-doping analyses at the Sochi Olympic and Paralympic Games 2014. Drug Test. Anal. 2014, 6, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Vorce, S.P.; Holler, J.M.; Cawrse, B.M.; Magluilo, J., Jr. Dimethylamylamine: A drug causing positive immunoassay results for amphetamines. J. Anal. Toxicol. 2011, 35, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Přibylka, A.; Švidrnoch, M.; Ševčík, J.; Maier, V. Enantiomeric separation of 1,3-dimethylamylamine by capillary electrophoresis with indirect UV detection using a dual-selector system. Electrophoresis 2015, 36, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.G.; Travis, J.; Pace, G.; Lieberman, H.R. Analysis of 1,3 dimethylamylamine concentrations in Geraniaceae, geranium oil and dietary supplements. Drug Test. Anal. 2014, 6, 797–804. [Google Scholar] [CrossRef]
- Cohen, P.A. DMAA as a dietary supplement ingredient. Arch. Intern. Med. 2012, 172, 1038–1039. [Google Scholar] [CrossRef]
- Kerpel dos Santos, M.; Walber, G.B.; Kreutz, T.; Soares, K.; Jacobi Danielli, L.; Mariotti, K.C.; Ritter, M.; Jackson, G.P.; Arroyo, L.E.; Pereira Limberger, R. Evaluation of the Presence of 1,3-Dimethylamylamine in Pelargonium Leaves and Essential Oils by Mass Spectrometric and Chromatographic Methods. Chromatographia 2019, 82, 875–883. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Gul, W.; Tolbert, C.; Elsohly, K.M.; Murphy, T.P.; Avula, B.; Chittiboyina, A.G.; Wang, M.; Khan, I.A.; Yang, M.; et al. Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi-centre investigation. Drug Test. Anal. 2015, 7, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Smillie, T.J.; Wang, Y.H.; Zweigenbaum, J.; ElSohly, M.A.; Khan, I.A. Fast identification of 1,3-dimethylamylamine using direct analysis in real time-QToF-MS. J. AOAC Int. 2015, 98, 757–759. [Google Scholar] [CrossRef]
- Zhang, Y.; Woods, R.M.; Breitbach, Z.S.; Armstrong, D.W. 1,3-Dimethylamylamine (DMAA) in supplements and geranium products: Natural or synthetic? Drug Test. Anal. 2012, 4, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Moro, E.; Dos Santos, A.; Uberti, F.; Restani, P. Could 1,3 dimethylamylamine (DMAA) in food supplements have a natural origin? Drug Test. Anal. 2013, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lisi, A.; Hasick, N.; Kazlauskas, R.; Goebel, C. Studies of methylhexaneamine in supplements and geranium oil. Drug Test. Anal. 2011, 3, 873–876. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Gul, W.; ElSohly, K.M.; Murphy, T.P.; Weerasooriya, A.; Chittiboyina, A.G.; Avula, B.; Khan, I.; Eichner, A.; Bowers, L.D. Pelargonium oil and methyl hexaneamine (MHA): Analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J. Anal. Toxicol. 2012, 36, 457–471. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, M.; Li, Z.C. Identification and quantification of dimethylamylamine in geranium by liquid chromatography tandem mass spectrometry. Anal. Chem. Insights 2012, 7, 47–58. [Google Scholar] [CrossRef]
- Fleming, H.L.; Ranaivo, P.L.; Simone, P.S. Analysis and confirmation of 1,3-DMAA and 1,4-DMAA in geranium plants using high performance liquid chromatography with tandem mass spectrometry at ng/g concentrations. Anal. Chem. Insights 2012, 7, 59–78. [Google Scholar] [CrossRef]
- Schilling, B.K.; Hammond, K.G.; Bloomer, R.J.; Presley, C.S.; Yates, C.R. Physiological and pharmacokinetic effects of oral 1,3-dimethylamylamine administration in men. BMC Pharmacol. Toxicol. 2013, 14, 52. [Google Scholar] [CrossRef]
- Gee, P. In reply. Ann. Emerg. Med. 2013, 61, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, M.C.; Hearne, E. “Plant or poison”: A netnographic study of recreational use of 1,3-dimethylamylamine (DMAA). Int. J. Drug Policy 2015, 26, 1279–1281. [Google Scholar] [CrossRef]
- Archer, J.R.H.; Dargan, P.I.; Lostia, A.M.; van der Walt, J.; Henderson, K.; Drake, N.; Sharma, S.; Wood, D.M.; Walker, C.J.; Kicman, A.T. Running an unknown risk: A marathon death associated with the use of 1,3-dimethylamylamine (DMAA). Drug Test. Anal. 2015, 7, 433–438. [Google Scholar] [CrossRef]
- Venhuis, B.; Kaste, D. Scientific Opinion on the Regulatory Status of 1,3-Dimethylamylamine (DMAA). Eur. J. Food Res. Rev. 2012, 2, 93–100. [Google Scholar]
- Liu, Y.; Santillo, M.F. Cytochrome P450 2D6 and 3A4 enzyme inhibition by amine stimulants in dietary supplements. Drug Test. Anal. 2016, 8, 307–310. [Google Scholar] [CrossRef]
- Lesiak, A.D.; Shepard, J.R. Recent advances in forensic drug analysis by DART-MS. Bioanalysis 2014, 6, 819–842. [Google Scholar] [CrossRef]
- Perrenoud, L.; Saugy, M.; Saudan, C. Detection in urine of 4-methyl-2-hexaneamine, a doping agent. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3767–3770. [Google Scholar] [CrossRef]
- O’Connor, F.G. Dietary supplements and warfighters: A challenge for military providers. Mil. Med. 2012, 177, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of compounds in preworkout supplements (“boosters”). Eur. J. Pharmacol. 2019, 859, 172515. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Gibbons, S.; Treble, R.; Setola, V.; Huang, X.P.; Roth, B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013, 700, 147–151. [Google Scholar] [CrossRef]
- Docherty, J.R.; Alsufyani, H.A. Cardiovascular and temperature adverse actions of stimulants. Br. J. Pharmacol. 2021, 178, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Alsufyani, H.A.; Docherty, J.R. Methylhexaneamine causes tachycardia and pressor responses indirectly by releasing noradrenaline in the rat. Eur. J. Pharmacol. 2019, 843, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Austin, K.G.; Farina, E.K. Surveillance of the armed forces as a sentinel system for detecting adverse effects of dietary supplements in the general population. Public Health Nutr. 2018, 21, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Harvey, I.C.; Farney, T.M.; Bell, Z.W.; Canale, R.E. Effects of 1,3-dimethylamylamine and caffeine alone or in combination on heart rate and blood pressure in healthy men and women. Physician Sportsmed. 2011, 39, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Farney, T.M.; McCarthy, C.G.; Canale, R.E.; Allman, R.J., Jr.; Bloomer, R.J. Hemodynamic and hematologic profile of healthy adults ingesting dietary supplements containing 1,3-dimethylamylamine and caffeine. Nutr. Metab. Insights 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Bloomer, R.; McCarthy, C.; Farney, T.; Harvey, I. Effect of Caffeine and 1,3-Dimethylamylamine on Exercise Performance and Blood Markers of Lipolysis and Oxidative Stress in Trained Men and Women. J. Caffeine Res. 2011, 1, 169–177. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Farney, T.M.; Harvey, I.C.; Alleman, R.J. Safety profile of caffeine and 1,3-dimethylamylamine supplementation in healthy men. Hum. Exp. Toxicol. 2013, 32, 1126–1136. [Google Scholar] [CrossRef]
- Whitehead, P.N.; Schilling, B.K.; Farney, T.M.; Bloomer, R.J. Impact of a dietary supplement containing 1,3-dimethylamylamine on blood pressure and bloodborne markers of health: A 10-week intervention study. Nutr. Metab. Insights 2012, 5, 33–39. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Farney, T.M.; Canale, R.E.; Alleman, R.J., Jr.; Bloomer, R.J. A finished dietary supplement stimulates lipolysis and metabolic rate in young men and women. Nutr. Metab. Insights 2012, 5, 23–31. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Canale, R.E.; Alleman, R.J., Jr.; Reed, J.P.; Bloomer, R.J. Biochemical and anthropometric effects of a weight loss dietary supplement in healthy men and women. Nutr. Metab. Insights 2012, 5, 13–22. [Google Scholar] [CrossRef]
- Powers, M.E. Acute stimulant ingestion and neurocognitive performance in healthy participants. J. Athl. Train. 2015, 50, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A. In reply. JAMA Intern. Med. 2013, 173, 595. [Google Scholar] [CrossRef]
- Guner, A.; Turkez, H. Examination of some toxicological parameters of dimethylamylamine when consumed alone or with caffeine. Arch. Biol. Sci. 2020, 72, 413–423. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Barberena, M.A.; Bisoffi, M.; Trujillo, K.; Conn, C.A. Treatment of human muscle cells with popular dietary supplements increase mitochondrial function and metabolic rate. Nutr. Metab. 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Zovico, P.V.C.; Curty, V.M.; Leal, M.A.S.; Meira, E.F.; Dias, D.V.; Rodrigues, L.C.D.M.; Meyrelles, S.D.S.; De Oliveira, E.M.; Vassallo, P.F.; Barauna, V.G. Effects of controlled doses of Oxyelite Pro on physical performance in rats. Nutr. Metab. 2016, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Zovico, P.V.C.; Klippel, B.F.; dos Santos, L.; Dias, D.V.; Barauna, V.G. Cardiovascular responses to different formulations of the OxyElite Pro supplement. Rbne-Rev. Bras. De Nutr. Esportiva 2019, 13, 182–194. [Google Scholar]
- Milton, R.M.; Kelly-Rehm, M.; Brahm, N.; Fox, M.D. Hypertension in an adolescent secondary to performance-enhancement supplement use. J. Pharm. Technol. 2014, 30, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Staub, B.A.; Natarajan, G.M.; Lasorda, D.M.; Poornima, I.G. Acute myocardial infarction associated with dietary supplements: Containing 1,3-dimethylamylamine and citrus aurantium. Tex. Heart Inst. J. 2014, 41, 70–72. [Google Scholar] [CrossRef]
- Eliason, M.; Deuster, P.; Adams, B.; Cancio, A.; Bestervelt, L.; Eichner, A. In response to letter to the editor: Re: “Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA)” (Mil Med 2012; 177(12): 1455-59). Mil. Med. 2013, 178, 4. [Google Scholar]
- Karnatovskaia, L.V.; Leoni, J.C.; Freeman, M.L. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin. J. Sport Med. 2015, 25, e23–e25. [Google Scholar] [CrossRef]
- Elgallab, J.; Glover, R.; Bhupali, D.; Gordon, D.; Kirchoff-Torres, K. Intracerebral Hemorrhage Associated With Dietary Supplement Containing DMAA (P5.134). Neurology 2014, 82, P5.134. [Google Scholar]
- McDermott, A.J. Unilateral mydriasis potentially associated with contact with a supplement powder mix. Mil. Med. 2012, 177, 359–360. [Google Scholar] [CrossRef]
- Armstrong, M. Atrial Fibrillation with Rapid Ventricular Response following use of Dietary Supplement Containing 1,3 Dimethylamylamine and Caffeine. J. Spec. Oper. Med. 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Durgam, R.G.; Thomas, S.; Drakes, S.; Lasak, A.M. Dietary Supplement Containing 1,3 Dimethylamylamine as a Cause of Necrotizing Myopathy: A Case Report. PM&R 2013, 5, S183. [Google Scholar] [CrossRef]
- Reedy, E.; Lyons, T.; Seguin, P.; Marzouk, A.; Franco, D.; Erdman, C. In response to: “Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA)” (Mil Med 2012; 177(12): 1455-59). Mil. Med. 2013, 178, 4–5. [Google Scholar]
- Willson, C. Sympathomimetic amine compounds and hepatotoxicity: Not all are alike—Key distinctions noted in a short review. Toxicol. Rep. 2019, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.B. Exposures to 1,3-dimethylamylamine-containing products reported to Texas poison centers. Hum. Exp. Toxicol. 2013, 32, 18–23. [Google Scholar] [CrossRef]
- Brown, J.A.; Buckley, N.A. Toxicity from bodybuilding supplements and recreational use of products containing 1, 3-dimethylamylamine. Med. J. Aust. 2013, 198, 414–415. [Google Scholar] [CrossRef]
- Dolan, S.B.; Gatch, M.B. Abuse liability of the dietary supplement dimethylamylamine. Drug Alcohol Depend. 2015, 146, 97–102. [Google Scholar] [CrossRef]
- Le, R.; Young, J.E.; Pesek, J.J.; Matyska, M.T. Separation of 1,3-dimethylamylamine and other polar compounds in a dietary supplement formulation using aqueous normal phase chromatography with MS. J. Sep. Sci. 2013, 36, 2578–2583. [Google Scholar] [CrossRef]
- Joshi, M.; Cetroni, B.; Camacho, A.; Krueger, C.; Midey, A.J. Analysis of synthetic cathinones and associated psychoactive substances by ion mobility spectrometry. Forensic Sci. Int. 2014, 244, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.; Assemat, G.; Martins, N.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J. Pharm. Biomed. Anal. 2016, 124, 34–47. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Ilse, M.; Hengen, J.; el-Atma, O.; Kuballa, T.; Kohl-Himmelseher, M.; Lachenmeier, D.W. Rapid assessment of the illegal presence of 1,3-dimethylamylamine (DMAA) in sports nutrition and dietary supplements using 1H NMR spectroscopy. Drug Test. Anal. 2014, 6, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Avila, V.; Zorio, M. Identification of methylhexaneamine by GC high-resolution TOFMS and soft ionization. Forensic Sci. Int. 2013, 231, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Geyer, H.; Schänzer, W.; Crone, C.; Kellmann, M.; Moehring, T.; Thevis, M. Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Anal. Bioanal. Chem. 2012, 403, 1279–1289. [Google Scholar] [CrossRef]
- Ocaña-Rios, I.; Araujo-González, F.; Olmos-Espejel, J.J.; Peña-Alvarez, A. Miniaturized Analysis of Methylhexanamine in Urine by Gas Chromatography Applying In Situ Derivatization. Chromatographia 2022, 85, 95–104. [Google Scholar] [CrossRef]
- Thevis, M.; Krug, O.; Geyer, H.; Schänzer, W. Expanding analytical options in sports drug testing: Mass spectrometric detection of prohibited substances in exhaled breath. Rapid Commun. Mass Spectrom. 2017, 31, 1290–1296. [Google Scholar] [CrossRef]
- Pavletic, A.J.; Pao, M. Popular Dietary Supplement Causes False-Positive Drug Screen for Amphetamines. Psychosomatics 2014, 55, 206–207. [Google Scholar] [CrossRef]
- Palmer, P.G. Deadly dimethylamylamine: “health” supplements are killing consumers while current regulations impede FDA action. J. Leg. Med. 2014, 35, 311–336. [Google Scholar] [CrossRef]
- Cancio, A.; Eliason, M.J.; Mercer, J.; Tran, T.; Deuster, P.A.; Stephens, M.B. Third-party certification of dietary supplements: Prevalence and concerns. Mil. Med. 2012, 177, 1460–1463. [Google Scholar] [CrossRef]
- Biliński, P.; HoŁownia, P.; Kapka-Skrzypczak, L.; WojtyŁa, A. Designer drug (DD) abuse in Poland; a review of the psychoactive and toxic properties of substances found from seizures of illegal drug products and the legal consequences thereof. part II-piperazines/piperidines, phenylethylamines, tryptamines and miscellaneous ‘Others’. Ann. Agric. Environ. Med. 2012, 19, 871–882. [Google Scholar] [PubMed]
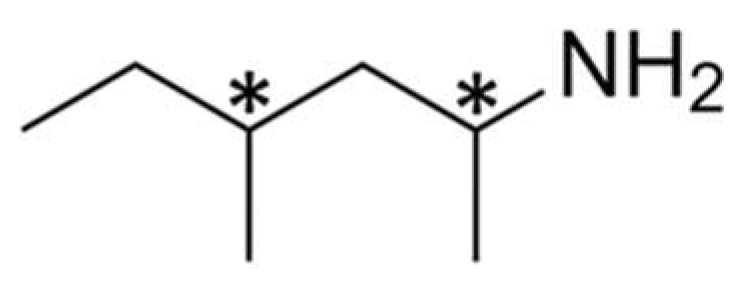
| Methylhexaneamine | 2-amino-4-methylhexane | Floradrene |
| 1,3-dimethylpentylamine | 2-hexanamine | Forthan |
| Dimethylamylamine | 4-methyl-2-hexanamine | Forthane |
| Dimethylpentylamine | 4-methyl-2-hexylamine | Fouramin |
| Geranium flower extract | 4-methylhexan-2-amine | Geranamine |
| Geranium oil | 2-aminoisoheptane | Geranium extract |
| Geranium stems and leaves | Metexaminum | Methexaminum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.N.; Dinis-Oliveira, R.J. Pharmacokinetic and Toxicological Aspects of 1,3-Dimethylamylamine with Clinical and Forensic Relevance. Psychoactives 2023, 2, 222-241. https://doi.org/10.3390/psychoactives2030015
Rodrigues AN, Dinis-Oliveira RJ. Pharmacokinetic and Toxicological Aspects of 1,3-Dimethylamylamine with Clinical and Forensic Relevance. Psychoactives. 2023; 2(3):222-241. https://doi.org/10.3390/psychoactives2030015
Chicago/Turabian StyleRodrigues, Afonso Nóbrega, and Ricardo Jorge Dinis-Oliveira. 2023. "Pharmacokinetic and Toxicological Aspects of 1,3-Dimethylamylamine with Clinical and Forensic Relevance" Psychoactives 2, no. 3: 222-241. https://doi.org/10.3390/psychoactives2030015
APA StyleRodrigues, A. N., & Dinis-Oliveira, R. J. (2023). Pharmacokinetic and Toxicological Aspects of 1,3-Dimethylamylamine with Clinical and Forensic Relevance. Psychoactives, 2(3), 222-241. https://doi.org/10.3390/psychoactives2030015







