Abstract
Background: Physician burnout is increasingly recognized as a problem in physician well-being and may negatively affect patient care outcomes. Burnout can begin at any point of training or practice, potentially as early as the first year of medical school. Thus, there is a need to characterize possible burnout in medical students as the first step to optimizing strategies for mitigation. Traditionally, burnout has been studied using survey-based variables; however, identifying novel physiological and molecular biomarkers could allow for the expansion of screening and intervention strategies. Methods: In this pilot prospective cohort study, we followed a group of preclinical 1st and 2nd year medical students (n = 9) at the University of Florida over one academic year of medical school. We collected survey responses (Maslach Burnout Inventory [MBI], Patient Health Questionnaire-9 [PHQ-9], and Perceived Stress Scale [PSS]) and measured a panel of candidate physiological biomarkers of burnout (Inflammatory Cytokine Panel, Heart Rate Variability [HRV], and Leukocyte Telomere Length). Results: In the study participants, MBI composite scores and PHQ-9 scores showed a statistically significant increase over the course of an academic year, indicating higher levels of medical student burnout. Additionally, respondents reported a statistically significant decrease in time devoted to exercise, and we measured a significant increase in body mass index (BMI) during the academic year. PSS scores showed an upward trend which was not statistically significant. Likewise, average leukocyte telomere length trended downward, but the change was not statistically significant. There were no measured changes in the serum concentration of pro-inflammatory cytokines, and time-domain heart rate variability metrics did not differ significantly between timepoints. Conclusions: This pilot study supports the notion that burnout can begin early in medical school and is detectable via survey instruments in first-year and second-year medical students even with a small sample size. Additionally, leukocyte telomere length could potentially be a useful biomarker of burnout with supporting data, but we did not observe any statistically significant changes in inflammatory cytokines or heart rate variability. Further investigation into these potential biomarkers with larger cohort sizes is required to fully characterize their clinical utility.
1. Introduction
Occupational burnout is a syndrome characterized by work-related symptoms of emotional exhaustion, feelings of cynicism, and depersonalization [1,2] and has been identified as a major problem in healthcare professionals [3,4]. Recent estimates of physician burnout have been at about 50% in 2016 [5], which increased to over 60% after the COVID-19 pandemic [6]. Compared to other professions, physicians are significantly more likely to experience symptoms of burnout during their careers [3]. Physician burnout is associated with other psychiatric comorbidities and can lead to a greater risk of depression, anxiety, and suicidal ideation [7]. Additionally, burnout can negatively affect patient interactions and patient care by leading to a greater risk of medical errors [8,9]. This is not surprising, since burnout and chronic stress can directly affect the higher order decision-making and critical-thinking capabilities of the prefrontal cortex that are essential for making complex medical decisions [10].
In the United States, prospective allopathic physicians finish four years of undergraduate classes prior to starting four years of medical school training, which are followed by 3–7 years of medical residency (Figure 1A). Medical school education is split into two years of preclinical studies and two years of clinical training through rotations on clinical wards. There is evidence that signs of burnout can occur early during medical training in ~36% of medical residents [11] and in ~37% of medical students [12]. Thus, there is a significant need to further assess and characterize the prevalence of burnout in medical trainees and its contributions to burnout in late-career physicians.
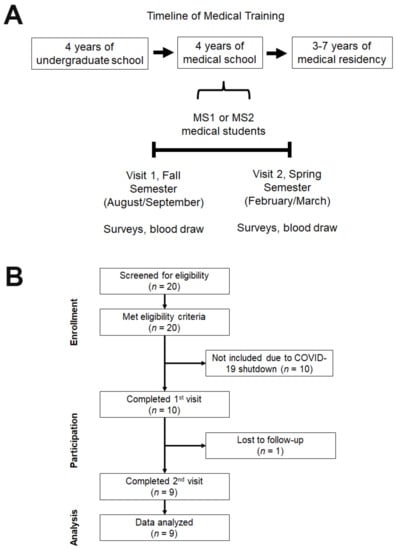
Figure 1.
Timeline and Study Flow Chart. (A) A timeline of the typical training periods for allopathic physicians in the United States, which traditionally consists of 4 years of undergraduate studies, 4 years of medical school, and 3−7 years of residency training. This study was conducted on first- or second-year allopathic medical students over the course of an academic year from fall to spring semester. (B) A CONSORT-style flowchart depicts single-arm, nonrandomized study design and shows participant screening based on inclusion/exclusion survey, general physical exam, and pregnancy test. This study was initially planned to enroll at least 20 participants, but participant enrollment was terminated at nine participants due to the COVID-19 shutdown.
Currently, the gold standard in burnout research is the Maslach Burnout Index (MBI), but there are many different definitions and cutoffs for determining burnout [4]. In this study, we wanted to use surveys for the early detection of changes in burnout symptoms using the MBI, perceived stress scale (PSS) for stress symptoms [13], and screening for potential overlap with depression symptoms via the nine-item patient health questionnaire (PHQ-9) [14,15]. However, surveys have inherent drawbacks for sensitive, subjective issues. Recently there has been interest in identifying objective measures associated with burnout such as biological, physiological, and molecular markers. These assessments would potentially allow for enhanced screening and early detection of burnout in medical students with the possibility for targeted interventions at both the individual and institutional levels.
One class of potential biomarkers for chronic stress is inflammatory cytokine levels, which has comparable effects on both psychosocial and physical stress via the inflammatory response [16]. Cytokines are defined as glycoproteins produced by activated immune cells that lead to the activation of other cells [17]. Under chronic psychosocial stress, the hypothalamic–pituitary–adrenal axis (HPA-axis) is continuously activated, which leads to persistent inflammation and the activation of various inflammatory cytokines. This constant inflammation may lead to a decreased stress response; however, this process is not yet well understood [17]. There is evidence that levels of several pro-inflammatory and anti-inflammatory cytokines are modified in conditions of psychosocial stress and burnout. The proinflammatory cytokine TNF-α has been found to increase with burnout in both teachers [18] and social insurance workers [19]. Likewise, the proinflammatory cytokine IL-1β has been found to increase with acute psychosocial stress [20]. IL-6, which has both proinflammatory and anti-inflammatory properties, increased in response to acute psychosocial stress [20]. The proinflammatory cytokine IL-8 was higher in adults with higher levels of perceived stress [21]. In contrast, the anti-inflammatory cytokine IL-4 was reduced in teachers with greater burnout [18]. Thus, we hypothesize that levels of these cytokines may be altered during preclinical medical education and can be correlated with any changes in surveys of burnout and depression.
Another potential marker of chronic stress in medical students is telomere length. Telomeres are short DNA repeats at the terminal ends of chromosomes which protect genetic information from degradation [22]. Telomerase is an enzyme that elongates telomeres by nucleotide addition and preserves telomere length [23]. Previous studies have shown that chronic stress and elevation in average cortisol levels can decrease telomerase activity in T-lymphocytes, leading to a subsequent decrease in telomere length [23]. This can be mediated by both perceived and chronic stress via the combined activities of stress-associated hormones and oxidative stress [24,25]. Transcriptomic data have also shown that individuals undergoing significant stressful life events who have a measurable decrease in telomere length also have altered gene expression of inflammatory and oxidative stress pathways [26]. Thus, we hypothesize that telomere length may be altered in preclinical medical students experiencing burnout.
Heart Rate Variability (HRV) is a proxy measure of autonomic nervous system activity and its effect on the interval between beats of the heart (the R-R interval). Historically, HRV has been used as an accepted prognostic measure for patients with cardiovascular disorders, potentially due to a diminished parasympathetic response secondary to stress [27]. Previous studies have shown that HRV is decreased in patients with acute and chronic stress [28]. Specifically, clinical burnout has been associated with decreased HRV measures. A study examining burnout in emergency medicine physicians demonstrated a consistent association between HRV measurements and burnout, especially with regards to emotional stressors [29]. Other studies have specifically investigated the relationship between HRV and major depressive disorder (MDD) and were able to create a logistical-regression model predictive of a diagnosis of MDD with 80% sensitivity and 79% specificity using HRV measures [30,31].
In this study, we investigate the potential utility of these above molecular markers in the detection of burnout by correlating measured values with results from gold standard surveys (MBI, PHQ-9, and PSS surveys). We seek to determine whether any tested biomarkers could predict clinical symptoms assessed by surveys of burnout and depression as a potential means for burnout screening in medical students.
2. Materials and Methods
2.1. Participant Selection and Exclusion Criteria
This study was approved by the University of Florida Institutional Review Board (IRB201700317), and informed consent was obtained from all participants prior to their participation in the study. Twenty first-year or second-year medical students at the University of Florida were screened for inclusion/exclusion, and all of them met the enrollment criteria. Ten subjects were enrolled into the study and completed the first clinic visit, and nine completed the second clinic visit at the completion of an academic year of medical school 6–7 months later (Figure 1). At each clinic visit, general demographic and lifestyle questionnaires were administered along with three additional health surveys: the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire-9 (PHQ-9), and the Perceived Stress scale (PSS). We also collected height and weight measurements, performed a blood draw, and obtained EKG measurements. Participants were compensated with $40 during the first visit and $40 upon completion of the second visit. While the remaining 10 eligible participants (and additional study participants) were initially planned for enrollment, the COVID-19 shutdown prevented their participation.
The exclusion criteria for this study were determined by consideration of participant groups that may have another medical condition which could contribute to chronic stress. We excluded several groups of students, including: 1. students currently under treatment for an active diagnosis of a psychiatric illness with recent treatment with therapy and psychiatric medications; 2. students with diagnosed autoimmune conditions or under treatment with oral or inhaled immunosuppressant medications (except for topical steroids or non-continuous treatment with nonsteroid anti-inflammatory medications such as NSAIDs or other analgesics); and 3. women who are pregnant or have plans to become pregnant during the study.
2.2. Questionnaires and Surveys
At the beginning of each visit, each participant completed the MBI, PHQ-9, and PSS surveys. The Maslach Burnout Inventory is divided into three subcategories: emotional exhaustion (MBI-EE), cynicism (MBI-C), and professional efficacy (MBI-PE). The MBI-PE is unique in that higher scores are reflective of a decreased risk of burnout, opposite to the MBI-EE and MBI-C. For this reason, the composite MBI score was the sum of the MBI-EE and MBI-C scores. Additionally, each participant completed a lifestyle questionnaire pertaining to the frequency and duration of leisure and exercise activities. At the first visit only, each participant also completed a demographics survey including questions on sex, gender identity, sexual orientation, age group, race, and ethnicity. All participant information and survey data were stored on the Research Electronic Data Capture (REDCap) secure web server and blinded to the study coordinators [32].
2.3. Inflammatory Cytokine Panel
At the end of each visit, 3 mL of blood was drawn into EDTA-coated tubes and immediately placed on ice. Samples were sent to an outside laboratory (ARUP Laboratories, Salt Lake City, UT, USA) for the measurement of a standardized inflammatory cytokine panel, which includes serum concentrations of TNF-ɑ, IL-1ꞵ, IL-2, sIL-2R, IL-6, IL-12, IFN-γ, IL-4, IL-5, IL-10, IL-13, and IL-17.
2.4. Heart Rate Variability
A Heal Force Prince 180D electrocardiogram (ECG) was used to record participants’ heart rhythm for 5 consecutive minutes using a six-lead setup. Participants were instructed to relax once recordings started. At 100 s after the start of recording, participants were instructed to speak random integers from 0–9 to the beat of a 1 Hz metronome to simulate a mental task [30,31]. After 100 s of the simulated mental task, the patients were again instructed to relax. R-to-R intervals were derived from these 5 min recordings. At each participant visit, standard deviation of R-R intervals (SDNN), root mean-square differences between consecutive R-R intervals (RMSSD), and the percentage of adjacent NN intervals differing by more than 50 ms (pNN50) were calculated from ECG measurements [33].
2.5. DNA Isolation
At each timepoint, participant blood was collected and stored at −80 °C. DNA was isolated from blood leukocytes by centrifugation and then extracted using a standard QIAamp DNA Blood Mini Kit according to the manufacturer’s instructions. 200 µL of blood from each participant was added to 20 µL of protease and mixed with Qiagen lysis buffer. The solution was incubated for 10 min at 56 °C and mixed with 200 µL of Ethanol. The final mixture was added to a QIAamp spin column and centrifuged at full speed for 1 min. The column was washed with Qiagen wash buffer twice and centrifuged at full speed for 1 min after each step. The final DNA was isolated and eluted from the column with 200 µL of Qiagen elution buffer and high-speed centrifugation.
2.6. Leukocyte Telomere Length Assay
After DNA extraction and isolation, leukocyte telomere length was calculated based on T/S ratios after quantitative polymerase chain reaction (qPCR) using previously validated methods from other studies [34,35,36]. A ratio of telomere to single copy gene (T/S ratio) can be used to reliably estimate telomere length and was calculated by comparing telomeres amplified by standard primers to a single copy reference gene. In this study, 36B4 (an acidic ribosomal phosphoprotein) was selected as the reference gene due to its stable single copy number and has been validated in prior studies [35,37,38].
Four replicates of each sample were loaded on two separate 96-well qPCR plates with either telomere primers or 36B4 gene primers. Primers used (written from 5′ to 3′) were:
telomere forward primer: CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT;
telomere reverse primer: GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT;
36B4 forward primer: CAGCAAGTGGGAAGGTGTAATCC;
36B4 reverse primer: CCCATTCTATCATCAACGGGTACAA [35,38].
For each amplification reaction, 20 ng of the sample template DNA and primers at final concentrations of 500 nM were added to SYBR green PCR master mix (ThermoFisher Scientific, Waltham, MA, USA), which contains SYBR Green dye, DNA polymerase, heat-labile Uracil-DNA glycosylase, dNTP blend, and other dyes/buffer components. The qPCR was run on SimpliAmp™ Thermal Cycler (ThermoFisher Scientific, Waltham, MA, USA) with PCR cycle settings of: 36B4 primers at 2 min of 95 °C, 15 s of 95 °C, and 1 min of 58 °C; telomere primers at 2 min of 95 °C, 15 s of 95 °C, and 1 min of 56 °C.
The average telomere length is estimated with the formula:
This calculation is based on T/S ratio correlations with reference to DNA lengths on Southern blots and provides a fairly accurate estimate of absolute telomere length [39,40,41].
2.7. Data Management and Statistical Analysis
All data were collected and stored on secure, encrypted servers. Participants selected a random identification number unknown to the study staff by drawing a paper strip from a bag. A participant identifier was then associated with each participant’s data and used to correlate data collected during the first and second visits, ensuring participant anonymity. All data are reported as mean ± standard error of the mean (SEM) unless otherwise noted. Statistical analysis was performed using the Graphpad Prism software (Version 9). Differences in mean scores between two groups were analyzed using Student’s paired t-test or Wilcoxon’s signed-rank test. Heart rate responsivity to mental tasks was analyzed by one-way repeated measures ANOVA.
3. Results
3.1. Participant Demographics
All participants in this study were preclinical (1st or 2nd year) medical students who primarily receive instruction through lectures and laboratory classes. Participants were followed over the course of an academic year (6−7 months). 20 participants filled out the survey, and all passed our exclusion/inclusion surveys (Figure 1B).
After enrollment, our study was paused early due to the COVID-19 shutdown and resulted in nine participants overall (Figure 1B). Seven participants (77.8%) were in the 18–24 age group, and two (22.2%) were older than 24. Four (44.4%) participants were female, and five (55.6%) were male. Seven (77.8%) were White, two (22.2%) were Asian, and one (11.1%) was Hispanic or Latino. Participant demographics are summarized in Table 1.

Table 1.
Summary of Demographic Data. This table shows a summary of the general characteristics of enrolled participants and is organized by sex, age, race, and ethnicity.
3.2. Survey Results for Burnout, Stress, and Depression
The Maslach Burnout Inventory General Survey for Students (MBI-SS) was used to detect burnout specifically in student groups (Figure 2). The MBI-SS is split into three different subscores for exhaustion, cynicism, and professional efficacy. An MBI composite score consisting of exhaustion and cynicism was calculated to estimate overall changes in burnout symptoms. The composite MBI score (the sum of exhaustion and cynicism subscores) showed a statistically significant increase from 18.89 ± 2.25 on visit 1 to 22.0 ± 2.6 on visit 2 (p = 0.0469, Wilcoxon signed-rank test). In addition, trends corresponding to an increase in burnout were observed in some of the individual MBI subscores, but these were not statistically significant individually: The MBI exhaustion subscore rose from 12.8 ± 1.6 (mean ± SEM) on visit 1 to 14.9 ± 2.0 on visit 2 (p = 0.1406, Wilcoxon signed-rank test); the MBI cynicism subscore increased from 6.1 ± 1.3 to 7.1 ± 1.2 (p = 0.3828, Wilcoxon signed-rank test); and the average MBI professional efficacy subscore was 26.22 ± 1.2 on visit 1 and was not significantly different from 27.0 ± 1.6 on visit 2 (p = 0.7031, Wilcoxon signed-rank test). In contrast, PSS scores for perceived stress were not significantly different between visit 1 at 18.6 ± 0.6 and visit 2 at 20.3 ± 1.1 (p = 0.2305, Wilcoxon signed-rank test; Figure 3A). Interestingly, PHQ-9 for depression symptoms significantly increased from 3.4 ± 1.0 on visit 1 to 5.9 ± 1.2 on visit 2 (p = 0.0273, Wilcoxon signed-rank test; Figure 3B).
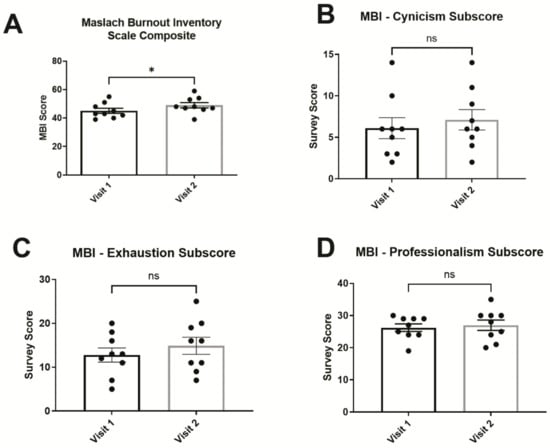
Figure 2.
Maslach Burnout Inventory (MBI) Scores for burnout. (A) MBI composite scores (sum of cynicism and exhaustion subscores) showed significant elevation after an academic year. Respective MBI subscores for (B) cynicism, (C) exhaustion, and (D) professionalism were not significantly changed when considered individually. Error bars in graphs show standard errors of the mean. * = p < 0.05, ns = not statistically significant.
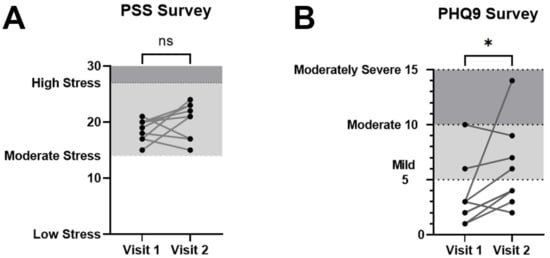
Figure 3.
Perceived Stress Scale (PSS) for stress and Patient Health Questionnaire—9 item survey (PHQ-9) for depression. (A) PSS survey scores were not significantly different between visits 1 and 2. (B) PHQ-9 survey as an estimation of depression symptoms showed a significant average increase on the second visit over the first. Cutoffs for stress level and depression symptom categories are shaded and shown for reference. Error bars in graphs show standard errors of the mean. * = p < 0.05, ns = not statistically significant.
3.3. Lifestyle Questionnaire
No changes were observed in reported time devoted to leisure activities; however, time devoted to exercise decreased by 0.6 days on average (4.9 ± 0.4 days/week on visit 1 vs. 4.3 ± 0.6 days/week on visit 2 (p = 0.0133, paired t test; Figure 4A). Average BMI also increased from 25.4 ± 1.1 on visit 1 to 26.0 ± 1.1 on visit 2 (p = 0.0236, paired t test; Figure 4B).
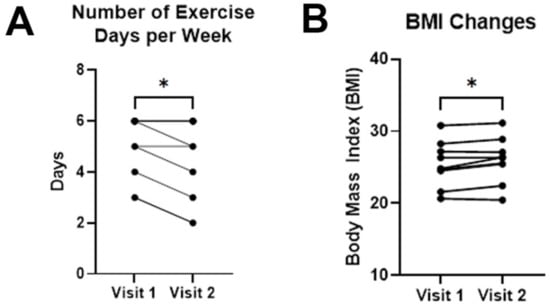
Figure 4.
Changes in exercise habits and BMI. (A) Medical students exercised fewer days per week over the course of a preclinical academic year. (B) Average BMI showed a statistically significant elevation between visits 1 and 2. Error bars in graphs show standard errors of the mean. * = p < 0.05, ns = not statistically significant.
3.4. Inflammatory Cytokines
A clinical cytokine panel was used to measure levels of selected inflammatory cytokines during study visits. Serum levels of IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-13 were below detection thresholds at both timepoints. Serum levels of TNF-α, IL-1β, IL-2, sIL-2R, IL-12, IL-17 did not significantly change from visit 1 to visit 2 (Table 2).

Table 2.
Inflammatory Cytokine Panel Results. This table summarizes the list of cytokines measured in the cytokine panel during both visits and the relative reference ranges. No statistically significant differences were detected between visit 1 and visit 2.
3.5. Heart Rate Variability
Our participant sample did not demonstrate a statistically significant difference in time-domain HRV metrics SDNN, RMSSD, or pNN50 between the first and second visits (Figure 5A–C). Participants demonstrated a favorable response to the mental task challenge, with a statistically significant increase in heart rate on the initial visit (Figure 5D). Interestingly, this effect was observable but not statistically significant on the second visit (Figure 5E).
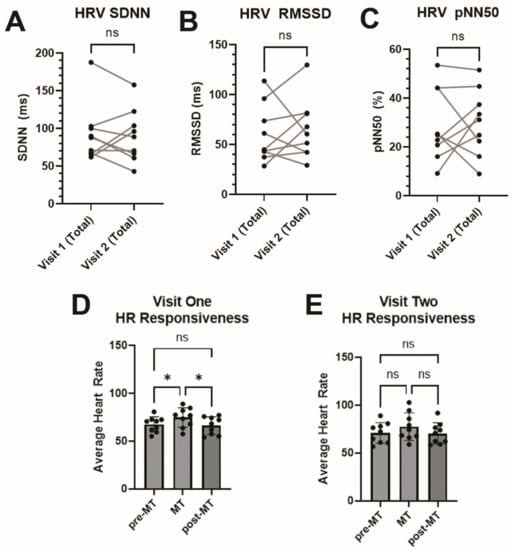
Figure 5.
Heart Rate Variability. Time-domain heart rate variability metrics were calculated between visits 1 and 2 using either (A) standard deviation of R-R intervals (SDNN), (B) root mean-square differences between consecutive R-R intervals (RMSSD), or (C) percentage of adjacent NN intervals differing by more than 50 ms (pNN50). (D) Heart rate during a mental task (MT) was significantly increased during visit 1 compared to pre- and post-MT, as measured by one-way repeated measures ANOVA, but (E) this was not statistically significant during visit 2. * = p < 0.05, ns = not statistically significant.
3.6. Leukocyte Telomere Length
As telomere lengths can vary depending on age, race, genetics, and many other factors [42,43], initial telomere lengths from each participant were used as that participant’s baseline value to calculate difference over an academic year. Average telomere lengths for visit one was an average of 5511 base pairs ± 183.4 (SEM) and 5393 base pairs ± 172.5 (SEM) for visit two. This led to an average decrease of 118.4 base pairs (p = 0.5617) over the course of an academic school year.
4. Discussion
The early detection and treatment of burnout syndrome in healthcare workers is vital to improving their well-being. The current standard in burnout syndrome screening and management is the administration of surveys/questionnaires designed to assess the subjective experiences associated with burnout; however, there are no reliable biomarkers capable of accurately predicting or tracking burnout syndrome. In this study, we present early pilot data on a small cohort of medical students within the course of an academic year, comparing results on validated surveys of burnout with a panel of measurements on several candidate biomarkers of burnout.
To detect burnout, stress, and depression, we used three different validated surveys: MBI, PSS, and PHQ-9. The Maslach Burnout Inventory is the gold standard for the measurement of burnout [1,2]. We specifically selected the MBI general survey for students (MBI-SS), which has been previously used in student populations and detects three different scales for exhaustion, cynicism, and professional efficacy. We used the MBI-SS survey at two different timepoints before and after an academic school year (6–7 months) in preclinical medical students. Even in such a short time frame, there was a significant difference in MBI composite score for exhaustion and cynicism. By comparison, the PSS survey did not show significant differences for the same population.
Another modifier of chronic stress is symptoms of depression and other related risk factors that could also impact the progression of burnout. In this study, we used the patient health questionnaire (PHQ-9) as a quick and highly sensitive tool to detect early symptoms of depression [14,15]. We found that even in the short period of a single academic year, medical students on average showed significant increases in clinical symptoms of depression based on PHQ-9 survey results. It is alarming to see that burnout and depression symptoms can be detected even in preclinical years. These data suggest that physician burnout likely has its roots early in medical school training, which may be exacerbated during later stages of medical and residency training.
Multiple factors contribute to well-being and the development of burnout, including physical and leisure activities that help to alleviate stress. We assessed exercise activities via lifestyle questionnaire and measured changes in body habitus via BMI changes over the study period. At the end of one academic year, medical students on average reported spending less time on exercise per week. This change in behavior corresponded to an increase in measured BMI over this period. Future studies could further explore the contribution of wellness activities such as exercise in a larger cohort of medical students and characterize the effects of motivational intervention.
Another goal of this study is to associate burnout and depression survey results with changes in biomarkers such as inflammatory cytokines and leukocyte telomere length. Over the course of an academic year, medical students did not display significant differences in levels of cytokines including TNF-ɑ, IL-1ꞵ, IL-2, sIL-2R, IL-6, IL-12, IFN-γ, IL-4, IL-5, IL-10, IL-13, and IL-17. This result may indicate that a longer duration of study with a larger study population is necessary to observe differences in cytokine levels in medical students undergoing burnout, or that other potential biomarkers would be better candidates for future study. Similarly, time-domain heart rate variability metrics did not significantly change between our participants, and a longer duration of study or larger sample size may be required to observe the effects of chronic stress on HRV. It appears that evidence of burnout and depression symptoms as evidenced by results on MBI and PHQ-9 survey results can occur prior to significant changes in cytokine panel or time-domain HRV measures. However, future studies are needed to clarify the potential clinical utility of these assays in burnout detection.
While leukocyte telomere length has been examined mostly in acute stress, several recent studies also looked at how chronic stress affects leukocyte telomere length. Recent stressful life events were associated with shorter telomere lengths in time periods as short as a year [44,45], which can also be found after longer time points of 4 and 6 years [46]. In medical residents, a recent study found significant telomere attrition after one year of medical residency training based on DNA collected from buccal swabs [40]. In our study on medical students, there was a notable downward trend in telomere length that was not statistically significant. However, this result may serve as a starting point for future studies that analyze the efficacy of blood-based telomere length assays as biomarkers of stress in medical students and medical professionals during different stages of training. Additionally, we observed less variance in telomere length of leukocytes isolated from whole blood compared to other methods such as buccal swabs.
The initial study design included the enrollment of cohorts of medical students spanning several classes of medical school students over 2–3 years. However, study recruitment was stopped early in 2020 due to the onset of the COVID-19 pandemic, significantly limiting participant enrollment and data collection. Multiple curriculum changes have persisted since this time, including the increased utilization of virtual lectures and labs as a secondary result of limitations of social distancing and scheduling difficulties [47,48]. The impact of the COVID-19 pandemic itself also posed a significant chronic source of stress [6] that would further confound the findings of this study, though these data were collected prior to the onset of the COVID-19 lockdown. Thus, we present data on chronic stress as a snapshot of a medical education curriculum prior to the COVID-19 pandemic and during a time when the USMLE Step 1 licensing examination was numerically scored.
While this study was performed largely within a single medical school curriculum at the University of Florida College of Medicine, a comparison of variations in medical curricula with different teaching models at diverse medical schools could better identify teaching models that are less likely to promote burnout.
5. Conclusions
In this pilot study, we found that MBI and PHQ-9 could detect early changes in symptoms of burnout and depression even for a small sample size. In this same time frame, inflammatory cytokines, leukocyte telomere length, and heart rate variability were not significantly different over the course of an academic year. However, follow-up studies could be performed to provide results with a higher statistical significance using longer study durations or larger sample sizes. The results of this initial pilot study will lay the foundation for future studies on burnout and chronic stress in medical students, residents, and medical professionals.
Author Contributions
Conceptualization, F.J.A., W.S.D., E.W.H., D.S., L.B.S. and Y.X.; data curation, F.J.A., W.S.D., E.W.H., D.S., C.D.H. and Y.X.; formal analysis, F.J.A., W.S.D., E.W.H., D.S. and Y.X.; funding acquisition, F.J.A., W.S.D., E.W.H., D.S., L.B.S., C.D.H. and Y.X.; validation, F.J.A., W.S.D., E.W.H., D.S., C.D.H. and Y.X.; investigation, F.J.A., W.S.D., E.W.H., D.S. and Y.X.; writing—original draft, F.J.A., W.S.D., E.W.H., D.S. and Y.X.; writing—review and editing, F.J.A., W.S.D., E.W.H., D.S., L.B.S., C.D.H. and Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
Funding support was provided by a clinical pilot grant from the Clinical and Translational Institute at the University of Florida, which was funded through the NIH National Center for Advancing Translational Sciences (Grant #UL1TR001427). Additional support was provided by the MD-PhD program at the University of Florida.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Florida (IRB201700317, 5 July 2018; Ame1_IRB201700317, 14 August 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data in this study can be made available upon reasonable request.
Acknowledgments
We want to express our deepest gratitude to the UF College of Medicine MD–PhD Directorate members and faculty that supported this project through both its inception and completion: W. Stratford May, Thomas A. Pearson, Mark S. Segal, Wayne T. McCormack, Kristianna M. Fredenburg, and Ali Zarrinpar. We thank the UF Clinical and Translational Science Institute (CTSI) staff who assisted with the administration and logistics of this project. We also want to thank other support staff for helping us with what would otherwise have been a hair-raising experience.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maslach, C.; Jackson, S.E.; Leiter, M.P. Maslach Burnout Inventory: Third edition. In Evaluating Stress: A Book of Resources; Scarecrow Education: Lanham, MD, USA, 1997; pp. 191–218. [Google Scholar]
- Maslach, C.; Jackson, S.E. The measurement of experienced burnout. J. Organ. Behav. 1981, 2, 99–113. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Boone, S.; Tan, L.; Dyrbye, L.N.; Sotile, W.; Satele, D.; West, C.P.; Sloan, J.; Oreskovich, M.R. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch. Intern. Med. 2012, 172, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Rotenstein, L.S.; Torre, M.; Ramos, M.A.; Rosales, R.C.; Guille, C.; Sen, S.; Mata, D.A. Prevalence of Burnout Among Physicians: A Systematic Review. JAMA 2018, 320, 1131–1150. [Google Scholar] [CrossRef]
- West, C.P.; Dyrbye, L.N.; Erwin, P.J.; Shanafelt, T.D. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet 2016, 388, 2272–2281. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; West, C.P.; Dyrbye, L.N.; Trockel, M.; Tutty, M.; Wang, H.; Carlasare, L.E.; Sinsky, C. Changes in Burnout and Satisfaction with Work-Life Integration in Physicians During the First 2 Years of the COVID-19 Pandemic. Mayo Clin. Proc. 2022, 97, 2248–2258. [Google Scholar] [CrossRef]
- Ryan, E.; Hore, K.; Power, J.; Jackson, T. The relationship between physician burnout and depression, anxiety, suicidality and substance abuse: A mixed methods systematic review. Front. Public. Health 2023, 11, 1133484. [Google Scholar] [CrossRef]
- Menon, N.K.; Shanafelt, T.D.; Sinsky, C.A.; Linzer, M.; Carlasare, L.; Brady, K.J.S.; Stillman, M.J.; Trockel, M.T. Association of Physician Burnout With Suicidal Ideation and Medical Errors. JAMA Netw. Open 2020, 3, e2028780. [Google Scholar] [CrossRef] [PubMed]
- Brunsberg, K.A.; Landrigan, C.P.; Garcia, B.M.; Petty, C.R.; Sectish, T.C.; Simpkin, A.L.; Spector, N.D.; Starmer, A.J.; West, D.C.; Calaman, S. Association of Pediatric Resident Physician Depression and Burnout with Harmful Medical Errors on Inpatient Services. Acad. Med. 2019, 94, 1150–1156. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Shanafelt, T. Physician Distress and Burnout, the Neurobiological Perspective. Mayo Clin. Proc. 2021, 96, 763. [Google Scholar] [CrossRef]
- Rodrigues, H.; Cobucci, R.; Oliveira, A.; Cabral, J.V.; Medeiros, L.; Gurgel, K.; Souza, T.; Gonçalves, A.K. Burnout syndrome among medical residents: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206840. [Google Scholar] [CrossRef]
- Almutairi, H.; Alsubaiei, A.; Abduljawad, S.; Alshatti, A.; Fekih-Romdhane, F.; Husni, M.; Jahrami, H. Prevalence of burnout in medical students: A systematic review and meta-analysis. Int. J. Soc. Psychiatry 2022, 68, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999, 282, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774. [Google Scholar] [CrossRef]
- Hänsel, A.; Hong, S.; Cámara, R.J.A.; von Känel, R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 2010, 35, 115–121. [Google Scholar] [CrossRef]
- Von Känel, R.; Bellingrath, S.; Kudielka, B.M. Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J. Psychosom. Res. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Perski, A.; Evengård, B.; Blomkvist, V.; Orth-Gomér, K. Physiological correlates of burnout among women. J. Psychosom. Res. 2003, 55, 309–316. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef]
- Marsland, A.L.; Sathanoori, R.; Muldoon, M.F.; Manuck, S.B. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behav. Immun. 2007, 21, 218–228. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Fauce, S.R.; Effros, R.B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 2008, 22, 600–605. [Google Scholar] [CrossRef]
- Mathur, M.B.; Epel, E.; Kind, S.; Desai, M.; Parks, C.G.; Sandler, D.P.; Khazeni, N. Perceived Stress and Telomere Length: A Systematic Review, Meta-Analysis, and Methodologic Considerations for Advancing the Field. Brain Behav. Immun. 2016, 54, 158. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Tosato, S.; Begni, V.; Tomassi, S.; Cattane, N.; Barcella, M.; Turco, G.; Ruggeri, M.; Riva, M.A.; Pariante, C.M.; et al. Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events. Transl. Psychiatry 2017, 7, e1042. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef]
- Lennartsson, A.K.; Jonsdottir, I.; Sjörs, A. Low heart rate variability in patients with clinical burnout. Int. J. Psychophysiol. 2016, 110, 171–178. [Google Scholar] [CrossRef]
- Kotov, A.V.; Revina, N.E. Heart rate variability during “alarm stage” of burnout syndrome in emergency doctors. Bull. Exp. Biol. Med. 2012, 153, 598–600. [Google Scholar] [CrossRef]
- Sun, G.; Shinba, T.; Kirimoto, T.; Matsui, T. An Objective Screening Method for Major Depressive Disorder Using Logistic Regression Analysis of Heart Rate Variability Data Obtained in a Mental Task Paradigm. Front. Psychiatry 2016, 7, 180. [Google Scholar] [CrossRef]
- Shinba, T.; Kariya, N.; Matsui, Y.; Ozawa, N.; Matsuda, Y.; Yamamoto, K.I. Decrease in heart rate variability response to task is related to anxiety and depressiveness in normal subjects. Psychiatry Clin. Neurosci. 2008, 62, 603–609. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ernst, G. Hidden Signals-The History and Methods of Heart Rate Variability. Front. Public. Health 2017, 5, 265. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere Length: A Review of Methods for Measurement. Nurs. Res. 2014, 63, 289. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR—Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 2019, 99, 271. [Google Scholar] [CrossRef]
- Akamine, R.; Yamamoto, T.; Watanabe, M.; Yamazaki, N.; Kataoka, M.; Ishikawa, M.; Ooie, T.; Baba, Y.; Shinohara, Y. Usefulness of the 5′ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J. Biochem. Biophys. Methods 2007, 70, 481–486. [Google Scholar] [CrossRef]
- Hudon, S.F.; Palencia Hurtado, E.; Beck, J.D.; Burden, S.J.; Bendixsen, D.P.; Callery, K.R.; Sorensen Forbey, J.; Waits, L.P.; Miller, R.A.; Nielsen, Ó.K.; et al. Primers to highly conserved elements optimized for qPCR-based telomere length measurement in vertebrates. Mol. Ecol. Resour. 2021, 21, 59–67. [Google Scholar] [CrossRef]
- Goglin, S.E.; Farzaneh-Far, R.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Whooley, M.A. Change in Leukocyte Telomere Length Predicts Mortality in Patients with Stable Coronary Heart Disease from the Heart and Soul Study. PLoS ONE 2016, 11, e0160748. [Google Scholar] [CrossRef]
- Ridout, K.K.; Ridout, S.J.; Guille, C.; Mata, D.A.; Akil, H.; Sen, S. Physician Training Stress and Accelerated Cellular Aging. Biol. Psychiatry 2019, 86, 725. [Google Scholar] [CrossRef]
- Lin, J.; Cheon, J.; Brown, R.; Coccia, M.; Puterman, E.; Aschbacher, K.; Sinclair, E.; Epel, E.; Blackburn, E.H. Systematic and Cell Type-Specific Telomere Length Changes in Subsets of Lymphocytes. J. Immunol. Res. 2016, 2016, 5371050. [Google Scholar] [CrossRef]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 51, 15. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 1816. [Google Scholar] [CrossRef]
- Verhoeven, J.E.; Van Oppen, P.; Puterman, E.; Elzinga, B.; Penninx, B.W.J.H. The Association of Early and Recent Psychosocial Life Stress with Leukocyte Telomere Length. Psychosom. Med. 2015, 77, 882–891. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Krauss, J.; Blackburn, E.H.; Epel, E.S. Determinants of telomere attrition over 1 year in healthy older women: Stress and health behaviors matter. Mol. Psychiatry 2015, 20, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Van Ockenburg, S.L.; Bos, E.H.; De Jonge, P.; Van Der Harst, P.; Gans, R.O.B.; Rosmalen, J.G.M. Stressful life events and leukocyte telomere attrition in adulthood: A prospective population-based cohort study. Psychol. Med. 2015, 45, 2975–2984. [Google Scholar] [CrossRef]
- Muntz, M.D.; Franco, J.; Ferguson, C.C.; Ark, T.K.; Kalet, A. Telehealth and Medical Student Education in the Time of COVID-19 and Beyond. Acad. Med. 2021, 96, 1655–1659. [Google Scholar] [CrossRef]
- Subramanian, T.; Rowland, K.J. Opportunities and Challenges in Medical Education During the COVID-19 Pandemic. Pediatr. Ann. 2022, 51, e319–e323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).