1. Introduction
In recent years, significant advancements have been made in macular hole management. However, refractory full-thickness macular holes (rFTMHs) continue to pose a clinical challenge for ophthalmologists and vitreoretinal surgeons. Despite the success of vitrectomy surgery with internal limiting membrane (ILM) peeling and gas tamponade as the standard treatment for most primary FTMHs, there are cases where closure is not achieved or persistent defects are present after surgery. The reported incidence of rFTMHs is 11.2% in 491 patients [
1] and 4.2% in 1078 eyes [
2] of patients who have undergone combined vitrectomy, ILM peeling, and gas tamponade. These cases present a unique set of challenges that call for innovative strategies to achieve successful closure and improve visual outcomes. Unlike primary surgery, there is no consensus on the optimal surgical approach for rFTMH repair. Multiple surgical techniques have been developed, but due to their relatively recent introduction into clinical practice, the supporting scientific literature remains limited.
This review involved a thorough search of PubMed, capturing all relevant studies published up to 24 December 2024. Eligible studies focused on persistent, reopened, secondary, recurrent, or recalcitrant macular holes, as well as cases of failed macular hole closure after initial surgery. The review explores the prevalence, causes, and pathogenesis of rFTMHs, along with surgical management strategies, including techniques and outcomes. Additionally, specific clinical questions related to macular hole surgical procedures were examined and are incorporated into this review paper.
2. Causes of Refractory Full-Thickness Macular Hole
Several factors contribute to the development of rFTMHs. These include the size [
3] and duration of the macular hole [
3,
4], with macular holes larger than 400 µm in diameter [
5,
6] or present for more than 6 months [
4] being more likely to be refractory. Ethnicity also plays a role, with Afro-Caribbean [
3] individuals being associated with rFTMHs. High myopia [
3,
4] is a risk factor for rFTMHs, as well as surgical factors such as incomplete vitrectomy [
3,
4], inadequate ILM peeling (ideally complete ILM peeling from arcade to arcade) [
3,
4], or insufficient gas tamponade [
3,
4]. Noncompliance with post-operative care [
3,
4], particularly in regard to head positioning, can also contribute to rFTMHs. Undergoing cataract surgery after the repair of a FTMH can increase the risk of hole reopening, especially in cases with CMO post-cataract operation up to 20% [
4,
7], and having concomitant macular disease [
4], such as drusen, can also increase the risk of rFTMHs.
3. Pathogenesis of Refractory Full-Thickness Macular Hole
The pathogenesis of rFTMHs involves multiple biomechanical [
8,
9] and biological factors [
9]. Persistent vitreomacular traction from incomplete vitreous separation or residual cortical vitreous may contribute to reopening [
3,
4]. Inadequate initial ILM peeling can fail to relieve tangential traction forces [
3,
4,
7], while excessive peeling may damage Müller cells that are crucial in hole closure. Epiretinal membrane re-proliferation [
10] can generate new traction, and post-operative cystoid macular edema [
7] may mechanically destabilize healed tissue. Gas tamponade failure [
3,
4] (due to poor positioning or short duration) may prevent proper apposition of hole edges. Underlying retinal stiffness in chronic cases (>6 months) or large holes (>400 µm) [
6] impairs plasticity for reattachment. In high myopia [
7], progressive macular stretch and atrophy create unfavorable conditions for healing. Additionally, post-operative inflammation [
7], RPE dysfunction, and disruption of glial cell migration pathways may impair the wound healing response, leading to hole persistence or recurrence despite initial surgical closure.
4. Current Surgical Treatment Options Available for Refractory Full-Thickness Macular Holes
There are numerous treatment options available for rFTMHs, including autologous tissue as a scaffolding for cell migration from the surrounding retina, such as inverted ILM flap, autologous ILM flap, and autologous ILM plug [
11]. Other options include implantation of a scaffold for glial cell proliferation, such as autologous or allogenic lens capsule flap transplantation and human amniotic membrane transplantation [
11], as well as surgical techniques to relieve macular hole stiffness and increase retinal compliance, such as macular subretinal fluid injection [
11]. Additionally, macular fibrin plug with autologous blood or autologous platelets concentrate preparation [
12,
13] and autologous retina transplantation are also treatment options [
11]. Each treatment option carries its own benefits and risks; the optimal treatment choice depends on the patient’s specific condition, the surgeon’s expertise, and the availability of specialized instrumentation and technology.
4.1. Inverted Internal Limiting Membrane Flap
The inverted ILM flap technique is a surgical method employed to treat large macular holes, particularly in cases of recurrent holes where remnants of ILM remain attached around the edges of the macular hole. This technique is advantageous as it offers a potential means to close the macular hole effectively. The rationale behind the inverted ILM flap technique is to alleviate the tractional forces on the macula, which are often a result of vitreous gel adhesion to the underlying retina [
14]. The ILM is a delicate acellular layer on the retinal surface; by peeling it away, the associated traction on the retinal surface is diminished. In this technique, a larger section of the ILM is peeled, leaving a portion intentionally attached to the retina at one edge to create a “flap” that covers the macular hole. This flap serves as a scaffold, facilitating retinal reattachment and promoting healing. The inverted ILM flap technique demonstrates excellent efficacy in repairing primary full-thickness macular holes, with reported closure rates of 89–98% [
15,
16,
17,
18] across varying hole sizes, including small (<400 µm) and very large (>1000 µm) defects [
15,
16,
17,
18]. Post-operative visual acuity consistently improved by ≥ 2 Snellen lines in these studies [
15,
16,
17,
18]. However, clinical outcomes for rFTMHs treated with this technique remain unreported in the current literature.
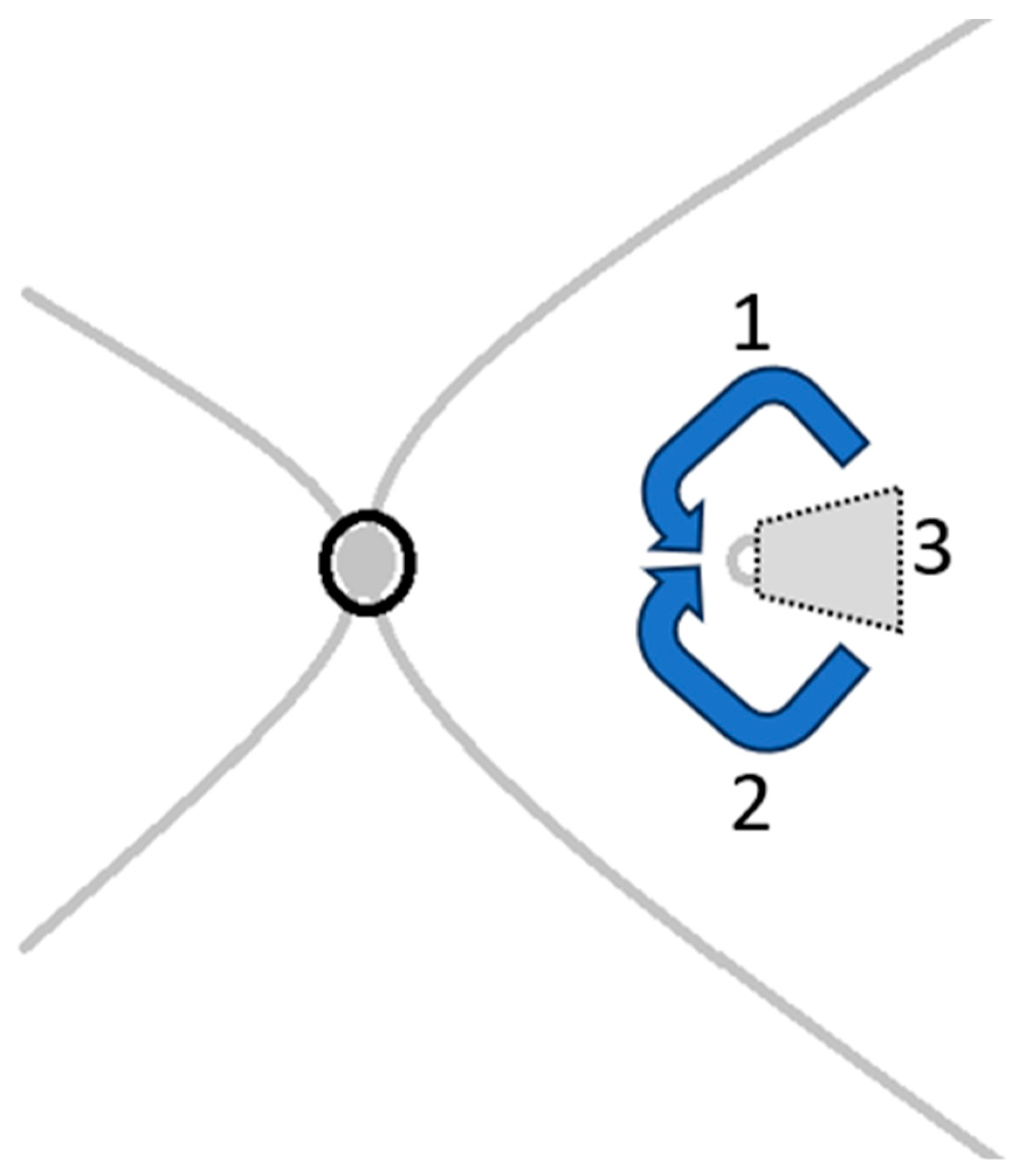
The surgical procedure (
Figure 1) is typically performed using a standard 3-port small-gauge vitrectomy. Following a complete vitrectomy, a biological dye such as Brilliant Blue G or Membrane Blue Dual is injected to highlight the remaining ILM and the area from which ILM has been previously removed. If sufficient ILM is available for an inverted flap, an ILM forceps or peeling hook is utilized to carefully detach the remaining ILM. It is important to peel a sufficiently large section to eliminate any residual traction while ensuring that the flap remains adequately sized (about 1.5 to 2 times of the hole size)—not too large to cause excessive traction, but large enough to cover the macular hole effectively. Once the attached edge of the ILM flap is elevated and inverted, the inner surface of the ILM (which was previously facing the vitreous) is positioned against the macular surface as illustrated in
Figure 2. A fluid–air exchange (FAX) is performed to facilitate adherence of the flap to the macular hole. Following the procedure, air is substituted with gas endotamponade. Patients are instructed to maintain a face-down posture for 3 to 7 days post-surgery to prevent any dislocation or displacement of the flap.
4.2. Autologous Internal Limiting Membrane Transplantation
The rationale for the repeated ILM peeling technique aligns closely with that of the inverted ILM flap technique. This procedure aims to totally remove any residual adherent vitreous cortex remnants and further weaken the tangential traction, which increases retinal elasticity and compliance [
14]. The ILM flap transplanted onto a macular hole also serves as a barrier preventing fluid from entering the macular hole and acts as a scaffold for glial cell proliferation on the surface of the macula, ultimately facilitating hole closure [
14]. Endotamponade is subsequently used to support the ILM flap and prevent dislocation. Among all endotamponade options, gas is preferred due to its high interfacial tension and buoyancy. However, silicone oil may be considered for patients who are unable to maintain a face-down position post-operatively, although it necessitates further surgical intervention for removal. Autologous transplantation of ILM for rFTMH has shown promising results, with a closure rate of 90–91% and significantly improved post-operative best-corrected visual acuity [
19,
20]. There are two techniques available for this procedure: the ILM free flap cover procedure and ILM plug procedure.
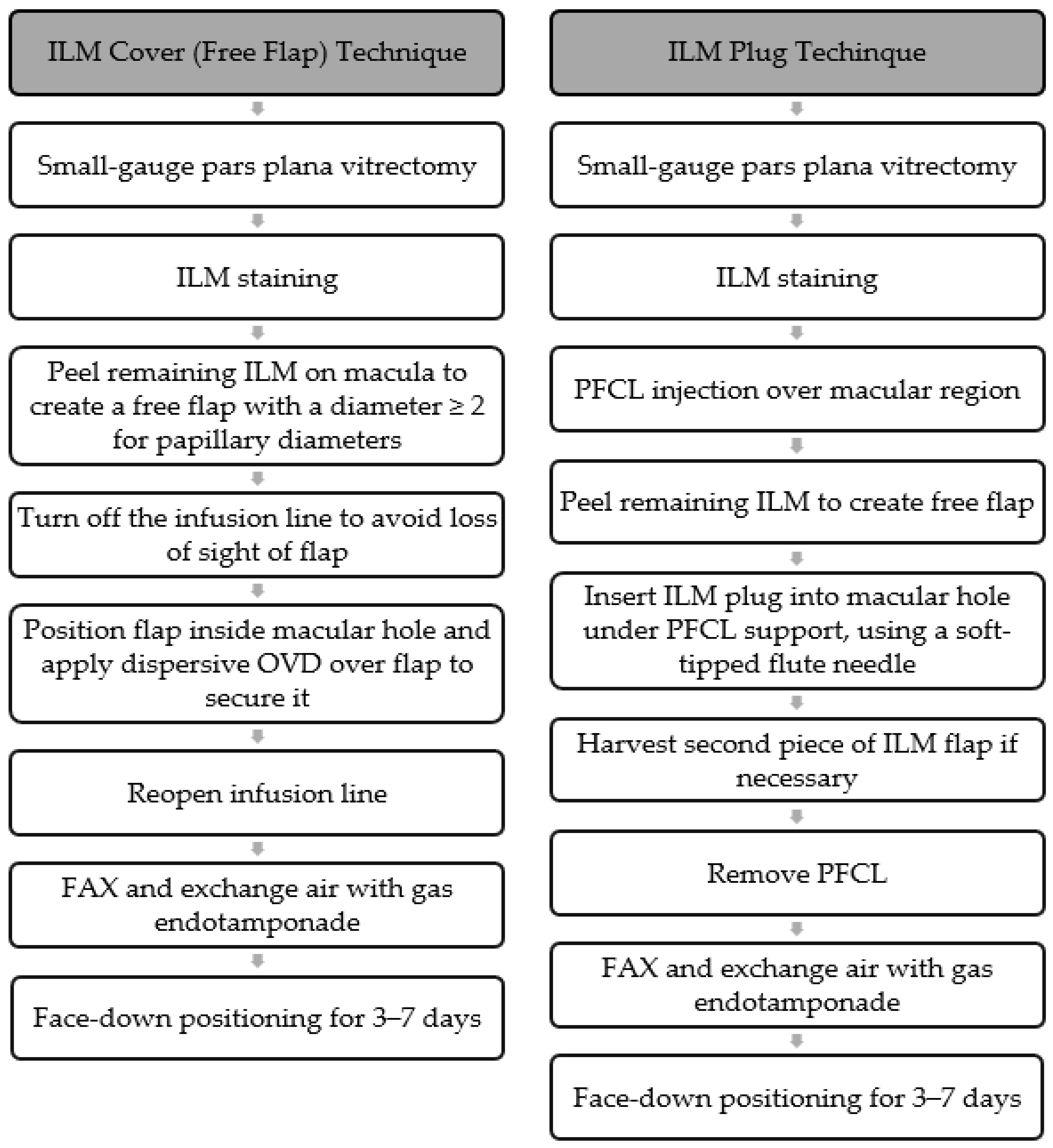
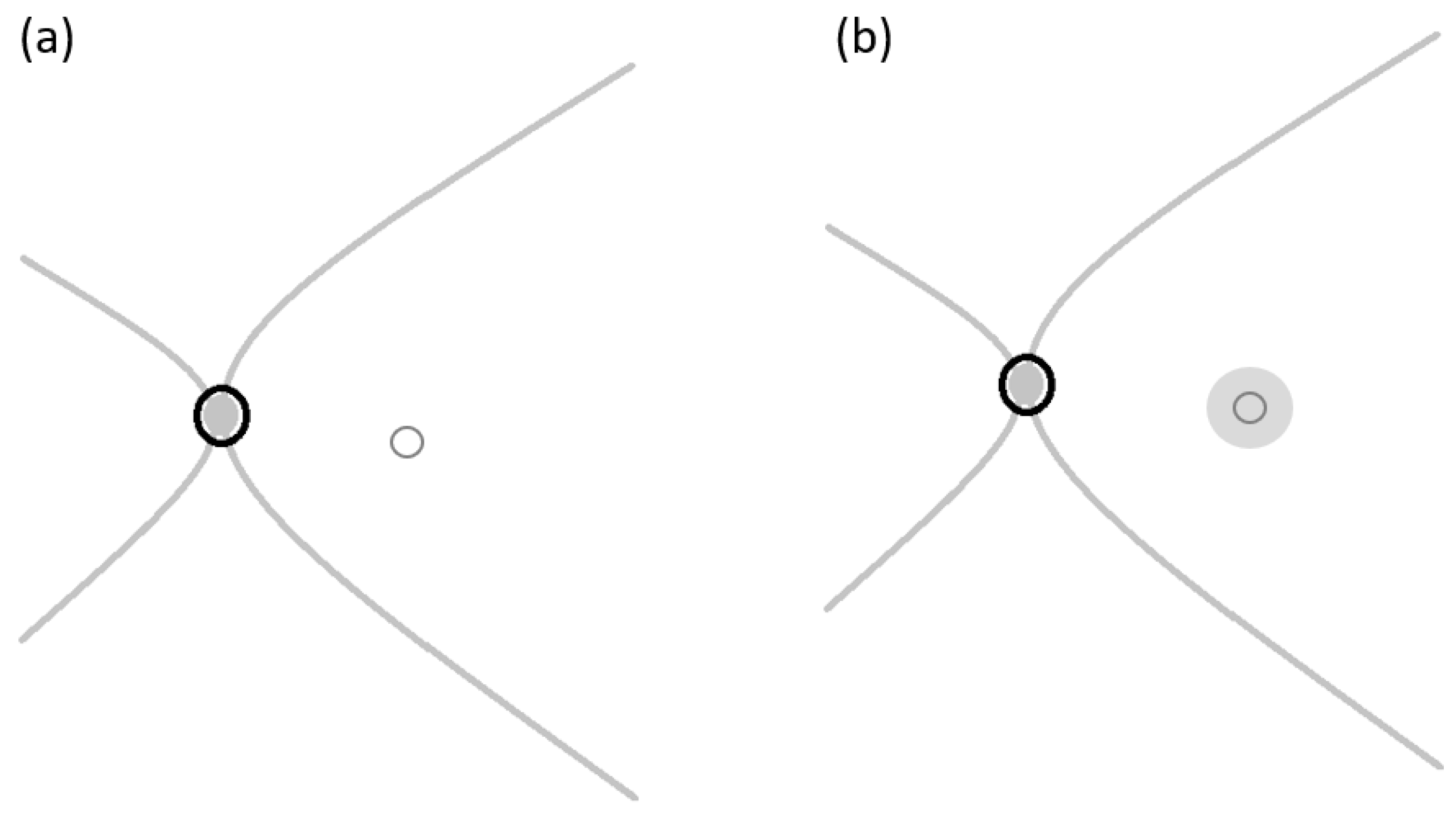
The ILM free flap cover procedure (
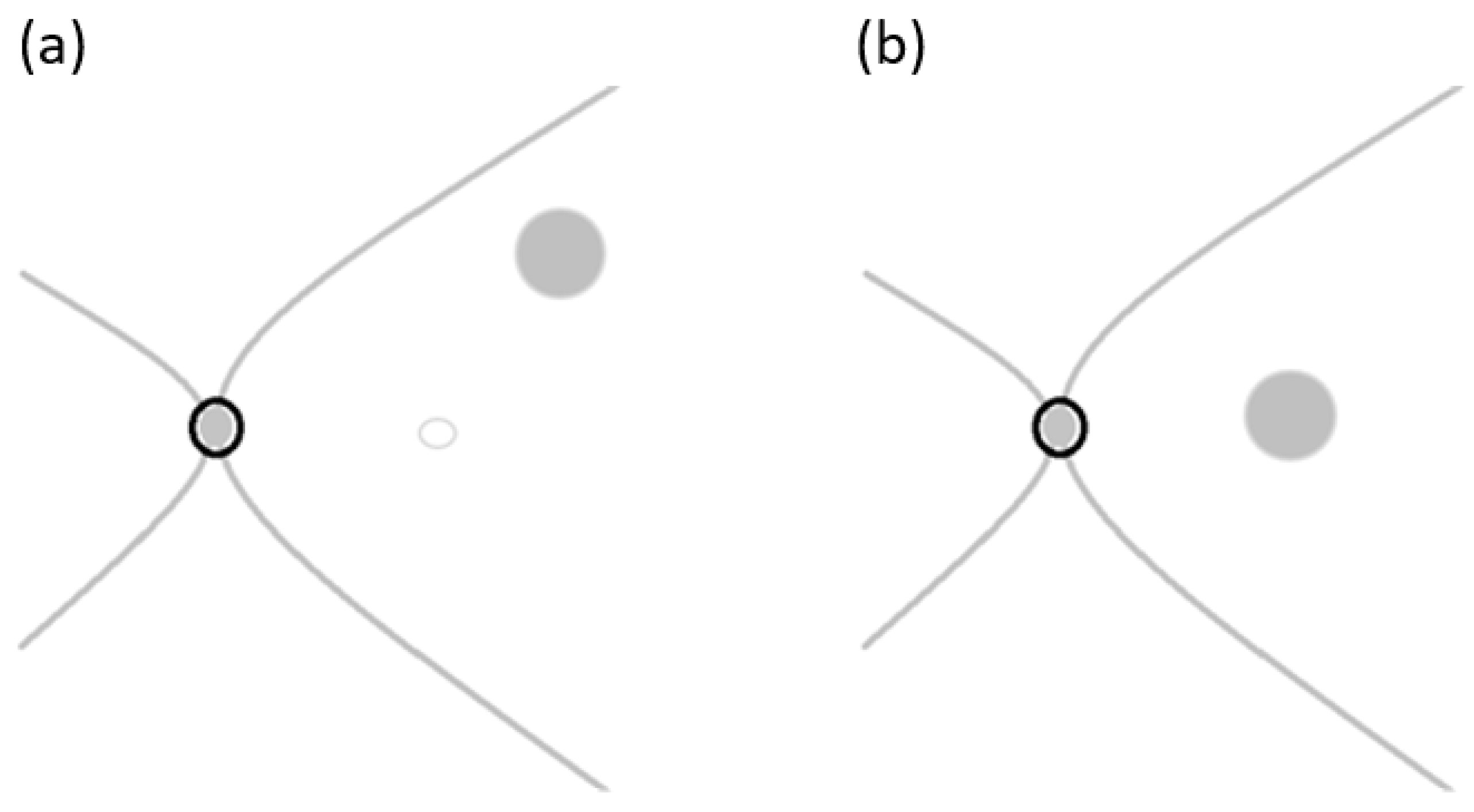
Figure 3) is performed with standard 3-port small-gauge vitrectomy. A biological dye is injected to pinpoint the remaining ILM and the area where the ILM has been previously removed. The remaining ILM is carefully peeled off to create a small free flap using a pinch-and-grasp technique from a region inside the vascular arcades [
21] as illustrated in
Figure 4a, ensuring a diameter of at least two papillary diameters in size to cover the macular hole [
22] as illustrated in
Figure 4b. To maintain visibility of the free ILM flap, the infusion line is temporarily turned off while the flap is positioned inside the macular hole [
20]. A dispersive Ophthalmic Viscosurgical Device (OVD) is then applied over the flap to secure it in place and left in the eye [
20]. Following this, fluid–air exchange is performed, taking care to keep the extrusion needle away from the macula [
20]. Subsequent to the procedure, the air is replaced with gas endotamponade, and patients are instructed to maintain a face-down position for 3 to 7 days post-surgery.
The ILM plug procedure (
Figure 3) begins with a standard 3-port small-gauge vitrectomy, during which a biological dye is used to locate the remaining ILM, as well as the area where it was previously removed. Perfluorocarbon liquid (PFCL) is then applied to cover the macular region [
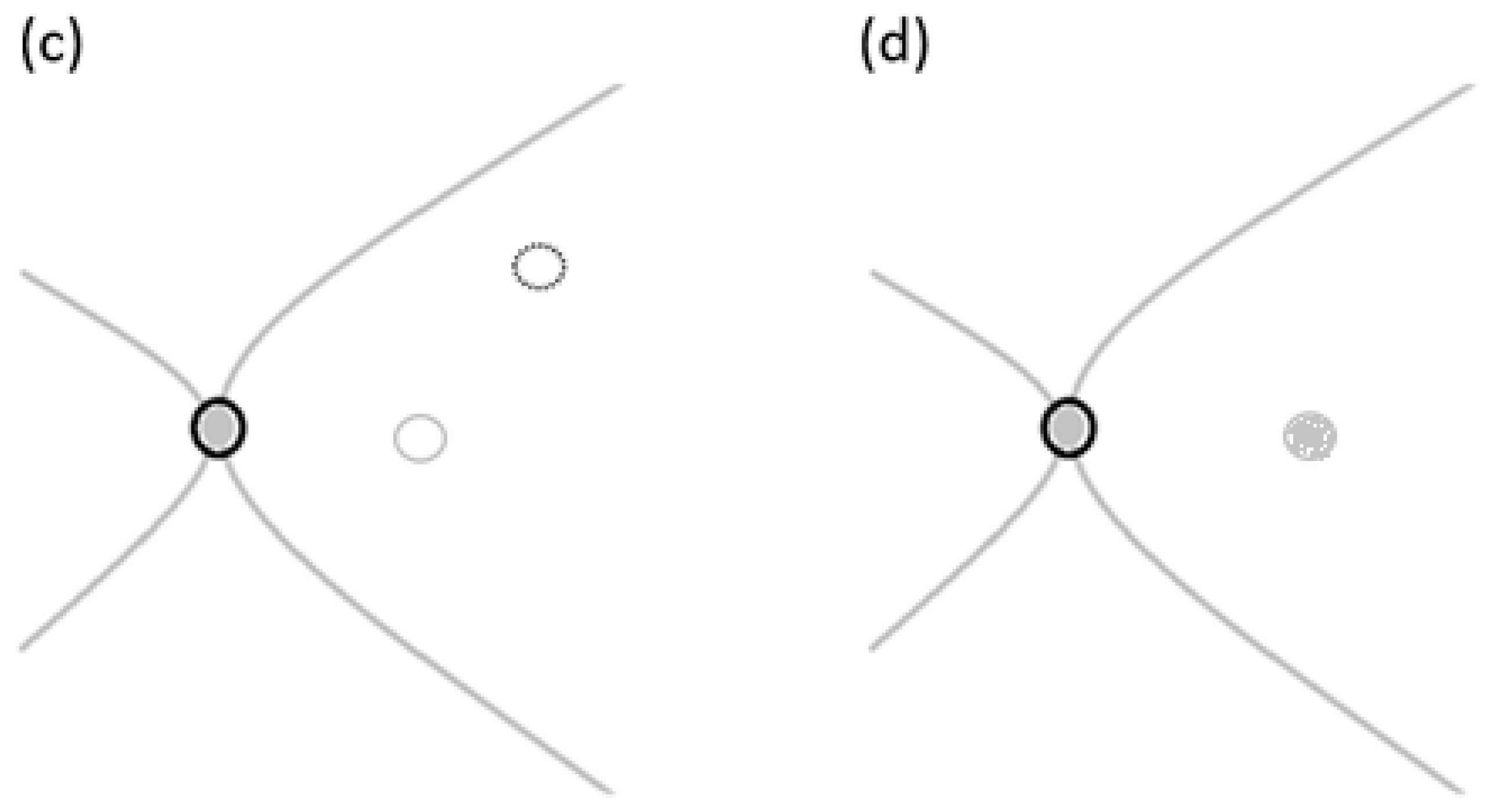
21]. The remaining ILM is delicately peeled off, creating a small free flap using a pinch-and-grasp technique from a specific area inside the vascular arcades as illustrated in
Figure 4c, with a diameter approximately the size of one disc diameter [
21]. The peeled ILM plug is inserted into the macular hole as illustrated in
Figure 4d under the PFCL support and gently positioned using a 23-gauge soft-tipped flute needle [
21]. If needed, a second piece of the ILM flap can be harvested using a similar technique to fill any remaining gap in the macular hole [
21]. The PFCL is subsequently removed, and fluid gas exchange is performed using gas tamponade. Post-operatively, patients are instructed to maintain a face-down position overnight and a prone position for the first 3 to 7 post-operative days to facilitate proper healing.
4.3. Lens Capsular Flap Transplantation
Conventional operative techniques such as vitrectomy with ILM peeling and gas tamponade have proven effective in promoting cell migration and facilitating the closure of macular holes [
23]. Studies on autologous lens capsular flap transplantation for macular holes have revealed positive immunoreactivity of macroglia and microglia cells within the transplanted posterior lens capsule [
24]. This finding suggests that the closure mechanisms may be similar between the ILM flap technique and lens capsule transplantation. The lens capsule, which is structurally akin to a basement membrane composed mainly of laminin and collagen Type IV, aids in bridging the retinal tissue above the macular hole while promoting the cellular migration necessary for closure [
25]. Due to its pliability and higher density compared to the ILM, the lens capsule adheres more readily to the retinal surface and can be precisely positioned over the macular hole [
5]. Notably, surgical outcomes vary depending on whether the anterior or posterior capsule is used; the anterior capsule, being thicker, adheres more firmly to the retina, thereby improving closure rates [
5]. Reported success rates for macular hole closure range from 75% to 100% [
4,
12,
26,
27], with higher efficacy observed when using an anterior rather than a posterior lens capsular flap [
11]. In view of the higher success rate of the anterior lens capsular flap, an allogenic lens capsular flap from a healthy donor undergoing cataract surgery on the same day can be used for the procedure [
27].
This surgery (
Figure 5) can be performed with a standard 3-port small-gauge vitrectomy. For phakic patients, the anterior lens capsule is stained with trypan blue and harvested, then introduced into the vitreous cavity using ILM forceps [
5]. For pseudophakic patients, the procedure involves staining the posterior lens capsule with trypan blue, followed by the creation of a posterior rhexis with an ILM forceps [
5]. Alternatively, an allogenic lens capsular flap harvested on the same day of operation from another patient can be utilized. The harvested flap is first placed in distilled water for at least 20 min to induce rapid cell lysis of the lens epithelial cells, thereby reducing the risk of post-operative proliferation of these cells on the macula [
27]. Subsequently, a biological dye is used to stain the remaining ILM around the macular hole, which is then peeled off [
5]. The harvested lens capsular flap is trimmed to a size at least 1 mm larger than the diameter of the macular hole [
5,
28] and introduced into the vitreous cavity using ILM forceps. It is gently positioned over the macular hole as illustrated in
Figure 6 with the outer surface of the harvested capsular flap facing down, as this side is smoother and lacks cells. Infusion is temporarily halted during the positioning of the capsular flap to minimize fluid turbulence, thereby reducing the risk of transplant dislocation or displacement [
5]. A small amount of dispersive OVD is then injected around the macular hole to secure the graft in place [
29]. Following the capsular flap transplantation, fluid–air exchange is carefully performed to prevent flap displacement. Subsequently to the procedure, air is replaced with gas endotamponade and patients are instructed to maintain a face-down posture for 3–7 days post-surgery to prevent flap dislocation. Alternatively, after fluid–air exchange, the eye can be filled with PFCL to prevent flap displacement [
5,
29]. If necessary, a direct exchange of PFCL with silicone oil can be performed to secure the unattached capsular flap [
5,
29]. Post-operatively, patients are advised to maintain a face-down position for 3–7 days to ensure optimal recovery [
29].
4.4. Preserved Human Amniotic Membrane Transplantation
The human amniotic membrane is the semi-transparent, three-layered avascular innermost structure of the human placenta with the thickness of 20–50 um [
30]. The basement membrane of the three layers is similar to other human basement membranes containing collagens IV, laminins, and fibronectin [
31]. Preserved human amniotic membrane transplantation for macular holes has become increasingly common due to its cost-effectiveness and high success rate in closing holes [
32]. The main advantage is that the product is readily available and can be used directly, avoiding the need for graft processing and the potential risk of infectious disease transmission associated with fresh amniotic membranes [
32]. However, one drawback is that preserved amniotic membranes provide fewer growth factors compared to fresh amniotic membranes, potentially impacting the healing process of macular holes [
32].
There are two different techniques currently being utilized for human amniotic membrane transplantation. The first technique involves using the membrane as a plug, inserting it into the subretinal space to plug the macular hole and promote closure [
33,
34]. However, this technique may result in potential mechanical damage to the retinal pigment epithelium during insertion of the membrane between the neurosensory retina and retinal pigment epithelium (RPE) during the surgery, leading to visual dysfunction [
32,
33,
34]. Additionally, the disordered structure of the macular area filling up with amniotic membranes post-closure may also impact macular function [
28,
33,
34]. On the other hand, the second technique involves using the preserved amniotic membrane as a cover for the macular hole, promoting closure at the anatomical level without affecting the outer structure of the retina [
32]. This approach theoretically provides maximum protection for the visual function of patients during the healing process [
32].
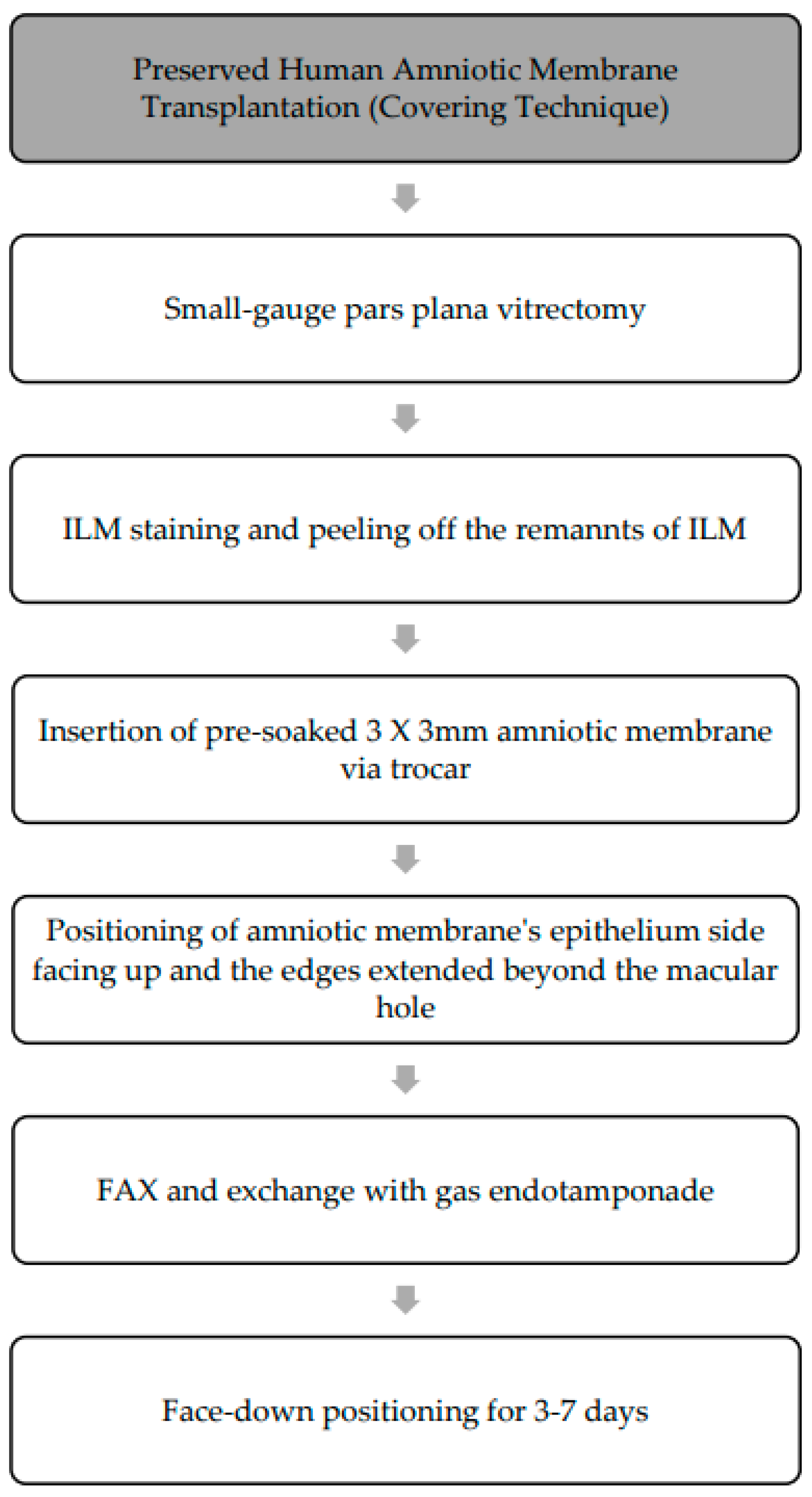
In view of the superior outcome of the covering technique using preserved human amniotic membrane, the focus of this method will be on the surgical technique in the subsequent segment. This procedure (
Figure 7) is performed using standard 3-port small-gauge vitrectomy. To ensure adequate peeling of ILM, biological dye can be applied to stain the ILM, allowing for the identification of any residual membrane around the macular hole from previous surgeries. During the procedure, a 3 mm × 3 mm preserved human amniotic membrane, pre-soaked in balanced salt solution, is meticulously introduced into the vitreous cavity through a small-gauge valved trocar (23- or 25-gauge trocar) [
25]. The amniotic membrane is positioned over the surface of the macular hole with the smooth epithelial side facing upwards [
35]. It is centered over the macula, ensuring that its edges extend beyond the border of the macular hole. Once the membrane is in place, any remaining liquid under the retina is removed through fluid–air exchange, and a flute needle is used to eliminate residual liquid around the membrane to ensure proper attachment to the retina. Following the procedure, air is replaced with gas endotamponade and patients are instructed to maintain a face-down position for 3–7 days to promote the closure of the macular hole. This procedure has shown promising results in achieving successful closure of macular holes with success rates ranging from 57.1% to 100% [
33,
36].
4.5. Macular Subretinal Fluid Injection
Macular subretinal fluid injection is a technique designed to release adhesions between the neurosensory retina and RPE. This is achieved by injecting fluid into the macula using a reflux mode of vitrectomy, which creates a temporary macular detachment [
11]. This approach aims to alleviate traction and tension at the edge of the macula, thereby increasing the compliance of the surrounding retina and facilitating closure of the macular hole [
11]. The reported success rate is 87.2% in closing large and chronic FTMHs [
37]. Other studies reported comparable success rate for macular hole closure with ranges from 83.3 to 100% [
4].
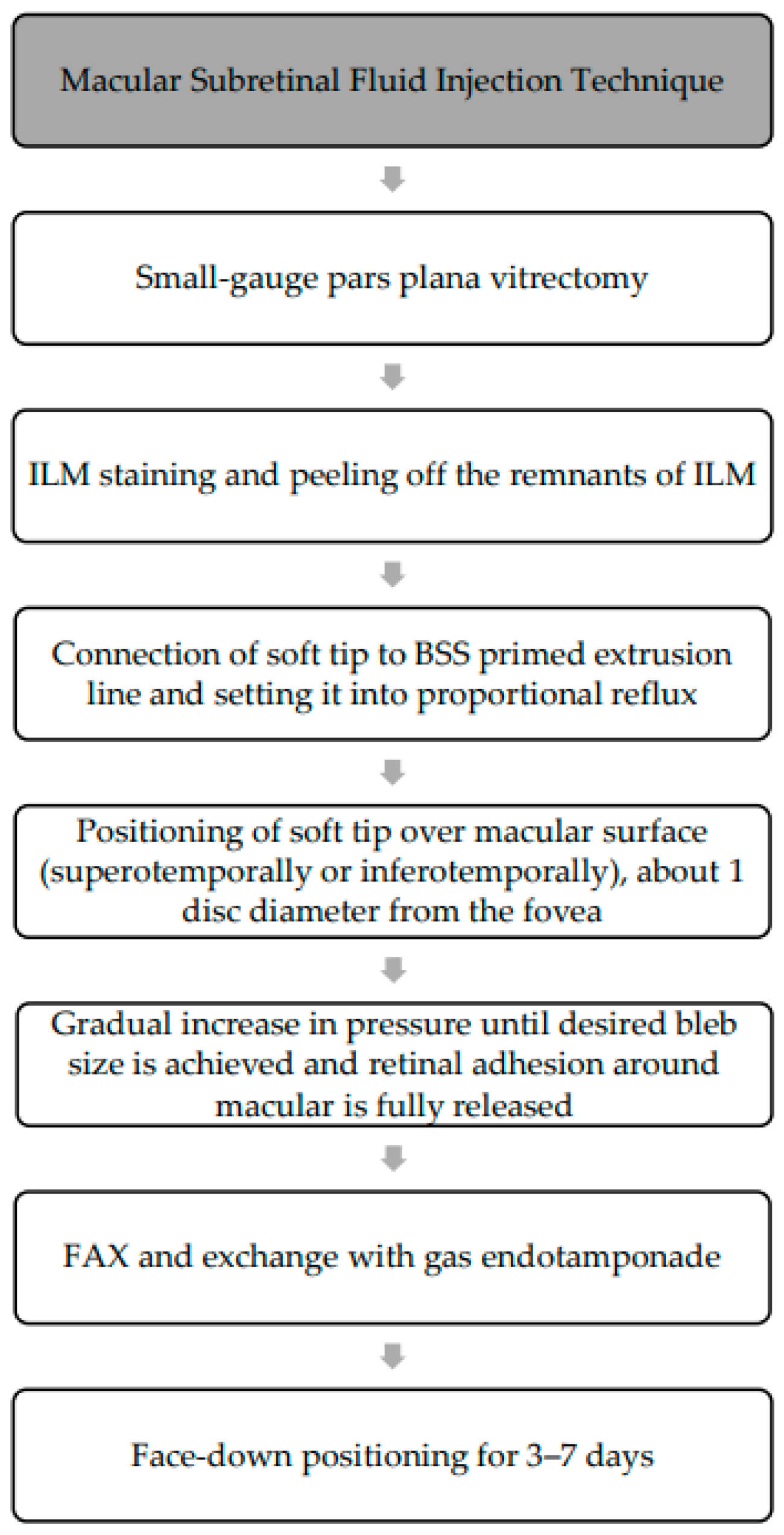
The procedure (
Figure 8) involves a standard 3-port small-gauge vitrectomy with the soft tip connected to the extrusion line primed with balanced salt solution (BSS) [
37]. The amount of fluid in the tubing may vary among manufacturers but is typically >10 mL [
38]. After vitrectomy, biological dye can be used to stain the ILM and assess for any residual ILM around the macular hole from previous surgeries. An extrusion line with a soft tip (e.g., a 30-gauge flute needle [
39]) is then set to proportional reflux [
37,
38], with standard settings ranging from 0 to 120 mmHg [
38]. The soft tip is positioned over the target area, typically over the macula superotemporally or inferotemporally, approximately 1 disc diameter from the fovea, and just touching the retinal surface [
38] as illustrated in
Figure 9. Pressure is gradually increased until the desired bleb size is achieved and retinal adhesion is released, typically requiring 30 to 40 mmHg (25 percent pedal depression) [
38]. Alternatively, the high end of reflux can be set to 40 mmHg (full pedal depression) to prevent potential RPE trauma from high-pressure jet streams [
38]. This technique can also be performed with a subretinal needle [
38] (e.g., 41-gauge subretinal cannula [
39]) on the extrusion line or connected to a 1 mL syringe filled with balance salt solution [
39], with this method requiring less pressure due to the stronger jet stream of fluid with a smaller diameter. The procedure is repeated until retinal adhesion around the macula is fully released. A complete gas–fluid exchange with gas endotamponade is performed [
37]. Post-operatively, patients were instructed to maintain a face-down position for 3 to 7 days.
4.6. Macular Autologous Blood or Fibrin Plug with Autologous Platelet Concentrates
Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are extensively utilized in regenerative medicine to promote tissue repair and regeneration [
12,
35]. These concentrates function by releasing growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) [
12,
40], which play crucial roles in cellular proliferation and healing. However, there are key differences between the two that should be considered when determining the best option for a patient. PRP, the first-generation platelet concentrate, involves injecting a concentrated form of platelets from the patient’s blood into damaged tissue such as the retinal layers of the macular hole. Limitations of PRP include the release of growth factors being closely linked with the clotting mechanism [
35] and the rapid release of growth factors upon activation with calcium chloride [
35] or thrombin [
13] (the activated form of PRP is called platelet-rich plasma gel) [
13]. PRF, the second-generation platelet concentrate, addresses these limitations by removing anticoagulants; thus, no activation with calcium chloride or thrombin is needed. It is created by spinning the patient’s blood in a centrifuge to create a thicker substance with a higher concentration of platelets and growth factors [
35]. PRF has shown a slower release of growth factors compared to PRP, potentially leading to longer-lasting effects and better outcomes [
35]. While both PRP and PRF are effective in promoting tissue healing, some studies suggest that PRF may have an advantage in promoting tissue regeneration and improving long-term outcomes [
13,
35]. However, PRF requires a more complex preparation process, which may result in higher costs and longer treatment times [
35].
On the day of the surgical procedure, a peripheral blood sample is collected from the patient. The blood can be transferred into a sterile syringe for direct injection onto the macular hole after fluid–air exchange or placed in a 10 mL test tube [
12,
13] for the preparation of platelet concentrates. The test tube can contain anticoagulants, such as citrate phosphate dextrose adenine (CPDA) [
12], acid citrate dextrose (ACD) [
12], or sodium citrate [
12,
13]. Blood in the test tube is then centrifuged to separate the various blood components and obtain the platelet concentrate [
13]. The PRP, located in the middle of the three distinct visible layers (platelet-poor plasma, PRP, and red blood cells) [
12,
13], is collected in a sterile syringe. If the centrifugation is performed on the test tube containing whole blood without an anticoagulant, then a clot containing platelets, known as PRF, will form [
12]. Conversely, centrifugation using a test tube containing an anticoagulant will result in liquid PRP. To activate the liquid PRP into PRP gel, an activator like calcium chloride, calcium gluconate, thrombin, batroxobin, or others can be added [
12].
This procedure (
Figure 10) involves a standard 3-port small-gauge vitrectomy. The adequacy of the previous ILM peeling is assessed after staining with biological dye, and fluid–air exchange is then performed. Depending on the type of blood product used, the next steps vary: with whole blood, 3–4 drops of blood are instilled inside the macular hole using a 25–30 gauge needle as illustrated in
Figure 11; with PRP, 3–4 drops of PRP are used [
12,
13]; with PRP gel, an activator is used to transform PRP into a gel before insertion; and with PRF, the fibrin clot is shaped and inserted into macular hole using forceps [
12,
13]. Fluid–air exchange is conducted and at the end of the procedure, the air is replaced with gas endotamponade. The patient is instructed to maintain a supine position for 1 h followed by a face-down position for 3–7 days [
12,
13]. The success rate of macular hole closure with this method ranges from 91 to 100% [
41,
42,
43]. Recent observations have indicated that using gas as endotamponade may not be essential in ensuring the stability of a fibrin clot in the macular hole. This finding is crucial for patients who may have difficulty maintaining a specific posture post-surgery.
4.7. Autologous Retinal Transplantation
Autologous retinal transplantation involves the harvesting of a small piece of healthy retina from a patient’s peripheral retina and transplanting it onto the macular hole area. This method aims to promote the closure of the macular hole by providing healthy retinal tissue to bridge the gap and facilitate healing. Retinal grafts have technical advantages in terms of size customization and ease of surgical handling, reducing trauma during surgery [
44]. Autologous retinal transplantation offers anatomical and functional benefits, with evidence suggesting that the graft can integrate into the host retina and improve visual function [
44,
45,
46,
47]. Ectopic synaptogenesis, retinal progenitor cells, and material transfer are potential mechanisms for retinal functional rehabilitation after transplantation [
44]. However, further research is essential to fully elucidate mechanisms involved in the integration of retinal grafts.
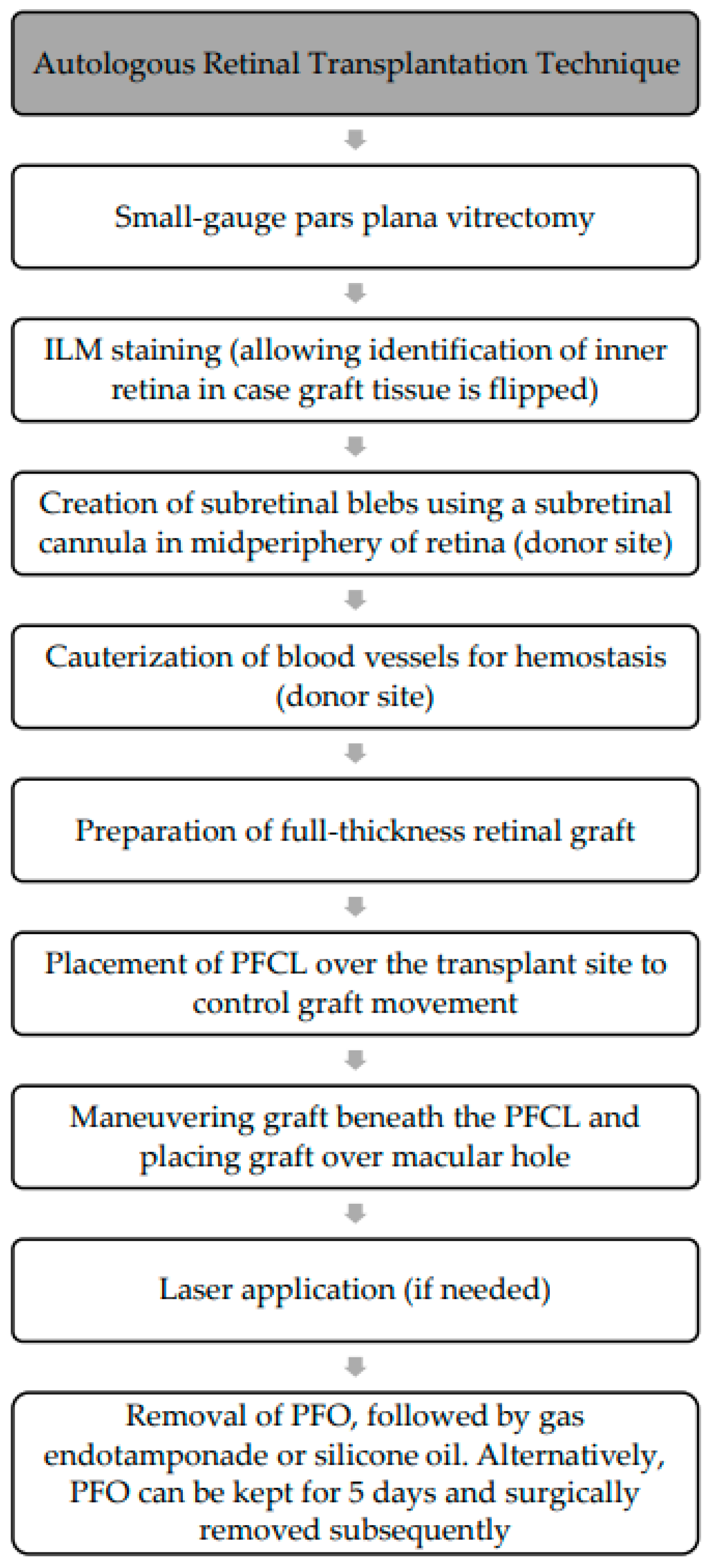
The procedure (
Figure 12) involves standard 3-port small-gauge vitrectomy with Chandelier endo-illumination for the bimanual components. Staining the ILM of the peripheral retina with a biological dye assists in identifying the inner retina in case the graft tissue is inadvertently flipped. A 39-gauge subretinal cannula attached to a viscous fluid control module is used to create a bleb of subretinal fluid in the midperipheral region as illustrated in
Figure 13a, followed by cauterization of blood vessels to achieve hemostasis. A bimanual technique with 25-gauge curved scissors and forceps is then used to create a 2–3 mm diameter macular graft, leaving a small attachment to prevent graft displacement. The PFCL is placed over the transplant site for control during graft movement. The graft is dissected, maneuvered beneath the PFCL, and placed over the macular hole as illustrated in
Figure 13b, and laser may be used to demarcate the harvest site and aid in graft adhesion. The PFCL can be left in place for 5 days post-operatively, and the patient is instructed to maintain a prone (face-up) position to promote adhesion; however, a second operation is needed to remove the heavy liquid. Alternatively, the PFCL can be removed and replaced with gas endotemponade or silicone oil. The success rate of autologous retinal transplantation in the treatment of refractory macular holes has been reported to range from 66.7 to 100% [
4,
46], and it has led to a number of patients experiencing improvements in functional vision, with a study showing a 3-line gain in vision in 36.6% of patients [
46]. Some studies have even reported a restoration of central vision in patients who previously had severe vision loss due to refractory macular holes.
5. Discussion and Expert Commentary
The decision to undergo reoperation for rFTMHs is supported by promising results from various surgical procedures. However, the lack of randomized controlled trials and large prospective studies makes it difficult to directly compare techniques and establish an evidence-based consensus on the best approach. Current studies have limitations such as methodological heterogeneity, retrospective designs, and small sample sizes. Based on the data presented in this review and real-life clinical experience, in cases of unsuccessful closure of primary macular hole or rFTMH, there is a strong trend towards repeated surgical repair in order to achieve good anatomical closure and visual improvement.
It is essential to carefully select the most suitable surgical technique in order to ensure a successful surgery for macular hole closure and visual recovery. This selection should be based on factors such as the patient’s specific factors and the characteristics of the macular hole, as illustrated in
Figure 14.
Post-operative posturing ability is first assessed in patients with rFTMH, as many surgical techniques require strict post-operative posturing. For patients unable to posture, a surgical technique using a macular plug with autologous platelet concentrates is preferred as it does not require post-operative endotamponade, eliminating the need for post-operative posturing. However, in institutions where plasma platelet concentrate preparation is limited, surgical options used to repair recurrent large macular hole (≥400 um) can be adopted with silicone oil injected as endotamponade. In patients who can posture post-operatively, residual ERM and ILM are assessed with wide-field optical coherence tomography. In cases where there is inadequate ERM or ILM peeling, the patient may undergo repeated vitrectomy with the extension of ILM peeling, the inverted ILM flap procedure, the ILM free flap cover procedure, or the ILM plug procedure, along with gas tamponade at the end of the procedure and face-down posture.
For patients with adequate ILM peeling (complete ILM peeling from arcade to arcade), the size of the macular hole is assessed. If the hole is ≤400 um, macular subretinal fluid injection to induce macular detachment, followed by fluid–air exchange and gas endotamponade, may be considered, while holes ≥400 um may require more complex surgical techniques such as anterior lens capsular transplantation, macular fibrin plug with autologous platelet concentrates, human preserved amniotic membrane, or autologous retinal transplantation. For phakic patients with cataracts, combined phacoemulsification with lens implantation and macular hole repair with anterior lens capsular implantation and gas endotamponade is recommended. In all phakic patients with cataracts, regardless of the type of macular hole surgical technique, cataract operation is recommended, as most procedures involve gas injection as endotamponade for a significant period of time, and the gas injected will definitely induce cataract progression. A second operation with cataract surgery after macular hole repair could potentially cause CMO secondarily to post-operative inflammation, and CMO could potentially lead to the reopening of the macular hole.
In pseudophakic patients, posterior lens capsular transplantation on the macular hole is not recommended due to lower success rates compared with anterior lens capsular transplantation. We do not have direct clinical experience in allogenic lens capsular transplantation, but we suggest considering contralateral anterior lens capsular transplantation as a potential option. Surgical treatment options such as macular fibrin plug and amniotic membrane cover techniques are preferred over autologous retinal transplantation in pseudophakic patients with large macular holes, with the macular fibrin plug and amniotic membrane cover showing better anatomical restoration and visual improvement compared to autologous retinal transplantation. However, when comparing macular fibrin plug and amniotic membrane cover techniques, the macular fibrin plug with greater regenerative properties is able to trigger outer retinal zone regeneration with ellipsoid line regeneration compared to preserved human amniotic membrane. In addition, the semi-transparency of the amniotic membrane is not optically clear, and when transplanted in the center part of the macular, it can hinder light transmission and potentially lead to poorer vision.
Despite significant advances in surgical techniques, the management of rFTMHs remains challenging. Future research should prioritize elucidating the pathobiology of macular hole persistence, including biomechanical factors like retinal stiffness and Müller cell dysfunction, as well as molecular pathways involving growth factors (VEGF, PDGF) and extracellular matrix remodeling, which could lead to pharmacologic adjuncts enhancing glial migration. Surgical optimization requires comparative studies of scaffold materials (ILM flaps, amniotic membrane, lens capsules), improved intraoperative traction management tools, and innovations in tamponades. Personalized approaches should integrate predictive models based on hole characteristics and biomarkers to guide technique selection, while hybrid strategies (e.g., ILM flap + platelet-rich fibrin) may synergize structural and biological repair. Addressing evidence gaps necessitates large multicenter trials with standardized outcomes and long-term (>5 years) functional data. Emerging technologies like intraoperative real-time OCT guidance and regenerative therapies (stem cell-derived patches, engineered scaffolds) hold promise for revolutionizing rFTMH repair. By bridging pathological insights and technical innovation, the next era of macular hole surgery can achieve higher success rates for complex cases.
6. Conclusions
In conclusion, these advanced surgical techniques offer a promising solution for patients with rFTMHs, providing ophthalmologists with more options to effectively treat these challenging cases and achieve anatomical closure and good functional success in visual improvement. However, it is important to tailor the choice of procedure to each patient’s unique circumstances, taking into account factors such as their ability to maintain the required posture post-surgery, the expertise of the surgeon, and the availability of specialized tools and technology. Personalized treatment planning based on these considerations will help optimize the risk–benefit balance for each individual case.