Abstract
(1) Background: It is estimated that 10% of dry eye disease (DED) occurs in patients with Sjogren’s syndrome (SS-DED) and represents a challenge when it comes to treatment. Both innate and adaptive immunity participate in the pathogenesis of SS-DED. Previous studies suggest that Th1 and Th17 cell immune responses are the main actors associated with the pathogenesis of this disease. Ocular surface mucins play a fundamental role in ocular surface homeostasis. In particular, the main transmembrane mucins, MUC1, MUC4 and MUC16, are dysregulated in DED and could be involved in the activation of pro-inflammatory cytokines at the ocular interface. Thus, the objective of this work was to analyze mucin and cytokine expression in ocular surface (OS) damage and correlate it with clinical symptoms.; (2) Methods: 18 patients with SS-DED and 15 healthy controls were included in the study. Samples of conjunctival cells were obtained through cytology impression. RNA was extracted from the collected samples and used to determine the expression of MUC1, 4 and 16 by qRT-PCR. Pro-inflammatory cytokines associated with DED pathogenesis (IL17 and IL-22) were also evaluated. The results were contrasted with the clinical findings on examination of the patients. (3) Results: We observed a significant increase in the expression of MUC1 and MUC4 in patients with SS-DED. MUC4 significantly correlated with both lower production and stability of the tear film, as well as greater superficial keratopathy. On the other hand, MUC1 and MUC16 were positively correlated with the presence of more severe DED symptoms. However, we could not reproduce an increase in IL-17 and IL-22 in DED patients as previously reported; (4) Conclusions: This work constitutes an approach to understanding how the gene expression of transmembrane mucins associates with SS-DED symptoms and clinical signs.
Keywords:
dry eye disease; Sjogren’s syndrome; transmembrane mucins; MUC1; MUC 4; MUC 16; pro inflammatory cytokines; IL 17; IL 22 1. Introduction
Dry eye disease (DED) is not only one of the most frequent ocular pathologies but also has the greatest impact on the quality of life of patients in the world today [1]. In addition, changes in habits, such as more extended use of screens [2], greater pollution [3] and population aging, mean that the projection is increasing. It is estimated that 10% of DED occurs in patients with Sjogren’s syndrome (SS-DED) [4]. This can be primary or secondary, which is associated with other connective tissue diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) or systemic sclerosis (SSc) [5]. SS-DED is of particular interest because it is generally severe and difficult to treat [5]. Despite its high prevalence, there is still a lack of knowledge of the molecular mechanisms involved in SS-DED.
Ocular surface (OS) mucins play a fundamental role in ocular surface homeostasis [6]. Mucin expression is dysregulated in DED and could be involved in the activation of pro-inflammatory cytokines at the OS [7]. However, the current knowledge on the expression of mucins at the OS in SS patients remains scarce and conflicting. In particular, in SS, the evidence on modifications in mucin expression is also contradictory. On the one hand, Argüeso et al. observed no significant changes in MUC1 and MUC4 gene expression in tears from patients with SS-DED, while MUC5AC mRNA levels were decreased when compared to tears from normal individuals [8]. On the other hand, Caffery et al. observed an increase in the expression of MUC1 and MUC16 in patients with SS autoimmune DED when compared to non-SS-DED and normal individuals [9,10]. In contrast, Jones et al. found a decrease in conjunctival MUC1 expression in patients with non-SS DED [11].
Both innate and adaptive immunity participate in the pathogenesis of SS-DED [12,13]. Indeed, previous studies reported that both Th1 and Th17 immune responses are associated with the pathogenesis of this disease [13] and are fundamental in maintaining the integrity of some epithelial barriers. For example, IL-17 stimulates the formation of tight junctions [14,15]. In addition, IL-22 has an important role in cell survival and proliferation [16], while IL-17 stimulates the production of antimicrobial peptides and chemokines that attract leukocytes when the epithelial barrier is penetrated [14]. Th1 and Th17 immune responses have been detected in a damaged ocular epithelium [17] and are significantly increased in the tears of patients with DED [17]. Furthermore, IL-17 and IL-22 levels have been reported to positively correlate with the OS Disease Index (OSDI) clinical questionnaire score and keratopathy score, while they negatively correlate with the tear film breakup time (TBUT) and the Schirmer I test [17]. This leads to an association of IL-17 and IL-22 levels with disease progression [17]. However, the favorable role of IL-17 in goblet cell proliferation has also been described, increasing mucus production in the tear [18]. Thus, the role of Th17 in DED is still not fully elucidated.
In this work, we aimed to investigate the levels of transmembrane mucins MUC1, MUC4 and MUC16, together with IL-17 and IL-22 levels, in the OS of patients with either primary or secondary SS-DED. Our study sheds further light on the role of the main transmembrane mucins (MUC1, 4 and 16) and the Th17 immune response in SS-DED.
2. Materials and Methods
2.1. Patient Recruitment
This study was approved by the Ethics Committee of the Hospital de Clínicas, Faculty of Medicine, University of the Republic, in Montevideo, Uruguay. All the patients were recruited in the autoimmune disease and ocular pathology clinic of the Department of Ophthalmology in conjunction with the Systemic Autoimmune Diseases Unit belonging to said hospital during the period between December 2017 and December 2019. Written informed consent was obtained from all the patients involved in the study. All the samples obtained from patients and healthy controls were numbered from 1 to 33 to identify the individuals and the clinical information collected and associate it with their biological samples, ensuring anonymity in the handling of data and clinical samples. Two groups of patients were obtained: (1) dry eye derived from primary (PSS-DED) or secondary (SSS-DED) Sjogren’s syndrome; (2) healthy controls without autoimmune diseases, without ocular pathologies, of the same age group as the patients, free of infectious diseases.
The inclusion criteria for the diagnosis of DED were the following: the presence of DED symptoms for at least the last three months; demonstration of keratopathy on the Oxford Scale greater than or equal to 1 [19]; a TBUT of less than 10 s; a Schirmer I test result of less than 13 mm; a score on the OSDI questionnaire [20] equal to or greater than 13 points; and a diagnosis of SSP or SSS. The criteria for the diagnosis of SS were based on those defined by the American–European Consensus. In the diagnosis of SSP, four points of the criteria detailed in references [19,20] (they must include histopathological and/or serological evidence). For the diagnosis of SSS, in addition to the diagnosis of SS, the presence of positive antinuclear antibodies, positive rheumatoid factor or the presence of antibodies associated with Ssc (anti-centromere, anti-Scl-70, anti-RNA polymerase III) was required.
The exclusion criteria were the following: patients under 18 years old, presenting anatomical anomalies (due to trauma or previous surgery); a history of eye surgery in the last 3 months; being on topical eye treatment (except artificial tears); having any ocular pathology in the last 3 months that affects the ocular surface (conjunctivitis, chalazion, etc.) or use of contact lenses; a history of head/neck radiotherapy; presenting systemic infections or active neoplastic involvement; and patients with concomitant use of anticholinergic drugs, who were excluded since these could confuse findings and/or symptoms.
2.2. Clinical Assessment of Patients
2.2.1. Tear Stability Test
The stability of the tear film was determined by measuring the time it took for the tear film over the cornea to break. Fluorescein staining was applied (2% fluorescein solution diluted in physiological saline) without topical anesthesia, and the time elapsed until the onset of signs of tear meniscus rupture was determined. The normal TBUT is equal to or greater than 10 s.
2.2.2. Tear Production Assessment
Tear production was assessed using the Schirmer type I test (without topical anesthesia), which was carried out by placing filter paper graduated in millimeters at the level of the lower palpebral fornix and measuring how wet it was after 5 min. A tear production greater than 20 mm is considered normal, a result less than 13 mm is considered insufficient, and an indeterminate or probably insufficient result is between 13 and 20 mm. For the 99% confidence interval, the mean values of tear secretion in individuals who were expected to have normal secretion were greater than 10 mm in 2 min and greater than 13 mm in 5 min (significance level less than 0.01).
2.2.3. OS Damage Assessment
Assessment of corneal and conjunctival surface damage was performed by applying lissamine green (1.5 mg strips of lissamine green (Hub Pharmaceuticals, LLC., Rancho Cucamonga, CA, USA). The strip was moistened with 1–2 drops of physiological saline and was subsequently applied at the level of the conjunctival fornix. Lissamine green only stains cells when their membrane is damaged, regardless of the integrity of the mucus that covers them. We used the Oxford Scale to grade the damage described in [19].
2.2.4. Dry Eye Symptom Assessment
The OSDI (Ocular Surface Disease Index) questionnaire [20,21], standardized for patients with DED, grades the severity of dry eye based on the answers to a set of different questions about the interference of symptoms in different activities. Symptoms with a score greater than or equal to 13 and less than 22 are considered mild dry eye, moderate is from 23 to 32, and severe is greater than 33.
2.3. Obtention of the Conjunctival Samples
Conjunctival epithelial cells were obtained using the impression cytology technique [19,20]. To this end, a 5 mm temporal ocular conjunctiva after application of topical anesthesia (0.5% tetracaine, Alcon China Ophthalmic Product Company Ltd., Beijing, China) was obtained. The two membranes from each patient were placed in sterile 1.5 mL tubes containing 700 µL of cell lysis buffer (Qiagen, Germantown, MD, USA). The tubes were stored at −80 °C to be used in subsequent tests.
2.4. Messenger RNA (mRNA) Extraction Method
mRNA was extracted using the RNeasy Micro Kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions.
2.5. Gene Expression by qRT-PCR
Total mRNA was quantified using a NanoDrop, and cDNA was subsequently synthesized using the SensiFAST cDNA Synthesis Kit (Bioline, London, UK), following the supplier’s recommendations. A reaction mix that included TransAmp Buffer, the reverse transcriptase enzyme at 200 U/µL, and 1 µg of RNA was used to generate cDNA in a Biometra T Gradient thermocycler, using the following program: 25 °C for 10 min, 42 °C for 15 min, 48 °C for 15 min and 85 °C for 5 min. For real-time PCR, the samples were analyzed in an Eco Real-Time PCR (Illumina, San Diego, CA, USA) using Fast SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA). The assays were performed using the Illumina Eco qPCR Kit (model 1010180, San Diego, CA, USA). A control without cDNA was included in all the assays to assess the contamination of the reagents used. The primers used in this study are listed in Table 1. The amplification conditions were 10 min at 95 °C, 15 s at 95 °C, 15 s at 62 °C and 15 s at 72 °C, with a final extension of 10 min at 95 °C. The melt curve consisted of cycles of 15 s at 95 °C, then 15 s at 55 °C and 15 s at 95 °C. Quantitative real-time PCR results were performed by the comparative threshold cycle method and normalized with gapdh as an internal control. The results were expressed as the relationship between each gene of interest and gapdh expression.

Table 1.
Primers used in RT-PCR in this study.
2.6. Statistical Analysis
The results were analyzed using a Student’s t-test with GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Correlation analyses were performed using simple linear regression. The results were considered significantly different when p < 0.05.
3. Results
3.1. Patient Population and Analysis of the Ocular Surface
A total of 18 patients with SS-DED along with 15 healthy controls were selected. All the patients and controls were women between 27 and 83 years old. The demographic data of age and sex are shown in Table 2. There were no significant differences in the age between the two groups (p = 0.923, p > 0.05). As expected, significant statistical differences (p < 0.05) were found between the two groups in the OSDI, TBUT, Schirmer I and Oxford Keratopathy Scale scores (Table 2).

Table 2.
Demographic and clinical data of the individuals recruited in this study.
The selected group of patients consisted of 50% with SSP and 50% with SSS, either secondary to SLE in four patients, RA in three patients or SSc in the remaining two patients. When comparing the clinical characteristics of the groups with SSP and SSS, we observed no significant differences in the OS parameters evaluated except for the result of the Schirmer I test, which was significantly higher, although still clearly below normality in the group with SSS. As expected, an inverse correlation was observed between TBUT, the OSDI score and the degree of superficial keratopathy [19,20]. In turn, the TBUT was significantly positively correlated with the result of the Schirmer I test when we considered all the recruited individuals, both controls and patients with DED (the higher the OSDI score, the greater the degree of keratopathy, the lower tear production estimated with the Schirmer I test and lower tear stability measured by TBUT). All these correlations were statistically significant with a value of p < 0.05.
3.2. Gene Expression of MUC1, MUC4 and MUC16 in Conjunctival Impression Samples in Patients with SS-DED versus Healthy Controls
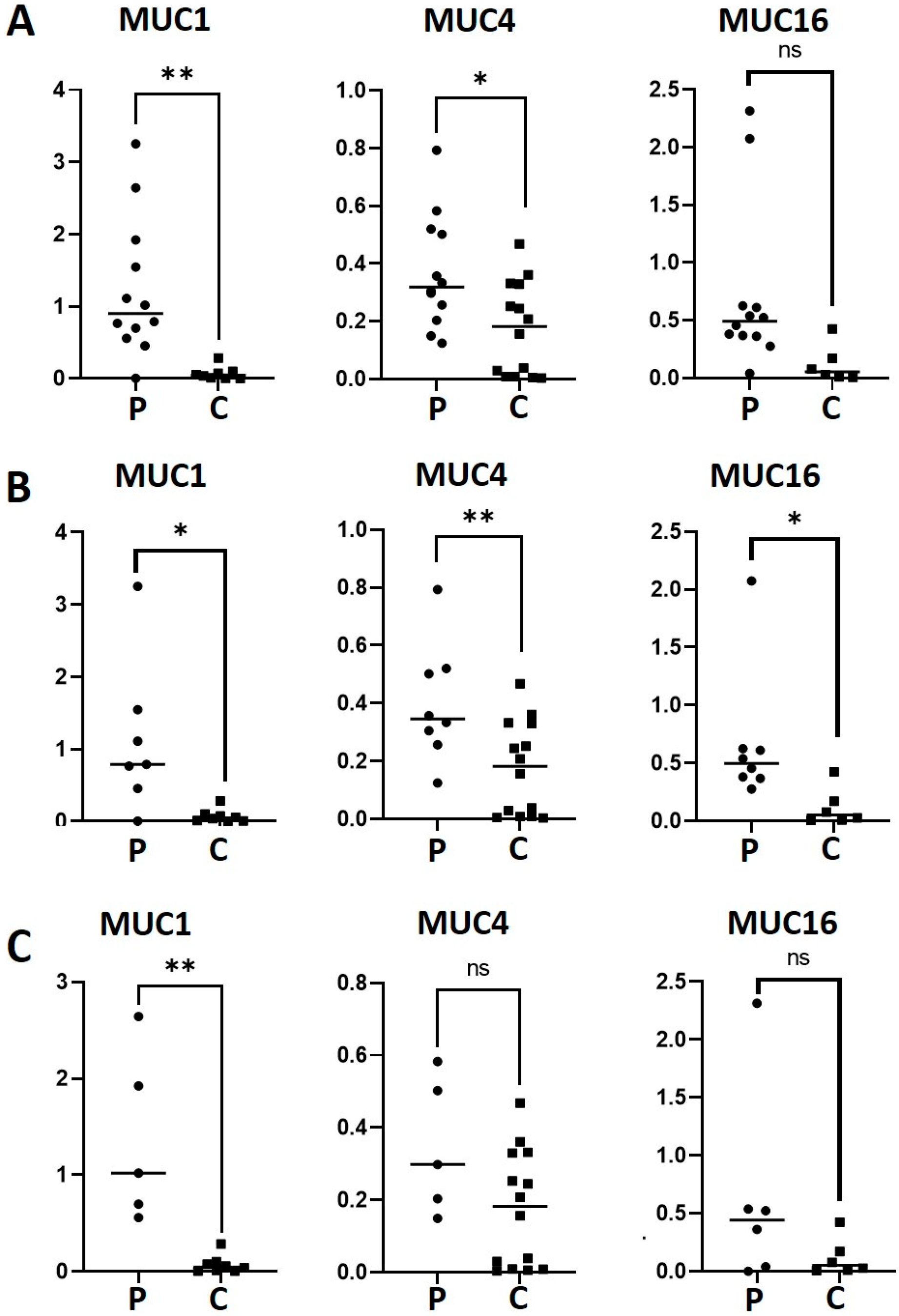
We evaluated the gene expression of MUC1, MUC4 and MUC16 in patients and controls by RT-qPCR (Figure 1A). A statistically significant difference was observed with a 17-fold higher expression of MUC1 (p = 0.0032) and 2.11-fold higher expression of MUC4 (p = 0.0104) in the patients compared to the healthy controls. However, there was a non-statistically significant increase in MUC16 expression (p = 0.0635) in the patients compared with the controls. We also analyzed the expression of mucins in the patients with SSP and SSS in relation to the healthy controls (Figure 1B,C). MUC1 expression was 15.7 and 19 times higher in the SSP group (p = 0.0136) and SSS (p = 0.0015) than the controls, respectively. MUC4 expression in the SSP group (p = 0.0095) was 2.29 times higher in relation to the controls, while no significant difference was found when comparing patients with SSS and the controls (p = 0.0641). Interestingly, MUC16 did demonstrate a significant difference (5.56 higher) between SSP patients and controls (p = 0.0465). Of note, the higher variation in MUC gene expression found in the patients compared to the controls could be attributed to the overlapping with other diseases or treatments.
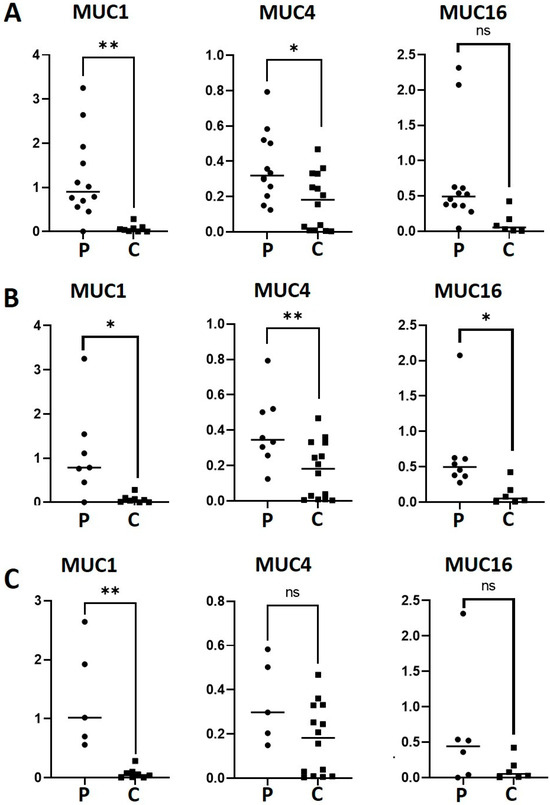
Figure 1.
mRNA levels of MUC1, MUC4 and MUC16 in patients with SS-DED (A), PSS-DED (B) and SSS-DED (C). mRNA was extracted from conjunctival cells obtained by conjunctival impression and amplified by RT-qPCR. Asterisks correspond to * p < 0.05 and ** p < 0.01; ns = no significant difference between the groups. The y-axis indicates the mucin fold expression in relation to gapdh. Squares correspond to healthy controls and circles to patients with DED.
3.3. Correlation of the Expression of MUC1, MUC4 and MUC16 in SS-DED and Controls
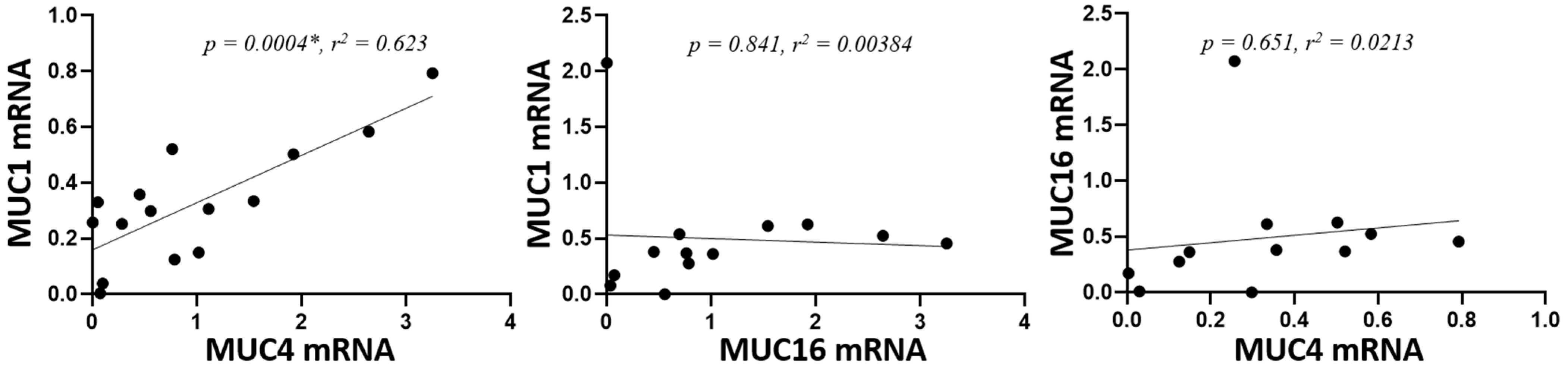
Next, we analyzed whether there was a statistical correlation in the gene expression of the studied mucins. A statistically significant correlation was observed between the expression of MUC1 and MUC4 (p = 0.0004), but not between MUC1 or MUC4 and MUC16 (p = 0.84 and p = 0.651, respectively) (Figure 2).
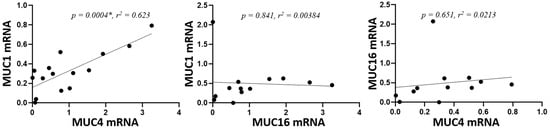
Figure 2.
Correlation of mucin mRNA levels. mRNA was extracted from conjunctival cells obtained by conjunctival impression and amplified by RT-qPCR (* = p < 0.05). The axes indicate the mucin fold expression in relation to gapdh.
3.4. Relationship between Mucin Gene Expression and Clinical Parameters of DED
3.4.1. TBUT and Mucin Expression
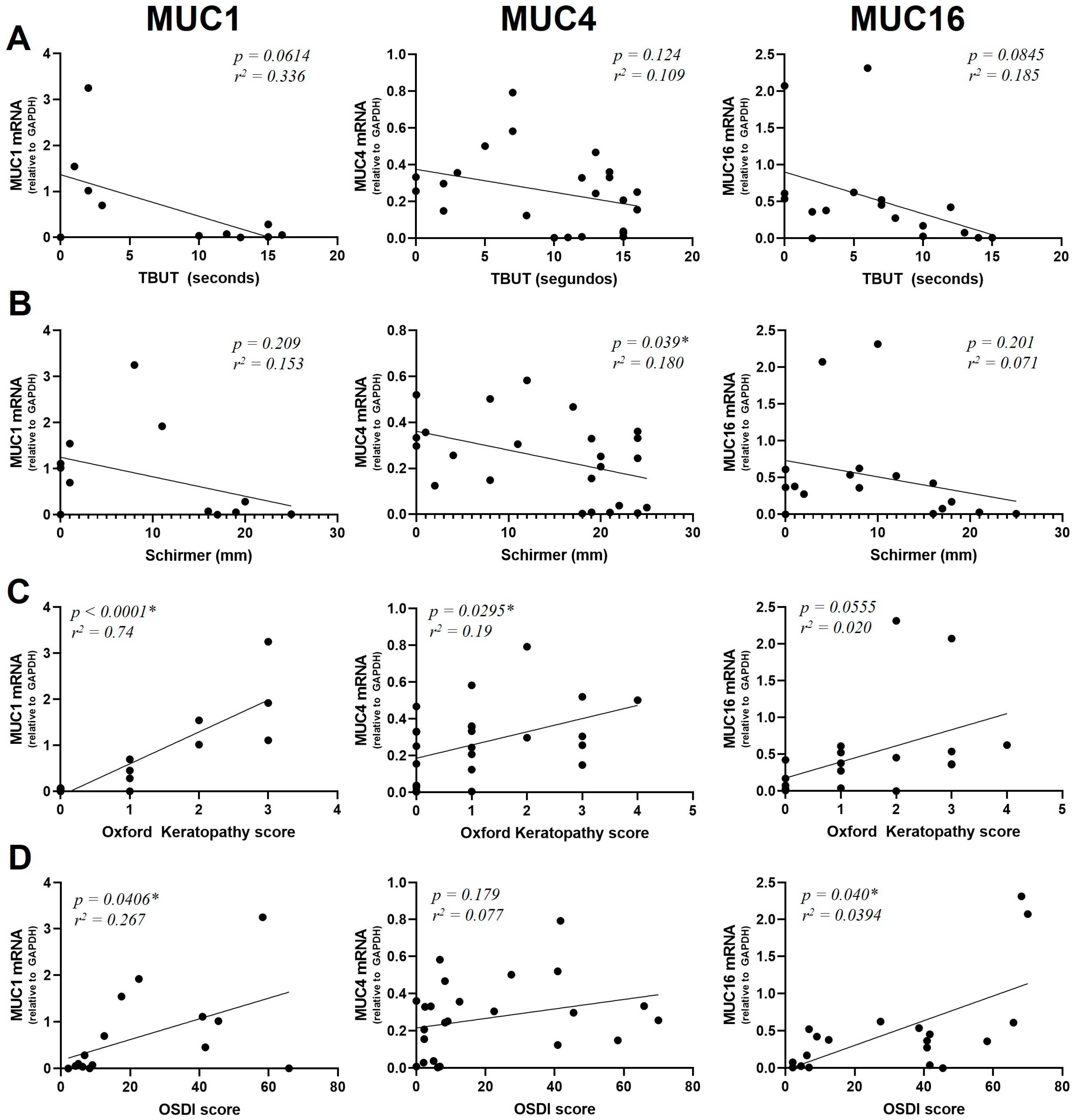
We were interested in analyzing how mucin gene expression was related to the patients’ OS parameters. Although TBUT was not significantly correlated to mucin expression (Figure 3A), a trend towards a negative correlation between mucins and TBUT was observed. In the case of MUC1: R2 = 0.34 and p = 0.0614; MUC4: R2 = 0.21 and p = 0.124; MUC16: R2 = 0.19 and p = 0.0845.
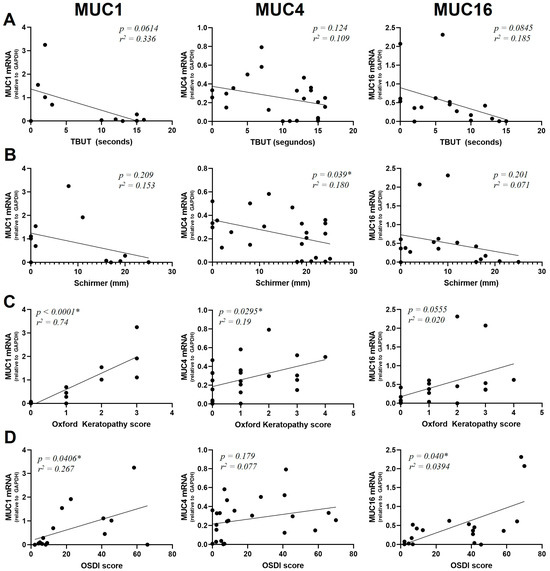
Figure 3.
Correlation of MUC1, MUC4 and MUC16 mRNA levels with TBUT score (A), Schirmer I (B), Oxford Keratopathy score (C) and OSDI score (D). mRNA was extracted from conjunctival cells obtained by conjunctival impression, extracted and amplified by RT-qPCR (* = p < 0.05). The y-axis indicates the mucin fold expression in relation to gapdh.
3.4.2. Schirmer I Test and Mucin Expression
A positive significant correlation between mucin expression and the Schirmer I test was found for MUC4 (R2 = 0.180 and p = 0.039) but not for MUC1 (R2 = 0.153, p = 0.209) or MUC16 (R2 = 0.0710, p = 0.301) (Figure 3B).
3.4.3. Superficial Keratopathy and Mucin Expression
MUC1 and MUC4 were expressed at higher levels in patients presenting a high degree of keratopathy (R2 = 0.74, p < 0.0001 for MUC1 and R2 = 0.19, p = 0.0295 for MUC4). In the case of MUC16, we found a statistically non-significant relationship between the degree of keratopathy and mucin expression (R2 = 0.2, p = 0.0553) (Figure 3C).
3.4.4. OSDI Score and Mucin Expression
MUC1 and MUC16 were found to have a significant positive correlation with the OSDI score (R2 = 0.267, p = 0.0406 for MUC1 and R2 = 0.394, p = 0.040 for MUC16). On the contrary, no significant correlation between MUC4 expression and the OSDI score was observed (R2 = 0.0770, p = 0.179) (Figure 3D).
3.5. Expression of IL-17 and IL-22 in SS-DED
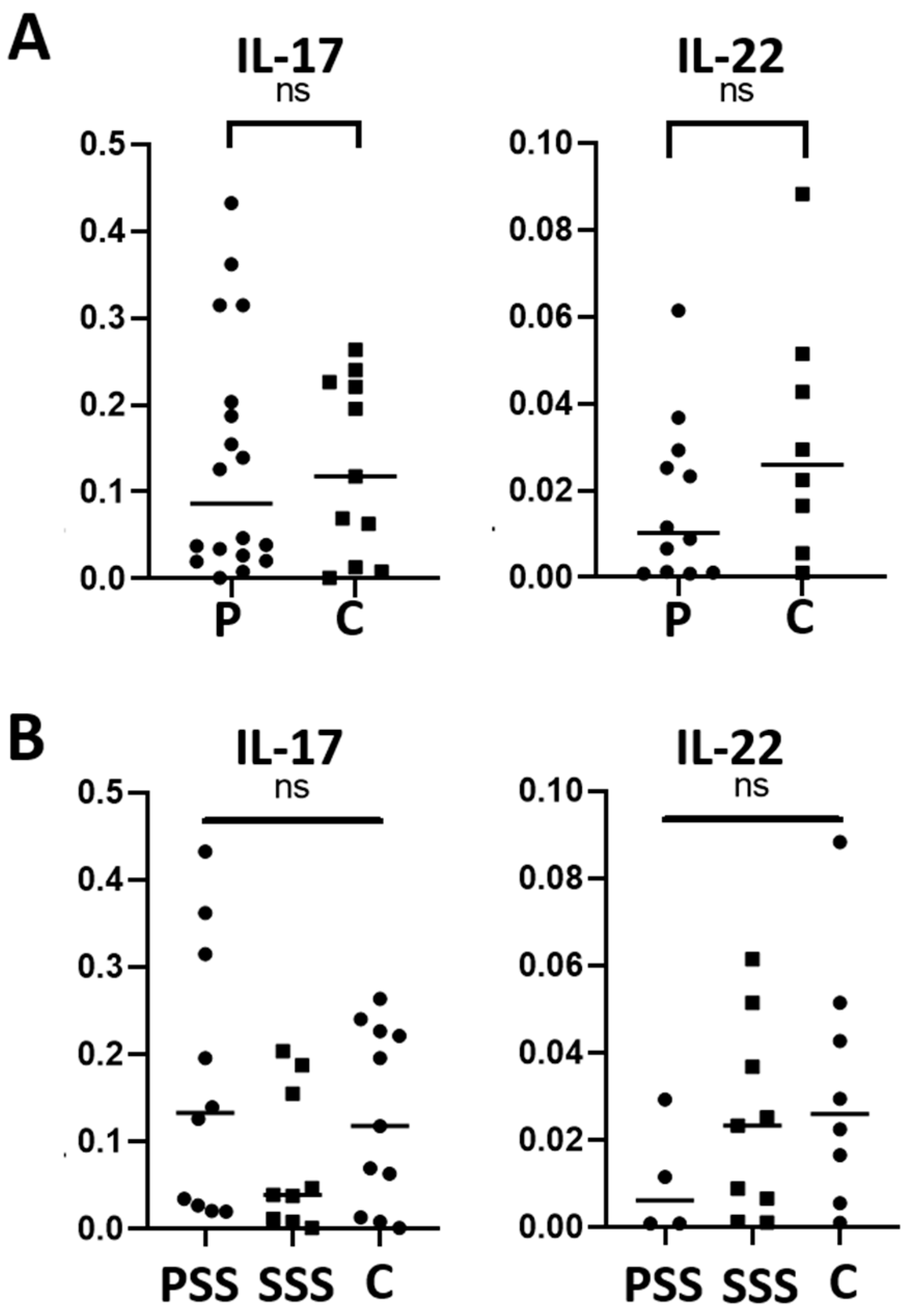
The expression of the pro-inflammatory cytokines IL-17 and IL-22 was also evaluated by RT-qPCR in the SS-DED patients and healthy controls, demonstrating to be similar between the two groups (p = 0.867 for IL-17 and p = 0.172 for IL-22) (Figure 4A). In addition, there was no significant difference when dividing the patients between SSS and PSS (Figure 4B).
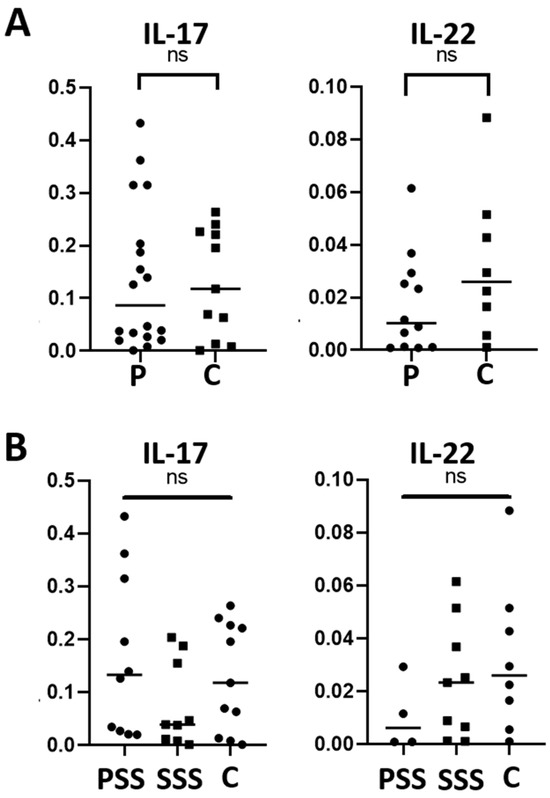
Figure 4.
IL-17 and IL-22 mRNA levels in conjunctival cells in patients with SS-DED and in healthy controls (A) and patients with SSS and PSS (B). Messenger RNA was extracted from conjunctival cells obtained using the conjunctival impression technique and amplified by RT-qPCR. The y-axis indicates the cytokine fold expression in relation to gapdh. ns = no significant difference between the groups.
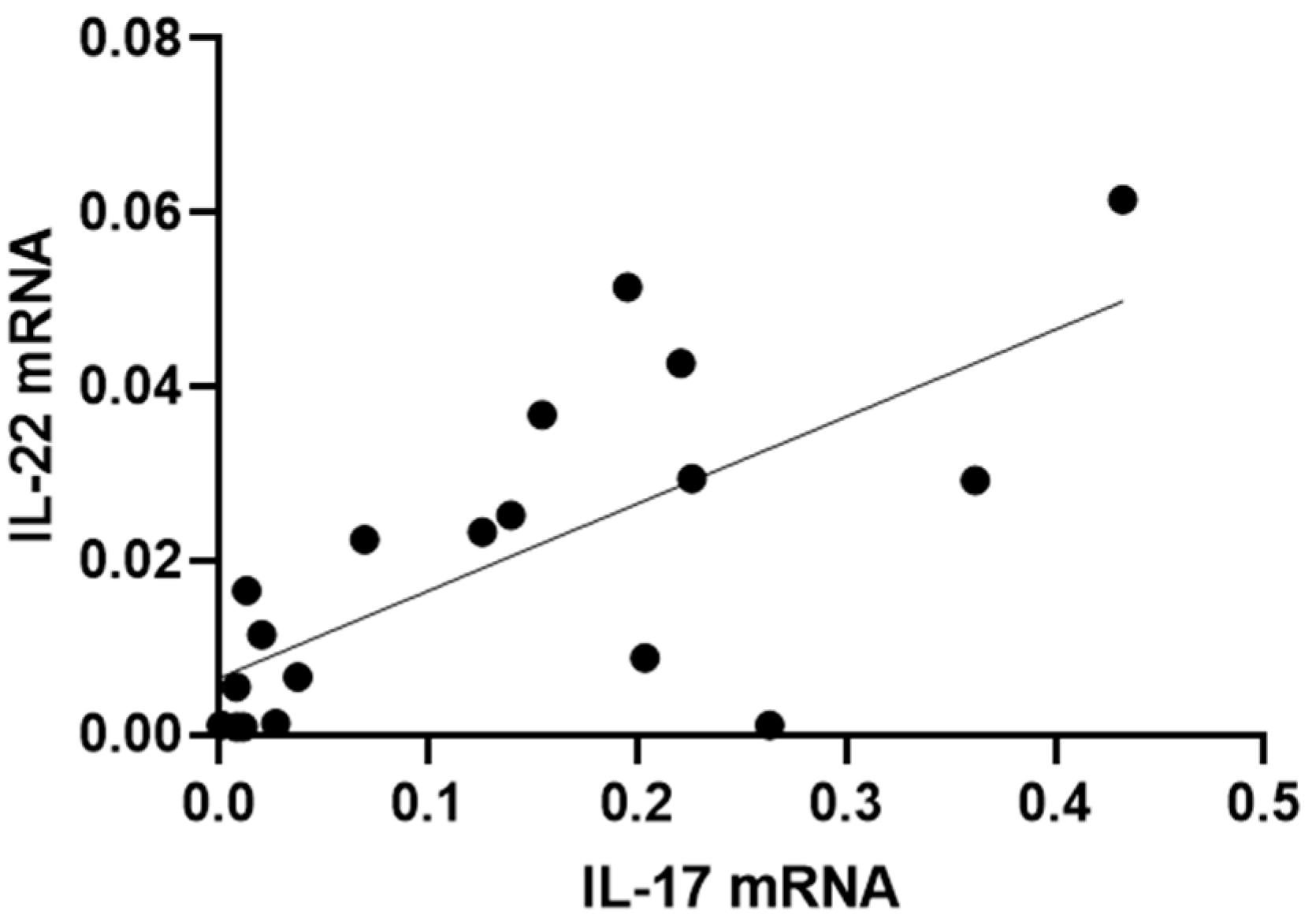
Furthermore, those patients with a higher expression of IL-17 also had a higher expression of IL-22 (R2 = 0.49, p = 0.0008) (Figure 5).
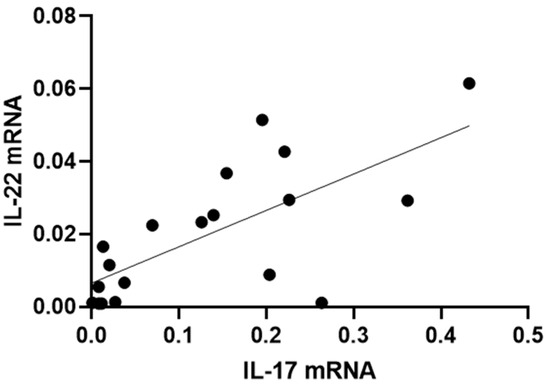
Figure 5.
Correlation between IL-17 and IL-22 mRNA levels in conjunctival cells in patients with SS-DED. Messenger RNA was extracted from conjunctival cells obtained using the conjunctival impression technique described in the Materials and Methods section.
3.6. Correlation of IL-17 and IL-22 with Clinical Parameters of Patients with SS-DED
The expression of IL-17 and IL-22 was then analyzed and correlated with the evaluated OS parameters in all the patients in the study. No significant correlations were observed between them and the OSDI, TBUT, Keratopathy or Schirmer test scores (p > 0.05).
4. Discussion
4.1. Differential Mucin Gene Expression in SS-DED
The current knowledge on the expression of OS mucins in patients with SS-DED remains scarce and conflicting, with some studies reporting similar expression of transmembrane mucins (MUC1, MUC4 and MUC16) [8] and others higher expression of these mucins [9] in tears from patients with SS-DED compared to healthy individuals. In our study, a significant increase in the expression of MUC1 and MUC4, as well as a trend towards an increase in MUC16 that was not statistically significant in all patients but was present when we analyzed the subgroup of patients with SSP, was found. In addition, a significant correlation was observed in MUC1 and MUC4 gene expression in patients with SS-DED. Eventually, a bigger sample size should be studied in order to statistically confirm these results.
Mucins are known for their protective role on epithelial surfaces and for trapping pathogens [22]. MUC1 is a membrane-bound mucin with a cytoplasmic tail, also expressed in secretory epithelial cells of the airways, gastrointestinal tract, and female reproductive tract [23]. Its expression is increased in inflammatory bowel syndrome [24] and fosters inflammatory conditions by activating the inhibitor of nuclear factor kappa beta (IKKb) and nuclear factor kappa beta (NF-kB) pathways [25]. In this sense, our results showing an increase in MUC1 expression could represent a response similar to other epithelia.
MUC4 is another membrane-bound mucin expressed in airway epithelial cells in a non-specific localization to basal, ciliated and goblet cells [26]. The published literature has reported that IL-4, IL-9 and neutrophil-secreted elastase increase MUC4 expression in airway epithelial cells [27,28,29]. Interestingly, MUC4 expression is increased in patients with colitis and in colorectal cancer [30]. Along the same line, MUC4−/− mice are resistant to the development of colitis, showing an upregulation in the expression of MUC2 and MUC3 [30].
MUC16 expression has also been observed to be upregulated in response to inflammatory factors, including LPS, IL-6, IL-8 and TNFα, in the reproductive epithelium of patients with ovarian cancer cells via NF-κB [31]. Interestingly, inflammatory stimuli such as IL-1α and IL-1β downregulate the expression and production of MUC16 by corneal epithelial cells [32]. In contrast, these cytokines, as well as IFNγ and TNFα, increase the shedding of MUC16 from cultured conjunctival epithelial cells [32]. As proinflammatory cytokine levels are upregulated in tears of patients with Sjogren’s syndrome [33], dysregulation of MUC16 could be a mechanism that contributes to the worsening of dry eye disease in these patients.
Interestingly, an increase in polypeptidyl-GalNAc-’Transferases is observed in cicatricial pemphigus, which is another autoimmune pathology [34]. These glycosyltransferases initiate the addition of O-glycans to the peptide backbone of mucins [35]. This suggests that the epithelial cells of the OS would have a compensatory response to the autoimmune attack by contributing to the synthesis of O-glycans on mucins to keep the surface moist at the level of the apical epithelium.
In the present work, we also observed that both MUC1 and MUC16, but not MUC4, were positively significantly correlated with a higher expression of dry eye symptoms. A positive correlation was seen between the expression of MUC1 and MUC4 and the grade of keratopathy on the Oxford Scale. In the case of MUC16, this relationship was marginally significant, with p = 0.055. The evaluation of a higher number of patients and healthy controls is needed in order to confirm these results.
The production of the aqueous component of tears, assessed using the Schirmer I test, was directly and significantly correlated only with MUC4. Those patients with lower tear production had higher levels of expression of this mucin. It is worth noting that patients with SSP had significantly lower tear production compared to patients with SSS. It is possible that in SSP there is greater involvement of the lacrimal gland in relation to SSS, thus generating less production of the aqueous component of the tear. However, this has not yet been assessed. The published literature has only established a decreased corneal sensitivity in patients with SS-DED versus non-SS-DED [36] but not differentiating between PSS and SSS; this would be interesting to further elucidate.
Interestingly, mucin expression was not correlated with TBUT in our study. However, it has been previously observed that certain patterns of tear breakage are associated with alterations in mucin expression. For example, lower TBUT has been observed in patients with lower conjunctival expression of MUC1, MUC16 and MUC20 in patients with non-SS DED [37]. The fact that our study was conducted in SS-DED could explain the obtained differences.
4.2. IL-17 and IL-22 Expression in SS-DED
Th17 cell polarization is initiated by dendritic cells in the lymph nodes draining the salivary and lacrimal glands, while in later phases of Sjogren’s disease, it also occurs locally in inflamed glandular tissue [38] in response to the production of TGFβ and IL-23. The ductal epithelial cells of these glands also produce cytokines important for Th17 polarization such as IL-1β [39]. Activated Th17 lymphocytes promote inflammation by stimulating the release of proinflammatory cytokines in exocrine glands, including IL-6 and TNFα, by secreting IL-17 and IL-22, which bind to their receptors expressed on epithelial and stromal cells [40]. IL-17 also promotes the production of matrix metalloproteases such as MMP-9, which is associated with acinar damage [41]. In the present work, we did not observe significant differences in IL-17 and IL-22 gene expression between SS-DED patients and healthy controls.
In a previous study, increased IL-17 and IL-22 levels were reported in patients with SS-DED when compared to non-SS-DED and healthy controls [17]. Moreover, the level of these cytokines positively correlated with the OSDI keratopathy questionnaire and negatively with the TBUT and the result of the Schirmer I test [17]. In our study, we observed, as expected, a strong correlation between the mRNA expression of both cytokines, that is, those patients with higher expression of IL-17 mRNA also had higher expression of IL-22, indicating a preferred Th17 profile. We also observed that those patients with higher expression of MUC16 had significantly higher expression of IL-22, in line with the study mentioned above [17]. However, we were unable to show a positive correlation between IL-17 and IL-22 with clinical parameters. We must highlight that the questionnaire used in the aforementioned study was not the OSDI questionnaire, and the keratopathy index was based on fluorescein staining as opposed to the lissamine green used in our study. This may generate a certain variability, although it should not be significant since they are valid alternatives for clinical evaluation. Furthermore, cytokines levels were evaluated by ELISA, which could also explain the different conclusions obtained in our study.
Last, we did not address in this study Th1-type cytokines such as IFNγ, which is involved in the death of goblet cells [42]. This plays a central role in conjunctival metaplasia and the decrease in the number of goblet cells. Furthermore, desiccation stress produces an increase in IFNγ, which in turn induces a decrease in the production of IL-13, which is associated with a protective role in CC [41]. It has been proposed that a constitutive expression of IL-13 would be a requirement for homeostatic control of goblet cells by stimulating the production of MUC5AC and MUC2 [43,44].
5. Conclusions
In this work, a significant increase in the expression of MUC1 and MUC4 was found in SS-DED patients when compared to controls. Furthermore, MUC4 significantly correlated with decreased tear production. On the other hand, MUC1 and MUC16 were positively correlated with the presence of more severe DED symptoms while MUC1 and MUC4 were significantly more expressed in patients with worse superficial keratopathy.
IL-22 gene expression correlated with the expression of MUC16, which in turn was increased in patients with worse DED symptoms. The results obtained in this work contribute to the further understanding of SS-DED and shed light on the use of mucins as biomarkers in DED.
Author Contributions
Conceptualization, T.F., M.E.V., E.C. and N.B.-B.; methodology, T.F., M.A., M.C. and N.B.-B.; software, N.B.-B.; validation, T.F. and N.B.-B.; formal analysis, N.B.-B. and M.C.; investigation, T.F. and N.B.-B.; resources, T.F. and N.B.-B.; data curation, T.F., M.A. and N.B.-B.; writing—original draft preparation, N.B.-B.; writing—review and editing, T.F., E.C. and M.A.; visualization, N.B.-B.; supervision, T.F., M.E.V. and N.B.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Universidad de la República.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital de Clínicas, Faculty of Medicine, University of the Republic, in Montevideo, Uruguay on 13 September 2017. The number of the protocol is 97-17, approved by the Ethical Committee of Hospital de Clínicas, UdelaR.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Data supporting reported results can be sent upon request to the corresponding author of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Papas, E.B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol. Opt. 2021, 41, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Mehra, D.; Galor, A. Digital screen use and dry eye: A review. Asia-Pac. J. Ophthalmol. 2020, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of Air Pollution and Weather on Dry Eye. J. Clin. Med. 2020, 9, 3740. [Google Scholar] [CrossRef]
- Akpek, E.K.; Amescua, G.; Farid, M.; Garcia-Ferrer, F.J.; Lin, A.; Rhee, M.K.; Varu, D.M.; Musch, D.C.; Dunn, S.P.; Mah, F.S. Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology 2019, 126, P286–P334. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjogren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Leger, A.J.S.; Caspi, R.R. Mucosal immunology of the ocular surface. Mucosal Immunol. 2022, 15, 1143–1157. [Google Scholar] [CrossRef]
- Argüeso, P.; Balaram, M.; Spurr-Michaud, S.; Keutmann, H.T.; Dana, M.R.; Gipson, I.K. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1004–1011. [Google Scholar]
- Caffery, B.; Heynen, M.L.; Joyce, E.; Jones, L.; Ritter, R., III; Senchyna, M. MUC1 expression in Sjogren’s syndrome, KCS, and control subjects. Mol. Vis. 2010, 16, 1720–1727. [Google Scholar]
- Caffery, B.; Joyce, E.; Heynen, M.L.; Jones, L.; Ritter, R., III; Gamache, D.A.; Senchyna, M. MUC16 expression in Sjogren’s syndrome, KCS, and control subjects. Mol. Vis. 2008, 14, 2547–2555. [Google Scholar]
- Jones, D.T.; Monroy, D.; Ji, Z.; Pflugfelder, S.C. Alterations of ocular surface gene expression in Sjögren’s syndrome. Adv. Exp. Med. Biol. 1998, 438, 533–536. [Google Scholar]
- Na, K.-S.; Hwang, K.-Y.; Lee, H.-S.; Chung, S.-H.; Mok, J.W.; Joo, C.-K. Wakayama symposium: Interface between innate and adaptive immunity in dry eye disease. BMC Ophthalmol. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Chivasso, C.; Sarrand, J.; Perret, J.; Delporte, C.; Soyfoo, M.S. The Involvement of Innate and Adaptive Immunity in the Initiation and Perpetuation of Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 658. [Google Scholar] [CrossRef]
- Dungan, L.S.; Mills, K.H. Caspase-1-processed IL-1 family cytokines play a vital role in driving innate IL-17. Cytokine 2011, 56, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Tan, X.; Sun, S.; Liu, Y.; Zhu, T.; Wang, K.; Ren, T.; Wu, Z.; Xu, H.; Zhu, L. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye 2014, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef]
- Oxford Grading System. Available online: https://www.aao.org/education/image/oxford-grading-system (accessed on 1 December 2023).
- Leanne Spiegle. How to Use Dry Eye Questionnaires in Your Practice. Available online: https://www.reviewofoptometry.com/article/how-to-use-dry-eye-questionnaires-in-your-practice (accessed on 1 December 2023).
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Bustos, N.A.; Ribbeck, K.; Wagner, C.E. The role of mucosal barriers in disease progression and transmission. Adv. Drug Deliv. Rev. 2023, 200, 115008. [Google Scholar] [CrossRef]
- Dhar, P.; McAuley, J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 117. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Buisine, M.-P.; Devisme, L.; Copin, M.-C.; Durand-Réville, M.; Gosselin, B.; Aubert, J.-P.; Porchet, N. Developmental mucin gene expression in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1999, 20, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Damera, G.; Xia, B.; Sachdev, G.P. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir. Res. 2006, 7, 39. [Google Scholar] [CrossRef]
- Damera, G.; Xia, B.; Ancha, H.R.; Sachdev, G.P. IL-9 modulated MUC4 gene and glycoprotein expression in airway epithelial cells. Biosci. Rep. 2006, 26, 55–67. [Google Scholar] [CrossRef]
- Fischer, B.M.; Cuellar, J.G.; Diehl, M.L.; Defreytas, A.M.; Zhang, J.; Carraway, K.L.; Voynow, J.A. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L671–L679. [Google Scholar] [CrossRef]
- Das, S.; Rachagani, S.; Sheinin, Y.; Smith, L.M.; Gurumurthy, C.B.; Roy, H.K.; Batra, S.K. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene 2016, 35, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Luo, N.; Liu, Q.; Liu, L.; Chen, D.; Cheng, Z.; Xi, X. Inflammatory signals induce MUC16 expression in ovarian cancer cells via NF-κB activation. Exp. Ther. Med. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F.; Jäger, K.; Worlitzsch, D.; Bräuer, L.; Schulze, U.; Schäfer, G.; Sel, S. Regulation of MUC16 by inflammatory mediators in ocular surface epithelial cell lines. Ann. Anat. Anat. Anz. 2008, 190, 59–70. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Argüeso, P.; Tisdale, A.; Mandel, U.; Letko, E.; Foster, C.S.; Gipson, I.K. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 86–92. [Google Scholar] [CrossRef]
- Cheng, P.W.; Radhakrishnan, P. Mucin O-glycan branching enzymes: Structure, function, and gene regulation. Adv. Exp. Med. Biol. 2011, 705, 465–492. [Google Scholar]
- Hoşal, B.M.; Örnek, N.; Zilelioğlu, G.; Elhan, A.H. Morphology of corneal nerves and corneal sensation in dry eye: A preliminary study. Eye 2005, 19, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yang, T.; Zhou, Y.; Ma, B.; Zhao, L.; Chen, J.; Qi, H. Comparison of mucin levels at the ocular surface of visual display terminal users with and without dry eye disease. BMC Ophthalmol. 2023, 23, 189. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Corneth, O.B.; Bootsma, H.; Kroese, F.G. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vakrakou, A.G.; Polyzos, A.; Kapsogeorgou, E.K.; Thanos, D.; Manoussakis, M.N. Impaired anti-inflammatory activity of PPARγ in the salivary epithelia of Sjögren’s syndrome patients imposed by intrinsic NF-κB activation. J. Autoimmun. 2018, 86, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Fogli, L.K.; Sundrud, M.S.; Goel, S.; Bajwa, S.; Jensen, K.; Derudder, E.; Sun, A.; Coffre, M.; Uyttenhove, C.; Van Snick, J.; et al. T Cell–Derived IL-17 Mediates Epithelial Changes in the Airway and Drives Pulmonary Neutrophilia. J. Immunol. 2013, 191, 3100–3111. [Google Scholar] [CrossRef]
- García-Posadas, L.; Hodges, R.R.; Li, D.; Shatos, M.A.; Storr-Paulsen, T.; Diebold, Y.; Dartt, D.A. Interaction of IFN-γ with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016, 9, 206–217. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Raince, J.K.; McClellan, A.J.; Shanmugam, K.P.; Pangelinan, S.B.; Volpe, E.A.; Corrales, R.M.; Farley, W.J.; Corry, D.B.; Li, D.-Q.; et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011, 4, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.T.; Coursey, T.G.; Corry, D.B.; De Paiva, C.S.; Pflugfelder, S.C. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4186–4197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).